Abstract
Severe liver failure is common and carries a high mortality risk in patients with both acute and acute-on-chronic liver failure. The failing liver constitutes a medical emergency, and in many cases liver transplantation is the only definite treatment. Extracorporeal liver support can be employed as a strategy for bridging to transplantation or recovery. This article focuses on options for artificial (nonbiological) extracorporeal treatment: single-pass albumin dialysis, fractionated plasma separation and adsorption (Prometheus®) and the molecular adsorbent recirculatory system. Their different principles, potential advantages and indications are discussed. Despite proven biochemical efficacy, there are little data regarding clinical end points. Thus far, molecular adsorbent recirculatory system therapy in acute and acute-on-chronic liver failure showed no survival benefit compared with standard medical therapy. Prometheus therapy showed reduced mortality in subgroups of higher severity of disease compared with standard medical therapy. Nevertheless, the value of extracorporeal liver support remains to be corroborated by further clinical studies that include the optimal timing, mode, intensity and duration of this treatment.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertgastrohep; (4) view/print certificate.
Release date: September 12, 2011; Expiration date: September 12, 2012
Learning objectives
Upon completion of this activity, participants will be able to:
• Describe indications for and rationale behind use of extracorporeal liver support
• Describe nonbiologic options for extracorporeal liver support
• Describe outcomes associated with use of extracorporeal liver support
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Editorial Director, Future Science Group, London, UK.
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CMEAUTHOR
Laurie Barclay, MD
Freelance writer and reviewer, Medscape, LLC.
Disclosure: Laurie Barclay has disclosed no relevant financial relationships.
AUTHOR AND CREDENTIALS
Sibylle Rademacher, MD
Department of Nephrology and Medical Intensive Care, Charité-Universitätsmedizin Berlin, Campus Virchow-Klinikum, Berlin, Germany.
Sibylle Rademacher, MD, has disclosed no relevant financial relationships.
Michael Oppert, MD
Department of Nephrology and Medical Intensive Care, Charité-Universitätsmedizin Berlin, Campus Virchow-Klinikum, Berlin, Germany.
Michael Oppert, MD, has disclosed no relevant financial relationships.
Achim Jörres, MD
Department of Nephrology and Medical Intensive Care, Charité-Universitätsmedizin Berlin, Campus Virchow-Klinikum, Berlin, Germany.
Achim Jörres, MD, has disclosed no relevant financial relationships.
Severe liver failure is common and carries a mortality rate of up to 60% Citation[1]. It occurs in patients with a critical loss of functional liver tissue. Although liver failure can present in various different ways, the usual end-stage phenomenology includes: hepatic encephalopathy (HE), jaundice, decreased protein production with coagulopathy, hemodynamic instability and hepato–renal syndrome (HRS). This may lead to enhanced susceptibility for severe infections and eventually multi-organ failure Citation[2].
Liver failure can occur as acute liver failure (ALF), chronic liver failure, or acute-on-chronic liver failure (ACLF). ALF, a syndrome in which patients without pre-existing liver disease sustain an acute liver injury with a rapid loss of hepatic function, is a rare but life-threatening condition, accounting for 0.1% of all deaths in the USA in the 1980s Citation[2]. Etiologies of ALF in patients without pre-existing liver disease differ by country: while infections (mainly acute viral hepatitis) dominate in Asia and central Europe, toxins or drugs, especially acetaminophen, are the main causes of liver failure in northern Europe and the USA; further causes are cardiovascular failure and metabolic reasons Citation[3,4]. It is also increasingly occurring in patients with or after resolved multi-organ failure due to severe sepsis and septic shock Citation[5].
Acute-on-chronic liver failure is defined as an acute deterioration of liver function in patients with a known underlying compensated chronic liver disease or stable decompensated cirrhosis, and is associated with a short-term mortality rate of 50–90% Citation[6,7]. In many cases deterioration of liver function in ACLF occurs following a precipitating event such as acute gastrointestinal bleeding, excessive alcohol intake, or severe infections and sepsis Citation[7,8]. The commonly accepted mechanism of liver failure is the inability of the impaired liver to metabolize various toxins. This hypothesis is supported by the fact that serum bilirubin levels (as a surrogate marker) correlate with the degree of liver failure. Citation[8,9]. Therefore, the main goal for the caring physician in the intensive care unit is to stabilize the patient and increase their ability to eliminate toxins. This may buy precious time for either the liver to recover by itself, or to find a suitable organ for transplantation. Although modern intensive care has improved substantially, orthotopic liver transplantation remains the only effective treatment in many patients with severe liver failure Citation[9]. Owing to persistent shortage of donor organs, however, extracorporeal strategies to support the liver function were developed to bridge patients with ALF or ACLF to either transplantation or recovery.
Modalities of artificial liver support
An ideal liver assist device should support the three main liver functions: detoxification, regulation and synthesis Citation[10]. The latter task can obviously only be taken over by systems incorporating functional liver cells. These biological or bio-hybrid systems are, however, highly complex and, at least at present, are not easily available and/or manageable in clinical routine. By contrast, the nonbiological systems are mainly based on existing technologies such as hemodialysis, hemofiltration and adsorption Citation[10], and can thus be more readily incorporated in the routines of modern intensive care.
Since the accumulation of toxins plays a critical role in persisting liver failure, the use of nonbiological systems that aid the detoxification processes appears to be a promising option. In liver failure, toxic metabolites vary, however, in physiochemical characteristics such as molecular size, water solubility and protein binding. While conventional extracorporeal procedures such as continuous veno-venous hemodiafiltration (CVVHDF) are highly effective in removal of small, water-soluble toxins such as ammonia and urea, they cannot eliminate large and/or protein-bound molecules. It is thus not surprising that their use did not entail a survival benefit in patients with liver failure Citation[11]. As a consequence, detoxification systems that have the ability to remove lipophilic substances such as bilirubin, bile acids, aromatic amino acids and medium-chain fatty acids were developed. The predominant carrier of such toxins in human blood is albumin, which is therefore considered as the main substrate for adsorption and is identified as being the best target for the detoxification procedure Citation[12]. Hence, the concepts of albumin dialysis and fractionated plasma separation and adsorption (FPSA) were introduced.
Plasmapheresis & high-volume plasmapheresis
During plasmapheresis therapy a patient’s plasma is removed by plasma filtration and replaced by fresh–frozen plasma. As the therapy also removes protein-bound toxic metabolites as well as inflammatory mediators, it has been tried also in advanced liver failure. While effective removal of cytokines and endotoxin as well as an improvement of HE was observed after high-volume plasmapheresis Citation[13], there is, however, little data regarding hard clinical end points. Disadvantages of this procedure are the risk of infection and allergic reactions Citation[14].
Single-pass albumin dialysis
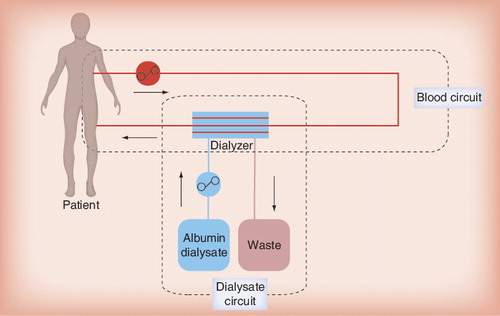
Single-pass albumin dialysis (SPAD) is the simplest form of albumin dialysis using the basic principles of hemodialysis or hemodiafiltration. It can be performed using a standard continuous veno-venous hemofiltration/CVVHDF machine for continuous renal-replacement therapy. The patient’s blood flows through a standard albumin-impermeable high-flux dialyzer or hemofilter and is dialyzed against an albumin-containing dialysate, thus enabling the removal of protein-bound molecules that are small enough to pass through the membrane pores . After passage of the dialyzer/hemofilter the dialysate is discarded (‘single-pass’), which constitutes the major difference to the molecular adsorbent recirculatory system (MARS®; Gambro, Germany) procedure (see later).
Various protocols for SPAD have been published using different dialysate albumin concentrations, treatment times and hardware Citation[15,16]. Our in-house protocol involves albumin dialysate in a conventional CVVHDF circuit. Anticoagulation is usually performed with either heparin or citrate. Standard bicarbonate-buffered dialysate is supplemented with albumin solution, resulting in a dialysate albumin concentration of 2% (20 g/l). For CVVHDF–SPAD, dialysate flow is set to 1 l/h in counter-directional flow. Albumin consumption thus approximates 480 g/24 h and renders this treatment fairly expensive.
In general, SPAD is easy to establish because it can be accomplished with standard dialysis equipment and is therefore widely applicable. A potential disadvantage may lie in the fact that, during the manufacturing process of human serum albumin, stabilizers such as caprylate (octanoate) or N-acetyl-tryptophanate are added. These stabilizers may diminish the binding capacity of albumin and thus decrease the efficacy of the SPAD procedure. Moreover, the stabilizers may be dialyzed back to the patients’ blood during the procedure. To date, however, no negative side effects on patients owing to the stabilizers have been reported to occur in the clinical setting Citation[15,16].
Molecular adsorbents recirculatory system
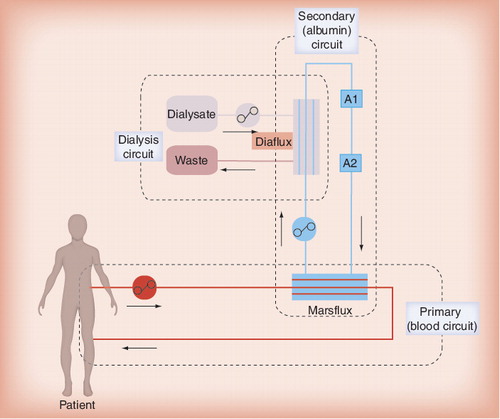
Molecular adsorbent recirculatory system is the most frequently applied artificial liver support, as well the best-studied detoxification system in liver failure to date. The MARS system, which was clinically introduced in 1993, is based on the principle of albumin dialysis. As in SPAD, the albumin plays the role of a molecular adsorber in the dialysate Citation[12]. To perform the procedure, a MARS monitor device and hemodialysis/hemofiltration machine are needed. In the primary circuit patient’s blood is passed through a hemofilter (Marsflux Filter) with a size selection threshold of less than 60 kDa, thus retaining albumin on the blood side. In a secondary circuit a 20% albumin solution is circulated, passing the filter in a counter-directional flow and acting as the dialysate. Toxins in the patient’s blood that dissociate from albumin binding may cross the membrane following the concentration gradient and bind to the albumin in the secondary circuit. As the Marsflux membrane is impermeable for albumin, only the free fraction of toxins can cross the membrane, which is the limiting factor for elimination of compounds with strong albumin binding such as unconjugated bilirubin Citation[17,18]. In the secondary circuit the ‘used’ albumin solution first undergoes dialysis using a high-flux filter to remove water-soluble toxins. Afterwards, the albumin solution is regenerated by passing two adsorbers: an anion exchanger resin (diaMARS IE250) and an uncoated charcoal adsorber (diaMARS AC250). The cleansed solution then re-enters the hemofilter of the primary circuit . One MARS session lasts 6–8 h, and after that time the albumin-regeneration capacity of the adsorbers decreases significantly.
No significant decrease in blood pressure has been observed under MARS therapy, but even a significant increase in blood pressure levels could be shown in comparison to Prometheus® (Fresenius, Germany) in a controlled study Citation[19]. This might be due to stable albumin levels compared with Prometheus Citation[17,20]. Besides, removal of vasoactive substances such as renin was observed during MARS treatment in the aforementioned study Citation[19]. A decline in platelet counts has been observed and activation of coagulation has been reported Citation[21–23]. Except for one retrospective study, no severe bleeding episodes were reported in association with MARS therapy Citation[23]. In summary, MARS is a safe procedure without significant occurrence of problems regarding hemodynamic or anticoagulation. Cytokines are not eliminated by MARS Citation[24,25].
Similar to SPAD and Prometheus, potential indications for MARS are ALF, ACLF, HE, Pruritus, drug intoxication and HRS Citation[26–28].
Prometheus FPSA
The Prometheus system is based on FPSA combined with hemodialysis. Its concept was first introduced in 1999 Citation[29].
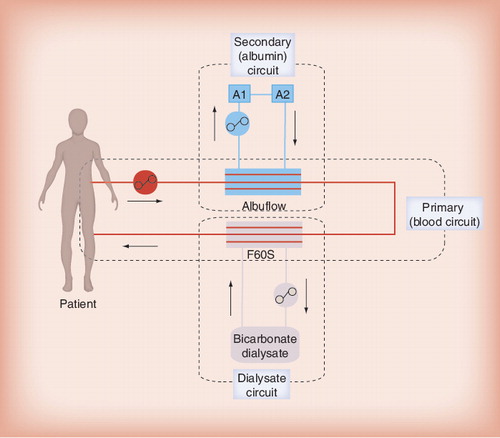
Using a standard dialysis catheter, the patient’s blood enters the primary extracorporeal circuit. In a first step the albumin fraction of the blood is selectively filtered through a specific polysulfone, albumin-permeable filter (Albuflow®) and enters a secondary circuit in which the filtered albumin-rich plasma fraction is treated by two adsorber columns: the first one is a neutral resin adsorber (Prometh® 01), and the second adsorber (Prometh® 02) is an anion exchanger for removal of negatively charged toxins. As the Albuflow filter has a size-selection threshold of 250 kDa, albumin-bound toxins may freely pass the Albuflow membrane by convection and bind to the adsorbers, resulting in effective removal, even of toxins with strong albumin binding. After passage of the two adsorbers, the cleansed albumin-rich plasma fraction re-enters the primary circuit. Here, in a second step, a conventional dialysis of the patients’ blood using a high-flux polysulfone dialyzer is performed to better eliminate water soluble compounds .
In the first published study on Prometheus treatment, a significant decrease in blood pressure during the session was observed, returning to baseline values afterwards Citation[30]. This finding could, however, not be reproduced in further studies Citation[31]. An initial reduction of blood pressure early into the procedure may, however, occur in some patients and can be explained by the acute reduction of intravascular blood volume due to the filling of the extracorporeal circuit. This phenomenon can be more pronounced in FPSA compared with SPAD or MARS owing to the filtration of the albumin-rich plasma fraction that fills the secondary circuit. Likewise, a lowering of systemic albumin levels with consecutive reduction of the colloid osmotic pressure was reported in some studies, as might be expected to occur owing to albumin filtration Citation[17,20]. However, no clinically relevant impairment of systemic hemodynamics was reported.
Furthermore, in Prometheus therapy, a reversible increase of white blood cell count without clinical signs of systemic infection was described, possibly occurring as a consequence of blood–membrane interaction Citation[30]. While no drop in platelet counts has been observed, clotting problems were occasionally reported, despite heparin anticoagulation. In such cases, an alternative management with citrate regional anticoagulation can be performed safely Citation[32]. Although a loss in pro- and anti-coagulant factors has been described during Prometheus therapy, no clinically relevant effects have been reported Citation[33]. Overall, Prometheus FPSA is a safe procedure that does not lead to clinically relevant changes in systemic hemodynamic or coagulation parameters. Moreover, several studies have confirmed its efficacy in toxin elimination Citation[20,30]. However, as with MARS, a decline in toxin elimination rates over time (>6 h) is observed, indicating saturation of the adsorber columns Citation[20]. In contrast, cytokines are not removed efficiently Citation[24,25].
In addition to its use as a treatment of ALF or ACLF, potential indications for Prometheus that were reported include HE, cholostatic pruritus, drug intoxications and HRS Citation[26–28]. Moreover, we have successfully used Prometheus FPSA as rescue therapy in patients with persistent ALF after recovery from multi-organ dysfunction syndrome. In these patients, isolated liver failure progressed despite successful therapy of septic or cardiogenic shock Citation[9]. Although these data were collected in our center in an uncontrolled, retrospective fashion, the results were encouraging, suggesting a new potential indication for extracorporeal liver support in the intensive care unit.
Therapeutic efficacy of artificial extracorporeal liver support therapy
Most data regarding clinical results with extracorporeal liver support therapy have been collected in ACLF patients. Data on ALF are limited and mostly based on case reports. Most of the studies published are limited by small patient numbers as well as by their design (case series or uncontrolled retrospective studies). Recently, however, three large prospective randomized studies evaluating patient survival in ALF or ACLF were completed (FULMAR, HELIOS and RELIEF) and became available in abstract form Citation[34–36].
Toxin removal (biochemical efficacy)
Several clinical studies underlined a higher efficiency of Prometheus in toxin removal compared with MARS. Effectiveness has been proven for both ALF and ACLF. A retrospective analysis confirmed the more effective toxin removal, showing a higher elimination rate of bilirubin, urea and creatinine Citation[20]. In a crossover study, similar results could be shown, with higher clearance rates of both water-soluble and water-insoluble toxins, especially bilirubin, in Prometheus therapy. Unconjugated bilirubin, a compound with tight albumin binding, was only eliminated by Prometheus, underlining the higher efficiency of the Prometheus system Citation[18]. In a controlled study a higher elimination rate of bilirubin by Prometheus could be underlined Citation[19]. In summary, an effective removal of toxins is shown for both systems; Prometheus being more efficient regarding tight albumin-bound and water-soluble toxins Citation[20].
HE & intracranial pressure
Significant improvement in HE could be demonstrated in several studies performing MARS in ACLF:
• Positive influence on HE was seen in a randomized, multicenter trial in chronic liver failure Citation[37];
• In a prospective randomized controlled study an improvement of HE without benefit in survival was found Citation[38];
• An open-label randomized controlled study showed an improvement of renal function and HE, as well as short-time survival under albumin dialysis Citation[39];
• An improvement of HE in ACLF by Prometheus was proven in another prospective trial Citation[26];
• In a case report HE was found to be improved by Prometheus therapy in ACLF Citation[40];
• In an uncontrolled study HE could not be improved by Prometheus in patients with ACLF Citation[30]. Data on ALF are limited in Prometheus and mostly based on case reports, also showing an improvement of HE Citation[41–43];
• Two animal studies have been published on decrease of intracranial pressure under extracorporeal liver support: in an animal study intracranial pressure in ALF in pigs was decreased by MARS therapy Citation[44]. Similar to MARS, a significant reduction in intracranial pressure by Prometheus therapy was observed in an experimental study on pigs with ALF Citation[27]. No human data on improvement of intracranial pressure are available thus far.
Hepato–renal syndrome
Hepato–renal syndrome is a severe complication of liver failure. Renal failure due to HRS is often quickly worsening and prognosis is poor. It mainly occurs late in cirrhosis, but may also be seen in ALF Citation[31,45,46]. Little data on influence of extracorporeal liver support on HRS are published thus far.
In a prospective randomized trial, improvement in kidney function as well as a 7-day survival benefit under MARS treatment in 13 patients with ACLF and HRS could be shown Citation[45]. In a study performing Prometheus therapy, it was shown that creatinine values improved significantly Citation[25]. In a subgroup analysis of the recently published HELIOS study, a survival benefit for patients with HRS could be shown under Prometheus treatment Citation[36]. A prospective randomized study on the impact of Prometheus therapy in HRS on outcome is presently ongoing in our center (LUTHER study; universal trial number U1111-1115-4645).
Cholestatic pruritus
Only uncontrolled data exist thus far regarding improvement of pruritus: in an animal study, removal of protein-bound drugs by MARS could be demonstrated Citation[47]. Improvement of cholestatic pruritus was described in numerous reports using MARS or Prometheus therapy Citation[28,48,49].
Patient survival
There have been no studies directly comparing the different systems regarding their potential impact on patient survival. Clinical improvement and better short-term survival have been shown for MARS treatment in small patient populations. A prospective case–control study of 13 patients showed no improvement in survival under MARS treatment, although toxins were removed efficiently and hemodynamic parameters improved significantly Citation[50]. A meta-analysis showed no significant survival benefit using MARS treatment in liver failure compared with standard therapy Citation[51]. In a retrospective analysis, Prometheus therapy led to a significant benefit in patients with ALF after survived multi-organ failure compared with patients with ACLF Citation[9]. The FULMAR study, a randomized controlled multicenter trial in 16 French centers, evaluated the efficacy and safety of MARS in 102 patients with fulminant and subfulminant hepatic failure, 38% of which had paracetamol poisoning. There was a trend of better 6-month survival in the MARS group: 84.9 vs 75.5%; although this did not reach statistical significance. The interpretation of results is made difficult by the fact that 68 of the 102 patients were transplanted with a median delay of 16.2 h between listing and incision, accordingly resulting in a short pretransplantation study phase in most patients Citation[34].
Recently, two prospective, randomized controlled multicenter studies comparing extracorporeal liver support and standard medical treatment in patients with ACLF were presented.
In the RELIEF study, 189 patients with ACLF were randomized to either SMT or additive treatment with MARS. MARS therapy was scheduled at a low dose over 21 days; in total, 156 patients were able to be analyzed. The primary end point was 28-day survival. There were no significant differences concerning baseline characteristics between the groups, although the number of patients with high model of end-stage liver disease (MELD) scores and spontaneous bacterial peritonitis was higher in the MARS group. In this study, age, MELD score and spontaneous bacterial peritonitis were independent risk factors for increased mortality. In the MARS group a higher reduction of creatinine and bilirubin levels was observed, and HE improved significantly in comparison to SMT. However, no survival benefit could be observed in the MARS group Citation[35].
In the HELIOS study, 145 patients with ACLF were randomized to either SMT or additive treatment with Prometheus. Prometheus therapy was initiated for 3 weeks, and the primary end points were 28- and 90-day survival. There were no significant differences in baseline characteristics concerning MELD score, age or etiology of underlying chronic liver disease. In the intention-to-treat analysis, survival on day 28 was 66 versus 63% (p = 0.70; SMT plus Prometheus vs SMT) and on day 90 was 47 versus 38% (p = 0.35), respectively. In a predefined subgroup analysis, a significant survival benefit was observed under FPSA therapy in patients with HRS type I (p = 0.04) or MELD score more than 30 (p = 0.02) Citation[36].
Expert commentary
Nonbiological extracorporeal liver support therapy effectively eliminates water-soluble and water-insoluble toxins in liver failure and carries no serious safety concerns. In clinical settings with interdisciplinary approach, implementation is feasible. Recent clinical trials showed no survival benefit of extracorporeal liver support therapy compared with standard therapy. However, for Prometheus therapy, patients with higher disease severity, such as presenting with HRS type I or a MELD score of more than 30, may be the most likely group to benefit from this treatment option. These findings, however, need to be corroborated by sufficiently powered randomized prospective trials.
Five-year view
Particular areas of uncertainty remain the timing, mode, intensity and duration of therapy of extracorporeal liver support. Further randomized prospective trials that are adequately powered to assess relevant clinical end points will be required to clarify these points and to identify the group(s) of patients who are most likely to benefit from treatment.
Key issues
• Severe liver failure is common and carries a high mortality.
• Orthotopic liver transplantation is the only definite treatment.
• Extracorporeal liver support is a method to bridge patients to transplantation or recovery.
• Molecular adsorbent recirculatory system and Prometheus® therapy are the most common techniques of nonbiological extracorporeal liver support systems.
• Molecular adsorbent recirculatory system and Prometheus therapy effectively eliminate water-soluble and nonsoluble toxins.
• No safety concerns are carried in clinical practice.
• While toxin elimination is proven to be effective, little data on hard clinical end points are available.
• Thus far no survival benefit compared with standard medical therapy could be demonstrated.
• Prometheus therapy showed a survival benefit in a subgroup of patients with higher severity disease; however, future studies are needed to confirm these findings.
• Modalities of start and timing of treatment, mode, intensity and duration need to be specified more clearly.
• The patient group with the highest benefit needs to be defined.
References
- Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am. J. Gastroenterol.102(11), 2459–2463 (2007).
- Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr. Gastroenterol. Rep.9(1), 66–73 (2007).
- Lee WM, Seremba E. Etiologies of acute liver failure. Curr. Opin. Crit. Care14(2), 198–201 (2008).
- Hadem J, Stiefel P, Bahr MJ et al. Prognostic implications of lactate, bilirubin, and etiology in German patients with acute liver failure. Clin. Gastroenterol. Hepatol.6(3), 339–345 (2008).
- Gimson AE. Hepatic dysfunction during bacterial sepsis. Intensive Care Med.13(3), 162–166 (1987).
- Katoonizadeh A, Laleman W, Verslype C et al. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut59(11), 1561–1569 (2010).
- Evenepoel P, Laleman W, Wilmer A et al. Detoxifying capacity and kinetics of Prometheus – an new extracorporeal system for the treatment of liver failure. Blood Purif.23(5), 349–358 (2005).
- Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif.20(3), 252–261 (2002).
- Oppert M, Rademacher S, Petrasch K, Jörres A. Extracorporeal liver support therapy with Prometheus in patients with liver failure in the intensive care unit. Ther. Apher. Dial.13(5), 426–430 (2009).
- Seige M, Kreymann B, Jeschke B, Schweigart U, Kopp KF, Classen M. Long-term treatment of patients with acute exacerbation of chronic liver failure by albumin dialysis. Transplant Proc.31(1–2), 1371–1375 (1999).
- Laleman W, Wilmer A, Evenepoel P, Verslype C, Fevery J, Nevens F. Review article: non-biological liver support in liver failure. Aliment. Pharmacol. Ther.23(3), 351–363 (2006).
- Stange J, Ramlow W, Mitzner S, Schmidt R, Klinkmann H. Dialysis against a recycled albumin solution enables the removal of albumin-bound toxins. Artif. Organs17(9), 809–813 (1993).
- Larsen FS, Hansen BA, Jørgensen LG, Secher NH, Kirkegaard P, Tygstrup N. High-volume plasmapheresis and acute liver transplantation in fulminant hepatic failure. Transplant Proc.26(3), 1788 (1994).
- Pless G. Artificial and bioartifical liver support. Organogenesis3(1), 20–24 (2007).
- Peszynski P, Klammt S, Peters E, Mitzner S, Stange J, Schmidt R. Albumin dialysis: single pass vs. recirculation (MARS). Liver22(Suppl. 2), 40–42 (2002).
- Mitzner S, Klammt S, Stange J, Schmidt R. Albumin regeneration in liver support – comparison of different methods. Ther. Apher. Dial.10(2), 108–117 (2006).
- Krisper P, Stauber R. Technology insight: artificial extracorporeal liver support – how does Prometheus compare with MARS? Nat. Clin. Pract. Nephrol.3(5), 267–276 (2007).
- Krisper P, Haditsch B, Stauber R et al.In vivo quantification of liver dialysis: comparison of albumin dialysis and fractionated plasma separation. J. Hepatol.43(3), 451–457 (2005).
- Laleman W, Wilmer A, Evenepoel P et al. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit. Care10(4), R108 (2006).
- Evenepoel P, Laleman W, Wilmer A et al. Prometheus versus molecular adsorbents recirculating system: comparison of efficiency in two different liver detoxification devices. Artif. Organs30(4), 276–284 (2006).
- Rifai K. Extracorporeal albumin dialysis. Hepatol. Res.38(S1), S41–S45 (2008).
- Doria C, Mandalà L, Smith JD et al. Thromboelastography used to assess coagulation during treatment with molecular adsorbent recirculating system. Clin. Transplant.18(4), 365–371 (2004).
- Bachli EB, Schuepbach RA, Maggiorini M, Stocker R, Müllhaupt B, Renner EL. Artificial liver support with the molecular adsorbent recirculating system: activation of coagulation and bleeding complications. Liver Int.27(4), 475–484 (2007).
- Stadlbauer V, Krisper P, Aigner R et al. Effect of extracorporeal liver support by MARS and Prometheus on serum cytokines in acute-on-chronic liver failure. Crit. Care10(6), R169 (2006).
- Rifai K, Ernst T, Kretschmer U, Haller H, Manns MP, Fliser D. Removal selectivity of Prometheus: a new extracorporeal liver support device. World J. Gastroenterol.12(6), 940–944 (2006).
- Kramer L, Gendo A, Funk G, Madl C, Falkenhagen D, Gangl A. Clinical experience with artificial liver support in chronic liver failure with encephalopathy. ASAIO J.46(2), 211 (2000).
- Ryska M, Laszikova E, Pantoflicek T, Ryska O, Prazak J, Koblihova E. Fractionated plasma separation and adsorption significantly decreases intracranial pressure in acute liver failure: experimental study. Eur. Surg. Res.42(4), 230–235 (2009).
- Rifai K, Hafer C, Rosenau J et al. Treatment of severe refractory pruritus with fractionated plasma separation and adsorption (Prometheus). Scand. J. Gastroenterol.41(10), 1212–1217 (2006).
- Falkenhagen D, Strobl W, Vogt G et al. Fractionated plasma separation and adsorption system: a novel system for blood purification to remove albumin bound substances. Artif. Organs23(1), 81–86 (1999).
- Rifai K, Ernst T, Kretschmer U et al. Prometheus – a new extracorporeal system for the treatment of liver failure. J. Hepatol.39(6), 984–990 (2003).
- Rifai K, Ernst T, Kretschmer U et al. The Prometheus device for extracorporeal support of combined liver and renal failure. Blood Purif.23(4), 298–302 (2005).
- Herget-Rosenthal S, Lison C, Treichel U et al. Citrate anticoagulated modified fractionated plasma separation and adsorption: first clinical efficiacy and safety data in liver failure. Presented at: The ASN Renal Week 2003. CA, USA, 12–17 November 2003.
- Meijers BK, Verhamme P, Nevens F et al. Major coagulation disturbances during fractionated plasma separation and adsorption. Am. J. Transplant.7(9), 2195–2199 (2007).
- Saliba F, Camus C, Durand F et al. Randomized controlled multicenter trial evaluating the efficacy and safety of albumin dialysis with MARS® in patients with fulminant and subfulminant hepatic failure. Presented at: The 59th Annual meeting of the American Association for the Study of Liver Diseases. CA, USA, 31 October–4 November 2008.
- Bañares R, Nevens F, Larsen FS et al. Extracorporeal liver support with the molecular adsorbent recirculating system (MARS) in patients with acute-on-chronic liver failure. The RELIEF trial. J. Hepatol.52(S1), S459–S460 (2010).
- Rifai K, Kribben A, Gerken G et al. Extracorporeal liver support by fractionated plasma separation and absorption (Prometheus®) in patients with acute-on-chronic liver failure (HELIOS study): a prospective randomized controlled multicenter study. J. Hepatol.52(S1), S3 (2010).
- Hassanein TI, Tofteng F, Brown RS Jr et al. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology46(6), 1853–1862 (2007).
- Sen S, Davies NA, Mookerjee RP et al. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl.10(9), 1109–1119 (2004).
- Heemann U, Treichel U, Loock J et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology36(4 pt 1), 949–958 (2002).
- Jung O, Asbe-Vollkopf A, Betz C, Caspary W, Geiger H, Faust D. Long-term therapy of acute chronic liver failure to successful transplantation with an extracorporeal liver support system. Z Gastroenterol.45(1), 21–24 (2007).
- Skwarek A, Grodzicki M, Nyckowski P et al. The use Prometheus FPSA system in the treatment of acute liver failure: preliminary results. Transplant. Proc.38(1), 209–211 (2006).
- Stadlbauer V, Fickert P, Lackern C et al. Hepatotoxicity of NONI juice: report of two cases. World J. Gastroenterol.11(30), 4758–4760 (2005).
- Kramer L, Bauer E, Schenk P, Steininger R, Vigl M, Mallek R. Successful treatment of refractory cerebral oedema in ecstasy/cocaine-induced fulminant hepatic failure using a new high-efficacy liver detoxification device (FPSA–Prometheus). Wien. Klin. Wochenschr.115(15–16), 599–603 (2003).
- Sen S, Rose C, Ytrebø LM et al. Effect of albumin dialysis on intracranial pressure increase in pigs with acute liver failure: a randomized study. Crit. Care Med.34(1), 158–164 (2006).
- Mitzner SR, Stange J, Klammt S et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl.6(3), 277–286 (2000).
- Guevara M, Ginès P. Hepatorenal syndrome. Dig Dis.23(1), 47–55 (2005).
- Sen S, Ytrebø LM, Rose C et al. Albumin dialysis: a new therapeutic strategy for intoxication from protein-bound drugs. Intensive Care Med.30(3), 496–501 (2004).
- Parés A, Herrera M, Avilés J, Sanz M, Mas A. Treatment of resistant pruritus from cholestasis with albumin dialysis: combined analysis of patients from three centers. J. Hepatol.53(2), 307–312 (2010).
- Bellmann R, Freistritzer C, Zoller H et al. Treatment of intractable pruritus in drug induced cholestasis with albumin dialysis: a report of two cases. ASAIO J.50(4), 387–391 (2004).
- Schmidt LE, Wang LP, Hansen BA, Larsen FS. Systemic hemodynamic effects of treatment with the molecular adsorbents recirculating system in patients with hyperacute liver failure: a prospective controlled trial. Liver Transpl.9(3), 290–297 (2003).
- Khuroo MS, Khuroo MS, Farahat KL. Molecular adsorbent recirculation system for acute and acute-on-chronic liver failure: a meta analysis. Liver Transpl.10(9), 1099–1106 (2004).
Artificial extracorporeal liver support therapy in patients with severeliver failure
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertgastrohep. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Your patient is a 46-year-old man with acute severe liver failure caused by acetaminophen toxicity. Based on the review by Dr Rademacher and colleagues, which of the following statements about indications for and rationale behind use of extracorporeal liver support is most likely correct?
□ A Mortality of severe liver failure is about 10%
□ B Mortality for acute liver failure is low, whereas mortality of acute-on-chronic liver failure is high
□ C Extracorporeal liver support is the only definitive treatment
□ D Extracorporeal liver support is a strategy for bridging to transplantation or recovery
2. Based on the review by Dr Rademacher and colleagues, which of the following statements is most likely correct regarding management options for the patient described in question 1?
□ A The most commonly used technique of nonbiologic extracorporeal liver support is single-pass albumin dialysis (SPAD)
□ B Fractionated plasma separation and adsorption (Prometheus® FPSA) therapy effectively eliminates water-soluble and nonsoluble toxins
□ C Harmful effects on hemodynamics and anticoagulation limit the safety of molecular adsorbent recirculatory system (MARS®)
□ D For MARS and Prometheus, toxin elimination rates increase from 6 to 12 hours of treatment
3. Based on the review by Dr Rademacher and colleagues, which of the following statements about outcomes associated with use of extracorporeal liver support is most likely correct?
□ A Optimal timing, mode, intensity, and duration of treatment are well-defined
□ B MARS significantly improves survival in patients with acute-on-chronic liver failure compared with medical therapy
□ C Prometheus vs standard medical therapy showed reduced mortality in patient subgroups with higher severity of disease
□ D No studies have assessed the effect of extracorporeal liver support on hepatorenal syndrome