Abstract
In recent years, acute-on-chronic liver failure has been recognized as a specific clinical form of liver failure associated with cirrhosis. The syndrome refers to an acute deterioration of liver function and subsequently of other end organs over a period of weeks following a precipitating event in a patient with previously well- or reasonably well-compensated cirrhosis. These precipitating events include either an indirect (e.g., variceal hemorrhage, sepsis) or a direct (e.g., drug-induced) hepatotoxic factor. The short-term mortality for this condition is more than 50%. At present, considerable efforts are ongoing to better characterize the syndrome, to gain further insight into its pathophysiology and to optimize therapy. This article aims to highlight the current concepts of these various aspects.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at www.medscape.org/journal/expertgastrohep; (4) view/print certificate.
Release date: July 22, 2011; Expiration date: July 22, 2012
Learning objectives
Upon completion of this activity, participants will be able to:
• Describe the definition and clinical characteristics of ACLF and its distinction from end-stage liver disease
• Describe the pathophysiology and clinical course of ACLF
• Describe therapeutic interventions that may be useful in ACLF
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Editorial Director, Future Science Group, London, UK.
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay, MD
Freelance writer and reviewer, Medscape, LLC.
Disclosure: Laurie Barclay has disclosed no relevant financial relationships.
AUTHOR AND CREDENTIALS
Wim Laleman
Department of Liver and Biliopancreatic Disorders, University Hospital Gasthuisberg, KU Leuven, Leuven, Belgium.
Disclosure: Wim Laleman has disclosed the following relevant financial relationships: Serves as a senior clinical investigator for Fund for Scientific Research – Flanders (Fundamenteel klinisch mandaat – Fonds voor Wetenschappelijk Onderzoek [FWO] Vlaanderen).
Len Verbeke
Department of Liver and Biliopancreatic Disorders, University Hospital Gasthuisberg, KU Leuven, Leuven, Belgium.
Disclosure: Len Verbeke has disclosed the following relevant financial relationships: serves as an aspirant-researcher for Fund of Scientific Research – Flanders (Aspirant Mandaat – FWO Vlaanderen).
Philippe Meersseman
Medical Intensive Care, University Hospital Gasthuisberg, KU Leuven, Leuven, Belgium.
Disclosure: Philippe Meersseman has disclosed no relevant financial relationships.
Joost Wauters
Medical Intensive Care, University Hospital Gasthuisberg, KU Leuven, Leuven, Belgium.
Disclosure: Joost Wauters has disclosed no relevant financial relationships.
Jos van Pelt
Department of Liver and Biliopancreatic Disorders, University Hospital Gasthuisberg, KU Leuven, Leuven, Belgium.
Disclosure: Jos van Pelt has disclosed no relevant financial relationships.
David Cassiman
Department of Liver and Biliopancreatic Disorders, University Hospital Gasthuisberg, KU Leuven, Leuven, Belgium.
Disclosure: David Cassiman has disclosed the following relevant financial relationships: serves as a senior clinical investigator for Fund for Scientific Research – Flanders (Fundamenteel klinisch mandaat – FWO Vlaanderen).
Alexander Wilmer
Medical Intensive Care, University Hospital Gasthuisberg, KU Leuven, Leuven, Belgium.
Disclosure: Alexander Wilmer has disclosed no relevant financial relationships.
Chris Verslype
Department of Liver and Biliopancreatic Disorders, University Hospital Gasthuisberg, KU Leuven, Leuven, Belgium.
Disclosure: Chris Verslype has disclosed no relevant financial relationships.
Frederik Nevens
Department of Liver and Biliopancreatic Disorders, University Hospital Gasthuisberg, KU Leuven, Leuven, Belgium.
Disclosure: Frederik Nevens has disclosed the following relevant financial relationships: serves as a senior clinical investigator for Fund for Scientific Research – Flanders (Fundamenteel klinisch mandaat – FWO Vlaanderen).
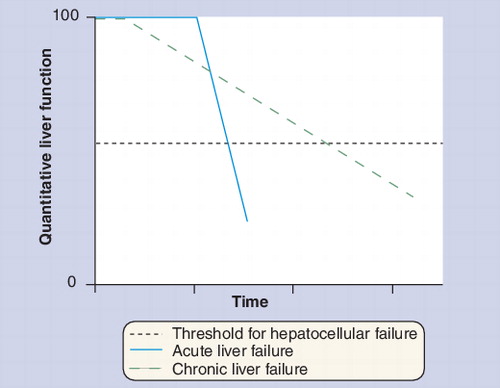
Liver failure results from the surpassing of a critical threshold of hepatocellular function, either acutely (acute liver failure) or due to progressive chronic liver disease (chronic liver failure).
(A) End-stage liver failure. (B) Acute-on-chronic liver failure.
MELD: Model for End-stage Liver Disease.
Adapted from Citation[11].
![Figure 3. 3-month mortality according to Model for End-stage Liver Disease score.MELD: Model for End-stage Liver Disease.Adapted from Citation[11].](/cms/asset/0afb1559-cf31-4733-9442-0bb852cfee18/ierh_a_11208992_f0003_b.jpg)
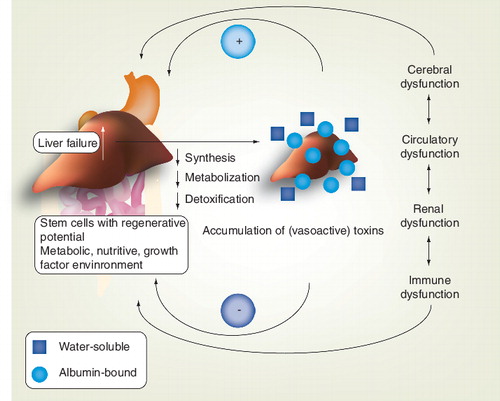
The combined actions of accumulated toxins and end-organ dysfunction further aggravate liver injury and incapacitate the regenerative environment.
NE: Norepinephrine; RAA: Renin–angiotensin II–aldosterone; VP: Vasopressin.
Modified from Citation[50].
![Figure 5. The pathophysiology of portal hypertensive syndrome.NE: Norepinephrine; RAA: Renin–angiotensin II–aldosterone; VP: Vasopressin.Modified from Citation[50].](/cms/asset/3cae8f92-4add-4888-b0a7-0836265db50b/ierh_a_11208992_f0005_b.jpg)
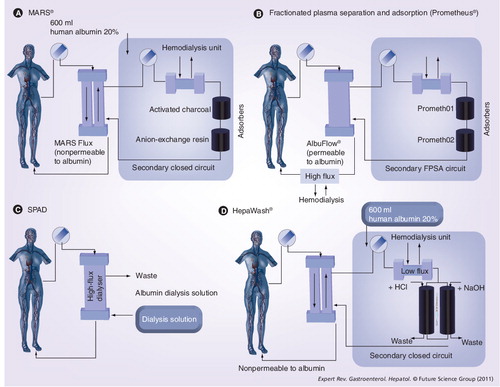
(A) A representive scheme of the MARS® device. Blood of the patient passes through a hollow-fiber dialysis module where it is dialyzed across an albumin-impregnated polysulfone membrane (MARS Flux dialyzer; membrane thickness: 100 nm; pore size: 50 kDa, surface area: 2.1 m2), which enables the exchange of water-soluble and protein-bound toxins by an albumin-coated membrane against a dialysate containing ‘recycled’ exogenous albumin. The membrane transiently absorbs and holds the toxins. The toxins are released upon contact with the membrane according to the concentration gradient and are carried to the other side of the membrane, where dialysis against the albumin-rich dialysate (600 ml of 20% human albumin) removes the toxins from the membrane. The toxin-enriched albumin solution is then first passed through another dialyzer countercurrent to a standard buffered dialysis solution, where clearance of water-soluble substances occurs by diffusion. The solution is then further purified on line from albumin-bound toxins by incorporation of unspecific adsorbents (an anion exchanger column and an uncoated charcoal column), resulting in regeneration (recycling) of the dialysate, allowing re-uptake of new toxins from the blood. (B) A representive scheme of the Prometheus® device. The first filter in the Prometheus device is the AlbuFlow®, which is made of polysulfone hollow fibers and is permeable to albumin (sieving coefficient: 0.6), and hence to albumin-bound substances. Subsequently, the albumin filtrate (and all molecules equal to the size of albumin) in the fractionated plasma separation and adsorption circuit is perfused through a column with neutral resin (prometh01) and a second column with an anion exchanger resin adsorber (prometh02), whereby the bound toxins are captured by direct contact with the high-affinity adsorbing material. The ‘native’ albumin is then returned to the patient. The FPSA recirculation circuit is driven by a roller pump at 300 ml/min. Finally, after passage through the AlbuFlow, the patient’s blood is dialyzed through a high-flux dialyzer (FX50), whereby water-soluble toxins are eliminated. (C) A representive scheme of the single-pass albumin dialysis device. A standard renal replacement therapy machine is used to run the circuit. Plasma from a reservoir is dialyzed against a low concentration albumin solution, which is disposed after a single pass. (D) A representive scheme of the HepaWash® device. Toxins in the patient blood dissociate from blood albumin, cross the filter membrane and bind to the exogenously added albumin in the dialysate of the secondary closed circuit. It first passes over a classic dialyzer countercurrent to a standard buffered dialysis solution, where clearance of water-soluble substances occurs by diffusion. Afterwards, albumin-bound toxins are removed over two absorbers, which is enhanced by pH and temperature changes through which the albumin can be regenerated. The recycled albumin can therefore be continuously re-used in one treatment session to eliminate toxins from the blood.
FPSA: Fractionated plasma separation and adsorption; MARS: Molecular adsorbent recirculating system; SPAD: Single-pass albumin dialysis.
Liver failure associated with cirrhosis: magnitude of the problem
The appearance of liver failure in a patient with cirrhosis represents a decisive time point both in terms of medical management and prognosis since this condition is frequently associated with rapidly evolving multiorgan dysfunction Citation[1,2]. The lack of liver detoxification, metabolic and regulatory functions and an altered immune response lead to life-threatening complications, such as renal failure, increased susceptibility to infection, hepatic coma and systemic hemodynamic dysfunction Citation[1,2]. Not surprisingly, the combined impact of these complications leads to mortality rates as high as 50–90% Citation[1–6].
Among liver diseases, cirrhosis is by far the most frequent reason for hospital admission or liver transplantation. It can be expected that this situation is unlikely to change in the next decade since recent WHO projections have demonstrated that cirrhosis will become the ninth most common cause of death in the Western world by 2015 Citation[201]. Currently, liver diseases are the tenth most common cause of death in the USA and account for an associated economic burden of approximately 1% of the total national healthcare expenditure (US$1.2 trillion) Citation[3,4]. Moreover, only 20% of patients with advanced cirrhosis globally can be treated with liver transplantation owing to the great imbalance between donation and potential recipients Citation[4].
The problem of cirrhosis is therefore of relevance to the general public and warrants further efforts both in terms of awareness, prevention, further optimization and renewal of the therapeutic armamentarium targeting the earliest stages of viral hepatitis, nonalcoholic fatty liver disease and alcohol-related liver disease, which represent the most prevalent chronic liver disorders.
(Re-)defining liver failure associated with cirrhosis: end-stage versus acute-on-chronic liver failure
Cirrhosis is a critical last phase in a relentless evolution of progressive parenchymal cell damage and death, nodular parenchymal regeneration and progressive fibrosis Citation[7]. However, only upon appearance of complete fibrous vascularized septa, which serve as intrahepatic shunt vessels and therefore compromise interaction with the hepatocyte, is the ‘true cirrhotic state’ achieved Citation[7]. Liver insufficiency in this context is considered a combination of progressive parenchymal extinction on the one hand and portosystemic shunting on the other hand, leading to the surpassing of a threshold of a critical functional liver cell mass Citation[1,2,7–9].
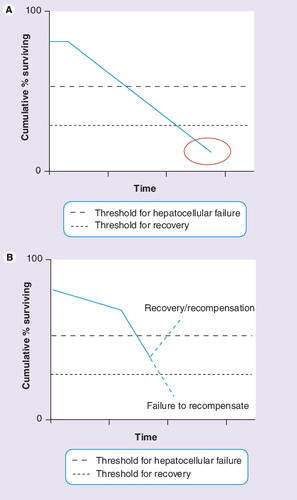
In recent years, the recognition of clinically different patterns of liver failure associated with cirrhosis has led to the demarcation of two different entities: ‘end-stage’ and ‘acute-on-chronic’ liver failure (AoCLF) Citation[1,2]. Although substantial overlap exists between these two entities in terms of clinical presentation (jaundice, hepatic encephalopathy, hyperdynamic circulatory state and/or hepatorenal syndrome), the main difference between them is the potential for recovery, the presence or absence of a precipitating event and the substantial evolution towards multiorgan failure . More specifically, while AoCLF refers to an acute deterioration of liver function and subsequently other end organs over a period of weeks following a precipitating event (by an indirect [e.g., variceal hemorrhage, sepsis] or a direct [e.g., drug induced, liver resection] hepatotoxic factor) in a patient with previously well- or reasonably well-compensated chronic liver disease, ‘end-stage liver disease’ refers to a chronically decompensated patient due to relentless progressive deterioration of the underlying chronic liver disorder .
The reasons why AoCLF syndrome has gained interest in recent years are likely to be twofold. First, the implementation of the Model for End-stage Liver Disease-(MELD) score, which is based on a logarithmic equation (0.957 × log [creatinine mg/dl] + 0.378 × log [bilirubine mg/dl] + 1.120 × log [international normalized ratio] + 0.643) as an organ allocation system in liver transplantation Citation[10]. This prognostic scoring system adequately reflects 3-month mortality and was put into practice in 2006 within the Eurotransplant zone Citation[11]. Before the implementation of the MELD score-based allocation policy, donor organs were basically allocated to recipients according to waiting time. As a result in this system, patients with rapidly evolving multiorgan failure, such as that typically seen in AoCLF, were highly unlikely to be granted a graft. By contrast, the MELD score-based allocation system relies on a ‘sickest first’ policy and therefore offers the opportunity for AoCLF patients to receive an allograft based upon disease severity. Moreover, this policy also raised the issue of bridging AoCLF patients to ‘salvage’ transplantation, which brings us to the second reason, which, more specifically, is a growing interest and focus on artificial liver support devices Citation[1,2,12–17]. This increased attention has further sharpened the duality of the therapeutic perspective between AoCLF and end-stage liver disease. In the case of end-stage liver disease, only liver transplantation will rescue the patient, whereas for AoCLF, any attempt to bring the decompensation back to the critical threshold of functional liver mass (even if temporary) is of interest. These attempts are strongly motivated by the earlier mentioned globally remaining disparity between the increasing number of patients qualifying for liver transplantation and the relatively static number of available donor organs. Moreover, even if a transplant is to be ‘reasonably’ expected based on the MELD-allocation system, this ‘reasonable’ time frame varies largely amongst cases and warrants therapeutic strategies to ‘bridge’ patients as multiorgan dysfunction further evolves while the patient is waiting and imperils the peri-operative and post-transplantation outcome.
Potential mechanisms involved in AoCLF: systemic inflammatory response, bacterial infections & organ dysfunction
In the past, the ‘toxin hypothesis’ was considered all-comprising Citation[1,2,18–21]. The ‘toxin hypothesis’ suggested the accumulation of toxins – as a result of impaired hepatic detoxification and/or metabolism – to be the culprit of multiorgan failure associated with liver failure . This theory was based on the observation that although severity and rapidity of organ dysfunction can vary, acute liver failure (i.e., without pre-existing liver disease), AoCLF and end-stage liver disease showed consistent signs of worsening liver function (as manifested by encephalopathy, jaundice, renal insufficiency and so on) The toxins considered to be involved (mainly water-insoluble and therefore albumin-bound) include, amongst others, aromatic amino acids, tryptophan, indoles, mercaptans, endogenous benzodiazepines, ammonia, prostanoids and nitric oxide Citation[18].
At present, the exact mechanisms leading from a compensated cirrhotic state to AoCLF are considered more complex than merely the accumulation of toxins, although this is still regarded as an important factor. In the current view, in the early phase of AoCLF there appears to be a dynamic and reciprocal interplay between enhanced intestinal permeability (leading to translocation of bacteria and their products), an imbalanced immune reaction and an aggravated intrahepatic microcirculatory dysfunction and subsequent worsened hyperdynamic state Citation[22–31]. In this concept, the accumulation of toxins might further enhance and perpetuate the ensuing end-organ dysfunction Citation[1,2].
Bacterial translocation (BT) is a frequent phenomenon in cirrhotic patients. Infection is the cause of admission in 30–50% of patients with cirrhosis Citation[23,24]. Increased BT and increased levels of circulating bacterial products are presumed to be a prerequisite to the development of AoCLF but this is insufficient to solely explain the whole phenomenom. More specifically, it needs to be accompanied by an inadequate immune response and subsequent aggravated vascular hypo-/hyper-reactivity (depending on the vascular territory).
The former, immune dysfunction, is a complex, highly interactive and multifactorial matter in cirrhotic patients Citation[23]. On the one hand, it begins with a decreased opsonization capacity (related to the decreased synthesis capacity of the cirrhotic liver), which is crucial in bacterial phagocytosis, and the bactericidal activity of these same phagocytic cells. In addition, the function of Kuppfer cells, which represent major effectors of the reticuloendothelial system (RES), is seriously impaired because of portosystemic shunting that leads to the evasion of portal and systemic bacteria to the action of the RES. Moreover, the latter also explains why other bacterial products, such as endotoxins and cytokines, fail to clear. Although the persistence of microbes, their toxins or injury are important triggers to an inflammatory reaction, the constellation of sepsis, or a syndrome resembling it, is primarily caused by the host’s imbalanced reaction to these initiating injuring factors Citation[25–36]. More specifically, upon presence of microbes or any form of tissue damage (e.g., hypoxia, ischemia or toxic agent), both a local proinflammatory and anti-inflammatory reaction are simultaneously initiated in an attempt to confine and control invading microbes locally, destroy damaged tissue and repair damage. If the initiating infectious or noninfectious factors overwhelm the local response or the local response becomes exaggerated, spilling of proinflammatory mediators into the systemic circulation results in recruitment of a systemic response, which we recognize clinically as the systemic inflammatory response (SIRS) Citation[16–18]. The severity of the disease syndrome resulting from this cascade of systemic mediators depends on the balance of SIRS and the compensatory anti-inflammatory syndrome Citation[29,31]. In parallel, and partially due to the unbalanced immune dysfunction, the hyperdynamic state typical of advanced cirrhosis (see later), is further aggravated and ultimately leads to the inability to maintain adequate perfusion pressure. This constellation leads to progressive ischemia and a self-perpetuating dysfunction of several end organs such as the kidney (hepatorenal syndrome) and the brain (hepatic encephalopathy), and finally leads to multiorgan failure and death.
Diagnostic & prognostic criteria for AoCLF: an ongoing quest
Although in theory the currently used working definition of AoCLF Citation[1,2,37,38] seems straightforward to use, medical practice is hampered by an often complex clinical picture, the absence of a clear precipitant, differentiation from a patient with end-stage liver disease and problems in determining the ideal moment and/or necessity or usefulness of certain therapeutic interventions Citation[38]. The additional lack of full elucidation of the pathophysiological pathways also adds weight in this matter as is the fact that the natural history of AoCLF patients remains poorly documented since there are only a handful prospective studies available Citation[38–40].
With regard to prognosis, AoCLF also poses problems since it coalesces both an acute potentially life-threatening insult and a severe chronic underlying disease. At present, two types of prognostic models are readily used. First, those evaluating the severity of disease, such as the Acute Physiology and Chronic Health Evaluation (APACHE) II and III and the Simplified Acute Physiology Score II. Second, those scores that aim to asses the number of organ dysfunctions and failures such as the Multiple Organ Dysfunction Score and Sequential Organ Failure Assessment (SOFA).
It is clear that our current liver-oriented scores (such as Child–Pugh and MELD) have limited value Citation[1,2,38,40]. Indeed, once extra-hepatic organ failure has began, mortality is determined by the degree of this end-organ dysfunction and not the severity of the liver disease (short-term mortality range: 46–89%) Citation[41–43]. As demonstrated in a recent cohort study assessing patients with cirrhosis in the intensive care unit (ICU) from 2005 until 2008, the in-patient mortality amounted to 70% in the presence of three or four nonhematologic organ failures at day 1 after admission whereas the presence of three organ failures or more after 3 days of treatment indicated a mortality of 89% Citation[44–46]. Organ failure scores such as APACHE II and SOFA therefore remain currently the most relevant Citation[38,40–46]. The quest for markers of early diagnosis or assessment of severity of disease nevertheless continues. We recently compared AoCLF patients to chronic decompensated end-stage patients with underlying alcoholic cirrhosis (n = 250 in total) and confirmed in a prospective comparative manner the clinical usefulness of positive SIRS criteria Citation[38]. SIRS criteria were described as a constellation of symptoms with the intent of creating a definition to bring together the multiple etiologies of organ dysfunction into a group of negatively synergistic responses to injury and/or infection that can collectively lead to microcirculatory dysfunction Citation[47]. In the context of AoCLF, we Citation[38] and others Citation[39,48,49] documented infection as the major precipitating factor for SIRS. This observation was further substantiated by the histopathological finding of ductular bilirubinostasis, which is the descriptive term for the presence of bile plugs in dilated ductules at the interface between the portal tract and parenchyma and is highly vulnerable to sepsis. In an attempt to solve and validate the aforementioned issues, a European, multicenter, observational prospective case-only study is currently recruiting: the Chronic Liver Failure (CLIF) Acute on Chronic Liver Failure in Cirrhosis (CANONIC) core study, which is conducted under the aegis of the CLIF Consortium, aims to better define the natural history of AoCLF and to evaluate prevalence, precipitating mechanisms, risk factors for its development, short-term and mid-term survival and the risk factors of mortality. For this purpose, this study aims to examine a cohort of approximately 1200 consecutive patients admitted to the hospital for more than 24 h for a complication of cirrhosis.
Clinical manifestations of AoCLF
Multiorgan failure is the central component in the AoCLF syndrome following hepatic decompensation. Liver insufficiency is typically associated with a decreased detoxification function as manifested by hyperbilirubinemia (with clinical jaundice), encephalopathy (see later) and reduction of synthetic function leading to hypoalbuminemia and a decrease in prothrombin time. In this specific setting, the lack of liver detoxification and metabolic and regulatory functions leads to life-threatening complications, which in the context of AoCLF typically involve systemic hemodynamic dysfunction, renal insufficiency, cerebral failure (hepatic encephalopathy) and increased susceptibility to infections.
Hemodynamics
Portal hypertension associated with cirrhosis is initiated by an increase in intrahepatic vascular resistance, both active (increased intrahepatic vascular tone) and passive (fibrosis), with an increase in splanchnic blood flow as a secondary phenomenon, which in turn gives rise to the hyperdynamic systemic state Citation[50–53]. For an extensive discussion of the pathogenesis of portal hypertension, the reader is referred to in-depth reviews and pivotal articles Citation[50–62]. Circulatory failure in AoCLF is typically characterized by an aggravated hyperdynamic state with the inability to obtain adequate perfusion pressure despite volume expansion with subsequent arising of lactate acidosis Citation[63–65]. To a different extent, the aggravation has been attributed either to sepsis related to the increased susceptibility to infections, to an impairment in cardiac systolic and/or diastolic function (see kidney) or to the so-called hepatoadrenal syndrome Citation[66–73]. With regard to the latter section, ample evidence suggests that a significant number of patients (˜50%) with chronic liver disease develop adrenal insufficiency in the case of concomitant sepsis Citation[69–71]. This condition impairs hemodynamic integrity, further worsens frequently encountered multiorgan failure and aggravates in-patient mortality risk (80 vs 35%). Recent but limited data suggest that relative adrenal insufficiency also occurs in liver disease without sepsis Citation[69,72–74]. This entity apparently relates to functional liver reserve Citation[69].
Kidney
Acute kidney injury is a frequent and prognostically important feature of AoCLF Citation[38–44,46,48]. The factors causing acute renal insufficiency relate to prerenal, renal and postrenal causes, with prerenal factors being more prevalent than the others (60, 39 and 1%, respectively) Citation[74]. Changes in renal blood flow already occur in early stage cirrhosis and culminate in progressive renal vasoconstriction in advanced cirrhosis as a result of the evolving systemic hemodynamic dysfunction, therefore predisposing cirrhotic patients to renal injury Citation[53–56]. Most intrarenal causes of acute renal insufficiency are due to ischemic acute tubular necrosis based on renal hypoperfusion. Prerenal factors range from obvious renal hypoperfusion in patients with acutely superposed intravascular volume depletion (caused by excessive diuretics, hemorrhage, vomiting or diarrhea) to more subtle renal hypoperfusion due to decreased arterial pressure (i.e., low renal perfusion pressure) and preglomerular vasoconstriction, such as that seen in patients with hepatorenal syndrome Citation[74]. Typically, two forms of hepatorenal syndrome are distinguished: type 1, the rapidly progressing form with 80% mortality at 2 weeks and type 2, which usually refers to reasonably steady chronic renal insufficiency in patients with refractory ascites Citation[75–78]. The diagnosis of type 1 hepatorenal syndrome is based on the exclusion of other expected causes of acute renal insufficiency in patients with decompensated cirrhosis (Box 1)Citation[74,75].
The causal factor that dramatically distinguishes between hepatorenal syndrome type 1 and 2 is thought to be insufficient cardiac output. It was suggested by Tristani and Cohn in 1967 Citation[78] that decreased cardiac output contributes to renal hypoperfusion but this was only recently confirmed Citation[66,67]. When cardiac output decreases, effective hypovolemia is further enhanced, leading to further recruitment of endogenous vasopressor systems, which in turn culminates in extreme further aggravation of the renal hypoperfusion and hepatorenal syndrome. These findings support the hypothesis that the hyperdynamic circulation is essential to maintain central blood volume and renal perfusion in cirrhosis. The mechanism leading to impaired or insuffient cardiac output in patients developing hepatorenal syndrome remains unknown. In recent years, a specific cardiac abnormality, ‘cirrhotic cardiomyopathy’, characterised by attenuated systolic and diastolic responses to stimuli, changes in repolarization and hypertrophy of the cardiac chambers, has been described and is being intensively studied Citation[68,79].
Brain
Metabolic encephalopathies are frequent in severely ill patients (>30%) and refer to the impairment of the brain’s integrated activity in the absence of structural abnormalities Citation[68,80]. The clinical manifestations correspond to a syndrome of global cerebral dysfunction induced by systemic stress and can vary from mild executive dysfunction or agitated delirium to deep coma with decerebration. Encephalopathy may either be a precipitating factor to or a consequence of AoCLF. Hyponatremic and hepatic encephalopathy are of the most common forms of neurological deterioration in decompensated cirrhosis and may even co-exist Citation[81]. The prognosis of patients admitted in the ICU for hepatic encephalopathy varies according to the presence or absence of other organ failure. A recent study has shown the in-patient mortality rate of cirrhotic patients admitted in the ICU with encephalopathy was approximately 10% in those with isolated encephalopathy compared with more than 80% in those with encephalopathy plus failures of other organs Citation[82]. Aspiration pneumonia and acute respiratory failure represent the most severe consequences of encephalopathy, indicating that airway protection is an absolute priority in advanced encephalopathy.
Increased susceptibility to infection
The rising of bacterial infections accounts for significant morbidity and mortality in patients with cirrhosis. Bacterial infections occur in 32–34% of hospitalized patients with cirrhosis and in approximately 45% of those admitted with upper gastrointestinal hemorrhage, which is significantly more than the incidence of 5–7% in their noncirrhotic counterparts Citation[23,24,83,84].
In a population-based study of more than 1300 individuals, cirrhotic patients were significantly more likely to have hospitalizations associated with sepsis (adjusted RR: 2.6) and to die from sepsis (adjusted RR: 2.0) Citation[85]. In this same study, cirrhotic individuals showed both more Gram-positive or Gram-negative infections listed concurrently on their hospital discharge information compared with noncirrhotic patients with bacteremia (1.44 vs 0.41% and 1.61 vs 0.53%, respectively) Citation[85]. The most common bacterial infections are spontaneous bacterial peritonitis (25%), urinary tract infections (20%), pneumonia (15%) and spontaneous bacteremia (12%) Citation[84].
The mortality of cirrhotic patients with infection ranged between 25 and 50% compared with 5–20% in patients with cirrhosis without infection and less than 5% in noncirrhotic patients with bacteremia. The risk of infection was shown to increase with upper gastrointestinal hemorrhage, advanced liver insufficiency, as reflected by lower serum albumin or higher Child–Pugh score Citation[23,24,83,84]. Moreover, not only bacterial infections are to be considered: fungemia, such as invasive aspergillosis, has also been shown to be an emerging and devastating infectious disease in cirrhotic patients in the ICU Citation[86].
Liver support systems in AoCLF
Current medical therapy involves the management of the precipitating event, supporting end organs and treatment of complications until the liver eventually recovers, leaving clinicians with no treatment options other than transplantation if these attempts fail (if considered eligible). However, the shortage of cadaveric organs and other transplant-related problems, have prompted the need for alternative methods to provide liver support. Since liver failure in AoCLF – by definition – is potentially reversible, considerable effort has been invested in the development of liver support systems. Currently, most of the experience is available for nonbiological support systems. In the following paragraphs, we aim to provide a brief summary of the principle, types and currently available data.
Rational basis for nonbiological liver support
The rational basis for the development of extracorporeal liver support was the ‘toxin theory,’ which was highlighted earlier. It was hypothesized that removal of the vasoactive, neuro- and hepatotoxic-toxins could not only lead to recovery of end-organ dysfunction but would also help to reconstitute the liver back to above the critical threshold of minimal functional liver cell mass by creating an improved intrahepatic environment for regenerative cells Citation[1,2,63–65].
Currently available devices
The molecular adsorbent recirculating system
The molecular adsorbent recirculating system (MARS®, Gambro, Stockholm, Sweden) device was developed by Stange and Mitzner in 1993 and applied for the first time in humans in 1996 Citation[87,88]. The system combines the efficacy of sorbents to remove albumin-bound molecules with the biocompatibility of a dialysis membrane. A schematic of the working mechanism of MARS is depicted in .
Prometheus® or fractionated plasma separation & adsorption
The Prometheus® (Fresenius Medical Care AG, Bad Homburg, Germany) system combines fractionated plasma separation and adsorption (FPSA [Fresenius Medical Care Allgemeine Gesellschaft, Bad Homburg, Germany]) with high-flux hemodialysis for the removal of both albumin-bound and water-soluble toxins and was introduced by Falkenhagen in 1999 Citation[89]. The device is schematically depicted in . The clearance of toxins is achieved by first separating all molecules of equal or lower size than albumin from blood by a novel capillary albumin dialyser, AlbuFlow® (Fresenius Medical Care AG, Bad Homburg, Germany), after which the solution is passed over two absorbers that cleaning the endogenous albumin, followed by the blood being passed over a high-flux dialyser. Maintenance and monitoring of the extracorporeal circuit is performed by a modified 4008 hemodialysis unit.
Single-pass albumin dialysis
The single-pass albumin dialysis (SPAD) system is comparable to MARS but differs in that a more diluted albumin solution (4.4% for SPAD as opposed to 20% for MARS) is used as dialysate. Moreover, the albumin-rich dialysate is not recycled since it is discharged after a single pass (hence the name). However, the advantage of SPAD is that less complex equipment is required (since a recirculation and recycling system is lacking) leading to a reduction in cost. With regard to detoxifying efficacy, data are conflicting and it is suggested that SPAD cannot fulfill its full potential without significantly increasing the costs Citation[90–93]. Currently, experiences with SPAD remain restricted to case reports or small series Citation[90–93].
HepaWash®
The HepaWash® (Hepa wash, Munich, Germany) system is very similar to the MARS device but differs by the fact that it regenerates (cleans) the exogenous albumin in the secondary circuit to at much larger extent, based on additional specific pH and temperature changes at the filter level Citation[94]. Owing to the technical approach described earlier and additional inventions, the treatment is expected to be more effective. The proof-of-concept has recently been demonstrated in a preclinical study with animals with acute liver failure. Human studies are currently on the way.
Clinical impact of liver support on survival
Human data are present only for MARS and Prometheus. The available studies, albeit often small and uncontrolled, clearly suggest biochemical and neurological improvement after MARS and Prometheus therapy with an additional positive hemodynamic effect only by MARS Citation[63–65,88,89,95–102]. The survival benefit that is supposed to result from these beneficial effects is, however, less obvious.
Survival benefit with MARS in patients with AoCLF was observed in a small study Citation[100]. In the absence of supportive large-sample randomized controlled data, the scientific community turned towards meta-analysis of the available data. Unfortunately, contrasting results were obtained in the two available meta-analyses regarding the effect on survival of nonbiological liver support in AoCLF. The first meta-analysis of four randomized and two selected nonrandomized trials in patients with AoCLF by Khuroo did not show any effect on mortality Citation[103]. On the other hand, explorative analysis (14 studies, 588 patients) by the Cochrane database group revealed a significant reduction of mortality in patients with AoCLF treated with nonbiological liver support as compared with the standard medical treatment group Citation[104]. However, it was questionable whether a meta-analysis could really help to answer the question of whether liver support improves survival or not in view of the low available sample sizes of patients enrolled in the individual studies and too much heterogeity in primary indication, primary end point and treatment protocols for intervention and standard treatment. It was concluded that the attempt to use a meta-analysis before there are sufficient data can therefore lead to wrong conclusions both in a negative or in positive sense. Owing to the aforementioned controversies, all hope was put on the concurrent multicenter randomized controlled trials with either MARS (RELIEF) trial MARS therapy of up to ten sessions of 6–8 h vs standard medical therapy. The trail enrolled 189 patients and had an average MELD of 28 Citation[105]. The second randomized contraolled trail (HELIOS) investigated Prometheus therapy of up to 11 treatments of minimal 4 h versus standard medical therapy. The study included 145 patients with an average MELD of 27 Citation[106]. Both studies are currently terminated and a primary analysis of both trials has been presented in abstract form. In the MARS-RELIEF trial, there was no effect on 28-day survival compared with standard medical therapy (59.2 vs 60%). No data were available with regard to later time points or subgroup analyses. In the HELIOS-trial, 28-day survival was comparable to the MARS-RELIEF and also nonsignificant (66 vs 63%). Reported predefined subgroup analyses here demonstrated a benefit of Prometheus in patients with hepatorenal syndrome type 1 (p = 0.004) and MELD >30 (p = 0.02). The full papers are awaited for further analysis and final conclusions.
Expert commentary & five-year view
Acute-on-chronic liver failure represents a newly-described complication of cirrhosis that has received considerable attention over the recent years and will undoubtfully continue to do so because of its impressive presentation, high mortality and impact on transplant allocation. At present, there is still an ongoing debate between ‘believers’ and ‘nonbelievers’; whether or not this syndrome really exists as an entity. Some even speak of an ‘identity crisis’. This is fed by the fact that there is only one consensus paper on diagnostic criteria (Asian Pacific Association for the Study of the Liver consensus) Citation[37] and because if one compares only clinical symptoms, such as jaundice, ascites and encephalopathy, there seems to be an overlap between AoCLF and chronic decompensated patients. However, similar clinical basic findings do not necessarily lead to the same disease constellation. One should take into account the whole frame of AoCLF and chronic hepatic decompensation considering clinical features, the potential impact of a precipitant, severity of concomitant end-organ dysfunction and the temporal relationship between liver dysfunction and end-organ dysfunction. For these reasons, a large multicenter prospective trial has been initiated in Europe (CANONIC–CLIF study) in an attempt to describe diagnostic criteria, natural history, mortality precipitating factors in a more evidence-based manner. Irrespective of ultimately globally accepted fine-tuned criteria, the core elements of AoLCF that have been consistently reported remain undisputably:
• The presence of a precipitating factor;
• A rapid deterioration in liver function;
• The subsequent initiation of organ failure;
• A high in-patient or early mortality (28 days).
In addition, translational studies exploring the pathogenesis of the early onset of AoCLF are urgently needed to identify potential targeted therapy that either holds or slows down the cytokine avalanche that follows from this initial hit.
Besides potential goal-directed pharmacological intervention, efforts to further develop liver support devices have also been stimulated. However, developing an effective liver assist technology has proven to be difficult because of the complexity of liver functions that must be replaced, as well as heterogeneity of the patient population. Initial experiences suggest a beneficial role of albumin dialysis, and in particular MARS, with regard to detoxification, hemodynamic improvement and renal recovery. The challenge upon us now is to learn how to best exploit these therapies to the patients’ advantage. We need to determine which biochemical and clinical parameters correlate best with important patient outcomes, such as overall survival, bridging to liver transplantation and prevention of multiorgan failure. This information will help determine which patients to select (or not) for liver support devices and which technical refinements are most likely to be beneficial and can guide future clinical studies. Different patient subgroups are likely to have different benefits from albumin dialysis. Trials should not combine acute liver failure and AoCFL, and different etiologies will have to be evaluated separately. The definite and published results of the recently terminated powered randomized controlled trials are urgently awaited.
Table 1. Systemic inflammatory response syndrome criteria.
Table 2. Molecular adsorbent recirculating system and Prometheus® in acute-on-chronic liver failure.
Box 1. Diagnostic criteria of hepatorenal syndrome.
• Cirrhosis with ascites
• Serum creatinine above 1.5 mg% (>1.33 mM)
• No improvement of serum creatinine (decrease to a level of 133 mmol/l) after at least 2 days with diuretic withdrawal and volume expansion with albumin. The recommended dose of albumin is 1 g/kg of bodyweight per day up to a maximum of 100 g/day
• Absence of shock
• No current or recent treatment with nephrotoxic drugs
• Absence of parenchymal kidney disease as indicated by proteinuria >500 mg/day, microhematuria (>50 red blood cells per high power field) and/or abnormal renal ultrasonography
Key issues
• Acute-on-chronic liver failure (AoCLF) is described as an acute and rapid deterioration of liver function accompanied by subsequent rapidly evolving multiple end-organ failure in a patient with previously well-compensated liver disease due to the effects of a precipitating event.
• The pathophysiology of AoCLF relates to intestinal permeability (leading to translocaton of bacteria and their products), an imbalanced immune reaction and aggravated microcirculatory dysfunction, as well as an accumulation of toxins.
• Infection is one of the most common causes of mortality in AoCLF patients.
• The global clinical and histological characteristics of AoCLF are ill-defined and few prospective, comparative data are available.
• The mortality of AoCLF is known to be high in in-patients (>50%) but no long-term data are available.
• Nonbiological liver support might represent a therapeutic tool in AoCLF to either recompensate or bridge the patient up to transplantation.
References
- Laleman W, Wilmer A, Evenepoel P, Verslype C, Fevery J, Nevens F. Review article: non-biological liver support in liver failure. Aliment. Pharmacol. Ther.23, 351–363 (2006).
- Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif.20, 252–261 (2002).
- Martin JA, Smith BL, Mathews TJ, Ventura SJ. Births and deaths: preliminary data for 1998. In: National Center for Health Statistics. National Vital Statistics Report. Vol. 47, No. 25. Hyattsville MD (Ed.). National Center for Health Statistics, USA (1998).
- Kim WR, Brown RS Jr, Terrault NA, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology36, 227–242 (2002).
- Leon DA, McCambridge J. Liver cirrhosis mortality rates in Britain from 1950 to 2002: an analysis of routine data. Lancet367, 52–56 (2006).
- Roberts SE, Goldacre MJ, Yeates D. Trends in mortality after hospital admission for liver cirrhosis in an English population from 1968 to 1999. Gut54, 1615–1621 (2005).
- Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J. Hepatol.40, 860–867 (2004).
- Vilstrup H, Iversen J, Tygstrup N. Glucoregulation in acute liver failure. Eur. J. Clin. Invest.16, 193–197 (1986).
- Wanless IR, Wong F, Blendis LM et al. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology21, 1238–1247 (1995).
- Wiesner R, Edwards E, Freeman R et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology124, 91–96 (2003).
- Desschans B, Van Gelder F, Van Hees D et al. Evolution in allocation rules for renal, hepatic, pancreatic and intestinal graft. Acta Chir. Belg.108, 31–34 (2008).
- Stange J, Mitzner S. A carrier-mediated transport of toxins in a hybrid membrane. Safety barrier between a patients blood and a bioartificial liver. Int. J. Artif. Organs19, 677–691 (1996).
- Sen S, Williams R. New liver support devices in acute liver failure: a critical evaluation. Semin. Liver Dis.23, 283–294 (2003).
- Sen S, Ytrebo LM, Rose C et al. Albumin dialysis: a new therapeutic strategy for intoxication from protein-bound drugs. Intensive Care Med.30, 496–501 (2004).
- Sen S, Williams R, Jalan R. Emerging indications for albumin dialysis. Am. J. Gastroenterol.100, 468–475 (2005).
- Stadlbauer V, Krisper P, Aigner R et al. Effect of extracorporeal liver support by MARS and Prometheus on serum cytokines in acute-on-chronic liver failure. Crit. Care10, R169 (2006).
- Krisper P, Stauber RE. Technology insight: artificial extracorporeal liver support – how does Prometheus compare with MARS. Nat. Clin. Pract. Nephrol.3, 267–276 (2007).
- Sen S, Jalan R, Williams R. Liver failure: basis of benefit of therapy with the molecular adsorbents recirculating system. Int. J. Biochem. Cell Biol.35, 1306–1311 (2003).
- Abouna GM. Cross-circulation between man and baboon in hepatic coma. Lancet2, 729–730 (1968).
- Hoofnagle JH, Carithers RL Jr, Shapiro C, Asscher N. Fulminant hepatic failure: summary of a workshop. Hepatology21, 240–252 (1995).
- Losser MD, Payen D. Mechanisms of liver damage. Sem. Liver Dis.16, 357–367 (1996).
- Thalheimer U, Triantos CK, Samonakis DN. Infection, coagulation, and variceal bleeding in cirrhosis. Gut54, 556–563 (2005).
- Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin. Liver Dis.28, 26–42 (2008).
- Navasa M, Fernandez J, Rodes J. Bacterial infections in liver cirrhosis. Ital. J. Gasteroenterol. Hepatol.31, 616–625 (1999).
- Antoniades CG, Berry P, Wendon J. The importance of immune dysfunction in determining outcome in acute liver failure. J. Hepatol.49, 845–861 (2008).
- Rosenbloom AJ, Pinsky MR, Bryant JL. Leukocyte activation in the peripheral blood of patients with cirrhosis of the liver and SIRS. Correlation with serum interleukin-6 levels and organ dysfunction. JAMA274, 58–65 (1995).
- Lin CH, Tsai IF, Ho YP. Endotoxemia contributes to the immune paralysis in patients with cirrhosis. J. Hepatol.46, 816–826 (2007).
- Wasmuth HE, Kunz D, Yagmur E et al. Patients with acute on chronic liver failure display ‘sepsis-like’ immune paralysis. J. Hepatol.42, 195–201 (2005).
- Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest112, 235–243 (1997).
- Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J. Hepatol.51, 475–482 (2009).
- Bone R. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit. Care Med.24, 1125–1128 (1999).
- Albillos A, Hera Ad Ade L, Reyes E et al. Tumour necrosis factor-α expression by activated monocytes and altered T-cell homeostasis in ascitic alcoholic cirrhosis: amelioration with norfloxacin. J. Hepatol.40, 624–631 (2004).
- Riordan SM, Skinner N, Nagree A et al. Peripheral blood mononuclear cell expression of toll-like receptors 2 and 4 in patients with liver cirrhosis. Hepatology37, 1154–1164 (2003).
- Leber B, Mayrhauser U, Rybczynski M, Stadlbauer V. Innate immune dysfunction in acute and chronic liver disease. Wien. Klin. Wochenschr.121, 732–744 (2009).
- Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int.26, 912–919 (2006).
- Fiuza C, Salcedo M, Clemente G et al. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J. Infect. Dis.182, 526–533 (2000).
- Sarin SK, Kumar A, Almeida JA et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol. Int.3, 269–282 (2009).
- Katoonizadeh A, Laleman W, Verslype C et al. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut59, 1561–1569 (2010).
- Duseja A, Chawla YK, Dhiman RK, Kumar A, Choudhary N, Taneja S. Non-hepatic insults are common precipitants in patients with acute on chronic liver failure (ACLF). Dig. Dis. Sci.55, 3188–3192 (2010).
- Karvellas CJ, Pink F, McPhail M et al. Bacteremia, acute physiology and chronic health evaluation II and modified end stage end liver disease are independent predictors of mortality in critically ill non-transplanted patients with acute on chronic liver failure. Crit. Care Med.38, 121–126 (2010).
- Aggarwal A, Ong JP, Younossi ZM, Nelson DR, Hoffman-Hogg L, Arroliga AC. Predictors of mortality and resource utilization in cirrhotic patients admitted to the medical ICU. Chest119, 1489–1497 (2001).
- Zimmerman JE, Wagner DP, Seneff MG et al. Intensive care unit admissions with cirrhosis: risk-stratifying patient groups and predicting individual survival. Hepatology23, 1393–1401 (1996).
- Wehler M, Kokoska J, Reulbach U, Hahn EG, Strauss R. Short-term prognosis in critically ill patients with cirrhosis assessed by prognostic scoring systems. Hepatology34, 255–261 (2001).
- Warrilow SJ. Predictions and outcomes for the critically ill patient with cirrhosis: is it time to settle on the SOFA and let jaundiced views on the outcome MELD away. Crit. Care Med.38, 2259–2260 (2010).
- Das V, Boelle PY, Galbois A et al. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit. Care Med38, 2108–2116 (2010).
- Cholongitas E, Senzolo M, Patch D et al. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short-term mortality in cirrhotic patients admitted to intensive care unit. Aliment. Pharmacol. Ther.23, 883–893 (2006).
- Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Concensus Conference definitions of the systemic inflammatory response syndrome (SIRS) and allied disorders in relation to critically injured patients. Crit. Care Med.25, 1789–1795 (1997).
- Thabut D, Massard J, Gangloff A et al. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology46, 1872–1882 (2007).
- Jalan R, Mookerjee RP. Acute-on-chronic liver failure: an early biopsy is essential? Gut59, 1455–1456 (2010).
- Laleman W, Van Landeghem L, Wilmer A, Fevery J, Nevens F. Portal hypertension: from pathophysiology to clinical practice. Liver Int.25, 1079–1090 (2005).
- Laleman W. Role of vasoactive substances and cellular effectors in the pathophysiology of cirrhotic portal hypertension: the past, the present and the future – Georges Brohée lecture. Acta Gastroenterol. Belg.72, 9–16 (2009).
- Bosch J, Garcia Pagan JC. Complications of cirrhosis. I. Portal hypertension. J. Hepatol.32(Suppl. 1), 141–156 (2000).
- Arroyo V, Jimenez W. Complications of cirrhosis. II. Renal and circulatory dysfunction. Light and shadows in an important clinical problem. J. Hepatol.32(Suppl. 1), 157–170 (2000).
- Fernandez Seara J, Prieto J, Quiroga J et al. Systemic and regional hemodynamics in patients with liver cirrhosis and ascites with and without functional renal failure. Gastroenterology97, 1304–1312 (1989).
- Maroto A, Gines P, Arroyo V et al. Brachial and femoral artery blood flow in cirrhosis: relationship to kidney dysfunction. Hepatology17, 788–793 (1993).
- Rivolta R, Maggi A, Cazzaniga M et al. Reduction of renal cortical blood flow assessed by Doppler in cirrhotic patients with refractory ascites. Hepatology28, 1235–1240 (1998).
- Guevara M, Bru C, Gines P et al. Increased cerebrovascular resistance in cirrhotic patients with ascites. Hepatology2, 839–844 (1998).
- Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A. Renal vasoconstriction in cirrhosis evaluated by duplex Doppler ultrasonography. Hepatology17, 219–224 (1993).
- Sugano S, Yamamoto K, Atobe T et al. Postprandial middle cerebral arterial vasoconstriction in cirrhotic patients. A placebo, controlled evaluation. J. Hepatol.34, 373–377 (2001).
- Dillon JF, Plevris JN, Wong FC et al. Middle cerebral artery blood flow velocity in patients with cirrhosis. Eur. J. Gastroenterol. Hepatol.7, 1087–1091 (1995).
- Henriksen JH, Kiszka-Kanowitz M, Bendtsen F. Review article: volume expansion in patients with cirrhosis. Aliment. Pharmacol. Ther.16(Suppl. 5), 12–23 (2002).
- Llach J, Gines P, Arroyo V et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology94, 482–487 (1998).
- Laleman W. Hemodynamic effects of albumin dialysis in patients with liver failure: for better or for worse? Ther. Apher. Dial.13, 384–392 (2009).
- Schmidt LE, Sorensen VR, Svendsen LB, Hansen BA, Larsen FS. Hemodynamic changes during a single treatment with the molecular adsorbents recirculating system in patients with acute-on-chronic liver failure. Liver Transpl.7, 1034–1039 (2001).
- Laleman W, Wilmer A, Evenepoel P et al. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit. Care10(4), R108 (2006).
- Ruiz del Arbol L. Urman J, Fernandez J et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology38, 1210–1218 (2003).
- Ruiz del Arbol L, Monescillo A, Arocena C et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology42, 439–447 (2005).
- Ma ZH, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology24, 451–459 (1996).
- Marik P, Gayowski T, Starzi T; Hepatic Cortisol Research and Adrenal Pathophysiology Study Group. The hepatoadrenal syndrome: a common yet unrecognized clinical condition. Crit. Care Med.33, 1254–1259 (2003).
- Tsai MH, Peng YS, Chen YC et al. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology43, 673–681 (2006).
- Fernández J, Escorsell A, Zabalza M et al. Adrenal insufficiency in patients with cirrhosis and septic shock: effect of treatment with hydrocortisone on survival. Hepatology44, 1288–1295 (2006).
- O’Beirne J, Holmes M, Agarwal B et al. Adrenal insufficiency in liver diease – what is the evidence? J. Hepatol.47, 418–423 (2007).
- Harry R, Auzinger G, Wendon J. The effects of supraphysiological doses of corticosteroids in hypotensive liver failure. Liver Int.23, 71–77 (2003).
- Moreau R, Lebrec D. Acute kidney injury: new concepts. Hepatorenal syndrome: the role of vasopressors. Nephron. Physiol.109, 73–79 (2008).
- Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut56, 1310–1318 (2007).
- Salerno F, Guevara M, Bernardi M et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int.30, 937–947 (2010).
- Gines A, Escorsell A, Gines P et al. Incidence, predictive factors and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology105, 229–236 (1993).
- Tristani FE, Cohn JN. Systemic and renal hemodynamics in oliguric hepatic failure: effect of volume expansion. J. Clin. Invest.46, 1894–1806 (1967).
- Moller S, Dumcke CW, Krag A. The heart and the liver. Expert Rev. Gastroenterol. Hepatol.3, 51–64 (2009).
- Bleck TP, Smith MC, Pierre-Louis SJ, Jares JJ, Murray J, Hansen CA. Neurologic complications of critical medical illnesses. Crit. Care Med.21, 98–103 (1993).
- Cordoba J, Garcia-Martinez R, Simon-Talero M. Hyponatremic and hepatic encephalopathies: similarities, differences and coexistence. Metab. Brain Dis.25, 73–80 (2010).
- Fichet J, Mercier E, Genee O et al. Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy. J. Crit. Care24, 364–370 (2009).
- Borzio M, Salerno F, Piantoni L et al. Bacterial infections in patients with advanced cirrhosis: a multicentric prospective study. Dig. Liver Dis.33, 41–48 (2001).
- Fernandez J, Navasa M, Gomez J et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology35, 140–148 (2002).
- Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the national hospital discharge survey. Chest124, 1016–1020 (2003).
- Meerseman W, Vandecasteele SJ, Wilmer A, Verbeken A, Peetermans WE, Van Wijngaerden E. Invasive aspergillosis in critically ill patients without malignancy. Am. J. Respir. Crit. Care Med.170, 621–625 (2004).
- Stange J, Ramlow W, Mitzner S, Schmidt R, Klinkmann H. Dialysis against a recycled albumin solution enables the removal of albumin-bound toxins. Artif. Organs17, 809–813 (1993).
- Stange J, Mitzner S. A carrier-mediated transport of toxins in a hybrid membrane. Safety barrier between a patients blood and a bioartificial liver. Int. J. Artif. Organs19, 677–691 (1996).
- Rifai K, Ernst T, Kretschmer U et al. Prometheus – a new extracorporeal system for the treatment of liver failure. J. Hepatol.39, 984–990 (2003).
- Peszynski P, Klammt S, Peters E et al. Albumin dialysis: single pass vs recirculation (MARS). Liver22(Suppl. 2), 40–42 (2002).
- Chawla LS, Georgescu F, Abell B et al. Modification of continuous venovenous hemodiafiltration with single-pass albumin dialysate allows for removal of serum bilirubin. Am. J. Kidney Dis.45, 51–56 (2005).
- Sauer IM, Goetz M, Steffen I et al. In vitro comparision of the molecular adsorbent recirculation system and single-pass albumin dialysis (SPAD). Hepatology39, 1408–1414 (2004).
- Kortgen A, Rauchfuss F, Götz M et al. Albumin dialysis in liver failure: comparison of molecular adsorbent recirculating system and single pass albumin dialysis – a retrospective analysis. Ther. Apher. Dial.13, 419–425 (2009).
- Al-Chalabi A, Matevossian E, Preissel AK et al. Survival improvement in pigs with liver failure and superimposed sepsis by a new liver support system (Hepa Wash®). Critical Care14, P508 (2010).
- Stadlbauer V, Davies NA, Sen S, Jalan R. Artificial liver support systems in the management of complications of cirrhosis. Semin. Liver Dis.28, 96–109 (2008).
- Stange J, Mitzner SR, Risler T et al. Molecular adsorbent recycling system (MARS): clinical results of a new membrane-based blood purification system for bioartificial liver support. Artif. Organs23, 319–330 (1999).
- Jalan R, Sen S, Steiner C, Kapoor D, Alisa A, Williams R. Extra-corporeal liver support with molecular adsorbents recirculating system in patients with severe acute alcoholic hepatitis. J. Hepatol.38, 24–31 (2003).
- Di Campli C, Zocco MA, Gaspari R et al. The decrease in cytokine concentration during albumin dialysis correlates with the prognosis of patients with acute on chronic liver failure. Transplant. Proc.37, 2551–2553 (2005).
- Mitzner SR, Stange J, Klammt S et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl.6, 277–286 (2000).
- Heemann U, Treichel U, Loock J et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology36, 949–958 (2002).
- Sen S, Davies NA, Mookerjee RP et al. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl.10, 1109–1119 (2004).
- Hassanein TI, Tofteng F, Brown RS Jr et al. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology46, 1853–1862 (2007).
- Khuroo MS, Farahat KL. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl.10, 1099–1106 (2004).
- Liu JP, Gluud LL, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for liver failure. Cochrane Database Syst. Rev.1, CD003628 (2004).
- Banares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M; RELIEF study group. Extracorporeal liver support with MARS in patients with acute-on-chronic liver failure. The RELIEF trial. J. Hepatol.52, S459 (2010).
- Rifai K, Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U; HELIOS-study group. Extracorporeal liver support by fractionated plasma separation and absorption (Prometheus) in patients with acute-on-chronic liver failure (HELIOS study): a prospective randomized controlled multicenter study. J. Hepatol.52, S3 (2010).
Website
- World Health Organization. Projections of mortality and burden of disease to 2030 www.who.int/healthinfo/statistics/bodprojections2030/en/index.html
Acute-on-chronic liver failure and artificial liver support
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertgastrohep. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For tech¬nical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) cred¬its are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Your patient is a 62-year-old man with chronic liver disease, previously fairly well compensated cirrhosis, and recent deterioration. Based on the above review by Dr. Laleman and colleagues, which of the following characteristics would make the diagnosis of acute-on-chronic liver failure (ACLF) vs end-stage liver disease most likely?
□ A Absence of a precipitating event
□ B Hepatic encephalopathy
□ C Rapidly evolving multiple end-organ failure
□ D Hyperdynamic circulatory state
2. The patient in question 1 is diagnosed with ACLF. Based on the above review, which of the following statements about the underlying pathophysiology and subsequent clinical course is most likely correct?
□ A Infection is not a likely cause of death
□ B Risk of in-patient mortality is about 25%
□ C The immune system and circulatory system are unlikely to be involved
□ D The pathophysiology includes intestinal permeability, leading to translocation of bacteria and their products
3. Based on the above review, which of the following statements about therapeutic interventions for the patient described in questions 1 and 2 is most likely correct?
□ A Nonbiological liver support plays no role in the treatment of ACLF
□ B Therapy involves managing the precipitating event, end-organ support, and treatment of complications
□ C The Molecular Adsorbent Recirculating System (MARS) combines fractionated plasma separation and adsorption (fractionated plasma separation and adsorption [FPSA]) with high-flux hemodialysis
□ D The Prometheus® system uses sorbents to remove albumin-bound molecules as well as a dialysis membrane
Notes
Adapted from Citation[75].