Abstract
Advances in insulin therapy have made a positive contribution to improving disease management in both Type 1 and Type 2 diabetes. The development of insulin analogs with time–action characteristics has made it easier to mimic physiological insulin secretion. The parallel improvement in delivery devices has also made insulin therapy more convenient, flexible and acceptable. The inevitable progression of Type 2 diabetes means that the majority of those people will also require insulin therapy at some point in their disease course. Current treatment options are many; when to initiate insulin and which regimen to choose are among the major questions confronting physicians in today’s rapidly evolving environment. This article summarizes the current strategies for initiating and optimizing the use of the basal insulin analog, insulin glargine, in Type 2 diabetes, leading to the intermediate stage of insulin therapy with the introduction of meal-related, rapid-acting insulin analogs in a stepwise manner prior to a full replacement basal-bolus regimen.
(A) Residual radioactivity at the injection site after injection of NPH insulin or glargine. (B) Plasma (free) concentrations and (C) glucose infusion rates after sc. injection of ultralente insulin, NPH insulin, glargine and CSII.
CSII: Continuous subcutaneous insulin infusion; NPH: Neutral protamine Hagedorn; sc.: Subcutaneous; SE: Standard error.
Panel (A) is reprinted with permission from Citation[8]. © 2000 American Diabetes Association.
Panels (B) and (C) are reprinted with permission from Citation[4]. © 2000 American Diabetes Association.
![Figure 2. Bioavailability and bioactivity of basal insulins.(A) Residual radioactivity at the injection site after injection of NPH insulin or glargine. (B) Plasma (free) concentrations and (C) glucose infusion rates after sc. injection of ultralente insulin, NPH insulin, glargine and CSII.CSII: Continuous subcutaneous insulin infusion; NPH: Neutral protamine Hagedorn; sc.: Subcutaneous; SE: Standard error.Panel (A) is reprinted with permission from Citation[8]. © 2000 American Diabetes Association.Panels (B) and (C) are reprinted with permission from Citation[4]. © 2000 American Diabetes Association.](/cms/asset/629f032f-06fe-4fa5-b0a7-e87bdd6aa28f/iere_a_11207935_f0002_b.jpg)
FPG: Fasting plasma glucose; HBA1c: Glycosylated hemoglobin; NPH: Neutral protamine Hagedorn insulin; PPG: Postprandial plasma glucose.
Adapted with permission from Citation[22]. © 2009 American Diabetes Association.
![Figure 3. An algorithm for the initiation and intensification of insulin therapy in Type 2 diabetes.FPG: Fasting plasma glucose; HBA1c: Glycosylated hemoglobin; NPH: Neutral protamine Hagedorn insulin; PPG: Postprandial plasma glucose.Adapted with permission from Citation[22]. © 2009 American Diabetes Association.](/cms/asset/463173aa-9f21-4ded-aabb-34e510ebaf2e/iere_a_11207935_f0003_b.jpg)
HbA1c: Glycosylated hemoglobin; NPH: Neutral protamine Hagedorn.
Reproduced with permission from Citation[35]. © 2007 Elsevier, Inc.
![Figure 4. Associations between end-of-study HbA1c and rates of hypoglycemia (confirmed by blood glucose <3.6 mmol/l) during treatment with either NPH insulin or glargine in people with Type 2 diabetes (p = 0.021 between treatments).HbA1c: Glycosylated hemoglobin; NPH: Neutral protamine Hagedorn.Reproduced with permission from Citation[35]. © 2007 Elsevier, Inc.](/cms/asset/74cfc246-e2dc-479d-9f91-645f81c4d560/iere_a_11207935_f0004_b.jpg)
Effects of insulinization with once-daily glargine or three-times-daily lispro. *p < 0.0001; **p = 0.0041; ***p = 0.0137.
Reproduced with permission from Citation[45]. © 2008 Elsevier, Inc.
![Figure 5. Eight-point blood glucose profiles.Effects of insulinization with once-daily glargine or three-times-daily lispro. *p < 0.0001; **p = 0.0041; ***p = 0.0137.Reproduced with permission from Citation[45]. © 2008 Elsevier, Inc.](/cms/asset/b4e35d2f-abbe-4a84-8088-47c0c80f4713/iere_a_11207935_f0005_b.jpg)
*p < 0.05; **p < 0.0001.
Reprinted with permission from Citation[50] © 2009 Blackwell Publishing Ltd.
![Figure 6. Eight-point blood glucose profiles. Effects of adding one dose of glulisine to ongoing glargine.*p < 0.05; **p < 0.0001.Reprinted with permission from Citation[50] © 2009 Blackwell Publishing Ltd.](/cms/asset/18705084-0c01-49a6-98e6-be1391b70fc4/iere_a_11207935_f0006_b.jpg)
Effects of adding multiple doses of glulisine to ongoing glargine.
*p < 0.005; **p = 0.0003; ***p < 0.0001.
Reprinted with permission from Citation[50]. © 2008 The Authors Journal Compilation and Blackwell Publishing Ltd.
![Figure 7. Eight-point blood glucose profiles.Effects of adding multiple doses of glulisine to ongoing glargine.*p < 0.005; **p = 0.0003; ***p < 0.0001.Reprinted with permission from Citation[50]. © 2008 The Authors Journal Compilation and Blackwell Publishing Ltd.](/cms/asset/793f496f-e3fc-488b-9d9b-724b1fd30e00/iere_a_11207935_f0007_b.jpg)
When Banting and Best first isolated insulin in 1922, their epoch-making discovery initiated a revolution in the management of diabetes Citation[1]. Thereafter, in the 20th century, major advances in insulin therapy were made and continued into the 21st century to meet new challenges. An estimated 366 million people worldwide were affected by diabetes in 2011, driven by a rapid increase in the prevalence of Type 2 diabetes, and this pattern is expected to continue into the foreseeable future Citation[2]. Projections indicate a 50% increase in the prevalence of diabetes worldwide by 2030, with the greatest burden likely to occur in low- and middle-income countries Citation[2]. Proinsulin therapy remains the most effective and consistent method of controlling blood glucose levels, and recent biotechnological advances have enabled the production of insulin analogs with characteristics that confer additional benefits to conventional insulins. With over 10 years of clinical experience, the basal insulin analog, insulin glargine (glargine), represents one of the most well-validated of the insulin analogs. The aim of this article is to provide an overview of the clinical evidence for the best strategies of initiating and intensifying insulin with a focus on glargine in people with Type 2 diabetes.
Insulin therapy: towards more physiological insulin delivery
The ultimate goal of exogenous insulin therapy is to replicate physiological insulin secretion and maintain blood glucose levels within a narrow range in order to reduce the risk of vascular complications associated with hyperglycemia while avoiding the deleterious effects of hypoglycemia. In healthy individuals, insulin is secreted in a basal and prandial pattern. In the fasting state, insulin levels are maintained at low ‘basal’ levels but rapidly increase in response to a meal– that is, prandial or bolus insulin. Within a few years of Banting and Best’s discovery, it was recognized that these two distinct components of insulin secretion were unlikely to be fulfilled by a single exogenous insulin preparation Citation[3]. The requirement for basal insulin could only be achieved with multiple daily injections of regular insulin, which stimulated the search for insulins with a more prolonged duration of action that could better replicate the physiological basal insulin secretory response.
Basal insulin: extending the action of insulin
The duration of action of regular human (or animal) insulin is 6–8 h when administered subcutaneously, and is thus not sufficient to provide all-night cover, resulting in fasting hyperglycemia. Increasing the dose not only lengthens the time–action profile but also increases the likelihood of hypoglycemia because of its peaked profile. The development of neutral (porcine) protamine Hagedorn (NPH) insulin in 1946 and the lente (porcine and bovine) series of insulins in the 1950s helped to overcome this limitation by offering prolonged effects due to delayed subcutaneous absorption. A single dose of ultralente or NPH insulin can persist at the site of injection for up to 48 h. However, both of these preparations are associated with substantial pharmacokinetic and pharmacodynamic variability, predominantly due to the fact that the preparations need to be resuspended prior to administration.
However, the protracted subcutaneous absorption of human NPH and human ultralente insulins does not necessarily translate into prolonged bioavailability beyond 24 h Citation[4]. Both the preparations may also increase the risk of hypoglycemia, particularly nocturnal hypoglycemia, because of their peak plasma concentrations that occur between 4 and 12 h after the subcutaneous administration. If the dose of ultralente/NPH insulin is markedly reduced, in an attempt to avoid hypoglycemia, the individual may experience the ‘dawn phenomenon’, whereby there is an insufficient level of insulin to suppress the early-morning increase in glucose output from the liver, resulting in hyperglycemia.
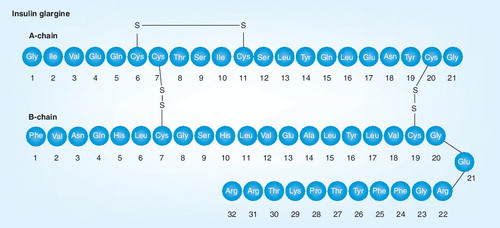
Greater understanding of the protein structure of insulin and the roles of key amino acids has opened up new avenues for the rational design of insulin analogs with more predictable absorption and time–action characteristics. Initial efforts to prolong the action included a diarginyl-insulin preparation (ArgB31 and ArgB32) Citation[5,6], and NovoSol Basal (GlyA21, ArgB27 and ThrB30) Citation[7], although both of these analogs ultimately proved to be unsatisfactory. Glargine was the first long-acting basal analog to be introduced into clinical practice and differs from human insulin by the replacement of A21 asparagine with glycine and the addition of two arginine residues at B31 and B32 (GlyA21, ArgB31 and ArgB32). These mutations endow glargine with an isoelectric point of 6.4–6.8, implying that it is easily soluble at acid pH and less soluble at neutral pH. As a result, upon subcutaneous injection, glargine forms an amorphous precipitate in the subcutaneous tissue, which slowly dissociates, providing a sustained release of insulin into the circulation Citation[8]. Once injected, glargine is metabolized quickly into two main active metabolites with in vitro activity similar to that of insulin, M1 (GlyA21) and M2 (GlyA21, des-ThrB30) Citation[9,10]. The M1 metabolite accounts for approximately 90% of the daily plasma insulin available Citation[11]. This protracted release of glargine from the subcutaneous depot translates into longer bioactivity than either human NPH or human ultralente insulin Citation[4,12]. Thus, glargine can be administered once daily, unlike the earlier ‘intermediate’/‘long-acting’ insulin preparations.
Another approach that has been used to protract the time–action profile of insulin is to conjugate the insulin molecule to a fatty acid, as with insulin detemir (detemir), which promotes binding to, and slow dissociation from, albumin, forming a ‘floating depot’ Citation[13,14]. Detemir must be administered at a fourfold higher concentration per unit relative to other insulin analogs because the added fatty acid of the detemir molecule is thought to interfere with insulin receptor (IR) binding, thus reducing potency Citation[15,101]. The time–action profile for detemir appears to be shorter than glargine in individuals with Type 1 diabetes, lasting between 12 and 24 h, which is dependent on the dose administered, implying that twice-daily injections may be necessary in individuals with Type 1 diabetes and a lesser number of people with Type 2 diabetes Citation[16–21].
Initiating insulin therapy
Type 2 diabetes is associated with a progressive loss of β-cell function, and one of the key challenges is in determining when to initiate insulin and how and when to intensify treatment. The consensus recommendations of the American Diabetes Association and the European Association for the Study of Diabetes are to initiate therapy with lifestyle interventions and metformin with the aim of achieving a treatment goal of glycosylated hemoglobin (HbA1c) <7.0% Citation[22]. Individuals failing to meet targets despite these interventions have the option to receive insulin Citation[23]. The consensus statement algorithm for the effective initiation and intensification of insulin therapy in Type 2 diabetes suggests targeting the fasting plasma glucose (FPG) component first, with a ‘basal’, ‘intermediate’ or ‘long-acting’ insulin preparation Citation[22].
Currently, NPH insulin, glargine and detemir are widely used, and many studies have demonstrated the efficacy of these basal insulins as starter insulins in Type 2 diabetes. Numerous studies have compared glargine with NPH insulin as initial insulin for Type 2 diabetes Citation[24–33]. A large proportion of subjects in these studies achieved target HbA1c (<7.0%) with glargine or NPH insulin, with similar improvements in glycemic control with either insulin. In the Treat-to-Target trial, HbA1c ≤7.0% was achieved by approximately 60% of individuals with either insulin Citation[24]. However, there was a significant difference in the number of individuals who achieved target without hypoglycemia, with this number being higher in the glargine group compared with the NPH insulin group.
The comparable treatment efficacy of glargine and NPH insulin is also reflected in a recent meta-analysis of 12 randomized controlled trials comprising 4385 subjects Citation[34]. Indeed, in that analysis, the mean net difference between NPH insulin and glargine in terms of HbA1c change was 0.08% (95% CI: -0.04–0.21). However, differences in FPG were more marked at 0.21 mmol/l (95% CI: -0.02–0.45), suggesting more favorable outcomes with glargine use. Clearly, the clinical relevance of this relatively small difference is debatable; what is more important is that glargine is consistently associated with a lower risk of hypoglycemia. Meta-analyses of pooled data by Mullins et al. based on six studies in Type 2 diabetes, associated glargine with a 13.7, 39.3 and 53.7% risk reduction (all p <0.05) for symptomatic, confirmed and severe hypoglycemia, respectively, when compared with NPH insulin. The lower risk of hypoglycemia with glargine was apparent at all levels of HbA1c achieved, with a visible trend towards a greater difference at lower levels of HbA1c suggesting that individuals nearer target (HbA1c <8%) would experience the greatest reduction in risk Citation[35]. A more recent meta-analysis has extended these findings to the risk of nocturnal hypoglycemia with glargine versus NPH insulin, in which the risk approximately halved with glargine (odds ratio = 0.44 for hypoglycemia with plasma glucose <2 mmol/l and 0.52 for hypoglycemia with plasma glucose <3.9 mmol/l). On the basis of this analysis, it was estimated that treating eight people with glargine instead of NPH insulin would prevent one person from experiencing a nocturnal symptomatic hypoglycemia event Citation[36].
Hypoglycemia is widely acknowledged as a barrier to insulin therapy and may limit the intensification of therapy to achieve and maintain optimal levels of glycemic control Citation[37,38]. Therefore, effective regimens associated with a lower risk of hypoglycemia should be preferred, particularly because severe hypoglycemia is a devastating experience with risk for serious outcomes, including death. The risk of hypoglycemia can be further reduced by the use of appropriate insulin titration regimens, with the aid of self-monitoring of blood glucose (SMBG), and progressive increases in insulin dose as demonstrated, for example, in the Treat-to-Target, LANMET, INSIGHT, GOAL A1c, AT. LANTUS and Glycemia Optimization Treatment studies Citation[24,25,39–42]. The Glycemia Optimization Treatment study showed a clear association between the intensity of the titration regimen and the magnitude of HbA1c improvement Citation[42]. Thus, there is a scope for individuals to select an appropriate titration regimen that balances risk of hypoglycemia with the required HbA1c improvement.
Detemir has also been shown to benefit over NPH insulin in clinical trials with significantly lower rates of hypoglycemia and lower weight gain Citation[43]. Relatively few randomized studies have compared glargine and detemir directly, but a recent Cochrane Collaboration meta-analysis provides a good overview of their relative benefits Citation[44]. Four studies were included in the analysis with a duration of 24–52 weeks and involving a total of 2250 people with Type 2 diabetes. Glargine was dosed once daily in the evening in all four studies, while detemir was initiated once daily in the evening, with the option of an additional dose in the morning in three studies and twice daily in the remaining study .
No statistically significant difference between the insulins was found in HbA1c levels at the end of the study or change in HbA1c from baseline to end point, and the proportion of patients achieving an HbA1c target was also not significantly different. End point FPG levels were significantly lower with glargine versus detemir, but the difference was marginal. The incidence of overall, nocturnal or severe hypoglycemia was comparable between the groups, again with no statistically significant difference.
Weight gain with both glargine and detemir was relatively low in this analysis; however, weight gain was significantly less with detemir compared with glargine (mean difference: -0.91 U/kg; 95% CI: -1.21 to -0.61). The mean daily basal insulin dose was significantly lower with glargine versus detemir (mean difference: 0.26 U/kg; 95% CI: 0.11–0.41), related to the difference in once- versus twice-daily dosing. Of those people randomized to once-daily detemir, 13.6–57.2% were injecting twice daily at the end of the study. Injection-site reactions also occurred in a significantly lower proportion of patients receiving glargine compared with detemir (0.4 vs 1.8%; risk ratio: 3.31; 95% CI: 1.13–9.73).
Tailoring insulin treatment
Targeting fasting blood glucose (FBG) is not the only option in Type 2 diabetes, and several studies have addressed the merits of targeting fasting and postprandial blood glucose, for example, the APOLLO study Citation[45], which compared once-daily glargine with three-times-daily lispro, and the 4-T study Citation[46], which compared once-/twice-daily detemir with three-times-daily aspart or twice-daily biphasic aspart. As anticipated, targeting postprandial hyperglycemia with either a rapid-acting insulin analog or a premixed/biphasic insulin elicited greater improvements in postmeal glucose levels, whereas basal insulins provided greater improvements in FBG. In the APOLLO study, HbA1c improved by 1.7% with glargine and by 1.9% with lispro, reaching similar levels of 7.0 and 6.8% at end point, respectively, after 44 weeks Citation[45]. Overall, 57% of those treated with glargine and 69% with lispro reached HbA1c ≤7.0%. However, the incidence of hypoglycemia was significantly lower with glargine at 5.2 versus 24.0 events per patient per year (p < 0.0001). Broadly comparable findings were reported in the 4-T study in terms of hypoglycemia, although only 27.8% of subjects treated with once- or twice-daily detemir achieved HbA1c ≤7.0%, compared with 48.7% with aspart and 41.7% with biphasic aspart at 1 year Citation[46]. Interestingly, in the 4-T study, after 3 years, 63.2, 67.4 and 49.4% of subjects, respectively, achieved an HbA1c ≤7.0%, which was at least partly attributable to the addition of a second type of insulin after 1 year Citation[19]. At 1 year, 17.9, 4.2 and 8.9% of subjects in the basal, prandial and biphasic insulin groups, respectively, received a second insulin, and this had risen to 81.6, 73.6 and 67.7%, respectively, at 3 years Citation[46]. These data suggest that initiating basal insulin and progressing to basal-bolus therapy by adding three-times-daily prandial insulin represents an effective method of achieving glycemic control while minimizing the risk of hypoglycemia compared with prandial or premixed/biphasic insulin strategies.
Clearly, prandial and fasting glucose levels offer important targets for intervention in diabetes. However, the evidence suggests that a tailored approach based on each individual’s needs may be most appropriate and that the blood glucose monitoring and HbA1c levels complement one another and should be used to guide therapy. People with marked postprandial hyperglycemia may benefit most from a rapid-acting insulin analog at mealtime, whereas individuals with predominantly fasting hyperglycemia may benefit most from initial treatment with a long-acting insulin at bedtime. In addition, progressive intensification of insulin therapy to multiple daily injections may offer a more effective approach with more scope for individualization.
For people with Type 2 diabetes needing cover for both fasting and postprandial hyperglycemia, there are two widely used approaches– namely, premixed insulin and basal-bolus therapy. In studies that compared glycemic control between fasting regimens (e.g., based on glargine) and premixed insulin, including LAPTOP and INITIATE, glycemic control was broadly comparable, but hypoglycemia was significantly lower with glargine than with premixed insulin Citation[47,48]. However, results of the 4-T study indicate that, after 12 months of biphasic aspart insulin therapy, only 41.7% of subjects reached HbA1c ≤7.0% Citation[46], which was only marginally increased to 49.4% at 3 years Citation[19]; this may reflect the limited scope for intensifying premixed insulin due to the fixed ratio of the short- and long-term components. By contrast, the basal insulin and prandial insulin groups showed marked increases in the proportions of subjects with HbA1c ≤7.0%, from 27.8 and 48.7%, respectively, at 1 year, to 63.2 and 67.4%, respectively, at 3 years. From year 1 to year 3, the proportions of subjects who did not require a second insulin decreased from 82.1 to 18.4% in the basal insulin group and from 95.8 to 26.4% in the prandial insulin group Citation[19,46].
These results indicate that, over time, simply targeting fasting or prandial glucose is not sufficient for most people with diabetes and that transfer to a more intensive regimen may be required to reach target. Traditionally, these individuals have been transferred to either a full basal-bolus regimen (basal insulin plus three prandial injections) or premixed insulin, although several studies have demonstrated advantages of a basal-bolus regimen Citation[49,50]. However, is transition to such intensive regimens necessary, or would a stepwise approach be better?
The OPAL study enrolled subjects already receiving basal glargine and randomized them to receive an additional single bolus of insulin glulisine (glulisine) at either breakfast or the main meal Citation[51]. In this study, glulisine given at breakfast was equally effective at controlling HbA1c, but the proportion reaching an HbA1c level ≤6.5% was slightly higher with glulisine given at the main meal (33.8 vs 27.8%); 52.2 and 36.5% of subjects reached HbA1c ≤7.0%. A second, proof-of-concept, study also assessed the ‘basal-plus’ intensification strategy, but with a run-in period during which the basal insulin was adjusted with the aim of optimizing FBG control prior to adding prandial insulin. Subjects with a HbA1c of 7.5–9.5% entered a 3-month run-in phase to optimize the glargine dose to achieve a FBG level ≤5.5 mmol/l using the treat-to-target algorithm Citation[52]. Those with HbA1c ≥7.0% were then randomized either to continue current therapy or to add a dose of glulisine before the main meal, and both treatments were continued for 3 months. Overall, 57 subjects were randomized to the control group and 49 to the bolus insulin group, and mean HbA1c at randomization was 7.9%. After 3 months of treatment, HbA1c improved by -0.11 and -0.37%, respectively (p = 0.0290). The results of these studies demonstrate that for most people with diabetes who are not at treatment goal (HbA1c <7%) with basal insulin alone, adding a single dose of prandial insulin is sufficient to improve control, while limiting the number of daily insulin injections.
For those individuals who are still unable to reach HbA1c with basal insulin plus one dose of bolus insulin (basal-plus), there is an opportunity to intensify therapy further by adding insulin prior to the other meals, progressing stepwise to a basal-bolus regimen.
Studies that have tested this approach include OSIRIS and the 1.2.3. Study Citation[53]. The 1.2.3. Study was a randomized, parallel-group, noninferiority study in people with Type 2 diabetes that compared basal glargine plus prandial glulisine administered once daily (n = 115), twice daily (n = 113) or three-times daily (n = 115) for 24 weeks following a 14-week insulin glargine run-in period Citation[54]. Eligible participants had previously received two or three different oral antidiabetic drugs for at least 3 months, had an HbA1c level ≥8.0% at baseline and a level >7.0% following the glargine run-in. In the once- and twice-daily arms, glulisine was administered at the meals that produced the greatest glycemic excursions, while dose adjustments were made weekly, targeting preprandial blood glucose values of 3.9–6.1 mmol/l and a bedtime concentration of 3.9–7.2 mmol/l, while avoiding ≥two hypoglycemic events per week.
The study showed that HbA1c reductions from randomization to the end of the study with glulisine once- or twice-daily were noninferior to glulisine three times daily (0.46, 0.48 and 0.56%, respectively). In terms of the proportion of people achieving glycemic targets, after the glargine run-in, approximately 37% of people achieved HbA1c <7%, while at the end of the study, an additional 39.5, 43.0 and 58.2% in the glulisine once-, twice- and three-times-daily groups, respectively, also achieved this target. Weight gain was reportedly low and was similar across all three treatment groups, while the rates of confirmed symptomatic hypoglycemia were 12.2, 12.9 and 17.1 events/person-year in the glulisine once-, twice- and three-times-daily groups, respectively. The mean total insulin dose was comparable across the three treatment groups at the end of the study (84 ± 27, 80 ± 50 and 81 ± 66 U/day, respectively). Ultimately, the 1.2.3. Study demonstrated noninferiority for once- or twice- versus three-times daily glulisine Citation[54].
The rationale for initiation of insulin therapy with a basal insulin, followed by stepwise progression to a basal-bolus regimen can be clearly demonstrated by reviewing profiles acquired by SMBG. In the APOLLO study, the addition of once-daily glargine or three-times daily lispro achieved expected effects on the eight-point blood glucose profiles Citation[45]. As shown in , glargine effectively shifted the entire profile downwards, although the glucose excursions remained unaltered. Meanwhile, lispro obtunded postprandial glucose (PPG) excursions, although fasting and preprandial hyperglycemia was still evident. In the OPAL study, the addition of a single bolus of glulisine reduced the glucose excursion at the appropriate meal Citation[51]. Extending this further, the addition of glargine plus glulisine at all three meals, as in the GINGER study, shifted the entire blood glucose profile downward, with superior glucose-lowering after each meal when compared with twice-daily premixed insulin Citation[50].
These profiles may also help to explain the results of the 4-T study, in which the initial basal insulin group showed the least number of subjects reaching the goal (HbA1c <7%) at 1 year compared with the prandial and biphasic groups Citation[19,46]. At the 3-year end point, more people who commenced basal insulin were more likely to reach their target, with less risk of hypoglycemia. This is the first reported evidence comparing these different regimens to support basal insulin over prandial as a starter regimen, only adding prandial as needed.
Finally, initiating and titrating to a full basal-bolus regimen is a major challenge for people with diabetes and may have apparent negative effects on quality of life or treatment satisfaction. However, in the APOLLO study, once-daily glargine was associated with greater improvements in overall treatment satisfaction compared with three-times-daily lispro Citation[45]. These findings were at least partly due to the significantly lower risk of hypoglycemia with glargine compared with lispro, and partly ascribed to the item reflecting ‘convenience with treatment’, which improved with glargine but deteriorated with lispro. Stepwise insulinization will allow subjects to gradually become accustomed to changes in therapy, as well as better controlling their diabetes in partnership with SMBG. Thus, one may anticipate that this will avoid these negative perceptions of insulin therapy.
Insulin glargine in special populations
One of the biggest advantages of glargine is it significantly reduces the risk of hypoglycemia compared with intermediate-acting insulins Citation[55]. This is especially important for certain patient populations where hypoglycemia poses a greater risk, such as the elderly. Studies have also suggested that Asian patients have lower insulin requirements than Western patients, potentially due to lower BMI, and may be at greater risk of hypoglycemia Citation[56,57].
Older patients
The treatment goals for older patients (>65 years of age) with Type 2 diabetes are essentially the same as those for younger patients. However, the approach to treatment may need to be modified to accommodate for comorbidities and psychosocial changes associated with aging, as well as differences in diet, eating patterns and activity levels compared with younger people Citation[58]. In terms of insulin therapy, advancing age is notable for a greater susceptibility to hypoglycemia, which can have serious clinical consequences in this population Citation[59,60]. Therefore, a treatment strategy that strikes the correct balance between effective glycemic control and minimized risk of hypoglycemia is particularly important in older patients.
Randomized controlled trials have consistently shown a lower risk of hypoglycemia with glargine compared with other forms of insulin, but there have been no such trials specifically looking at outcomes in older adults. A recent meta-analysis of individual participant data from five large-scale, randomized trials with similar designs has compared outcomes with glargine versus NPH insulin in people with Type 2 diabetes aged ≥65 years who were poorly controlled on oral agents (n = 604) Citation[61]. In this age group, glargine was associated with significantly greater reductions in both HbA1c (-1.4 vs -1.1%) and FBG (-4.7 vs -4.1 mmol) compared with NPH insulin (p < 0.001 for both the groups) over a 24–28-week treatment period. There were comparable increases in insulin dose for both the groups over the treatment period (17 U/day for both groups), although the insulin dose at baseline was higher for older adults randomized to glargine versus NPH insulin (15 vs 12 U/day). The significant improvement in glycemic control with glargine achieved by older adults was not associated with an increase in hypoglycemic events. Rates of daytime hypoglycemia were comparable between glargine and NPH insulin, and there were numerically lower rates of nocturnal hypoglycemia with glargine, although this was not statistically significant. This meta-analysis suggests that a regimen based on glargine plus oral agents represents an effective treatment option to meet therapeutic targets for older adults with Type 2 diabetes, without increasing the risk of hypoglycemia.
Asian patients
Studies by Kawamori et al. Citation[62] and Pan et al. Citation[28] first showed that the benefits of glargine demonstrated in Western populations were consistent among people with Type 2 diabetes of Asian descent. The study by Pan et al. randomized a total of 443 insulin-naive individuals from ten Asian countries to once-daily glargine or NPH insulin administered at bedtime and titrated to target a FBG level of ≤120 mg/dl, both in combination with a 3 mg fixed dose of glimepiride Citation[28]. Over 24 weeks of treatment, superiority in terms of glycemic control was demonstrated for glargine, with HbA1c levels in the per-protocol population reduced by 1.1% (9.00–7.90%) with glargine compared with 0.9% (9.05–8.13%) with NPH insulin. The proportion of patients reaching the HbA1c target of <7.5% was higher with glargine versus NPH insulin, although this was not significant (38.1 vs 30.3%, respectively). Individuals receiving glargine experienced a significantly lower number of hypoglycemic episodes compared with those receiving NPH insulin (682 vs 1019, respectively). Analysis of hypoglycemia by time of day revealed that approximately twofold fewer symptomatic nocturnal hypoglycemic episodes occurred with glargine compared with NPH insulin, with no increase in daytime hypoglycemia.
This study used a relatively high FBG target of ≤120 mg/dl compared with the value of ≤100 mg/dl that is more typically used in the studies on glargine on the basis of the possible higher risk of hypoglycemia in Asian patients Citation[61]. The author noted that a greater response rate may have been achieved with more aggressive titration of glargine. A similar pattern was also observed in clinical practice in the FINE Asia study, despite low rates of hypoglycemia, and this suggests that basal insulin may be underutilized at present Citation[56].
Kanazawa et al. evaluated the long-term efficacy of glargine as part of a basal-bolus regimen in Japanese people, including 60 people with Type 2 diabetes Citation[63]. Individuals included in the study had been treated with once-daily NPH insulin in combination with a rapid-acting or regular insulin for at least 1 year, with fair-to-poor control (HbA1c ≥6.5%) for at least 3 months prior to inclusion. Their current NPH and prandial insulin were optimized during an initial 3-month run-in phase. Basal insulin was then switched from NPH insulin to an equivalent dose of glargine, which was adjusted to target a FBG level of 100–140 mg/dl. The rapid-acting or regular insulin was continued as previously with a PPG target of 140–170 mg/dl. Mean HbA1c levels were significantly improved within 3 months of the switch to glargine, and this effect persisted at 18 months (HbA1c baseline: 8.2%; 18 months: 7.7%; p < 0.01 for the difference). The daily basal insulin dose significantly increased with the switch to glargine, while the total bolus insulin dose was not significantly different. Bodyweight increased slightly but significantly, while the frequency of hypoglycemia was slightly lower, although not significantly, after the switch to glargine.
Basal insulin in combination with non-insulin therapies
For some individuals, the limitations of hypoglycemia and weight gain associated with insulin therapy are more acute. One method of overcoming these issues is to combine insulin with non-insulin-based therapies that have complementary efficacy and safety profiles. The glucose-lowering effect of metformin monotherapy is associated with limited hypoglycemia and modest weight loss or weight neutrality, indicating that it may be complementary to insulin therapy Citation[22]. By contrast, sulfonylureas (SUs) are associated with higher rates of hypoglycemia and weight gain. Fonseca et al. recently reported data from a pooled analysis of 11 randomized trials of glargine therapy in combination with oral agents showing that adding glargine to metformin was associated with a significantly greater reduction in HbA1c levels versus adding glargine to a SU (-2.0 vs -1.7%; p = 0.0001), and a greater proportion of patients achieving an HbA1c target ≤7.0% (68.1 vs 50.4%; p = 0.0001) Citation[64]. Importantly, the benefits of glargine plus metformin versus glargine plus SU were achieved with significantly less weight gain (1.6 vs 2.3 kg) and significantly lower rates of hypoglycemia (1.81 vs 4.88 events/person-year). It should be noted that people receiving glargine plus metformin were younger (52.5 vs 61.2 years) and had a shorter disease duration (6.0 vs 9.1 years), and that the observed improvements were likely to reflect, in part, the benefits of earlier initiation of glargine therapy in the metformin group.
Other potentially complementary agents to insulin include the amylin analogs and incretin-based therapies (GLP-1 receptor mimetics and DPP-4 inhibitors). These agents show pronounced glucose-lowering effects and a relatively low risk of hypoglycemia and weight gain; furthermore, some agents in this group also have the added advantage of being able to target PPG excursions to varying degrees, making them a potentially attractive option for combination with the FPG-targeting basal insulins. A 4-week, randomized, active-controlled, proof-of-concept study by Arnolds et al. compared the effect of adding exenatide or sitagliptin to glargine plus metformin in 48 people with poorly controlled Type 2 diabetes (HbA1c 7–10%) Citation[65]. The primary outcome measure was 6-h postprandial blood glucose excursion (area under the curve blood glucose0–6 h) after ingestion of a standardized meal, and addition of exenatide or sitagliptin to glargine plus metformin was associated with a comparable and significant improvement. Statistically significant improvements in HbA1c, seven-point blood glucose profiles and FBG levels were also observed. Over 4 weeks, bodyweight was slightly but significantly lower with exenatide plus glargine plus metformin (-0.9 kg) and remained essentially unchanged with sitagliptin plus glargine plus metformin and glargine plus metformin alone (+0.4 and +0.1 kg, respectively). Rates of hypoglycemia were low and were comparable across the groups.
Subsequently, there has been a great deal of interest in the combination of basal insulins and GLP-1 analogs. A randomized, placebo-controlled study reported by Buse et al. demonstrated that the addition of exenatide to glargine plus metformin with or without pioglitazone was associated with significant improvements in HbA1c levels over 30 weeks of treatment Citation[66]. HbA1c levels decreased by -1.74% with exenatide and -1.04% with placebo (between-group difference: -0.69% [95% CI: -0.93 to -0.46]; p < 0.001). Bodyweight decreased by -1.8 kg with the addition of exenatide but increased by +1.0 kg with placebo (between-group difference: -2.7 kg [95% CI: -3.7 to -1.7]). Average increases in insulin dosage with exenatide and placebo were 13 and 20 U/day, respectively. The estimated number of hypoglycemic events per participant per year was low and did not differ significantly between the groups (1.4 vs 1.2; p = 0.49). Lixisenatide, a second GLP-1 analog with potent PPG-lowering effects, is also in development and is being evaluated as add-on therapy to glargine in three Phase III studies: GetGoal-Duo, GetGoal-L and GetGoal-L Asia. Preliminary data from the GetGoal-L Asia study have recently been reported and showed that adding once-daily lixisenatide for patients insufficiently controlled on basal insulin, with or without a SU, significantly improved HbA1c compared with placebo, with particularly pronounced reductions in PPG levels Citation[67].
Insulin glargine & cancer risk
There is growing awareness of an association between diabetes and cancer. Epidemiological studies show that Type 2 diabetes is an independent risk factor for a number of different cancers, including bladder, breast, colorectal, endometrium, liver and pancreatic cancer Citation[68]. Risk factors common to both cancer and diabetes include age, obesity, physical activity, diet, alcohol and smoking, and several diabetes-related pathophysiological mechanisms have also been implicated in increasing cancer risk, including hyperinsulinemia, hyperglycemia and inflammation Citation[69]. Insulin is known to have mitogenic properties Citation[70] through activation of the insulin receptor and direct or indirect activation of the insulin-like growth factor receptor Citation[71]. It has been postulated that insulin analogs may have an increased risk of cancer relative to human insulin due to altered affinities for the insulin receptor or insulin-like growth factor receptor Citation[72,73]. However, a recent review of in vitro and in vivo data by Ciaraldi et al. found that while glargine may stimulate mitogenic activity in some cell lines at supraphysiological concentrations, the same effect was not seen in animal studies Citation[71]. Furthermore, glargine in vivo is rapidly transformed into its metabolites M1 and M2, the metabolic and mitogenic characteristics of which have been shown to be broadly equivalent to human insulin Citation[74,75].
Nevertheless, concerns regarding a possible link between the use of glargine and an increased risk of cancer have been raised following the publication of four epidemiological studies in the journal Diabetologia in 2009 Citation[76–79]. An initial German study analyzed approximately 127,000 insulin-treated individuals from an insurance database Citation[78]. For all cancers, the unadjusted hazard ratio (HR) for glargine compared with human insulin was 0.85 (95% CI: 0.79–0.93), indicating a significant decrease in overall cancer risk with glargine. However, an increased risk of cancer with glargine was suggested following a subanalysis adjusting for insulin dose (HR: 1.14; 95% CI: 1.05–1.24) with a dose-dependent increase in risk. However, subjects were not classified by dose group when they were enrolled, questioning the validity of the dose-relationship analysis Citation[80]. Major flaws in this study included: a lack of information on potentially relevant factors such as insulin resistance, body mass index, smoking and duration of diabetes; removal of people who changed insulin type during the study; and a higher proportion of subjects in the human insulin ‘high-dose’ group than in the glargine ‘high-dose’ group (46.0 vs 13.5%). The accompanying editorial cautioned against overinterpretation of these results in light of findings from three additional observational studies using databases from Sweden, Scotland and the UK Citation[81].
The Swedish study followed 114,841 people with diabetes using linked data from multiple registries Citation[79]. The findings showed no detectable difference in the risk of cancer between glargine and other insulins for all malignancies combined (rate ratio [RR]: 1.07; 95% CI: 0.91–1.27). In a subanalysis of cancer types, there was no significant relationship between glargine and all cancer subtypes evaluated, with the exception of breast cancer, which showed an increased risk for users of glargine alone (RR: 1.99; 95% CI: 1.31–3.03). However, no increase in risk was observed for users of glargine in combination with another insulin (RR: 1.10; 95% CI: 0.77–1.56). These data are difficult to interpret, and the author suggests that the short duration from the start of glargine use to the increased incidence rate for breast cancer may be due to random fluctuation.
Using a national clinical database, the Scottish Diabetes Research Network Epidemiology Group showed that, overall, there was no increase in cancer risk when all individuals receiving glargine (n = 3959) were considered (HR: 1.02 for all cancers; 95% CI: 0.77–1.36) Citation[75]. However, a secondary analysis of those receiving glargine alone again showed an overall increase in cancer risk (HR: 1.55; 95% CI: 1.01–2.37), with more cases of breast cancer compared with people receiving non-glargine insulins (HR: 3.39; 95% CI: 1.46–7.85). However, as with the Swedish study, the number of cases of breast cancer was small. The author concludes that the findings are unlikely to reflect an effect of glargine itself, but are more likely to be the result of allocation bias, with the less healthy individuals more likely to receive a simple once-daily regimen of glargine.
The fourth study examined The Health Information Network database, which includes data from approximately 300 general practices in the UK Citation[77]. For people receiving basal insulin alone versus glargine alone, there was no increase in cancer risk (HR: 1.24; 95% CI: 0.90–1.70). In addition, analysis of glargine versus all other insulins as a single comparator group revealed no difference with respect to progression of breast cancer (HR: 0.86; 95% CI: 0.42–1.75).
Findings from more recent observational studies on the short- and long-term effects of glargine and the risk of breast cancer also failed to show a clear relationship Citation[82,83]. Data from a UK study of 15,277 women with Type 2 diabetes using insulin observed for up to 8 years suggested that there was no increased risk with short-term glargine use (<5 years; overall HR: 0.9; 95% CI: 0.6–1.3) but that long-term use may increase the risk of breast cancer, particularly in women with long-term use of other insulins prior to staring glargine (HR: 2.7; 95% CI: 1.1–6.5) Citation[82]. The authors propose several hypotheses for this disparity, but discount long-term insulin exposure as the underlying mechanism. A short-term cohort study of cancer occurrence in insulin users in Sweden observed an increase in the incidence rate of breast cancer with glargine use in 2006 and 2007, but not in 2008. The author commented that the short duration of glargine use in this analysis suggest that the observed increased risk of breast cancer could be due to random fluctuation and that data for additional years are needed to determine whether there is a genuine association between glargine use and breast cancer Citation[83].
Collectively, it is difficult to draw conclusions from these data. The results within and between studies are inconsistent, the duration of observation being short relative to the natural history of cancer, with limited information available on the insulin products used, and the study populations are not always comparable. Indeed, observational studies are limited by the need to make statistical adjustments for unmatched comparisons. Prospective randomized controlled trials are required to answer the question of whether treatment with a specific therapy is associated with an increased risk of cancer.
More recently, findings from a 5-year, open-label, randomized controlled study showed no evidence of a greater risk of the development or progression of diabetic retinopathy with glargine versus NPH insulin Citation[84]. In addition, this study provided an opportunity to assess the number of neoplasms that occurred during this period, which showed a similar risk of malignancy for glargine and NPH insulin Citation[85]. The ORIGIN trial included cancer incidence as a secondary outcome and found that there was no increased incidence of all cancers combined, any organ-specific type of cancer (including breast, lung, colon, prostate, melanoma or other) or cancer mortality in the glargine group versus the standard-care group, over a median follow-up period of 6.2 years Citation[86]. A recent meta-analysis from the Northern European Database Study, which comprises data from both observational studies and randomized clinical trials based on 907,008 patients and 2,597,602 person-years, was recently reported Citation[87]. This provided evidence that risk of cancer (all forms combined), as well as any organ-specific type of cancer, is not increased among users of glargine compared with other insulins. Additional reports from this study, as well as other large database studies reported at the American Diabetes Association 2012 Scientific Sessions (the Kaiser Permanente Collaboration and a US database analysis) will provide further details on the lack of association between glargine and cancer risk. In addition, results from the International Study of Insulin and Cancer are anticipated Citation[88].
In the absence of adequate supporting evidence, there are concerns that inappropriate interpretation of some or all of the four initial epidemiological studies described earlier could cause unwarranted alarm Citation[80,89]. Response statements from key regulatory and professional bodies have aimed to put these findings into perspective and have advised, without exception, that people should continue using their insulin glargine treatments and consult their doctor if they are concerned Citation[102,103]. The US FDA issued an updated Drug Safety Communication in January 2011 following their review of the four observational studies. They stated that the evidence presented is inconclusive due to limitations in the study designs and the available data for analysis and that people could continue to use glargine Citation[102].
Conclusion
The soluble insulin glargine is a pharmacokinetically optimized basal insulin that overcomes many of the clinical limitations of traditional suspensions of intermediate and basal insulins. In people with Type 1 diabetes, once-daily glargine as part of a multiple daily injection strategy is an effective method of achieving glycemic control with lower rates of hypoglycemia than NPH insulin and at a considerably lower cost than continuous subcutaneous insulin infusion. In people with Type 2 diabetes, initiating treatment with once-daily glargine with a simple treat-to-target algorithm improves glycemic control with lower rates of hypoglycemia and less weight gain than premixed or prandial insulin strategies. The stepwise addition of prandial insulin to basal therapy may have advantages over progression to full basal-bolus therapy, although more studies are needed to confirm the efficacy of this approach in everyday clinical practice. With more than 10 years of clinical experience, glargine is now established as the gold-standard reference for basal insulin in the treatment of diabetes Citation[45]. Efforts should now be focused on optimizing the initiation, titration and combination of glargine with prandial insulin in order to maximize its potential and improve outcomes for people with insulin-requiring diabetes.
Expert commentary
The main challenges facing diabetes healthcare professionals today are when to initiate insulin, which insulin to use and which treatment/titration algorithm to adopt. Guidelines recommend an initial focus on the fasting component of the blood glucose profile with an intermediate- or long-acting basal insulin. Hypoglycemia is considered to be one of the major barriers to initiating insulin therapy and is often a deciding factor when selecting an insulin regimen. Therefore, it makes clinical sense to adopt a treatment regimen that minimizes this risk. Glargine has been shown to result in fewer hypoglycemic events than NPH insulin, along with comparable glycemic control, and also reports less weight gain than premixed or prandial insulin strategies in Type 2 diabetes. The question of how to intensify therapy for people no longer controlled on basal insulin alone is an area where progress is now also being made. Glargine has been relatively well studied as the basis of ‘stepwise’ regimens for the intensification of insulin therapy, whereby a single prandial insulin injection is added, stepwise, to an ongoing basal regimen and gradually increased to a full basal-bolus regimen as required. Allowing a gradual increase in prandial insulin dosing in this manner, rather than resorting immediately to a basal-bolus strategy, is a further move toward individualizing treatment. Such tailoring of insulin therapy is likely to play a key role in effective disease management.
To date, there is no conclusive clinical evidence to suggest a link between glargine therapy and cancer, and current users should continue with their glargine therapy in full confidence Citation[87,90–92]. Longer-term, large-scale prospective study data are required, and such studies are being undertaken, to provide conclusive evidence of a clinically meaningful association.
Five-year view
As our understanding of the pathogenesis of diabetes deepens and the scope of insulin therapy continues to evolve, the next few years should provide us with answers to some outstanding and important questions in diabetes management. The results of the ORIGIN trial have recently been reported. This was a long-term, prospective, large-scale, randomized controlled trial of basal insulin therapy targeting fasting normoglycemia in people with early Type 2 diabetes or prediabetes (impaired fasting glucose or impaired glucose tolerance; approximately 12% of the trial population) at high risk of cardiovascular events Citation[86]. In 12,537 people, followed up for a median of 6.2 years, it was found that there was no difference in cardiovascular outcomes between the glargine and standard -care groups, with patients in the glargine group achieving the target median FPG (≤94 mg/dl [5.2 mmol/l]). There was a modest increase in weight (+1.6 vs -0.5 kg, respectively) and low hypoglycemic rates (rate of severe hypoglycemia: 1.00 vs 0.31 per 100 person-years) with glargine. Individuals with prediabetes at randomization treated with glargine were 28% less likely to develop diabetes over the course of the study. Further subanalyses of the ORIGIN trial are awaited and an extension of this study will be forthcoming (ORIGINALE study), which should provide further insight into the benefits of early initiation of insulin. In more advanced disease, current evidence is favorable for stepwise intensification of insulin therapy, but will this translate into a new insulin treatment paradigm that encourages more people to initiate and adhere to insulin therapy in the long term? The potential is certainly there, although additional studies are required to further validate this process. We could also have clarification on the critical question of whether or not there is a direct link between cancer and glargine use. Recent studies have created considerable debate on this topic; however, data from large database studies reported at the American Diabetes Association 2012 Scientific Sessions (Northern European Database Study, Kaiser Permanente Collaboration and US database study) suggest that there is no link. Full publication of these studies is eagerly anticipated. The ultimate goal for insulin therapy in diabetes would be to see improved glycemic control, with more people meeting their individualized targets and experiencing fewer hypoglycemic events and long-term complications. Tailoring treatment to the individual, simplifying titration algorithms, optimizing the use of currently available insulin analogs and validating more patient-friendly regimens, for example, minimal daily injections, would contribute considerably to achieving this goal.
Table 1. Overview of studies comparing insulin detemir and insulin glargine.
Key issues
• Intensive research and development concerning the molecular structure and action of endogenous insulin has facilitated the development of human analogs with different time–action characteristics.
• Long-acting analogs, such as glargine and detemir, better mimic the basal component of insulin secretion than neutral protamine Hagedorn insulin, while rapid-acting analogs, such as lispro, aspart and glulisine, with their quicker onset and shorter duration of action than regular human insulin, mimic meal-related insulin secretion.
• Currently available long- and rapid-acting analogs can be combined to closely mimic normal physiological insulin secretion in people with diabetes.
• The once-daily insulin analog, glargine, with its protracted release from subcutaneous depots after injection, is effective and well tolerated in both Type 1 and Type 2 diabetes.
• Glargine has comparable efficacy to neutral protamine Hagedorn, but with fewer hypoglycemic events, including nocturnal hypoglycemia, and has less weight gain than premixed or prandial insulin strategies in Type 2 diabetes.
• Glargine has proven efficacy as a ‘starter insulin’ in Type 2 diabetes.
• Guidelines recommend improving the fasting component of the blood glucose profile, when first initiating insulin therapy, with a basal, intermediate- or long-acting insulin, such as glargine.
• Tailoring of insulin therapy to suit individual people’s needs is critical for effective glycemic control.
• Fear of hypoglycemia may limit insulin intensification and potentially compromise effective disease management; the use of regimens associated with low risk of hypoglycemia and appropriate titration algorithms may reduce this risk.
• A stepwise approach to insulin intensification with the gradual introduction of a prandial rapid-acting insulin to an established basal regimen may provide scope for further individualization of insulin therapy.
• Optimizing the initiation, titration and combination of glargine with a prandial insulin may help in tailoring individual insulin therapy programs, which may improve outcomes in people with diabetes.
Financial & competing interests disclosure
DR Owens has received lecture fees/honoraria from sanofi-aventis and Roche Diagnostics. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial support was provided by Huw Jones, PhD, Medicus International, and was funded by sanofi-aventis in the production of this manuscript.
References
- Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus. Can. Med. Assoc. J. 12(3), 141–146 (1922).
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 94(3), 311–321 (2011).
- Lawrence RD, Madders K. Employment of diabetics. Br. Med. J. 2(4064), 1076–1077 (1938).
- Lepore M, Pampanelli S, Fanelli C et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 49(12), 2142–2148 (2000).
- Zeuzem S, Stahl E, Jungmann E, Zoltobrocki M, Schöffling K, Caspary WF. In vitro activity of biosynthetic human diarginylinsulin. Diabetologia 33(2), 65–71 (1990).
- Monti LD, Poma R, Caumo A et al. Intravenous infusion of diarginylinsulin, an insulin analogue: effects on glucose turnover and lipid levels in insulin-treated type II diabetic patients. Metab. Clin. Exp. 41(5), 540–544 (1992).
- Jørgensen S, Vaag A, Langkjaer L, Hougaard P, Markussen J. NovoSol Basal: pharmacokinetics of a novel soluble long acting insulin analogue. BMJ 299(6696), 415–419 (1989).
- Owens DR, Coates PA, Luzio SD, Tinbergen JP, Kurzhals R. Pharmacokinetics of 125I-labeled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care 23(6), 813–819 (2000).
- Kuerzel G, Sandow J, Seipke G, Lang A, Maas Skrzipczyk HJ. Kinetic and metabolite profile of insulin glargine. Diabetologia 44(Suppl. 1), A798 (2001).
- Kuerzel GU, Shukla U, Scholtz HE et al. Biotransformation of insulin glargine after subcutaneous injection in healthy subjects. Curr. Med. Res. Opin. 19(1), 34–40 (2003).
- Lucidi P, Portcellati F, Rossetti P et al. Metabolism of insulin glargine after sc injection of therapeutic dose in Type 2 diabetes mellitus. Presented at: 71st Scientific Sessions of the American Diabetes Association. San Diego, CA, USA, 24–28 June 2011 (Poster 071-OR).
- Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time–action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 23(5), 644–649 (2000).
- Hamilton-Wessler M, Ader M, Dea M et al. Mechanism of protracted metabolic effects of fatty acid acylated insulin, NN304, in dogs: retention of NN304 by albumin. Diabetologia 42(10), 1254–1263 (1999).
- Kurtzhals P, Havelund S, Jonassen I, Markussen J. Effect of fatty acids and selected drugs on the albumin binding of a long-acting, acylated insulin analogue. J. Pharm. Sci. 86(12), 1365–1368 (1997).
- Vigneri R, Squatrito S, Sciacca L. Insulin and its analogs: actions via insulin and IGF receptors. Acta Diabetol. 47(4), 271–278 (2010).
- Brunner GA, Sendhofer G, Wutte A et al. Pharmacokinetic and pharmacodynamic properties of long-acting insulin analogue NN304 in comparison to NPH insulin in humans. Exp. Clin. Endocrinol. Diabetes 108(2), 100–105 (2000).
- Heinemann L, Sinha K, Weyer C, Loftager M, Hirschberger S, Heise T. Time–action profile of the soluble, fatty acid acylated, long-acting insulin analogue NN304. Diabet. Med. 16(4), 332–338 (1999).
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with Type 2 diabetes. Diabetologia 51(3), 408–416 (2008).
- Holman RR, Farmer AJ, Davies MJ et al.; 4-T Study Group. Three-year efficacy of complex insulin regimens in Type 2 diabetes. N. Engl. J. Med. 361(18), 1736–1747 (2009).
- Porcellati F, Rossetti P, Busciantella NR et al. Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in Type 1 diabetes: a double-blind, randomized, crossover study. Diabetes Care 30(10), 2447–2452 (2007).
- Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with Type 2 diabetes. Clin. Ther. 30(11), 1976–1987 (2008).
- Nathan DM, Buse JB, Davidson MB et al.; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in Type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32(1), 193–203 (2009).
- Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycaemia in Type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association of Diabetes (EASD). Diabetologia 55, 1577–1596 (2012).
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of Type 2 diabetic patients. Diabetes Care 26(11), 3080–3086 (2003).
- Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M et al. Insulin glargine or NPH combined with metformin in Type 2 diabetes: the LANMET study. Diabetologia 49(3), 442–451 (2006).
- Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in Type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 24(4), 631–636 (2001).
- Yki-Järvinen H, Dressler A, Ziemen M; HOE 901/300s Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in Type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care 23(8), 1130–1136 (2000).
- Pan CY, Sinnassamy P, Chung KD, Kim KW; LEAD Study Investigators Group. Insulin glargine versus NPH insulin therapy in Asian Type 2 diabetes patients. Diabetes Res. Clin. Pract. 76(1), 111–118 (2007).
- Eliaschewitz FG, Calvo C, Valbuena H et al.; HOE 901/4013 LA Study Group. Therapy in Type 2 diabetes: insulin glargine vs. NPH insulin both in combination with glimepiride. Arch. Med. Res. 37(4), 495–501 (2006).
- Fritsche A, Schweitzer MA, Häring HU; 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with Type 2 diabetes. A randomized, controlled trial. Ann. Intern. Med. 138(12), 952–959 (2003).
- Fonseca V, Bell DS, Berger S, Thomson S, Mecca TE. A comparison of bedtime insulin glargine with bedtime neutral protamine hagedorn insulin in patients with Type 2 diabetes: subgroup analysis of patients taking once-daily insulin in a multicenter, randomized, parallel group study. Am. J. Med. Sci. 328(5), 274–280 (2004).
- Massi Benedetti M, Humburg E, Dressler A, Ziemen M. A one-year, randomised, multicentre trial comparing insulin glargine with NPH insulin in combination with oral agents in patients with Type 2 diabetes. Horm. Metab. Res. 35(3), 189–196 (2003).
- HOE 901/2004 Study Investigators Group. Safety and efficacy of insulin glargine (HOE 901) versus NPH insulin in combination with oral treatment in Type 2 diabetic patients. Diabet. Med. 20(7), 545–551 (2003).
- Bazzano LA, Lee LJ, Shi L, Reynolds K, Jackson JA, Fonseca V. Safety and efficacy of glargine compared with NPH insulin for the treatment of Type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet. Med. 25(8), 924–932 (2008).
- Mullins P, Sharplin P, Yki-Jarvinen H, Riddle MC, Haring HU. Negative binomial meta-regression analysis of combined glycosylated hemoglobin and hypoglycemia outcomes across eleven Phase III and IV studies of insulin glargine compared with neutral protamine Hagedorn insulin in Type 1 and Type 2 diabetes mellitus. Clin. Ther. 29(8), 1607–1619 (2007).
- Home PD, Fritsche A, Schinzel S, Massi-Benedetti M. Meta-analysis of individual patient data to assess the risk of hypoglycaemia in people with Type 2 diabetes using NPH insulin or insulin glargine. Diabetes. Obes. Metab. 12(9), 772–779 (2010).
- Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in Type 2 diabetes. Diabet. Med. 25(3), 245–254 (2008).
- Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 57(12), 3169–3176 (2008).
- Gerstein HC, Yale JF, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with Type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet. Med. 23(7), 736–742 (2006).
- Kennedy L, Herman WH, Strange P, Harris A; GOAL AIC Team. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with Type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) Trial. Diabetes Care 29(1), 1–8 (2006).
- Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R; ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled Type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care 28(6), 1282–1288 (2005).
- Tanenberg R, Zisman A, Stewart J. Glycemia optimization treatment (GOT): glycemic control and rate of severe hypoglycemia for five different dosing algorithms of insulin glargine (GLAR) in patients with Type 2 diabetes mellitus (T2DM). Diabetes 55(Suppl. 1), abstract 567-P (2006).
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with Type 2 diabetes. Diabetes Care 29(6), 1269–1274 (2006).
- Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for Type 2 diabetes mellitus. Cochrane Database Syst. Rev. 7, CD006383 (2011).
- Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with Type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 371(9618), 1073–1084 (2008).
- Holman RR, Thorne KI, Farmer AJ et al.; 4-T Study Group. Addition of biphasic, prandial, or basal insulin to oral therapy in Type 2 diabetes. N. Engl. J. Med. 357(17), 1716–1730 (2007).
- Janka HU, Plewe G, Riddle MC, Kliebe-Frisch C, Schweitzer MA, Yki-Järvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for Type 2 diabetes. Diabetes Care 28(2), 254–259 (2005).
- Raskin P, Allen E, Hollander P et al.; INITIATE Study Group. Initiating insulin therapy in Type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care 28(2), 260–265 (2005).
- Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in Type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 31(1), 20–25 (2008).
- Fritsche A, Larbig M, Owens D, Häring HU; GINGER study group. Comparison between a basal-bolus and a premixed insulin regimen in individuals with Type 2 diabetes–results of the GINGER study. Diabetes. Obes. Metab. 12(2), 115–123 (2010).
- Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in Type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes. Obes. Metab. 10(12), 1178–1185 (2008).
- Owens D, Sert-Langeron C, Riddle MC. Adding a single dose of insulin glulisine to basal insulin glargine plus oral antihyperglycemic drug therapy improves glycemic control in Type 2 diabetes: a 6-month proof-of-concept study [abstract]. Diabetes 58(Suppl. 1), A122 (2009).
- Raccah D. Stepwise intensification of prandial insulin versus basal−bolus insulin therapy in patients with Type 2 diabetes mellitus. Presented at: 46th Annual Meeting of the European Association for the Study of Diabetes. Stockholm, Sweden, 20–24 September 2010 (Abstract 958).
- Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with Type 2 diabetes mellitus and basal insulin treatment failure. Endocr. Pract. 17(3), 395–403 (2011).
- Rosenstock J, Dailey G, Massi-Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced hypoglycemia risk with insulin glargine: a meta-analysis comparing insulin glargine with human NPH insulin in Type 2 diabetes. Diabetes Care 28(4), 950–955 (2005).
- Tsai ST, Pathan F, Ji L et al. First insulinization with basal insulin in patients with Type 2 diabetes in a real-world setting in Asia. J. Diabetes 3(3), 208–216 (2011).
- McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care 27(1), 66–69 (2004).
- Shahar D, Fraser D, Shai I, Vardi H. Development of a food frequency questionnaire (FFQ) for an elderly population based on a population survey. J. Nutr. 133(11), 3625–3629 (2003).
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch. Intern. Med. 157(15), 1681–1686 (1997).
- Greco D, Angileri G. Drug-induced severe hypoglycaemia in Type 2 diabetic patients aged 80 years or older. Diabetes Nutr. Metab. 17(1), 23–26 (2004).
- Lee P, Chang A, Blaum C, Vlajnic A, Gao L, Halter J. Comparison of safety and efficacy of insulin glargine and neutral protamine hagedorn insulin in older adults with Type 2 diabetes mellitus: results from a pooled analysis. J. Am. Geriatr. Soc. 60(1), 51–59 (2012).
- Kawamori R, Kadawaki T, Iwasaki M. Efficacy and safety of insulin glargine in concurrent use with oral hypoglycemic agents for the treatment of Type 2 diabetic patients. J. Clin. Therap. Med. 19(5), 445–464 (2003).
- Kanazawa Y, Igarashi Y, Komiya K et al. Long-term efficacy of insulin glargine after switching from NPH insulin as intensive replacement of basal insulin in Japanese diabetes mellitus. Comparison of efficacy between Type 1 and Type 2 diabetes (JUN-LAN Study 1.2). Endocr. J. 54(6), 975–983 (2007).
- Fonseca V, Gill J, Zhou R, Leahy J. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes. Metab. 13(9), 814–822 (2011).
- Arnolds S, Dellweg S, Clair J et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care 33(7), 1509–1515 (2010).
- Buse JB, Bergenstal RM, Glass LC et al. Use of twice-daily exenatide in basal insulin-treated patients with Type 2 diabetes: a randomized, controlled trial. Ann. Intern. Med. 154(2), 103–112 (2011).
- Seino Y, Min K, Niemoller E, Takami A. 0278-OR lixisenatide significantly improves glycemic control in Asian patients with T2DM insufficiently controlled on basal insulin ± SU. Diabetes Obes. Metab. (doi: 10.1111/j.1463-1326.2012.01618.x) (2011) (Epub ahead of print).
- Giovannucci E, Harlan DM, Archer MC et al. Diabetes and cancer: a consensus report. Diabetes Care 33(7), 1674–1685 (2010).
- Johnson JA, Pollak M. Insulin, glucose and the increased risk of cancer in patients with Type 2 diabetes. Diabetologia 53(10), 2086–2088 (2010).
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 8(12), 915–928 (2008).
- Ciaraldi TP, Sasaoka T. Review on the in vitro interaction of insulin glargine with the insulin/insulin-like growth factor system: potential implications for metabolic and mitogenic activities. Horm. Metab. Res. 43(1), 1–10 (2011).
- Le Roith D. The insulin-like growth factor system. Exp. Diabesity Res. 4(4), 205–212 (2003).
- LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 3(3), 302–310 (2007).
- Fawcett J, Tsui BT, Kruer MC, Duckworth WC. Reduced action of insulin glargine on protein and lipid metabolism: possible relationship to cellular hormone metabolism. Metab. Clin. Exp. 53(8), 1037–1044 (2004).
- Sommerfeld MR, Müller G, Tschank G et al. In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PLoS ONE 5(3), e9540 (2010).
- Colhoun HM; SDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 52(9), 1755–1765 (2009).
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in Type 2 diabetes. Diabetologia 52(9), 1766–1777 (2009).
- Hemkens LG, Grouven U, Bender R et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 52(9), 1732–1744 (2009).
- Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies–a population-based follow-up study in Sweden. Diabetologia 52(9), 1745–1754 (2009).
- Pocock SJ, Smeeth L. Insulin glargine and malignancy: an unwarranted alarm. Lancet 374(9689), 511–513 (2009).
- Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia 52(9), 1699–1708 (2009).
- Suissa S, Azoulay L, Dell’Aniello S, Evans M, Vora J, Pollak M. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia 54(9), 2254–2262 (2011).
- Ljung R, Talbäck M, Haglund B, Jonasson JM, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies – a three-year population-based observation. Acta Oncol. 50(5), 685–693 (2011).
- Rosenstock J, Fonseca V, McGill JB et al. Similar progression of diabetic retinopathy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with Type 2 diabetes: a long-term, randomised, open-label study. Diabetologia 52(9), 1778–1788 (2009).
- Rosenstock J, Fonseca V, McGill JB et al. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with Type 2 diabetes: findings from a 5 year randomised, open-label study. Diabetologia 52(9), 1971–1973 (2009).
- The ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. doi: 10.1056/nejmoa1203858 (2012) (Epub ahead of print).
- Boyle P, Koechlin A, Boniol M, Robertson C, Bolli GB, Rosenstock J. Updated meta-analysis of cancer risk among users of insulin glargine. Diabetes 61(Suppl. 1), A345 (1332-P) (2012).
- Grimaldi-Bensouda L, Marty M, Pollak M et al. The international study of insulin and cancer. Lancet 376, 769–770 (2010).
- Garg SK, Hirsch IB, Skyler JS. Insulin glargine and cancer – an unsubstantiated allegation. Diabetes Technol. Ther. 11(8), 473–476 (2009).
- Blin P, Lassalle R, Dureau-Pournin C et al. Insulin glargine and risk of cancer: a cohort study in the French National Healthcare Insurance Database. Diabetologia 55(3), 644–653 (2012).
- Lind M, Fahlen M, Eliasson B, Oden A. The relationship between the exposure time of insulin glargine and risk of breast and prostate cancer: an observational study of the time-dependent effects of antidiabetic treatments in patients with diabetes. Prim. Care Diabetes 6(1), 53–59 (2012).
- van Staa TP, Patel D, Gallagher AM, de Bruin ML. Glucose-lowering agents and the patterns of risk for cancer: a study with the General Practice Research Database and secondary care data. Diabetologia 55(3), 654–665 (2012).
- Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with Type 2 diabetes. Diabetes Metab. Res. Rev. 25(6), 542–548 (2009).
- Swinnen SG, Dain MP, Aronson R et al. A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with Type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care 33(6), 1176–1178 (2010).
Websites
- Levemir: Epar European Medical Association. Levemir: EPAR – Scientific Discussion. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000528/WC500036658.pdf (Accessed 22 April 2011)
- Us Food and Drug Administration. FDA Drug Safety Communication: Update to ongoing safety review of Lantus (insulin glargine) and possible risk of cancer. www.fda.gov/Drugs/DrugSafety/ucm239376.htm (Accessed 23 February 2011)
- European Medicine Agency. European Medicines Agency update on safety of insulin glargine. www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/2011/WC500010326.pdf (Accessed 5 June 2011)