Abstract
The science of reproductive endocrinology/in vitro fertilization (IVF) has moved forward considerably since the first IVF baby was born in 1978. IVF was originally indicated for women with tubal factor infertility, but it has now become the treatment for couples with unexplained subfertility, male subfertility, cervical factor, failed ovulation induction, endometriosis or unilateral tubal pathology. IVF was initially performed with the single dominant ovarian follicle produced during a spontaneous menstrual cycle. This was very inefficient and pregnancy rates were dismal. Consequently, superovulation protocols using parenteral gonadotrophins to induce maturation of multiple follicles were soon adopted worldwide. In addition, any supernumerary embryos remaining after embryo transfer may be cryopreserved for future embryo transfers without the need for another fresh IVF cycle. A greater understanding of IVF endocrinology has led to improved IVF pregnancy outcomes and satisfaction for the anxious parents. However, with the greater success of IVF treatment, new complications associated with the treatment arise, namely the ovarian hyperstimulation syndrome. Ovarian hyperstimulation can be associated with severe morbidity and may be even fatal. Ovarian hyperstimulation syndrome is an iatrogenic condition secondary to medical stimulation of the ovary, and was virtually unknown until IVF treatment was initiated. This article will discuss the recent developments in IVF treatment endocrinology and protocols, as well as prevention/treatment of its complications.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertendo (4) view/print certificate.
Release date: 30 July 2012; Expiration date: 30 July 2013
Learning objectives
Upon completion of this activity, participants will be able to:
• Compare the use of gonadotropin-releasing hormone agonists and antagonists in IVF
• Analyze the use of gonadotropins in IVF
• Assess treatment of the luteal phase during IVF
• Evaluate other endocrine issues in IVF
Financial & competing interests disclosure
PUBLISHER
Elisa Manzotti
Publisher, Future Science Group, London, UK.
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Charles P Vega, MD
Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine, CA, USA.
Disclosure: Charles P Vega, MD, has disclosed no relevant financial relationships.
AUTHORS AND CREDENTIALS
Chun Ng, MRCOG
Consultant in Reproductive Medicine and Surgery, Hammersmith Hospital, Du Cane Road, London, W12 0HS, UK.
Disclosure: Chun Ng, MRCOG, has disclosed no relevant financial relationships.
Geoffrey Trew, MRCOG
Consultant in Reproductive Medicine and Surgery, Hammersmith Hospital, Du Cane Road, London, W12 0HS, UK.
Disclosure: Geoffrey Trew, MRCOG, has disclosed no relevant financial relationships.
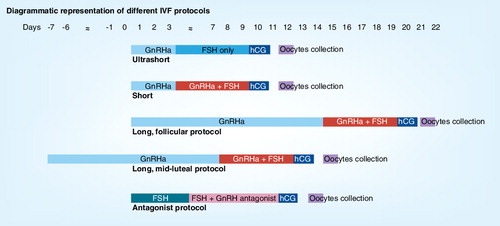
IVF protocols using gonadotrophin-releasing hormone agonist
In vitro fertilization (IVF) cycles generally aim to produce several embryos to improve the chances of success. Initially, the ovaries are stimulated with individualized doses of gonadotrophins alone in order to produce more than one oocyte. However, there is a risk of a premature surge of luteinizing hormone (LH), which could disrupt normal follicle and oocyte development, making IVF treatment less successful. The inclusion of gonadotrophin-releasing hormone agonists (GnRHa) in ovarian stimulation protocols has been used to reversibly block pituitary function and prevent a premature LH surge Citation[1]. The use of GnRHa has resulted in improvements in IVF treatment, including decreased cancellation of treatment cycles and higher pregnancy rates Citation[2].
There are three main protocols involving the administration of GnRH: short, ultrashort and long protocols . The short and ultrashort protocols involve the administration of GnRHa from day 1 or day 2 of the cycle for 3 days in the ultrashort protocol (hence using only the flare-up effect) and until human chorionic gonadotrophin (hCG) injection in the short protocol. Another alternative scheme using the flare-up effect is the microdose flare protocol. The long protocol involves the administration of GnRHa for at least 14 days to achieve ovarian activity suppression before the gonadotrophin is administered Citation[1]. There are two long protocol regimens: the long luteal phase protocol starts GnRHa from the mid-luteal phase of the previous menstrual cycle, whereas the long follicular phase protocol starts GnRHa from the first day of the menstrual cycle. The GnRHa and gonadotrophins are continued until sufficient ovarian follicles are available, while hCG is administered before oocyte collection.
Various studies have been published to determine the most effective GnRHa protocol as an adjuvant to gonadotrophins in IVF cycles. The overall suggestion from systematic review and meta-analysis is that the use of the long protocol is associated with a statistically significant higher clinical pregnancy rate per cycle started than with a short protocol. There is also evidence of an increased number of oocytes obtained in the long protocol compared with the short protocol. However, a higher dose of gonadotrophins was required in the long protocol Citation[1]. When choosing between the two long protocols (either the mid-luteal or the follicular phase protocol), a study by Jenkins demonstrated the initiation of the follicular phase of the long protocol to be associated with a higher risk of functional cysts Citation[3]. There is controversy over whether cysts are associated with poorer outcome Citation[1]. For the poor responders, the microdose flare protocol has been frequently advocated. The basic hypothesis is to administer the minimal dose of GnRHa necessary to induce gonadotrophin release while minimizing premature ovulation Citation[4,5]. However, the number of trials comparing microdose flare regimen to other approaches are limited. A small randomized trial by Kahraman et al. has shown that the microdose flare protocol has similar efficacy to that of the multiple-dose GnRH antagonist protocol in poor responders Citation[6].
IVF protocols using GnRH antagonists
The long GnRHa protocol has proven to be an effective IVF protocol, and has played an important role in reducing the incidence of premature LH surges by reversibly blocking pituitary gonadotrophin secretion. However, the long protocol requires approximately 2–3 weeks for ovarian desensitization to succeed, with relatively high financial and timely cost owing to an increased requirement for gonadotrophin injections and the need for hormonal and follicular ultrasonographic measurements Citation[7].
GnRH antagonists have emerged as an alternative in preventing premature LH surges. GnRH antagonists can be used to prevent a LH surge during controlled ovarian hyperstimulation without the hypo-estrogenic side effects or long downregulation period associated with GnRHa Citation[8]. GnRH antagonists act by binding competitively to the GnRH receptors, thereby preventing endogenous GnRH from exerting its stimulatory effects on the pituitary cells. The competitive blockade of the receptors leads to an immediate arrest of gonadotrophin secretion. This property allows their use at any time during the follicular phase.
Initially, two general approaches emerged in GnRH antagonist administration: the single-dose protocol, in which one cetrorelix injection (Cetrotide® 3 mg [Merck Serono S.A., Geneva, Switzerland]) is administered later in the follicular phase around stimulation day 7 or 8, and the multiple-dose regimen (cetrorelix or ganirelix 0.25 µg), in which the antagonist is administered daily from stimulation day 6 onwards (fixed) Citation[9]. Subsequently, further variations were introduced. In order to reduce the number of antagonist injections and the duration of stimulation, flexible protocols were developed. Instead of starting with the GnRH antagonist on a fixed day, timing of administration was made dependent on the follicular size. GnRH antagonist injections were started as soon as the follicles reached a size of more than 14, 15 or 16 mm in diameter after 5 days of stimulation Citation[9]. The initiation of the GnRH antagonist protocol relies on the occurrence of spontaneous menses, which may make it difficult to schedule the IVF treatment to meet the organizational needs of both the patients and the treatment centre, which might have important economic and practical implications. To counter this particular problem, oral contraceptive pill pretreatment in GnRH antagonist cycles has been advocated for scheduling oocyte retrieval to the convenience of all concerned Citation[10].
In general, most investigators have found more favorable results with the multiple-dose protocol than with the single-dose protocol Citation[11]. The single-dose protocol may result in greater suppression of serum LH than multiple-dose protocol, and was further associated with shorter duration of gonadotrophin use, smaller number of developing follicles, lower serum estradiol levels on the day of hCG administration and a smaller number of zygotes Citation[12]. When the fixed GnRH antagonist protocol was compared with a flexible protocol, a meta-analysis has shown no statistically significant difference in pregnancy rate per women randomized, although there was a trend towards higher pregnancy rate with the fixed protocol Citation[13]. However, there was a statistically significant reduction in the number of antagonist ampoules and the amount of gonadotrophins used in the flexible protocol.
The earliest reported results of comparative studies with long GnRHa protocols and meta-analysis indicated 5% less clinical pregnancies in the antagonist group Citation[14]. Together with the results of a large national database, evaluations were not in favor of the GnRH antagonist, making it the second choice for many clinicians Citation[15]. However, GnRH antagonists were found to be associated with significantly less OHSS Citation[14,16,17].
However, the most recent systematic review and meta-analysis (2011) have found that there are no significant differences demonstrated in live birth rate and ongoing pregnancy rate between GnRHa and GnRH antagonists downregulation protocols Citation[8]. With regards to safety, GnRH antagonists continue to result in a significantly reduced incidence of OHSS. Changes in the findings of recent meta-analyses can possibly be explained by factors that may have changed over time, such as the type of GnRH antagonist protocols used, the incidence of LH instability, oral contraceptive pill pretreatment, or better patient selection or allocation. Furthermore, improved learning curve, with the relatively new GnRH antagonists over recent years and extensive experience in large studies leading to more favorable outcomes, may have influenced pregnancy outcomes Citation[8].
Gonadotrophins used in IVF
The first phase of IVF treatment consists of ovarian hyperstimulation to produce multiple ovarian follicles. This is done using either human menopausal gonadotrophins (hMGs), produced from the urine of menopausal women or recombinant follicle-stimulating hormone (rFSH), produced using recombinant technology. The most important difference between these two gonadotrophins is that, unlike rFSH preparations, hMG contains both FSH and LH activity Citation[18]. Since the 1980s, a variety of urinary gonadotrophins have been produced, such as purified FSH that contains less than 1 international unit (IU) of LH per 75 IU of FSH. The third-generation urinary gonadotrophins are highly purified FSH with less than 0.1 IU of LH per 75 IU of FSH Citation[19]. While both hMG and rFSH have been shown to achieve follicular development in ovarian stimulation for IVF, much remains to be understood about the differential effects between the two gonadotrophins’ preparations Citation[18]. The clinical issue of whether there is any real difference in live birth rates between hMG and rFSH in ovarian stimulation for IVF using the most commonly used protocols, has been widely discussed Citation[20].
Data from the European and Israeli Study Group Citation[21] suggest that the additional LH activity with hMG may improve ongoing pregnancy rates in IVF Citation[21], while evidence from the MERiT trial indicates that LH activity contributes towards an endocrine profile that is conducive to better embryo quality and/or endometrial receptivity Citation[22]. However, the latest Cochrane review comparing rFSH to any of the other gonadotrophins, irrespective of the downregulation protocol used, did not result in any evidence of a statistically significant difference in live birth rate, OHSS or clinical pregnancy as a whole Citation[18]. It therefore appears that all available gonadotrophins are equally effective. The choice of one or the other product depends upon the availability of the product, the convenience of its use and the associated cost Citation[18].
The ovarian stimulation regimens described above require daily injections of gonadotrophins by the patient for up to 3 weeks. This can be inconvenient and may contribute to high levels of stress and anxiety for the patient. Corifollitropin α (Elonva®) was developed by Schering-Plough Corp. (NJ, USA) as an injectable long-acting FSH agonist. It is a fusion product of human FSH and the C-terminal peptide of the β-subunit of hCG produced by recombinant DNA. It has the same pharmacologic activity as FSH but with slower absorption and a twofold longer half-life, thus requiring less frequent dosing Citation[23,24]. This allows a single injection of corifollitropin α to effectively replace the seven daily injections of FSH Citation[23]. Corifollitropin α is indicated as a multifollicular stimulant for women undergoing controlled ovarian stimulation using GnRH antagonist protocols.
In the Phase III trial of corifollitropin α, a single subcutaneous injection of corifollitropin α was no less effective as a multifollicular stimulant than seven once-daily injections of rFSH when used as part of a GnRH antagonist-assisted controlled ovarian stimulation cycle Citation[24]. The mean number of oocytes retrieved per cycle demonstrated corifollitropin α to be equivalent to rFSH. The median duration of stimulation was between 9 and 11 days Citation[23]. Compared with rFSH, patients using corifollitropin α required a further 2 days of stimulation with rFSH on top of corifollitropin α prior to triggering oocyte maturation with the administration of hCG.
In the most recent systematic review and meta-analysis, there was no evidence of a statistically significant difference in ongoing pregnancy rate for corifollitropin α versus rFSH. There was evidence of increased ovarian response with significantly higher numbers of oocytes, and obtained embryos, and a smaller dose of FSH used compared with women stimulated with daily rFSH injections Citation[25]. However, corifollitropin α was associated with a higher cycle cancellation rate than daily rFSH. The risk of cancellations due to high ovarian response and the risk of OHSS were fivefold higher in the corifollitropin α group than in the rFSH group Citation[25]. The higher ovarian response and risk of OHSS with corifollitropin α could be explained by the sustained and higher FSH immuno-reactivity concentrations and the inability for dose adjustment after treatment with a single dose of corifollitropin α compared with the daily rFSH regimen, leading to a rapid increase of serum estradiol and inhibin levels and the development of more medium-sized follicles with corifollitropin α Citation[23,26]. Furthermore, more multiple pregnancies were found in the corifollitropin α group compared with that of the daily rFSH group, although the differences were not statistically significant Citation[25]. In general, corifollitropin α was well tolerated with no significant difference. The most commonly reported side effects were pelvic pain, discomfort, headache and drug-related local or systemic reactions Citation[23,25].
However, the current trials do not provide information regarding the use of corifollitropin α in anticipated hyper- and poor-responders to gonadotrophin stimulation. Further data on long-term complications and congenital malformation for the fetus or newborn are lacking. It is, therefore, currently unknown whether the child or mother may experience any consequences in the future. Corifollitropin α is not used in long agonist protocols because a larger cohort of follicles is expected with a higher risk of OHSS and possibly higher risk of cancellations Citation[24]. Another main problem with corifollitropin α is that dose adjustments cannot be made in patients with a low response or in patients with a risk of high response such as women with polycystic ovarian syndrome (PCOS).
Despite the availability of evidence concerning the usage of rFSH alone in IVF treatment, the role of LH in the follicular phase of IVF cycles remains a matter of controversy. There are debates still persisting with regard to the inadequacy of rFSH alone in ensuring a satisfactory IVF cycle. According to the two-cell, two-gonadotrophin theory, only FSH is essential for triggering antral follicle formation and follicular growth, but some LH is essential in the preantral stage (follicle size <10 mm) to stimulate secretion of androgens by thecal cells Citation[27]. Meanwhile, recombinant LH (rLH) entered the market, which can serve in combination with rFSH as an alternative to hMG. rLH is analogous to endogenous LH and is characterized by high purity, precision of dosing and consistency. When administered by subcutaneous injection, rLH has a half-life of 24 h and exhibits modest accumulation with an accumulation ratio of 1.6 ± 0.8 Citation[28].
In a subcategory of patients with hypogonadotropic hypogonadism, the requirement of LH add-back was evidently demonstrated by Shoham et al. for an adequate steroidogenesis, fertilization and implantation Citation[29]. In normogonadotropic patients, evidence from meta-analysis suggests that low endogenous LH levels that occur during ovarian stimulation for IVF using GnRHa protocols are not associated with a decreased probability of ongoing pregnancy beyond 12 weeks Citation[30]. However, there are some data from early studies suggesting that exogenous LH may offer added clinical benefits to certain subgroups of patients using GnRHa, such as women aged >35 years and patients with an initial suboptimal response to gonadotrophin stimulation Citation[31–34]. However, randomized controlled trials have shown that exogenous LH does not offer any benefit on birth rate, clinical pregnancy rate, ongoing pregnancy rate or number of retrieved oocytes in patients undergoing IVF with GnRH antagonist protocols Citation[35–37]. A recent randomized trial has shown that rLH significantly increased the implantation rate in patients aged between 36 and 39 years. A better ongoing pregnancy rate per cycle was observed, although this is not statistically significant. Patients younger than 36 years of age did not obtain any benefit from rLH administration Citation[38].
Mild ovarian stimulation for IVF
Current regimens for ovarian stimulation in IVF are complex and expensive, requiring several weeks of daily injections of medications, and intense ovarian response monitoring is usually required to ensure patient safety and successful treatment. Other negative effects associated with ovarian stimulation include emotional stress, high drop-out rates and abdominal discomfort Citation[39]. Concerns were expressed regarding contemporary ovarian stimulation approaches for IVF, and there have been calls for the use of milder stimulation protocols Citation[40]. The International Society for Mild Approaches in Assisted Reproduction defined mild ovarian stimulation for IVF as the method when FSH or hMG is administered at lower doses, and/or when oral compounds (antiestrogens or aromatase inhibitors) are used, either alone or in combination with gonadotrophins. hCG injection and luteal support are also administered. The aim is to collect two to seven oocytes (based on clinical experience and results of mild IVF within the International Society for Mild Approaches in Assisted Reproduction consensus group) Citation[41].
In mild ovarian stimulation, lower doses and fewer days of gonadotrophins are used, consequently resulting in a less complex treatment, hence improving compliance. This diminishes patient distress and complications such as OHSS, and may reduce the need for frequent visits to the clinic resulting from intense monitoring of ovarian response Citation[42]. Mild stimulation protocols have been shown to decrease drop-out rates and to allow higher acceptance of repetitive IVF cycles Citation[43]. Medication costs per cycle were also significantly less, but this should be viewed in the context of reduced pregnancy rates per cycle. Over 1 year of treatment, medication costs were similar comparing mild versus standard stimulation. When mild stimulation is combined with a single-embryo transfer policy, costs associated with pregnancy complications were dramatically decreased Citation[44].
However, despite the above advantages of mild ovarian stimulation protocols, progress in their use has been slow and the concept has been accepted by only a handful of practitioners Citation[42]. Mild stimulation protocols were found to be associated with lower pregnancy rates per cycle, although term birth rates after 1 year were similar Citation[45]. There are also pressures on clinicians and patients alike to achieve the best results in each stimulation cycle. Another barrier to the uptake of mild stimulation protocols is the ever-increasing age of women with infertility who seek IVF. Dose-finding trials for the stimulation of gonadotrophins generally do not involve older women; mild ovarian stimulation has not yet been tested in women who are older than 38 years of age Citation[42]. The funding of IVF cycles is also a barrier to mild stimulation. The majority of IVF cycles are paid for per stimulated cycle. As it takes more IVF cycles to achieve an equivalent live birth rate, the financial burden, either publically or privately funded, dissuades the financier from adopting the mild stimulation cycle Citation[42].
Final oocyte maturation triggering in IVF
The LH surge is essential in the final stages of oocyte maturation and for triggering follicle rupture with expulsion of the oocyte from the ovarian follicle and its capture by the fimbria of the fallopian tube. In addition, the LH surge promotes luteinization to form an active corpus luteum. These effects of LH are essential for conception to occur Citation[19]. In an IVF treatment cycle, urinary hCG has been used to mimic the endogenous LH surge. There are structural similarities between hCG and human LH and hence both hormones stimulate the same receptor Citation[46]. However, early urinary preparations were associated with a number of disadvantages that include being an uncontrolled source, lack of purity and batch-to-batch variation in activity, leading to variable results Citation[47]. Recently, recombinant technology has allowed the production of recombinant hCG and rLH with high purity and batch-to-batch consistency. However, a review of the efficacy and safety of the recombinant hCG compared with urinary hCG in patients undergoing IVF treatment cycles found no evidence of statistically significant differences between recombinant hCG or rLH and urinary hCG with respect to the ongoing pregnancy or live birth rate, clinical pregnancy rate, miscarriages and the number of retrieved oocytes or incidence of OHSS Citation[19].
When GnRH antagonists were introduced for the prevention of a premature LH surge in IVF treatment, it became possible to trigger final oocyte maturation and ovulation with a single bolus dose of GnRHa as an alternative to hCG. The GnRH antagonist occupies the GnRH receptor without causing downregulation, and once GnRHa displaces the GnRH antagonist from the receptor, the receptor becomes activated, inducing a release of gonadotrophins. Several studies have demonstrated the feasibility of inducing an endogenous LH surge by administering a bolus dose of GnRHa in a GnRH antagonist protocol Citation[48,49]. This may be particularly useful in patients who are at high risk for OHSS.
A Cochrane systematic review and meta-analysis found oocyte triggering with GnRHa to be associated with a significantly reduced risk of OHSS compared with hCG triggering. However, this reduction of OHSS was at the expense of pregnancy outcome. The GnRHa triggering was associated with a significantly lower live birth rate, a reduced ongoing pregnancy rate and a higher miscarriage rate in fresh autologous embryo transfer cycles Citation[50]. The group also found that luteal phase support using different regimens could not compensate for these lower reproductive outcomes in GnRHa-triggered cycles.
Luteal phase in IVF
Following ovulation, the luteal phase of a natural cycle is characterized by the formation of a corpus luteum, which secretes steroid hormones including progesterone and estrogen Citation[51]. Progesterone in turn induces the secretory transformation of the endometrium, preparing it for implantation by thickening and increasing vascularization, in order to facilitate implantation. If conception and implantation occur, the developing trophoblastic tissue of the placenta secretes hCG, which in turn maintains the corpus luteum and its secretion Citation[52,53].
The luteal phase in all stimulated IVF cycles is deficient Citation[54]. Currently, the etiology of luteal phase deficiency in IVF cycles is thought to be due to high steroid levels Citation[55]. The steroid levels are high because of the multiple corpus luteum, which results in higher cumulative steroids than in a natural cycle. This leads to a negative feedback effect on the pituitary gland and lowers LH levels, resulting in premature luteolysis. The consequence of premature luteolysis is reduced chances in pregnancy Citation[56].
Adequate luteal phase support is therefore necessary during IVF to ensure implantation and pregnancy. There have been various methods suggested, but there is no general agreement on the best protocol Citation[55]. These methods include administering hCG Citation[57,58] or progesterone orally Citation[59], vaginally Citation[60,61], rectally Citation[62] or intramuscularly Citation[63]. Meta-analysis and systematic reviews have shown that progesterone administrated via the of vaginal, rectal and intramuscular routes give comparable results for implantation and pregnancy rates in fresh IVF cycles Citation[55,56,62]. Oral micronized progesterone was not found to be efficient for supporting the luteal phase of stimulated IVF cycles due to reduced bioavailability, as it is subjected to first-pass prehepatic and hepatic metabolism Citation[55].
Other authors further raised the possible role of adding estrogen to the progesterone to improve implantation rates Citation[51]. In natural cycles, the corpus luteum not only produces progesterone, but also estrogen and other steroid hormones. Under progesterone supplementations, it has been shown that mid-luteal estradiol concentrations decrease in a proportion of patients, and this may be associated with a concomitant decrease in pregnancy rates Citation[64]. However, evidence has shown that routine addition of estrogen to progesterone-supported luteal phases in GnRHa and GnRH antagonist cycles does not improve IVF outcomes Citation[56,65,66].
Other suggested methods of luteal phase support include hCG and GnRHa. A meta-analysis by Nosarka et al. demonstrated that the use of hCG seemed to be superior compared with that of progesterone Citation[57]. However, the use of hCG also results in a significant increase in OHSS compared with other treatments Citation[51]. Therefore, due to the hyperstimulation risk, luteal support with hCG should be avoided if the specific patient is at risk of hyperstimulation syndrome Citation[56,67,68].
GnRHa was previously suggested as a novel luteal phase support that may act at the level of pituitary gonadotrophs, the endometrium and the embryo itself Citation[69]. It is hypothesized that GnRHa may support the corpus luteum by stimulating the secretion of LH by gonadotroph cells or by acting directly on the endometrium through the locally expressed GnRH receptors Citation[70]. A randomized trial by Tesarik et al. demonstrated that GnRHa administered for 6 days following intracytoplasmic sperm injection in both GnRHa and GnRH antagonist cycles resulted in significant improvement of implantation and live birth rates compared with placebo Citation[69]. Luteal phase GnRHa administration also increased luteal phase serum hCG, estradiol and progesterone concentrations. However, there are concerns regarding adverse effects on the oocytes and, more importantly, the embryos Citation[71]. Further trials are awaited in this regard.
PCOS & IVF
PCOS is one of the most common endocrinopathies, affecting 5–10% of women of reproductive age Citation[72–74].
PCOS is surrounded by controversies regarding both its diagnosis and treatment Citation[75]. To overcome the inconsistencies surrounding the definition of PCOS, experts in PCOS from all over the world convened in Rotterdam, The Netherlands, in 2003 and arrived at a consensus regarding the diagnosis of the syndrome. The meeting was endorsed by the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine, and its proceedings were published in Fertility and Sterility and in Human Reproduction Citation[72,76]. Although significant progress has been made towards the development of universally accepted diagnostic criteria for PCOS, the optimal treatment for infertile women with PCOS has not yet been defined. Various interventions have been proposed for patients with PCOS in general; however in patients undergoing IVF, the interventions represented a therapeutic challenge. IVF is indicated in women with PCOS who have associated pathologies, or following failed ovulation induction or unsuccessful intrauterine insemination cycles. These pathologies include tubal damage, severe endometriosis, preimplantation genetic diagnosis and male factor infertility Citation[75].
The response of women with PCOS to IVF treatment is often different from women with normal ovaries. Several stimulation protocols have been published for the treatment of patients with PCOS undergoing IVF. The most standard protocol that has been used is the long desensitization GnRHa protocol associated with FSH Citation[75,77]. However, a meta-analysis has shown that the cycle cancellation rate is significantly increased in patients with PCOS Citation[78]. Duration of stimulation is significantly longer in patients with PCOS, even when the daily dose of FSH is similar to that of women without PCOS. Significantly more oocytes per retrieval were obtained in PCOS patients, but fertilization rates were similar to with women without PCOS. With regard to the probability of pregnancy, the clinical pregnancy rate per cycle started was similar between PCOS and non-PCOS patients Citation[75]. The same was true for pregnancy rates per oocyte retrieval and embryo transfer.
Over the past 15 years, it has become increasingly recognized that insulin resistance is central to the pathogenesis of PCOS Citation[73]. Metformin, a biguanide insulin-lowering agent, has been extensively investigated in the management of PCOS. Suppression of insulin levels with metformin may reduce the hyperinsulinemia and hyperandrogenism suppression of the ovarian response Citation[79]. Previous systematic reviews have demonstrated that metformin improves the reproductive function of some women with PCOS Citation[80,81]. However, studies have shown that for PCOS patients undergoing IVF treatment, short-term cotreatment with metformin neither enhances the response to the stimulation nor improves the fertilization rate, but does improve the pregnancy outcome Citation[82]. In addition, more recent studies and reviews have shown that cotreatment with metformin before or during IVF cycles does not improve live birth rates, pregnancy rates or severe OHSS Citation[79,83]
OHSS & IVF
One of the problems encountered by PCOS patients undergoing IVF treatment is the higher risk of developing OHSS. OHSS is a serious iatrogenic complication of ovarian stimulation that is triggered by exogenous administration and/or endogenous production of hCG. The high sensitivity of PCOS patients to gonadotropic stimulation is probably related to the fact that polycystic ovaries twice the number of available FSH-sensitive antral follicles in their follicular cohort than normal ovaries Citation[84]. OHSS may result in significant morbidity and, very rarely, mortality Citation[85]. The pathophysiology of OHSS remains unclear. OHSS affects approximately 14% of women undergoing controlled ovarian hyperstimulation Citation[86]. Severe OHSS affects approximately 2–5% of women, and is characterized by growth of multiple, large follicles with a massive extravascular protein-rich fluid shift. This may lead to hypovolemia, hemoconcentration, oliguria and electrolyte disturbance Citation[87]. Prevention of OHSS can be attempted at different points of IVF treatment. Primary prevention could be implemented before the treatment, by identifying known risk factors and thus high-risk patients who can benefit from specific preventive strategies. Primary prevention could also be proposed before treatment by careful selection of appropriate stimulation protocols and dosage of the gonadotrophins in patients with an identified risk of high response. Secondary prevention can be undertaken during treatment by applying preventive measures when an exaggerated ovarian response occurs Citation[88].
The choice of the gonadotrophin starting dose is an important parameter to prevent the onset of OHSS. The use of low-dose stimulation protocols seems to reduce the incidence of OHSS in patients with PCOS Citation[89]. The starting dose of gonadotrophins is usually chosen based on the clinical judgment and on parameters known to influence the ovarian response such as age, BMI, presence of PCOS and history of OHSS or high response Citation[88]. The choice of urinary hMG or rFSH does not seem to influence the incidence of OHSS Citation[90]. Cotreatment with metformin for PCOS patients undergoing IVF treatment has already been discussed.
The introduction of the GnRH antagonist protocol in the general IVF population has been shown to be associated with a significantly lower risk of OHSS compared with the long GnRHa protocol Citation[8]. Due to these findings, the GnRH antagonist protocol has been suggested as a safer treatment option for women with PCOS who are at high risk for OHSS. The most recent systematic review and meta-analysis of a GnRH antagonist compared with the long GnRHa protocol in women with PCOS undergoing IVF confirmed the association of a significantly lower risk of pooled moderate and severe cases of OHSS when the antagonist protocol was used Citation[91]. The use of GnRHa triggers in antagonist cycles, (thereby replacing the use of hCG), as a trigger in high-risk patients is also an additional strategy to reduce rates of severe OHSS.
Anti-Müllerian hormone & IVF
Anti-Müllerian hormone (AMH), also known as Müllerian inhibiting substance, is a homodimeric glycoprotein linked by disulfide bonds with a molecular weight of 140 kDa Citation[92]. The hormone belongs to the TGF-β superfamily. In females, AMH is produced by the granulosa cells of follicles – from the stage of the primary follicle to the initial formation of the antrum. In female neonates, AMH is virtually undetectable but increases gradually until puberty and remains relatively stable thereafter and throughout the reproductive period Citation[93]. AMH levels measured through a full menstrual cycle do not show fluctuation patterns, in contrast to levels of FSH, LH and estradiol Citation[94,95]. Serum AMH levels show a reduction throughout the reproductive life cycle and are undetectable after menopause Citation[96].
It is widely accepted that the reduction of AMH levels in serum is the first indication of a decline in the follicular reserve of the ovaries Citation[95]. AMH levels highly correlate with the follicle numbers and therefore constitute an important marker for individual ovarian reserve assessment Citation[97]. Systematic reviews and meta-analyses have shown that AMH is a reliable predictor of poor response to ovarian stimulation for IVF Citation[98–103]. It has been demonstrated that AMH is a better marker in the prediction of poor response than basal FSH, but performs equally well compared with the antral follicle count Citation[97]. AMH has also been shown to be a good predictor of an excessive ovarian hyperstimulation response in IVF cycles when compared with female age, BMI and basal FSH or inhibin B Citation[102,104,105]. However, AMH has been shown to be a poor predictor of pregnancy outcome and therefore, it is unsuitable to be used clinically in this respect Citation[98]. Another use of AMH in clinical situations is to tailor the stimulation protocol to the patient. One study has shown that AMH can be used to individualize FSH dosage in IVF cycles. This usage of AMH could result in reduced risk of OHSS and decreased treatment burden, while still maintaining pregnancy rates Citation[106]. It also provides guidance for a more appropriate higher dose of gonadotrophin for patients with a reduced AMH level.
Growth hormone & IVF
Growth hormone is a biological peptide hormone that is synthesized, stored and secreted by somatotroph cells located in the anterior pituitary gland. Growth hormone can be synthetically produced using recombinant DNA technology and is licensed to be used in the human population. Growth hormone is reported to modulate the action of FSH on granulosa cells by upregulating the local synthesis of IGF-1 Citation[107]. This interest has been stimulated by animal studies that suggest that growth hormone may increase the intraovarian production of IGF-1 Citation[108]. IGF-1 displays growth hormone dependence both in vivo and in vitro Citation[109]. The addition of IGF-1 to gonadotrophins in granulosa cell cultures increased the gonadotrophin activity on the ovary by several mechanisms, including augmentation of aromatase activity, 17β-estradiol and progesterone production, and LH receptor formation Citation[110].
Homburg et al. have shown that cotreatment with biosynthetic human growth hormone sensitizes the human ovary to the stimulatory effect of treatment with gonadotrophins Citation[111]. They later reconfirmed the positive role of growth hormone in augmentation of ovarian response by a randomized, double-blind, placebo-controlled trial Citation[112]. However, other studies have failed to show a significant influence of growth hormone supplementation on follicular recruitment and estradiol secretion by mature follicles and oocyte yield Citation[113].
A recent meta-analysis by Kolibianakis et al. has shown that the addition of growth hormones increases the probability of clinical pregnancy and live birth in poor responders undergoing ovarian stimulation with GnRH analoges and gonadotrophins for IVF Citation[114]. The improvement in clinical pregnancy and live birth rates was also demonstrated in a previous meta-analysis published by the Cochrane group in 2003 Citation[115]. It is not clear by what mechanism the growth hormone addition increases the probability of pregnancy. A plausible explanation might be that growth hormone addition is associated with an increased proportion of patients who reach embryo transfer and are thus exposed to the chance of pregnancy Citation[114]. Further systematic review and meta-analysis by the most recent Cochrane group demonstrated no difference in IVF outcome measures and adverse events in the routine use of growth hormone adjuvant therapy in IVF Citation[107]. However, the group demonstrated a statistically significant difference in both birth rates and pregnancy rates favoring the use of growth hormone adjuvant therapy in IVF women who are considered poor responders without increasing adverse events. The report further emphasized the difficulty in defining the subgroup of poor responders that may benefit the most from growth hormone adjuvant therapy in IVF, owing to the diverse definitions of poor responder used in the studies. The subgroup analysis demonstrated a statistically significant difference in pregnancy rates favoring the use of growth hormone adjuvant therapy in women who are considered poor responders owing to previous suboptimal response following controlled ovarian stimulation without increasing adverse events.
The conclusion and implication for practice from the Cochrane group is that in women undergoing IVF, who are not considered poor responders, there is no evidence from randomized controlled trials to support the use of growth hormone. In women who are considered poor responders, the use of growth hormone has been shown to significantly improve the live birth and pregnancy rates. However, the group advised caution in interpreting their result, due to the small number of trials analyzed, and each trial was small with significant clinical heterogeneity.
Dehydroepiandrosterone & IVF
Casson et al. first suggested the therapeutic benefit of dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve Citation[116]. They observed that DHEA does appear to augment ovulation induction in poor responders, particularly in patients who are 35–40 years of age and have normal FSH concentrations. They also suggested that in micronized form the androgen offers potential for postmenopausal steroid replacement, adjunctive to estrogen Citation[117].
The initial therapeutic use of DHEA in patients with diminished ovarian reserve was motivated by observed increases in IGF-1 after DHEA supplementation Citation[118]. As growth hormone had been suggested to improve the yield of oocytes via IGF-1, DHEA has been hypothetized to be able to achieve the same effect Citation[119]. Unfortunately, a randomized clinical trial of DHEA in the USA (ClinicalTrials.gov identifier: NCT00419913 Citation[201]) had to be abandoned due to unwillingness of patients to participate in the trial. A multicenter European trial involving centers in Austria, Switzerland and the Czech Republic, designed in follow-up to the cancelled US trial, also had to be abandoned due to the same reason Citation[119]. This is hardly surprising, as the patients have a very limited time left for conception to occur. However, an Israeli group managed to complete a small, prospective, randomized study Citation[120]. Most other published data on DHEA supplementation are mainly comprised of case series or case reports. There is evidence showing that DHEA treatment increases oocyte and embryo numbers Citation[121]. A review of all of the published data on DHEA by Gleicher et al. suggests that DHEA improves ovarian function, increases pregnancy chances and, by reducing aneuploidy, lowers miscarriage rates Citation[119]. Over time, DHEA also appears to objectively improve ovarian reserve. However, a very recent systematic review and meta-analysis by Bosdou et al. has shown that DHEA has no significant effect on clinical pregnancy and live birth rates in poor responders undergoing IVF Citation[122]. Animal data support support the fact that androgens promote preantral follicle growth and reduction in follicle atresia Citation[123]. Improvement of oocyte or embryo quality with DHEA supplementation potentially suggests a new concept of ovarian aging, where ovarian environments, but not oocytes themselves, age. DHEA may therefore, represent a first agent beneficially affecting the aging ovarian environment Citation[119]. Considering the absence of significant side effects and, at least within the USA, the availability of DHEA as a food supplement, the data are leaning towards a favorable effect in diminished ovarian reserved population and IVF patients. The possible side effects of DHEA include acne, deepening of the voice and facial hair growth Citation[124].
Further data on the effect of DHEA in IVF patients are awaited, and further definition of the best-suited patient population for such treatment, maximizing effective treatment protocols and best delivery systems, are needed before DHEA is universally accepted as a treatment in the IVF population.
Conclusion
Success rates in IVF continue to improve as we gain greater insight into the medication used and the endocrinology of IVF. Other parameters in IVF treatment such as cryopreservation and oocytes’ donation also continue to be better understood and improved. Mild ovarian stimulation protocols should also be considered in the future, although difficult economic times may counter that consideration. Overall, these improvements will have a cumulative effect in the success of treatment cycles.
Expert commentary
This review has aimed to evaluate the evidence of recent developments in the IVF treatment protocol, focusing mainly on the hormonal medications used in IVF. Each protocol was dissected and each stage in the IVF treatment was evaluated to give the evidence available for best practice. The first phase of IVF involved ovarian stimulation, and evidence showed that all available gonadotrophins are equally effective. The standard long GnRHa protocol was the most efficient IVF protocol, but new evidence is emerging indicating the GnRH antagonist protocol to be equally effective. The GnRH antagonist protocol is also found to be associated with fewer incidences of OHSS, which is associated with debilitating morbidity and occasional mortality. The final oocyte maturation process is done by administering hCG; both urinary and recombinant hCG are equally effective, but the recombinant hCG is easier to use and less prone to errors as it is in a prefilled syringe. For patients at risk of OHSS, luteal support should be with progesterone and not hCG. PCOS patients undergoing IVF should be informed and warned about OHSS, for which the GnRH antagonist protocol could be considered a treatment option. Clinicians should be cautious and wary of OHSS if their patients have PCOS. For older patients who may have declining ovarian reserve, Anti-Müllerian hormone measurement may be advocated, as it is a reliable predictor of poor response to ovarian stimulation in IVF. In poor responders, there is some evidence showing growth hormone addition to be associated with improved clinical pregnancy and live birth rates. There is also some initial evidence that DHEA may help poor responders, although further research has not substantiated DHEA, and further evidence is needed before it is universally advocated.
Five-year view
More patients will be seeking IVF treatment in the future, as women continue to delay pregnancy. This is despite the period of austerity and uncertain economic future, as individuals will want to ensure career foothold and financial stability before embarking on pregnancy. Success rates in all aspects of IVF, especially the clinical pregnancy and live birth rates, will continue to improve as we gain greater insight into the endocrinology and further improvements in the medication used in IVF. GnRH antagonist protocols will be the most favored protocols for IVF. The advantages of antagonist protocol include shorter treatment times and less risk of OHSS. Other parameters in IVF treatment such as cryopreservation and oocytes donation also continued to be better understood and improved. Mild ovarian stimulation protocol may also see greater usage, although difficult economic times may counter that consideration. Overall, these improvements will have a cumulative effect in the success of treatment cycles. Individualized assessment prior to IVF treatment with AMH may become universal, especially with improvements in their assay and cost, and this will allow safer and more effective cycles. It is likely that over the next 5 years, orally active peptides will be introduced to replace some of the injectable gonadotrophin preparations that are presently used. Patient convenience will be improved during the treatment protocols. It is also likely that eventually the eggs will be grown in vitro from small pieces of ovarian cortical tissue, so that the patient does not require any stimulation at all.
Key issues
• New evidence shows that fixed gonadotrophin-releasing hormone antagonist protocols are as efficacious as gonadotropin-releasing hormone agonist protocols.
• A GnRH antagonist cycle is associated with significantly less incidence of ovarian hyperstimulation syndrome, and therefore patients with polycystic ovarian syndrome should be preferentially considered for this protocol.
• Human menopausal gonadotrophins and recombinant follicle-stimulating hormones are equally effective.
• The final oocyte maturation process is achieved by administering human chorionic gonadotrophin (hCG); both urinary and recombinant hCGs are equally effective.
• Luteal support can be given either by progesterone or hCG; hCG should be avoided if women are at risk of ovarian hyperstimulation syndrome.
• Serum anti-Müllerian hormone measurements should be considered to individualize the dose of follicle-stimulating hormone before ovarian stimulation, especially for patients who are likely to respond poorly to ovarian stimulation.
References
- Maheshwari A, Gibreel A, Siristatidis CS, Bhattacharya S. Gonadotrophin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database Syst. Rev. 8, CD006919 (2011).
- Hughes EG, Fedorkow DM, Daya S, Sagle MA, Van de Koppel P, Collins JA. The routine use of gonadotropin-releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta-analysis of randomized controlled trials. Fertil. Steril. 58(5), 888–896 (1992).
- Jenkins J. The influence, development and management of functional ovarian cysts during IVF cycles. J. Br. Fertil. Soc. 1(2), 132–136 (1996).
- Loutradis D, Drakakis P, Vomvolaki E, Antsaklis A. Different ovarian stimulation protocols for women with diminished ovarian reserve. J. Assist. Reprod. Genet. 24(12), 597–611 (2007).
- Surrey ES. Management of the poor responder: the role of GnRH agonists and antagonists. J. Assist. Reprod. Genet. 24(12), 613–619 (2007).
- Kahraman K, Berker B, Atabekoglu CS et al. Microdose gonadotropin-releasing hormone agonist flare-up protocol versus multiple dose gonadotropin-releasing hormone antagonist protocol in poor responders undergoing intracytoplasmic sperm injection-embryo transfer cycle. Fertil. Steril. 91(6), 2437–2444 (2009).
- Olivennes F, Fanchin R, Bouchard P et al. The single or dual administration of the gonadotropin-releasing hormone antagonist cetrorelix in an in vitro fertilization–embryo transfer program. Fertil. Steril. 62(3), 468–476 (1994).
- Al-Inany HG, Youssef MA, Aboulghar M et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst. Rev. 5, CD001750 (2011).
- Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF? Hum. Reprod. 22(11), 2805–2813 (2007).
- Griesinger G, Venetis CA, Marx T, Diedrich K, Tarlatzis BC, Kolibianakis EM. Oral contraceptive pill pretreatment in ovarian stimulation with GnRH antagonists for IVF: a systematic review and meta-analysis. Fertil. Steril. 90(4), 1055–1063 (2008).
- Lee TH, Wu MY, Chen HF, Chen MJ, Ho HN, Yang YS. Ovarian response and follicular development for single-dose and multiple-dose protocols for gonadotropin-releasing hormone antagonist administration. Fertil. Steril. 83(6), 1700–1707 (2005).
- Ng EH, Ho PC. Use of gonadotrophin releasing hormone (GnRH) antagonist (cetrotide) during ovarian stimulation for in vitro fertilization treatment: multiple doses and single dose. J. Obstet. Gynecol. Res. 27(5), 261–265 (2001).
- Al-Inany H, Aboulghar MA, Mansour RT, Serour GI. Optimizing GnRH antagonist administration: meta-analysis of fixed versus flexible protocol. Reprod. Biomed. Online 10(5), 567–570 (2005).
- Al-Inany H, Aboulghar M. GnRH antagonist in assisted reproduction: a Cochrane review. Hum. Reprod. 17(4), 874–885 (2002).
- Griesinger G, Felberbaum R, Diedrich K. GnRH antagonists in ovarian stimulation: a treatment regimen of clinicians’ second choice? Data from the German National IVF Registry. Hum. Reprod. 20(9), 2373–2375 (2005).
- Al-Inany H, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst. Rev. 4, CD001750 (2001).
- Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst. Rev. 3, CD001750 (2006).
- van Wely M, Kwan I, Burt AL et al. Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles. Cochrane Database Syst. Rev. 2, CD005354 (2011).
- Youssef MA, Al-Inany HG, Aboulghar M, Mansour R, Abou-Setta AM. Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane Database Syst. Rev. 4, CD003719 (2011).
- Afnan M. Identifying real differences in live birth rates between HMG and rFSH in IVF. Reprod. Biomed. Online 18(Suppl. 2), 25–30 (2009).
- European and Israeli Study Group on Highly Purified Menotropin versus Recombinant Follicle-Stimulating Hormone. Efficacy and safety of highly purified menotropin versus recombinant follicle-stimulating hormone in in vitro fertilization/intracytoplasmic sperm injection cycles: a randomized, comparative trial. Fertil. Steril., 78(3), 520 (2002).
- Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum. Reprod. 21(12), 3217–3227 (2006).
- Seyhan A, Ata B. The role of corifollitropin α in controlled ovarian stimulation for IVF in combination with GnRH antagonist. Int. J. Womens. Health 3, 243–255 (2011).
- Croxtall JD, McKeage K. Corifollitropin α: a review of its use in controlled ovarian stimulation for assisted reproduction. BioDrugs 25(4), 243–254 (2011).
- Mahmoud Youssef MA, van Wely M, Aboulfoutouh I, El-Khyat W, van der Veen F, Al-Inany H. Is there a place for corifollitropin α in IVF/ICSI cycles? A systematic review and meta-analysis. Fertil. Steril. 97(4), 876–885 (2012).
- Fauser BC, Alper MM, Ledger W, Schoolcraft WB, Zandvliet A, Mannaerts BM; Engage Investigators. Pharmacokinetics and follicular dynamics of corifollitropin α versus recombinant FSH during ovarian stimulation for IVF. Reprod. Biomed. Online 21(5), 593–601 (2010).
- Shoham Z. The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation. Fertil. Steril. 77(6), 1170–1177 (2002).
- le Cotonnec JY, Loumaye E, Porchet HC, Beltrami V, Munafo A. Pharmacokinetic and pharmacodynamic interactions between recombinant human luteinizing hormone and recombinant human follicle-stimulating hormone. Fertil. Steril. 69(2), 201–209 (1998).
- Shoham Z, Balen A, Patel A, Jacobs HS. Results of ovulation induction using human menopausal gonadotropin or purified follicle-stimulating hormone in hypogonadotropic hypogonadism patients. Fertil. Steril. 56(6), 1048–1053 (1991).
- Kolibianakis EM, Collins J, Tarlatzis B, Papanikolaou E, Devroey P. Are endogenous LH levels during ovarian stimulation for IVF using GnRH analogues associated with the probability of ongoing pregnancy? A systematic review. Hum. Reprod. Update 12(1), 3–12 (2006).
- Humaidan P, Bungum M, Bungum L, Yding Andersen C. Effects of recombinant LH supplementation in women undergoing assisted reproduction with GnRH agonist down-regulation and stimulation with recombinant FSH: an opening study. Reprod. Biomed. Online 8(6), 635–643 (2004).
- Marrs R, Meldrum D, Muasher S, Schoolcraft W, Werlin L, Kelly E. Randomized trial to compare the effect of recombinant human FSH (follitropin α) with or without recombinant human LH in women undergoing assisted reproduction treatment. Reprod. Biomed. Online 8(2), 175–182 (2004).
- Ferraretti AP, Gianaroli L, Magli MC, D’angelo A, Farfalli V, Montanaro N. Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil. Steril. 82(6), 1521–1526 (2004).
- De Placido G, Alviggi C, Perino A et al.; Italian Collaborative Group on Recombinant Human Luteinizing Hormone. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Hum. Reprod. 20(2), 390–396 (2005).
- Sauer MV, Thornton MH 2nd, Schoolcraft W, Frishman GN. Comparative efficacy and safety of cetrorelix with or without mid-cycle recombinant LH and leuprolide acetate for inhibition of premature LH surges in assisted reproduction. Reprod. Biomed. Online 9(5), 487–493 (2004).
- Griesinger G, Shapiro DB. Luteinizing hormone add-back: is it needed in controlled ovarian stimulation, and if so, when? J. Reprod. Med. 56(7–8), 279–300 (2011).
- Levi-Setti PE, Cavagna M, Bulletti C. Recombinant gonadotrophins associated with GnRH antagonist (cetrorelix) in ovarian stimulation for ICSI: comparison of r-FSH alone and in combination with r-LH. Eur. J. Obstet. Gynecol. Reprod. Biol. 126(2), 212–216 (2006).
- Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Pellicer A. Impact of luteinizing hormone administration on gonadotropin-releasing hormone antagonist cycles: an age-adjusted analysis. Fertil. Steril. 95(3), 1031–1036 (2011).
- Fauser BC, Devroey P. Why is the clinical acceptance of gonadotropin-releasing hormone antagonist cotreatment during ovarian hyperstimulation for in vitro fertilization so slow? Fertil. Steril. 83(6), 1607–1611 (2005).
- Edwards RG, Lobo R, Bouchard P. Time to revolutionize ovarian stimulation. Hum. Reprod. 11(5), 917–919 (1996).
- Nargund G, Fauser BC, Macklon NS, Ombelet W, Nygren K, Frydman R; Rotterdam ISMAAR Consensus Group on Terminology for Ovarian Stimulation for IVF. The ISMAAR proposal on terminology for ovarian stimulation for IVF. Hum. Reprod. 22(11), 2801–2804 (2007).
- Fauser BC, Nargund G, Andersen AN et al. Mild ovarian stimulation for IVF: 10 years later. Hum. Reprod. 25(11), 2678–2684 (2010).
- Verberg MF, Eijkemans MJ, Heijnen EM et al. Why do couples drop-out from IVF treatment? A prospective cohort study. Hum. Reprod. 23(9), 2050–2055 (2008).
- Polinder S, Heijnen EM, Macklon NS, Habbema JD, Fauser BJ, Eijkemans MJ. Cost–effectiveness of a mild compared with a standard strategy for IVF: a randomized comparison using cumulative term live birth as the primary endpoint. Hum. Reprod. 23(2), 316–323 (2008).
- Heijnen EM, Eijkemans MJ, De Klerk C et al. A mild treatment strategy for in vitro fertilisation: a randomised non-inferiority trial. Lancet 369(9563), 743 (2007).
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 50, 465–495 (1981).
- Zegers-Hochschild F, Fernández E, Mackenna A, Fabres C, Altieri E, Lopez T. The empty follicle syndrome: a pharmaceutical industry syndrome. Hum. Reprod. 10(9), 2262–2265 (1995).
- Beckers NG, Macklon NS, Eijkemans MJ et al. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J. Clin. Endocrinol. Metab. 88(9), 4186–4192 (2003).
- Fauser BC, de Jong D, Olivennes F et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J. Clin. Endocrinol. Metab. 87(2), 709–715 (2002).
- Youssef MA, Van der Veen F, Al-Inany HG et al. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist assisted reproductive technology cycles. Cochrane Database Syst. Rev. 1, CD008046 (2011).
- Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P. An update of luteal phase support in stimulated IVF cycles. Hum. Reprod. Update 13(6), 581–590 (2007).
- Penzias AS. Luteal phase support. Fertil. Steril. 77(2), 318–323 (2002).
- Pabuccu R, Akar ME. Luteal phase support in assisted reproductive technology. Curr. Opin. Obstet. Gynecol. 17(3), 277–281 (2005).
- Humaidan P, Papanikolaou EG, Kyrou D et al. The luteal phase after GnRH-agonist triggering of ovulation: present and future perspectives. Reprod. Biomed. Online 24(2), 134–141 (2012).
- Fatemi HM. The luteal phase after 3 decades of IVF: what do we know? Reprod. Biomed. Online 19(Suppl. 4), 4331 (2009).
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst. Rev. 10, CD009154 (2011).
- Nosarka S, Kruger T, Siebert I, Grové D. Luteal phase support in in vitro fertilization: meta-analysis of randomized trials. Gynecol. Obstet. Invest. 60(2), 67–74 (2005).
- Pritts EA, Atwood AK. Luteal phase support in infertility treatment: a meta-analysis of the randomized trials. Hum. Reprod. 17(9), 2287–2299 (2002).
- Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: results of a randomised study. J. Steroid Biochem. Mol. Biol. 97(5), 416–420 (2005).
- Zarutskie PW, Phillips JA. A meta-analysis of the route of administration of luteal phase support in assisted reproductive technology: vaginal versus intramuscular progesterone. Fertil. Steril. 92(1), 163–169 (2009).
- Simunic V, Tomic V, Tomic J, Nizic D. Comparative study of the efficacy and tolerability of two vaginal progesterone formulations, Crinone 8% gel and Utrogestan capsules, used for luteal support. Fertil. Steril. 87(1), 83–87 (2007).
- Tay PY, Lenton EA. The impact of luteal supplement on pregnancy outcome following stimulated IVF cycles. Med. J. Malaysia 60(2), 151–157 (2005).
- Leeton J, Trounson A, Jessup D. Support of the luteal phase in in vitro fertilization programs: results of a controlled trial with intramuscular Proluton. J. In Vitro Fert. Embryo Transf. 2(3), 166–169 (1985).
- Sharara FI, McClamrock HD. Ratio of oestradiol concentration on the day of human chorionic gonadotrophin administration to mid-luteal oestradiol concentration is predictive of in vitro fertilization outcome. Hum. Reprod. 14(11), 2777–2782 (1999).
- Jee BC, Suh CS, Kim SH, Kim YB, Moon SY. Effects of estradiol supplementation during the luteal phase of in vitro fertilization cycles: a meta-analysis. Fertil. Steril. 93(2), 428–436 (2010).
- Kolibianakis EM, Venetis CA, Papanikolaou EG, Diedrich K, Tarlatzis BC, Griesinger G. Estrogen addition to progesterone for luteal phase support in cycles stimulated with GnRH analogues and gonadotrophins for IVF: a systematic review and meta-analysis. Hum. Reprod. 23(6), 1346–1354 (2008).
- Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization–embryo transfer cycles. Fertil. Steril. 73(4), 761–766 (2000).
- Buvat J, Marcolin G, Guittard C, Herbaut JC, Louvet AL, Dehaene JL. Luteal support after luteinizing hormone-releasing hormone agonist for in vitro fertilization: superiority of human chorionic gonadotropin over oral progesterone. Fertil. Steril. 53(3), 490–494 (1990).
- Tesarik J, Hazout A, Mendoza-Tesarik R, Mendoza N, Mendoza C. Beneficial effect of luteal-phase GnRH agonist administration on embryo implantation after ICSI in both GnRH agonist- and antagonist-treated ovarian stimulation cycles. Hum. Reprod. 21(10), 2572–2579 (2006).
- Pirard C, Donnez J, Loumaye E. GnRH agonist as novel luteal support: results of a randomized, parallel group, feasibility study using intranasal administration of buserelin. Hum. Reprod. 20(7), 1798–1804 (2005).
- Lambalk CB, Homburg R. GnRH agonist for luteal support in IVF? Setting the balance between enthusiasm and caution. Hum. Reprod. 21(10), 2580–2582 (2006).
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81(1), 19 (2004).
- Tsilchorozidou T, Overton C, Conway GS. The pathophysiology of polycystic ovary syndrome. Clin. Endocrinol. 60(1), 1–17 (2004).
- Balen AH. Is metformin the treatment of choice for anovulation in polycystic ovary syndrome? Nat. Clin. Pract. Endocrinol. Metab. 3(6), 440–441 (2007).
- The Saloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum. Reprod. 23(3), 462–477 (2008).
- The Rotterdam EAsPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 19(1), 41–47 (2004).
- Griesinger G, Diedrich K, Tarlatzis BC, Kolibianakis EM. GnRH-antagonists in ovarian stimulation for IVF in patients with poor response to gonadotrophins, polycystic ovary syndrome, and risk of ovarian hyperstimulation: a meta-analysis. Reprod. Biomed. Online 13(5), 628–638 (2006).
- Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum. Reprod. Update 12(1), 13–21 (2006).
- Tso LO, Costello MF, Albuquerque LE, Andriolo RB, Freitas V. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2, CD006105 (2009).
- Costello MF, Eden JA. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil. Steril. 79(1), 1–13 (2003).
- Lord J, Flight I, Norman R. Insulin sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, d-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst. Rev. 2, CD003053 (2003).
- Tang T, Glanville J, Orsi N, Barth JH, Balen AH. The use of metformin for women with PCOS undergoing IVF treatment. Hum. Reprod. 21(6), 1416–1425 (2006).
- Swanton A, Lighten A, Granne I et al. Do women with ovaries of polycystic morphology without any other features of PCOS benefit from short-term metformin co-treatment during IVF? A double-blind, placebo-controlled, randomized trial. Hum. Reprod. 26(8), 2178–2184 (2011).
- Van Der Meer M, Hompes PG, De Boer JA, Schats R, Schoemaker J. Cohort size rather than follicle-stimulating hormone threshold level determines ovarian sensitivity in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 83(2), 423–426 (1998).
- Braat DD, Schutte JM, Bernardus RE, Mooij TM, van Leeuwen FE. Maternal death related to IVF in The Netherlands 1984–2008. Hum. Reprod. 25(7), 1782–1786 (2010).
- Aboulghar MA, Mansour RT, Serour GI, Amin Y. Ultrasonically guided vaginal aspiration of ascites in the treatment of severe ovarian hyperstimulation syndrome. Fertil. Steril. 53(5), 933–935 (1990).
- Aboulghar MA, Mansour RT, Serour GI, Amin YM. Moderate ovarian hyperstimulation syndrome complicated by deep cerebrovascular thrombosis. Hum. Reprod. 13(8), 2088–2091 (1998).
- Olivennes F. Ovarian hyperstimulation syndrome prevention strategies: individualizing gonadotropin dose. Semin. Reprod. Med. 28(6), 463–467 (2010).
- Marci R, Senn A, Dessole S et al. A low-dose stimulation protocol using highly purified follicle-stimulating hormone can lead to high pregnancy rates in in vitro fertilization patients with polycystic ovaries who are at risk of a high ovarian response to gonadotropins. Fertil. Steril. 75(6), 1131–1135 (2001).
- Daya S. Updated meta-analysis of recombinant follicle-stimulating hormone (FSH) versus urinary FSH for ovarian stimulation in assisted reproduction. Fertil. Steril. 77(4), 711–714 (2002).
- Pundir J, Sunkara SK, El-Toukhy T, Khalaf Y. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod. Biomed. Online 24(1), 6–22 (2012).
- Cate RL, Mattaliano RJ, Hession C et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell 45(5), 685–698 (1986).
- Lee MM, Donahoe PK, Hasegawa T et al. Müllerian inhibiting substance in humans: normal levels from infancy to adulthood. J. Clin. Endocrinol. Metab. 81(2), 571–576 (1996).
- Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J. Clin. Endocrinol. Metab. 91(10), 4057–4063 (2006).
- Karkanaki A, Vosnakis C, Panidis D. The clinical significance of anti-Müllerian hormone evaluation in gynecological endocrinology. Hormones (Athens). 10(2), 95–103 (2011).
- van Rooij IA, Tonkelaar I, Broekmans FJ et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause 11(6 Pt 1), 601–606 (2004).
- Broer SL, Mol B, Dólleman M, Fauser BC, Broekmans FJ. The role of anti-Müllerian hormone assessment in assisted reproductive technology outcome. Curr. Opin. Obstet. Gynecol. 22(3), 193–201 (2010).
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum. Reprod. Update 12(6), 685–718 (2006).
- Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Müllerian hormone measurement in a routine IVF program. Hum. Reprod. 23(6), 1359–1365 (2008).
- Riggs RM, Duran EH, Baker MW et al. Assessment of ovarian reserve with anti-Müllerian hormone: a comparison of the predictive value of anti-Müllerian hormone, follicle-stimulating hormone, inhibin B, and age. Am. J. Obstet. Gynecol. 199(2), 202.e1–202.e8 (2008).
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil. Steril. 93(3), 855–864 (2010).
- Nardo LG, Gelbaya TA, Wilkinson H et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil. Steril. 92(5), 1586–1593 (2009).
- Barad DH, Weghofer A, Gleicher N. Comparing anti-Müllerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertil. Steril. 91(4), 1553 (2009).
- Lee TH, Liu CH, Huang CC et al. Serum anti-Müllerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum. Reprod. 23(1), 160–167 (2008).
- Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Prediction of high ovarian response to controlled ovarian hyperstimulation: anti-Müllerian hormone versus small antral follicle count (2–6 mm). J. Assist. Reprod. Genet. 26(6), 319–325 (2009).
- Nelson SM, Yates RW, Lyall H et al. Anti-Müllerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum. Reprod. 24(4), 867–875 (2009).
- Duffy JM, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A. Growth hormone for in vitro fertilization. Cochrane Database Syst. Rev. 1, CD000099 (2010).
- Yoshimura Y, Ando M, Nagamatsu S et al. Effects of insulin-like growth factor-I on follicle growth, oocyte maturation, and ovarian steroidogenesis and plasminogen activator activity in the rabbit. Biol. Reprod. 55(1), 152–160 (1996).
- Blumenfeld Z, Amit T. The role of growth hormone (GH), GH-receptor and GH-binding protein in reproduction and ovulation induction. J. Pediatr. Endocrinol. Metab. 9(2), 145–162 (1996).
- Mason HD, Martikainen H, Beard RW, Anyaoku V, Franks S. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cells in vitro. J. Endocrinol. 126(3), R1–R4 (1990).
- Homburg R, Eshel A, Abdalla HI, Jacobs HS. Growth hormone facilitates ovulation induction by gonadotrophins. Clin. Endocrinol. (Oxf). 29(1), 113–117 (1988).
- Homburg R, West C, Torresani T, Jacobs HS. Cotreatment with human growth hormone and gonadotropins for induction of ovulation: a controlled clinical trial. Fertil. Steril. 53(2), 254–260 (1990).
- Dor J, Seidman DS, Amudai E, Bider D, Levran D, Mashiach S. Adjuvant growth hormone therapy in poor responders to in vitro fertilization: a prospective randomized placebo-controlled double-blind study. Hum. Reprod. 10(1), 40–43 (1995).
- Kolibianakis EM, Venetis CA, Diedrich K, Tarlatzis BC, Griesinger G. Addition of growth hormone to gonadotrophins in ovarian stimulation of poor responders treated by in vitro fertilization: a systematic review and meta-analysis. Hum. Reprod. Update 15(6), 613–622 (2009).
- Harper K, Proctor M, Hughes E. Growth hormone for in vitro fertilization. Cochrane Database Syst. Rev. 3, CD000099 (2003).
- Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum. Reprod. 15(10), 2129–2132 (2000).
- Buster JE, Casson PR, Straughn AB et al. Postmenopausal steroid replacement with micronized dehydroepiandrosterone: preliminary oral bioavailability and dose proportionality studies. Am. J. Obstet. Gynecol. 166(4), 1163–1168; discussion 1168 (1992).
- Casson PR, Santoro N, Elkind-Hirsch K et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil. Steril. 70(1), 107–110 (1998).
- Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod. Biol. Endocrinol. 9, 67 (2011).
- Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum. Reprod. 25(10), 2496–2500 (2010).
- Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum. Reprod. 21(11), 2845–2849 (2006).
- Bosdou JK, Venetis CA, Kolibianakis EM et al. The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Hum. Reprod. Update 18(2), 127–145 (2012).
- Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol. Endocrinol. 24(7), 1393–1403 (2010).
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J. Clin. Pharmacol. 39(4), 327–348 (1999).
Website
- Clinicaltrials.gov. A trial of Dehydroepiandrosterone (DHEA) treatment for in vitro fertilization (IVF). http://clinicaltrials.gov/ct2/show/NCT00419913
Endocrinological insights into different in vitro fertilization treatment aspects
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertendo. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. You are seeing a 38-year-old nulliparous woman who has failed to conceive a child with her husband after 18 months of trying. She presents for consideration for in vitro fertilization (IVF). What should you consider regarding the use of gonadotropin-releasing hormone (GnRH) agonists and GnRH antagonists for this patient?
□ A There is no difference in pregnancy rates per cycle in comparing long and short protocols for GnRH agonists
□ B Single-dose protocol for GnRH antagonist use results in a higher number of zygotes compared with a multiple-dose protocol
□ C GnRH antagonists are associated with a significantly higher live birth rate vs GnRH agonists
□ D GnRH antagonists are less likely to promote ovarian hyperstimulation syndrome (OHSS) compared with GnRH agonists
2. What should you consider regarding gonadotropins to use for this patient?
□ A Human menopausal gonadotropins (HMG) have follicle-stimulating hormone (FSH) activity but lack luteinizing hormone (LH) activity
□ B Recombinant FSH (rFSH) results in higher live birth rates compared with HMG
□ C Corifollitropin alfa improves the pregnancy rate compared with rFSH
□ D Corifollitropin alfa is associated with a higher risk of OHSS compared with rFSH
3. The patient is treated successfully with gonadotropins and enters the luteal phase. Which of the following statements regarding the luteal phase in IVF is most accurate?
□ A It is deficient in all stimulated IVF cycles
□ B Only intramuscular progesterone is effective to improve pregnancy rates
□ C Adding estrogen to progesterone will improve pregnancy rates
□ D hCG is a good choice for women at risk of OHSS
4. What should you consider regarding other endocrine issues during IVF?
□ A Anti-Müllerian hormone (AMH) is a poor marker of ovarian reserve
□ B AMH is best used as a predictor of pregnancy outcome after implantation
□ C This patient should receive growth hormone even if responding well to treatment
□ D Supplementation with dehydroepiandrosterone (DHEA) cannot be recommended for routine use during IVF at this time