Abstract
Pediatric and adolescent gynecology focuses on a unique subset of gynecologic disorders among younger females. In the pediatric patient, gynecologic issues often present as vulvar and vaginal problems, while in the adolescent patient, complaints of abdominopelvic pain and abnormal menstrual bleeding commonly result in a gynecologic evaluation. This article focuses on two common vulvovaginal problems in the pediatric patient: vulvovaginitis and accidental genital trauma. Common infectious pathogens and treatments are reviewed along with other dermatologic and chemical causes of vulvovaginitis. The review of genital trauma focuses on various types of injury: straddle, penetrating and lacerations, and includes indications for surgical intervention. Pain and bleeding are the most common reasons for adolescents to frequent the gynecologist office, and this review focuses on these two topics, specifically menorrhagia and endometriosis. Etiologies of menorrhagia are reviewed with specific attention to anovulation and coagulation disorders. Hormonal therapy for these patients is addressed. The review of endometriosis provides insight into both medical and surgical management for optimum treatment of this disease.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 1.5 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at www.medscapecme.com/journal/expertob; (4) view/print certificate.
Learning objectives
Upon completion of this activity, participants should be able to:
• Describe the difference between gynecologic problems in female children and adolescents
• Identify the most common pathogens involved in prepubertal vulvovaginitis
• Compare the accuracy of tests for gonorrhea and Chlamydia in children
• Describe dermatologic gynecologic conditions in children
• Describe the etiology and presentation of vaginal foreign bodies in children and adolescents
Financial & competing interests disclosure
Editor
Elisa Manzotti, Editorial Director, Future Science Group, London, UK.
Disclosure:Elisa Manzotti has disclosed no relevant financial relationships.
CME Author
Désirée Lie, MD, MSEdClinical Professor, Family Medicine, University of California, Irvine, Orange, California; Director of Research and Patient Development, Family Medicine, University of California, Irvine, Medical Center, Rossmoor, CA, USA.
Disclosure:Désirée Lie, MD, MSEd, has disclosed the following relevant financial relationship: she served as a nonproduct speaker for: ‘Topics in Health’for Merck Speaker Services.
Authors and Credentials
Paige Hertweck, MDDepartment of Obstetrics, Gynecology and Women’s Health, University of Louisville School of Medicine, Louisville, KY, USA
Disclosure:Paige Hertweck, MD, has served as a speaker or a member of a speakers bureau for Merck & Co., Inc.
Jennie Yoost, MDDepartment of Obstetrics, Gynecology and Women’s Health, University of Louisville School of Medicine, Louisville, KY, USA
Disclosure:Jennie Yoost, MD, has disclosed no relevant financial relationships.
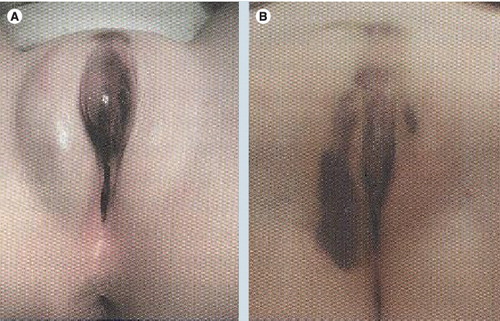
(A) Straddle injury in 6-year-old who fell while climbing over a chair sustaining a stable periclitoral hematoma. (B) Same patient 6 days later with extravasation of hematoma.
Reprinted with permission from Diane Merritt, Washington University, St Louis, MO, USA.
Reprinted from Citation[122].
![Figure 3. Black endometriosis.Reprinted from Citation[122].](/cms/asset/7229cb9d-2646-471b-88c6-2008770fefd0/ierb_a_11205111_f0003_b.jpg)
Reprinted from Citation[122].
![Figure 4. Blue endometriosis.Reprinted from Citation[122].](/cms/asset/6c4f8f5d-2d24-44c7-a76f-3c70ceaf72b9/ierb_a_11205111_f0004_b.jpg)
Reprinted from Citation[122].
![Figure 5. Red–pink endometriosis.Reprinted from Citation[122].](/cms/asset/8927f3ac-0475-444d-b595-33f268d9210e/ierb_a_11205111_f0005_b.jpg)
Reprinted from Citation[122].
![Figure 6. Clear endometriosis.Reprinted from Citation[122].](/cms/asset/542a2ff2-0048-44b6-9f3d-6ffd2a04d614/ierb_a_11205111_f0006_b.jpg)
Reprinted from Citation[122].
![Figure 7. White endometriosis.Reprinted from Citation[122].](/cms/asset/69c582d3-b023-4587-a8be-25ff995ae21d/ierb_a_11205111_f0007_b.jpg)
Vulvovaginitis
Inflammation of the vulvar and vaginal tissues and complaints of vaginal discharge are common gynecologic problems. In children, the hypoestrogenic state of the vagina contributes to the susceptibility of infection. The vaginal mucosa is thin and the vagina has an alkaline pH, making it different from the vagina of adolescents and adults. Children are also at an increased risk from behavioral aspects, such as poor perineal hygiene leading to fecal contamination, poor hand washing, and frequent play that can allow dirt or sand to cause irritation or infection. A child may present with discharge, irritation, pruritis, urinary symptoms, abnormal odor or erythema of the vulva Citation[1,2]. In adolescents, the increase in estrogen further develops the vaginal epithelium, making it more resistant to infection. Vaginitis, cervicitis and salpingitis are therefore more common than vulvovaginitis in this age group Citation[3].
Initial history should include home remedies, prescription creams or other medications that may have already been used. Medical history of eczema, contact dermatitis, other recent upper respiratory, pharyngeal or gastrointestinal infections, and diabetes should also be assessed. The question of sexual abuse should not be overlooked, and when indicated, a review of the social situation should be conducted to record who is involved or comes in contact with the child on a daily basis Citation[1]. After a complete physical examination, a genital examination can be performed in the frog-leg or knee-chest position, and using a sterile moist swab, samples should be obtained for Gram stain, wet mount, and aerobic and anaerobic cultures Citation[4]. When obtaining samples, a smaller caliber urethral swab is better tolerated than a typical cotton-tipped swab. In children who are not cooperative with examination, an examination under anesthesia with or without vaginoscopy can help facilitate the diagnosis Citation[5].
Etiologies
Most cases of vulvovaginitis in the pediatric population are nonspecific in nature and have no significant bacterial cause. These cases are most commonly present with a history of vulvovaginal irritation and erythema for months to years that may have responded to broad-spectrum antibiotics, but then recur. In many cases, the neutral pH of the vagina allows rectal bacteria to overgrow in the vagina and create these symptoms even when there is an attempt at adequate hygiene. These cases are best treated with symptomatic relief with one to two soaks in warm tub water daily and postbath use of a barrier cream such as zinc oxide or another diaper rash ointment. If there is not relief within 48–72 h, or there is a discharge, a specific cause should be ruled out with a culture (Box 1).
Vulvovaginitis caused by specific organisms can be divided into nonsexually and sexually transmitted etiologies. Isolation of the predominant organism is important, and testing for the presence of gonorrhea and chlamydia should be performed when appropriate.
Normal vaginal flora includes mixed aerobes and anaerobes, commonly Staphylococcus epidermidis, diptheroids, lactobacilli and anaerobic bacteria such as bacteroide Citation[6]. Typically, no treatment is indicated for this mixed picture of commonly occurring organisms. The predominant bacterial flora isolated in cases of vulvovaginitis include Streptococcus pyogenes, Staphylococcus aureus, Proteus mirabilis, Neisseria meningitides, Streptococcus pneumonia, Enterococci, Escherichia coli and Gardnerella vaginalis. Bacterial-associated vaginitis is best treated by antibiotics directed toward the specific organism Citation[7].
In one prospective study evaluating vulvovaginitis in patients 12 months to 12 years of age, infections from streptococcus, particularly S. pyogenes, were the most common infection diagnosed Citation[8]. In another study of 80 prepubertal girls with vulvovaginitis, S. pyogenes was the pathogen in 59% Citation[9]. This infection should respond to appropriate oral antibiotic therapy, such as penicillin or ampicillin. Recurrent vulvovaginitis with S. pyogenes has been documented from asymptomatic bacterial carriage within the nasopharynx Citation[10,11].
Other studies have shown that the evaluation of constipation should be included as part of the management of vulvovaginitis. Constipation is commonly present in children with vulvovaginitis, and may contribute to vulvar and vaginal colonization of coliform bacteria. Painful vaginitis may also lead to the child withholding bowel movements, which further complicates the problem Citation[12]. When compared with women of childbearing age and menopausal women, the highest occurrence of vaginal Gram-negative bacteria, mainly E. coli, was in prepubescent girls Citation[13].
Other rare bacterial infections can occur. Shigella can produce acute vaginal infections in children, and should be specifically tested for in the child that presents with a personal or family history of diarrheal illness preceding vaginal infection or in the child presenting with vaginal bleeding of unknown origin Citation[14]. Haemophilus influenzae is not a commonly found vaginal flora. This pathogen in childhood can cause a vaginitis that many times coexists with another infection such as otitis, bronchitis or adenitis. The spread of these respiratory bacteria is from hand to perineal contact Citation[2].
Candida albicans can be found in healthy patients on the skin, mouth, colon and vaginal mucosa. This fungus is responsible for diaper dermatitis that presents with erythematous skin in the diaper area involving the inguinal folds. There may also be scattered satellite lesions. Fungal vulvovaginitis is much more common in the postadolescent female. In children, candidal infections are uncommon outside of the diaper period. Presenting symptoms of candidiasis includes pruritis, erythematous mucosa and thick white vaginal discharge. Inflammation may be severe enough to produce erosive changes to the vulva that cause burning with urination. Diagnosis is made by visualization of hyphae or budding yeast on potassium hydroxide preparation. Treatment consists of topical antifungal creams including nystatin, miconazole, clotrimazole or terconazole creams. If erythema is intense, adding a topical hydrocortisone preparation may be helpful. Children who are immunosuppressed can receive oral fluconazole suspension, via a 4.5-mg/kg single dose Citation[15].
Sexually transmitted infections include Neisseria gonorrhea, Chlamydia trachomatis and Trichomonas vaginalis. Trichomonas is rare in children or adolescents unless they have been sexually abused, as this pathogen generally prefers a vagina exposed to the action of estrogens Citation[16]. Diagnosis is made by wet mount and treated with metronidazole 15 mg/kg/day three-times daily for 7 days. In sexually abused children, the incidence of gonococcal infections ranges from 5 to 20%, while the incidence of chlamydial infections ranges from 2 to 13% Citation[1].
Chlamydia trachomatis can be a coexistant infection in patients with gonorrhea. One study showed that, among children with gonorrhea, there was a 27% incidence of concurrent chlamydial infection. The purulent discharge associated with gonorrhea infection may cease after treatment and be replaced by serous discharge from persisting chlamydial infection Citation[17]. Vaginal colonization can occur in newborn infants with ocular or respiratory tract infection Citation[18]. There are reports of perinatal transmission that can persist for at least 3 years Citation[19]. Sexual abuse, however, must still be considered. Acute gonorrhea typically causes severe vaginitis with purulent discharge. All children with gonococcal infections should be evaluated for sexual abuse Citation[8]. The standard for diagnosing gonorrhea and chlamydia in children was previously by culture; however, several studies have reviewed the role of nucleic acid amplification tests (NAATs) on both urine and vaginal swab specimens. A study in 2009 completed in four US cities compared urine and genital swabs for chlamydia and gonorrhea collected from children evaluated for sexual abuse. The prevalence of infection among 485 female children was 2.7% for chlamydia and 3.3% for gonorrhea by NAAT. The sensitivity of urine NAATs for chlamydia and gonorrhea relative to culture is reported to be 100%, and suggests that NAATs on urine with confirmation are adequate for use as a new forensic standard for diagnosis for chlamydia and gonorrhea in children suspected for sexual abuse. This is helpful in younger patients who may not tolerate a vaginal swab Citation[20,21]. Chlamydia is treated with erythromycin 50 mg/kg/day four-times daily for 14 days, or if the patient is over 8 years of age, azithromycin 1 g orally for one dose or doxycycline 100 mg twice daily for 7 days. For those under 45 kg in weight, gonorrhea is treated with one dose of ceftriaxone 125 mg intramuscularly, plus treatment for chlamydial infection Citation[4].
Chemical/irritant (or allergic) vulvovaginitis
The lower one-third of the vagina, along with the vulvar tissue, is most commonly associated with nonspecific vaginitis. This includes reactions to local irritants such as soaps, bubble bath, nylon underwear, cosmetics, perspiration, sand and dirt. The underlying cause of irritation must be eliminated, and teaching of proper perineal hygiene should be given to the parent or child. Pruritis can be treated with daily sitz baths and application of hydrocortisone cream. In adolescents experimenting with feminine sprays, douches, tampons or pads, careful history and avoidance of the irritant is all that is necessary for resolution Citation[1,5,7].
Dermatologic conditions
Lichen sclerosus can produce vulvovaginitis and vaginal bleeding Citation[7]. Lesions are small, pink, flat papules that coalesce into plaques that can become atrophic and often present in a classic figure-of-eight configuration of parchment-like thin epithelium that includes both the vulva and perianal area. Associated subepithelial hemorrhages may be confused with vulvar trauma and abuse. Excoriation and secondary infection may complicate the clinical picture. Etiology may be linked to autoimmune disorders. Lichen sclerosis commonly presents with pruritis, and treatment is symptomatic with application of topical steroids. Chronic constipation is present in more than 60% of these cases, and failure to address this may further aggravate the dermatologic condition Citation[12,22].
Lichen simplex chronicus (LSC) is a chronic eczematoid inflammatory disorder presenting with pruritic lichenified plaques that can also cause vaginal bleeding, mainly from excoriation Citation[7]. LSC can be a primary condition, or secondary as a reaction to another vulvar disease, such as lichen sclerosus or lichen planus. Primary LSC is more common and may be due to exposure to an irritating substance, such as a laundry product, which results in chronic irritation and scratching. This creates persistence of an isolated area of pruritis that is fairly well localized on the labial skin. Treatment includes topical steroids and antihistamines to cease the itch–scratch cycle Citation[22].
Lichen planus is a less common dermatosis that presents as reticular or lacy white patterns of erosions of the vulva and the oral mucosa. The oral lesions may not be symptomatic. Affected patients may complain of hair loss and have a history of papular and puritic lesions on other skin surfaces such as the ankles, dorsal surfaces of the hands, and flexor surfaces of the wrists and forearms.
Most of these dermatoses will respond to the use of a 2-week tapering dose of steroids followed by maintenance barrier creams (i.e., clobetasol propionate ointment twice daily for 2 weeks, followed by fluticasone ointment twice daily for 2 weeks, then alclometasone ointment twice daily for 2 weeks, and lastly hydrocortisone 1% ointment twice daily for 2 weeks).
Anatomic abnormalities
An ectopic ureter should be included in the differential diagnosis of a child with persistent vaginal discharge. The discharge can either be thick and white or purulent, or present with constant dampness. An ectopic ureter describes an anomalous opening of the ureter into the vagina, perineum or distal urethra. An intravenous pyelogram is used for diagnosis Citation[12] and is helpful when the affected kidney is functional. MRI with urography sequences may provide better delineation of urologic structures and serve a dual function to detect coexisting reproductive tract malformations, as well as localizing a small nonfunctioning kidney Citation[23].
Congenital Mullerian anomalies, which typically present in the adolescent with pain secondary to menstrual obstruction, can rarely present in the prepubertal female with persistent foul smelling vaginal discharge due to partially obstructed vaginal canals, resulting in bacterial overgrowth. Genitograms and MRI may be helpful in delineating the anatomy in these unusual cases Citation[24]. Chylous lymphatic drainage site has also been reported. It is a rare anomaly that occurs with lymphatic obstruction Citation[5].
Foreign body
A foreign body in the vagina can present with bleeding or foul malodorous discharge. A total of 79% of all foreign bodies in the vagina are wads of toilet paper Citation[7]. In the adolescent population, the most common foreign body is a tampon Citation[1]. In girls presenting with persistent vulvovaginal complaints, a foreign body has been found in up to 45% in girls under the age of 6 years Citation[25], in 4% of patients under 13 years of age Citation[26], and up to 15% of patients with bloody discharge Citation[25].
Many patients will present with recurrent or persistent vaginal discharge. To rule out a foreign body, a careful clinical examination must be performed with possible need for examination under anesthesia, vaginal irrigation or vaginoscopy. Vaginal irrigation has been described using a pediatric Foley catheter or a pediatric feeding tube, a 60-ml syringe and normal saline. Approximately 200 ml of fluid can be injected when the Foley tip is inserted in the vagina. The moderate pressure irrigates the vagina. This method can be performed in the clinic in older, cooperative children. Under anesthesia, vaginoscopy can be performed using a pediatric cystoscope or small hysteroscope. Normal saline is used to distend the vagina, and the labia can be held closed for better distention and visualization. Vaginoscopy under anesthesia is more successful in the identification of foreign bodies not discernable under clinical examination Citation[5].
There is a large spectrum of diagnoses that cause vulvovaginitis. Careful history and examination should be performed to rule out vaginitis from sexual abuse, or from chemical or dermatologic causes. Vaginitis from specific organisms can be treated with appropriate antibiotics, and in persistent cases an examination under anesthesia is indicated to rule out the presence of a foreign body or anatomic cause of the discharge.
Trauma
Genital trauma in the pediatric patient can be from accidental injury or from intentional assault Citation[27]. The location of the injury, however, can cause great caregiver anxiety over concerns of future gynecologic and sexual development Citation[28]. Perineal injuries represent 0.2% of all injuries in children 15 years of age and younger Citation[29]. In the prepubertal child, unestrogenized tissue lacks distensibility and is more fragile, which can, in rare cases of blunt vehicular trauma, result in vaginal lacerations as the result of extreme pelvic compressive forces Citation[30].
Abrasions, contusions, lacerations and hematomas can all result from trauma to the genital area. The most common etiology of genital trauma in girls under the age of 14 years is straddle injury to the vulva. A 3.5-year retrospective review of unintentional genital trauma presenting to a pediatric referral center emergency department evaluating 105 girls revealed that 81% were found to have straddle injuries from a variety of common objects such as the arms of chairs, countertops, ledges of pools and bathtubs, bicycle crossbars, and ladder rungs Citation[28]. Another study of 358 girls revealed that most injuries were in children younger than 10 years of age. Assaults were more common in children 0–4 years of age, falls and bicycle injuries common in children 5–9 years of age and car accidents were responsible for the majority of genital trauma over the age of 15 years Citation[31]. The need for surgical intervention in pediatric and adolescent genital trauma is low. Patients requiring surgical repair is reported at only 15–20% Citation[28,32]. In patients who sustain only blunt trauma, need for surgical repair has been reported at only 9% Citation[31]. Healing can be achieved with conservative measures such as sitz baths and decreased physical activity, especially for the first 48–72 h to ensure the area is not reinjured Citation[28].
Despite the low risk for operative care, the examination of children and adolescents with genital injuries must be thorough. Conscious sedation or anesthesia may be needed owing to patient intolerance of examination from either pain or fear. Injuries should be described in detail, including appearance, location and size. Photographs for documentation can also be helpful Citation[33]. Although sexual assault will not be reviewed in detail, it is imperative to consider assault in any child presenting with genital injury. The history given by the child, caregivers and witnesses are the most important element in the initial assessment. It is helpful to question parties separately for corroboration. Clues of sexual abuse can include a nonambulatory child or any infant younger than 9 months of age, perianal, vaginal or hymenal injury without history of penetrating trauma, extensive or severe trauma, presence of nongenital trauma, and lack of correlation between history and physical findings Citation[28,32,34].
Straddle injuries
As noted previously, straddle injuries are the most common accidental female genital trauma, defined as a fall where the subject straddles an object compressing the soft tissue of the vulva between the object and the underlying bones of the pelvis. Examples of these objects can be the arms of chairs, bathtubs, bicycle crossbars, ladder rungs and playground equipment Citation[28,32,34]. In a retrospective review, the majority of cases occurred between the ages of 2 and 6 years, and straddle injuries were more common in the summer months Citation[32]. Straddle injuries most commonly involve the external genitalia, vestibule, perineum or posterior fourchette. Injuries extending outside the genital and anal areas are uncommon Citation[33].
Most straddle injuries are nonpenetrative injuries and present with ecchymosis or hematoma formation over the labia or mons . Vaginal bleeding due to vulvar hematomas can be profuse. If hematoma formation is near the urethra, the patient’s ability to urinate may be compromised and a Foley catheter should be placed until the swelling resolves. If the hematoma becomes progressively larger, serial hemoglobins should be checked. In the majority of cases, observation and cold compresses are all that is indicated. With falling hematocrit or patient instability, surgery is necessary. Surgical intervention focuses on incising the hematoma and ligating any bleeding vessels. Blood transfusion may also be necessary Citation[7]. A recent study reported the case of a Word catheter, traditionally used for Bartholin’s cysts, used to treat a large vulvar hematoma in an adolescent patient. The Word catheter was left in place for 8 days after incision and drainage of the hematoma, allowing for effective healing Citation[35].
Penetrating injuries
Penetrating injuries are defined as a piercing injury of the genitourinary (GU) or anorectal tissues. These types of injury are usually more extensive and more commonly involve trauma to the hymen and vagina, and visceral injury is possible even in the absence of significant bleeding and symptoms Citation[32,33]. Both straddle and water jet penetrating injuries in the vagina by high insufflation pressures have been described Citation[32,34].
Penetration injuries can present with hematuria, vaginal bleeding, vaginal discharge, pain or rectal bleeding. These cases require a thorough examination and may need to be performed with assistance from conscious sedation or anesthesia. Abdominal radiography, anoscopy and sigmoidoscopy can also be indicated. If the injury is above the hymen or if the patient is not hemodynamically stable, a thorough examination under anesthesia with vaginoscopy is indicated, and if there are concerns about intraperitoneal injury a laparoscopy or laparotomy should be performed Citation[36].
Hematuria is a common finding after blunt trauma to the genital area or GU tract. However, based on a retrospective review of patients sustaining blunt trauma presenting to a tertiary trauma center at a children’s hospital, microscopic hematuria alone is a relatively nonspecific finding, and is not a good predictor of GU injury. Not only did two-thirds of patients presenting with some degree of hematuria have no GU injury Citation[37], in this study, hematuria defined as less than 20 red blood cells/high-powered field as an indication for evaluation of the GU tract would have missed approximately one third of cases with a GU tract injury or anomaly. The need for GU tract evaluation in pediatric trauma patients is based as much on clinical judgement and signs such as positive physical abdominal findings, head injury and pelvic fracture as on hematuria.
In penetrating injuries, GU injury is more common, and gross hematuria is the most important indicator of severe GU injury. CT scan is the recommended imaging as it gives the most information about abdominal injuries Citation[38].
Lacerations
Lacerations to the vagina can be seen with a straddle or penetrating injury, forceful abduction of the legs, assault or consensual sexual activity Citation[28,36]. Motor vehicle accidents resulting in pelvic fracture can result in bone shards causing lacerations. In children with pelvic fractures, 17% will also have GU injuries Citation[34]. Risk factors for laceration during consensual sexual activity include first coitus, insertion of foreign objects during sex, congenital vaginal abnormalities, and coitus associated with drug and alcohol use Citation[28].
Lacerations of the vagina frequently extend into the fornix. An examination under anesthesia must be performed to determine the extent of the lesion to rule out involvement of the rectovaginal septum or extension of the laceration into the peritoneal cavity Citation[36]. Vaginoscopy with a small hysteroscope or cystoscope has proven beneficial to visualize the vaginal walls, fornices and cervix. Vaginoscopy can also assist in the repair of lacerations or coagulation of bleeding Citation[39]. Tetanus prophylaxis should be considered if the laceration or penetrating injury was from an unknown object or metal device Citation[40].
Labial & clitoral strangulation
Hair-thread tourniquet syndrome involves fibers of a hair or thread wrapped tightly around an appendage producing tissue necrosis. Strangulation of the fingers toes, uvula and penis have been described. This syndrome can also affect female external genitalia, labia minora and the clitoris. Labial and clitoral tourniquet syndromes have been described in older age groups (aged 7–11 years) as opposed to other appendages. The patient’s head hair is the most likely tourniquet source, and these cases are inadvertent and accidental. Patients present with symptoms of perineal pain and burning. Hair is an ideal tourniquet because it is thin and has high tensile strength. The swollen tissue surrounding the hair may embed it into the tissue, making diagnosis difficult. This tourniquet constriction causes lymphatic obstruction, venous outflow obstruction and decreased vascular perfusion. This syndrome must be recognized early and the hair removed to avoid necrosis of the tissue and eventual amputation. Early removal of the tourniquet results in successful reperfusion and is the only treatment indicated. Antibiotic treatment can be used for cases where necrosis or infection is found Citation[41–44].
Summary
Accidental trauma is commonly seen in the prepubertal child, with straddle injuries the most prevalent. The majority of cases require no surgical intervention; however, penetrating injuries or hemodynamic instability require immediate laparotomy or laparoscopy. Sexual abuse must always be a consideration in the evaluation.
Dysfunctional uterine bleeding: menorrhagia in adolescents
Dysfunctional uterine bleeding refers to endometrial bleeding that is prolonged, excessive or irregular, and not attributed to an anatomic lesion of the uterus (Box 2)Citation[45]. Heavy menses, or menorrhagia, is defined as menstrual loss greater than 80 ml Citation[46]. Anovulation and bleeding disorders make up the vast majority of menorrhagia in adolescents, which differs from an adult population, where pelvic pathology such as fibroids and polyps are more common Citation[47].
According to a committee opinion from the American College of Obstetricians and Gynecologists, normal menstruation in adolescents occurs between 11 and 14 years of age, the normal cycle length is 21–45 days, and the length of the period is 7 days or less Citation[48]. The events of the menstrual cycle should occur in an orderly and sequential pattern due to the effect of the hypothalamic–pituitary–ovarian (HPO) axis. In adolescents, the HPO axis takes time to mature after menarche, which can lead to anovulation. In the first 2 years after menarche, 55–82% of cycles are anovulatory, and by the fourth and fifth year this decreases to 20% of cycles being anovulatory. Approximately 95% of dysfunctional uterine bleeding in adolescents is due to anovulation Citation[49–51].
Without ovulation, progesterone is not subsequently produced from the corpus luteum of the ovary. This leads to a state of unopposed estrogen. Unopposed estrogen stimulates endometrial growth, which ultimately outgrows its blood supply. The endometrium becomes excessively thickened and unstable, and the lining breaks down irregularly and unpredictably. This leads to heavy bleeding and prolonged menstruation Citation[49,50,52].
Heavy, prolonged, recurrent menses may also represent an underlying coagulation disorder. This is especially true in those adolescents with sufficient bleeding to cause anemia Citation[53,54]. A normal number of platelets, normal platelet function and normal levels of clotting factors are all needed for hemostasis to occur. Bleeding disorders can be inherited or acquired, and can be due to thrombocytopenia, platelet function disorders and clotting factor deficiencies. von Willebrand Disease (VWD) is the most common inherited bleeding disorder, estimated to affect 1% of populations studied Citation[53,54]. VWD is due to either a qualitative or quantitative defect in von Willebrand factor (VWF). VWF is required to adhere platelets to exposed subendothelium and protect Factor VIII from proteolysis Citation[55]. One study showed that the most common coagulation disorders based on hospital admission over a 10-year period were VWD, idiopathic thrombocytopenic purpura and leukemia Citation[56]. The prevalence of menorrhagia in women with VWD ranges from 32 to 100% Citation[57], while the prevalence in platelet disorders such as Bernard–Soulier syndrome and Glanzmann’s thrombasthenia have been reported at 51 and 98%, respectively Citation[58,59]. The prevalence of menorrhagia among hemophilia carriers, who have variably reduced levels of Factor VIII or Factor IX, is reported to be 10–57% Citation[55].
Among adolescents with menorrhagia, the prevalence of VWD is 5–36%, the prevalence of platelet dysfunction is 2–44%, the prevalence of clotting factor deficiency is 8%, and the prevalence of thrombocytopenia is 13–20% Citation[55]. As coagulation disorders are the second most common cause of menorrhagia among adolescents, laboratory screening for these disorders is recommended in women under the age of 18 years who present with menorrhagia Citation[60,61] (see later under ‘Laboratory assessment’).
Other causes of menorrhagia, such as pregnancy and infection, should be considered in the sexually active adolescent. Sexually transmitted infections, especially chlamydia endometritis, can result in heavy menses. Infection causes inflammation of the endometrium, making the endometrial lining fragile Citation[47]. Adolescents are more likely than adults to contract a sexually transmitted infection, become reinfected and to have partners that are likely to have a sexually transmitted disease Citation[49]. Most adolescents are also sexually active for at least 6 months before they present for contraception, which increases their risk of abnormal bleeding due to a threatened abortion, incomplete abortion or ectopic pregnancy. In cases of heavy bleeding, adolescent pregnancy should always be ruled out Citation[62,63].
History & physical examination
The evaluation of the adolescent with menorrhagia should begin with a detailed history. Age of menarche, frequency of menstruation, and duration and character of flow should be recorded. Adolescent’s perception, complaints of heavy menstrual bleeding and understanding of what constitutes menorrhagia can be variable owing to their limited knowledge of what is considered normal. Asking about the number of pads or tampons used is often inaccurate, and it is more helpful to ask details such as number of overflow pads or number of hours each pad or tampon can be worn Citation[49]. It is also necessary to assess the impact on the adolescents’ life by determining the number of school days missed or inability to participate in sports or social activities Citation[47]. Specific historical information that may lead to the diagnosis of a coagulation defect includes prolonged bleeding from wounds, surgery or dental procedures, easy bruising with minimal trauma, nosebleeds lasting more than 10 min or requiring medical attention, unexplained bleeding from the GI tract or anemia requiring transfusion. Menorrhagia with bleeding disorders tends to present at menarche. It is also important to assess family history of known bleeding disorders Citation[55,63].
Upon physical examination, height, weight, BMI, orthostatic blood pressure and pulse should be recorded. Examination of the major body systems gives insight to endocrinologic or systemic disease. Petechial hemorrhages and ecchymosis give evidence of a bleeding disorder. Tanner stage of breast and pubic hair should also be noted. In sexually active adolescents, a complete pelvic examination should be performed, including testing for sexually transmitted diseases Citation[49].
Laboratory assessment
Laboratory assessment should begin with a pregnancy test and a complete blood count with platelet count to assess for anemia and thrombocytopenia. Coagulation studies including prothrombin time and partial thromboplastin time are also generally indicated Citation[60]. On occasion, a patient may have heavy bleeding that is not reflected in the complete blood count, and the patient may present on initial assessment with a normal hemoglobin, hematocrit and platelet count. Therefore, a serum ferritin or reticulocyte count can be helpful, as a low ferritin with a normal hemoglobin count suggests depleted iron stores, and an elevated reticulocyte count can indicate increased blood loss Citation[63]. The partial thromboplastin time should be normal in patients with a platelet dysfunction and may also be normal in patients with VWD. If history indicates a bleeding disorder, if bleeding is severe, prolonged or associated with menarche, or if the initial screen is abnormal, specific laboratories for coagulation defects should be tested. These include VWF antigen, Factor VIII activity, Factor XI antigen, ristocetin C cofactor and platelet aggregation studies Citation[49,60]. Estrogens will increase VWF, so obtaining laboratory testing prior to initiating hormonal treatment is important. Furthermore, platelet aggregation testing results are not accurate unless the hemoglobin count is approximately 10 gm/dl. Attention to these details when ordering and interpreting laboratory testing is crucial with regards to patient diagnosis and management. Referral to a hematology specialist may be needed for investigation beyond these laboratories to determine the presence of rarer factor deficiencies in a cost-effective manner Citation[47].
Treatment
The goal of initial assessment is to determine which adolescent needs treatment and which adolescent can be observed until the maturation of the HPO axis results in regular normal menses. If there is no anemia, patient stress is minimal and the flow is only slightly to moderately increased, observation is appropriate. A menstrual calendar or pictorial blood assessment chart can also be used to prospectively record the exact menstrual pattern Citation[52,64].
Hormonal treatment is the most effective therapy, with more than 93% of adolescents responding to some form of hormonal treatment Citation[65]. Estrogen provides hemostasis and progesterone stabilizes the endometrium. The easiest way to administer these hormones is by use of combined oral contraceptive pills (OCPs). Monophasic pills containing at least 30–35 µg of ethinyl estradiol should be used initially and can be given once daily in the adolescent who is not actively bleeding. If there is heavier or active bleeding an OCP taper can be used. There are several regimens documented, but a common approach is to give one pill four-times daily until bleeding stops, then one pill three-times daily for 3 days, then one pill twice daily for 2 days, followed by one pill once daily. Antiemetics may be required when multiple pills are given. After completing the OCP taper, the patient can continue cycling OCPs for 6 months. If contraception is needed, the pills are continued long term. Prescribing OCPs in a continuous, rather than cyclic fashion, is another option to suppress menses Citation[49,50,63]. Any time hormonal methods are prescribed, possible side effects of hormonal therapy must be explained, including nausea, vomiting, spotting, breakthrough bleeding and the rare possibility of passing a decidual cast or having a venous thromboembolic event Citation[66].
Hormonal therapy is often successful in treating menorrhagia in adolescents with bleeding disorders, and OCPs can be used as first-line treatment. OCPs have been specifically shown to reduce menstrual blood loss in patients with VWD Citation[67], and can possibly increase VWF and Factor VIII levels Citation[55].
If combined OCPs are contraindicated, or the patient or family does not wish to start oral contraception, cyclic progestins can be used. Oral medroxyprogesterone 10 mg or norethindrone acetate 5 mg can be given for 10–14 days each month to induce a withdrawal bleed that is cyclic and predictable. This pattern is continued for 3–6 months Citation[45]. Medroxyprogesterone acetate injection can also be used to reduce endometrial proliferation and blood loss. Medroxyprogesterone acetate is now available in a subcutaneous form, which is a good alternative in patients with bleeding disorders in which an intramuscular injection may result in bleeding Citation[55].
The levonorgestrel intrauterine device (IUD) is effective in decreasing menstrual blood loss, and should be considered in both nulliparous and parous adolescents Citation[68]. A recent study using the levonorgestrel IUD in 16 women with coagulopathies showed that, after 9 months of use, all women had a significant increase in hemoglobin concentration. Pictoral bleeding assessment charts (PBACs) were also used in these patients. A PBAC score of over 100 is significant for menorrhagia, and the initial median PBAC score among these patients was 213. After 9 months of IUD use, PBAC scores significantly improved to a median of 47. A total of 54% of subjects became amenorrheic with IUD use Citation[69]. There is only one study of the use of levonorgestrel IUD in adolescents; a retrospective study that revealed that 17% of those users did so for the treatment of menorrhagia. However, the study was not designed to investigate the therapeutic efficacy for the device. The 1-year continuation rate was 85% in these adolescents and there was a low expulsion rate Citation[70].
The addition of NSAIDs can also offer improvement of symptoms. Naproxen sodium and mefenamic acid decrease menstrual blood loss by 46 and 47%, respectively Citation[71]. These should be avoided, however, when there is high suspicion of a bleeding disorder. Antifibrinolytics, such as tranexamic acid and aminocaproic acid, can reduce menstrual loss by 50%, but have side effects of nausea, diarrhea, headaches and abdominal pain Citation[72]. These drugs can be successfully used in patients with bleeding disorders to inhibit fibrinolysis. Tranexamic acid is better tolerated, but not yet available in the USA Citation[55].
1-deamino-8-d-arginine vasopressin (DDAVP) has an established role treating patients with abnormal bleeding in VWD, mild-to-moderate hemophilia A, and some cases of platelet dysfunction. DDAVP increases levels of VWF and Factor VIII from endothelial cells, and is given intranasally or intravenously. Side effects include water retention and hyponatremia, so use is limited to 48 h Citation[55].
In cases of severe bleeding, clinical signs of blood loss may be present and the patient should be admitted to the hospital. Hormonal therapy is the mainstay of treatment for menstrual cessation, most commonly with use of a tapering dosage of combination OCPs (every 6 h until bleeding ceases followed by a taper in dose over several days). In cases of intractable nausea, either intravenous premarin 25 mg every 4 h can be given or placement of hormonal contraceptive patches. In one study, intravenous premarin stopped severe uterine bleeding after two doses in 72% of patients Citation[73]. If bleeding does not stop within 24 h, gynecologic consultation is needed Citation[45,50]. In patients with bleeding disorders that present with severe bleeding, clotting factor concentrates such as fresh–frozen plasma or cryoprecipitate can be given. VWF concentrates can be given to patients with VWD, recombinant Factor VIII or Factor IX given to hemophila carriers, and platelet transfusions given in cases of severe thrombocytopenia Citation[55]. In acute life-threatening situations, the use of a Foley balloon to tamponade the uterine cavity, and the use of uterine packing, uterine artery embolization and endometrial balloon ablation have all be described in adolescents Citation[74–76].
In some cases, thrombocytopenia or myelosuppression can be anticipated, such as in adolescents undergoing chemotherapy. Preventative measures can be taken by giving a gonadotropin-releasing hormone (GnRH) agonist to induce amenorrhea. A retrospective study compared depot medroxyprogesterone acetate, a GnRH agonist and placebo, in women undergoing myelosuppressive chemotherapy. No menorrhagia was found in the women receiving the GnRH agonist, compared with 21% in the medroxyprogesterone group and 40% in the placebo group Citation[77].
Careful assessment and prompt recognition is important in the adolescent with menorrhagia. Anovulation is more common and will often abate as the HPO axis matures. By contrast, diagnosing an underlying coagulation disorder or an otherwise asymptomatic cervicitis (i.e., chlamydia) can lead to specific treatment options. No single therapy or treatment is universal, and it must be tailored to the adolescent and clinical situation. For more information on treatment options, see the ‘Information resources’ section.
Endometriosis
Many adolescents presenting for adolescent gynecologic evaluation present with a complaint of chronic pelvic pain, and endometriosis is a possible etiology. Endometriosis is defined by the presence of endometrial glands and stroma outside the uterus Citation[78]. The etiology of endometriosis has been debated, and there are several theories regarding this matter. Sampson’s theory suggests endometriosis occurs in the pelvis by retrograde menstruation through the fallopian tubes Citation[79]. This theory is supported by the observation that implants occur most commonly in the dependent portions of the pelvis. Meyer’s theory states that undifferentiated cells of the peritoneal surface differentiate into endometrial tissue, while Halban’s theory suggests that endometrial tissue is spread through vascular or lymphatic channels Citation[78]. More recent studies report that immunologic defects contribute to endometriosis, allowing ectopic endometrial tissue to proliferate and not be cleared by the immune system Citation[80,81].
The incidence of endometriosis among adolescents with pelvic pain is estimated at 25–38% Citation[82], while in adolescents refractory to medical treatment for pelvic pain, endometriosis is found in 67% of cases at laparoscopy Citation[83]. Endometriosis has been described in premenarchal girls Citation[84,85], and has also been documented to occur within 1 month of menarche Citation[86]. Endometriosis, when diagnosed in the adolescent versus an adult patient, is more likely to be associated with a Mullerian anomaly. One study showed Mullerian anomalies in 11% of 74 teenagers studied with endometriosis Citation[87]. Another difference from an adult population is that, at the time of diagnosis, approximately 74–80% of adolescents will have stage I or mild disease Citation[88,89], whereas adults will present with higher stages of disease. Endometriosis is a progressive disease; therefore, young adolescents will probably not have deep infiltrating endometriotic lesions Citation[90,91]. Similarly, endometriomas are not often seen in adolescents as they do not typically present until the mid-20s Citation[78].
History & physical examination
A detailed history in the adolescent with chronic pelvic pain can reveal cyclic or acyclic pain. Some patients have an increase in pain mid-cycle and again with menses. The patient should be asked about the location of pain, pain severity, quality and timing of the pain Citation[92]. An abdominal examination should be performed as well as a pelvic examination when appropriate. In young adolescent patients or virginal patients a pelvic examination can be omitted Citation[93]. In adults with endometriosis, pelvic examination can reveal tender nodularity, a fixed and nonmobile uterus, or adnexal masses such as endometriomas. The adolescent pelvic examination is more likely to reveal only mild-to-moderate tenderness Citation[78] as endometriomas are not common. While endometriotic implants cannot be seen on any imaging study, and endometriomas are uncommon in the adolescent, a pelvic ultrasound should be performed to rule out a uterine anomaly, other pelvic pathology or the rare endometrioma Citation[94].
Medical management
The treatment for adolescents has been adapted from adult cases of endometriosis. Medical management is the initial approach in the adolescent patient. Surgical therapy should be reserved for the patient with persistent pain despite medical treatment. The goal of therapy is to treat the pain, cease progression of the disease and preserve fertility Citation[89].
Nonsteroidal anti-inflammatory drugs are the first-line treatment in adolescents and work by inhibiting the cyclooxygenase enzyme pathway that produces prostaglandins and leukotrienes Citation[95]. NSAIDs have been shown to decrease menstrual loss and significantly improve dysmenorrhea Citation[95].
Adolescents with symptoms that do not respond to NSAID treatment for three menstrual periods can be offered hormonal therapy. The use of continuous OCPs, GnRH agonists or progestin therapy are used to suppress the proliferation of endometriotic implants that depend on ovarian steroids for growth. Continuous OCPs have been shown to suppress endometriosis Citation[96], and when compared with cyclic OCP therapy, a Cochrane review of six randomized controlled trials demonstrated continuous OCP use to be more effective in treating menstrual pain Citation[97].
One randomized controlled trial also noted that women using continuous OCPs had significantly fewer days of bleeding, less hygiene product use and less expense. Side effects of continous OCPs include breakthrough bleeding, which decreases over time, nausea and breast tenderness Citation[98]. OCPs can be used concurrently with NSAIDs, and continuous hormone therapy similar to OCPs can be obtained using the transdermal patch (Ortho Evra®; norelgestromin/ethinyl estradiol transdermal system, Ortho–McNeil–Jannsen Pharmaceuticals, Inc., NJ, USA) or vaginal ring (Nuva Ring®; etonogestrel/ethinyl estradiol vaginal ring, Organon USA, NJ, USA) Citation[78].
Continuous progestins can also be effective in reducing pain from endometriosis by inducing amenorrhea Citation[99]. Regimens include norethindrone acetate 15 mg daily, medroxyprogesterone acetate 30–50 mg daily or medroxyprogesterone acetate 150 mg intramuscularly every 3 months. Side effects most notably include irregular menses, weight gain, bloating, acne and headaches. Progestins can be used in patients who do not tolerate OCP therapy, or in whom OCP therapy is contraindicated Citation[78]. Similarly, a levonorgestrel-releasing IUD can be beneficial in suppressing menses, and should be another consideration in the adolescent population. IUDs have been shown to be safe and effective in adolescents, with no increased risk of pelvic inflammatory disease or ectopic pregnancy Citation[100,101]. There are reports of the etonogestrel implant being used successfully in adults to treat pain symptoms associated with endometriosis Citation[102]. The levonorgestrel IUD has also been shown to be as effective as a GnRH analog in the treatment of chronic pain associated with endometriosis in adults Citation[103].
Gonadotropin-releasing hormone agonists work by downregulating the pituitary release of gonadotropins, which results in a hypoestrogenic state Citation[78]. A GnRH agonist is appropriate to use in adolescents who have failed the primary treatment regimens described previously. With initial treatment there is a stimulatory phase prior to the downregulation that may cause a worsening of symptoms initially prior to suppressing pain. One study demonstrated that 3.75 mg of depot leuprolide given intramuscularly every month for 6 months improved pelvic pain in 85% of patients, compared with only 43% receiving placebo Citation[104]. Another study reported 81% improvement with use of depot leuprolide compared with 36% in the placebo group after only 3 months, with improvement in dysmenorrhea, pelvic pain, deep dyspareunia, pelvic tenderness and in duration. They recommended surgical intervention by laparoscopy at 3 months for those not responding to GnRH therapy Citation[105].
A worrisome side effect of GnRH agonist therapy is its negative effect on bone mineral density. As older adolescents have attained more of their peak bone density, GnRH therapy is used more commonly in older adolescents both as empiric (in girls >18 years of age) and post-surgical therapy (in girls >16 years of age) for those with endometriosis. When GnRH agonists are used for 6 months, the trabecular bone loss is 7% and may not return to baseline levels following cessation of treatment Citation[106]. To combat this loss, daily add-back therapy with either estradiol (0.625 mg daily), norethindrone (5 mg daily) or medroxyprogesterone acetate (5 mg daily) has been shown to be effective in the adult population to help preserve BMD and not interfere with the affect of the GnRH agonist on relieving pelvic pain Citation[107–109]. A recent study evaluated BMD in adolescents undergoing GnRH treatment with add-back therapy of daily norethindrone acetate (5 mg daily). They found normal BMD at the hip in 27 out of 36 patients, with only two subjects exhibiting a low BMD Z-score Citation[110]. This study supports the use of GnRH agonists with add-back therapy in adolescents. The use in adolescents is recommended for approximately 6 months followed by continous OCP use. If GnRH therapy is prolonged for more than 9 months, BMD should be performed every 6 months, then every 2 years when stable Citation[78].
Complementary therapy
There is a case report and some preliminary data indicating that the use of Japanese acupuncture may be effective in the treatment of endometriosis-related pain, and that it is safe and well tolerated. More definitive trials are needed Citation[111,112].
Surgical management
Confirmation of histologic diagnosis from a surgical biopsy is the gold standard for diagnosing endometriosis. Concern must be taken for initiating surgical intervention at a young age for chronic pelvic pain, which may lead to multiple surgeries later in life and increased perioperative morbidity Citation[90]. However, in patients who are refractory to medical treatment, surgery is indicated. As stated previously, most adolescents will have mild disease and the appearance of the implants is not the typical powder burn blue–black, chocolate or fibrotic lesions more typical of the deeper, more progressive disease seen in adults . Adolescents predominately have clear vesicular lesions, pearly granular punctations (white implants), and/or small hemorrhagic or petechial spots of the pelvic peritioneum . These lesions are classically seen as white vesicles or red lesions Citation[78,90]. The clear vesicular lesions may be best identified by filling the culdesac with fluid and placing the laparoscope under the fluid. Laparoscopic excision of lesions consistent with endometriosis allows for histologic diagnosis and complete removal of disease. Fulguration of lesions will treat superficial disease, but sometimes spares deeper disease. The appearance of normal peritoneum is reliable for absence of endometriosis, and there is no need to biopsy in these cases to rule out microscopic endometriosis Citation[113]. Despite most adolescents presenting with mild disease, radical excision of severe endometriotic lesions has been reported in adolescents with good success Citation[114]. The recurrence rate of pain after surgery, however, has been reported to range from 30 to 51% Citation[115]. Therefore, medical treatment following surgery with regimens discussed previously is recommended to treat lesions that could not be surgically removed. One study in adults showed a decreased recurrence of dysmenorrhea 1 year postoperatively when adjuvant long-term OCP treatment was administered Citation[116]. Recently, the simultaneous use of a levonorgestrel IUD and an etonogestrel subdermal implant has been reported to be successful for the treatment of an adolescent with debilitating endometriosis Citation[117] who had previously undergone surgical and other medical therapy.
While endometriosis is a common cause of pelvic pain in adolescents, there is a great deal of overlap with other etiologies such as musculoskeletal pain and interstitial cystitis (IC). One study reviewing 63 patients with chronic pelvic pain reported that 11% of patients had both musculoskeletal pain and endometriosis as final diagnoses. Physical therapy is the treatment of choice for pelvic pain of musculoskeletal origin Citation[118]. Similarly, pelvic pain from IC can be found with endometriosis. IC symptoms, such as irritative voiding or suprapubic pain, can be aggravated prior to or during menses. A recent study performed concomitant cystoscopy and laparoscopy in patients aged 13–25 years. A total of 25% of subjects were diagnosed with both IC and endometriosis Citation[119]. This study argues the case for performing cystoscopy at the time of laparoscopy in adolescents with refractory pelvic pain.
Pelvic pain in the adolescent can be complex, and frequent visits, careful listening and response to concerns are all a part of initial treatment. A combination of medical and surgical therapy can then be used to reduce disease burden and provide durable symptomatic relief, which is crucial to prevent disease progression.
Expert commentary
Pediatric and adolescent gynecology (PAG) is an evolving field that intersects the fields of pediatrics, dermatology, urology, endocrinology, surgery, oncology, psychiatry, genetics and physical therapy. Many gynecologic problems encountered in infants and children are unique to these age groups and involve physician skills differing from those used with adult patients. Many practicing obstetricians–gynecologists are often unaware of the uniqueness of these gynecologic issues in this age population, and are often uncomfortable with evaluating and managing these young patients. As a result, PAG was created as a new nonboarded subspecialty incorporating the expertise of gynecologists, pediatricians, urologists, pediatric surgeons, endocrinologists, geneticists, adolescent medicine physicians and psychiatrists as a way to meet the needs of this population.
The establishment of medical education, training, research and dissemination of information in the field of PAG has been an international endeavor. Organizations such as the North American Society for PAG (NASPAG), the Latin American Association of Obstetrics and Gynecology, Child and Youth (ALOGIA), the European Association of Paediatric and Adolescent Gynaecology (EURAPAG), the British Society for Paediatric and Adolescent Gynaecology (BritsPAG) and the Federation Intertationale de Gynecologie Infantile et Juvenile (FIGIJ) all aim to provide a forum for education, research and communication among health professionals who provide gynecologic care to children and adolescents. FIGIJ goes even further by having created an examination to create international recognition for those physicians of different specialties that develop the PAG. This specialty has academic recognition by few universities, but many university centers give this training. As the field of PAG is an emerging one, primarily since the late 1980s, there is much collaboration and learning to be completed to further our understanding in this unique field of medicine. Additional research is needed in all aspects of PAG to determine the best care for all PAG patients, especially when defining optimal treatment.
Five-year view
As previously mentioned, there is much to learn in this emerging field of PAG. With respect to vulvovaginitis in prepubertal girls, a clinical review of the available electronic literature and Cochrane database information on the etiology and management of prepubertal vulvovagnitis notes that most existing studies of the microflora of the prepubertal vagina are flawed by the lack of control subjects, the combination of prepubertal and peripubertal patients, small numbers or lack of comprehensive cultures for a wide variety of microorganisms Citation[120]. The authors state and are correct that we need well-designed, adequately powered, high-quality studies to evaluate the vaginal microflora in asymptomatic prepubertal children and in those with symptoms of vulvovaginitis, as well as randomized controlled treatment studies for symptomatic prepubertal vulvovaginitis patients.
The optimal treatment of menorrhagia associated with coagulation disorders is not known. In the coming years, randomized hormonal, antifibrinolytic and combination treatment protocols at various hemophilia centers will be able to shed light on how best to approach these complex management issues.
Many clinicians are beginning to use extended cycling of combined hormonal contraceptives in adolescents to induce amenorrhea and treat menorrhagia, dysmenorrhea and endometriosis, but further study is needed to determine the best regimen to treat each subset of patients and meet their individual needs Citation[121]. Similarly, the levonorgestrel IUD is being used on an increasing basis for both on- and off-label indications. The paucity of data on their use in the adolescent begs for additional study to monitor the safety and efficacy in adolescents, and the management for off-label indications.
Finally, the area of endometriosis in the adolescent is completely open with respect to research. Almost all of the adolescent treatment recommendations are extrapolated from available adult data. Specific drug studies in adolescents and the use of newer drugs such as aromatase inhibitors in the adolescent population are needed. Now that many PAG researchers are coming up on decades of practice, hopefully many retrospective studies will come forth regarding not only treatment regimens and pain outcomes, but also long-term fecundability studies.
Unfortunately, confounding these research needs there is the existing confusion about adolescent participation in research owing to uncertainty about the need for parental permission and what constitutes appropriate protection for adolescents as research subjects. Hopefully, this will be clarified to enable the appropriate research to take place to answer the many questions in PAG that are still unanswered.
Information resources
• Asociación Latinoamericana de Obstetricia y Ginecología Infantil y de la Adolescentia (ALOGIA): www.alogia.cl
• The British Society for Paediatric and Adolescent Gynaecology: www.britspag.org
• Federation Intertationale de Gynecologie Infantile et Juvenile (FIGIJ): www.figij.org
• North American Society for Pediatric and adolescent gynecology (NASPAG): www.naspag.org
Box 1. Contact/irritant vulvitis: key elements.
History:
• Exposure to urine or incontinence products, chlorine, moisture, heat, bath products, laundry products, and so on.
• External vulvar burning, stinging, soreness, redness and pruritis
• Blood staining in undergarments (if severe skin breakdown)
• Activities and clothing that trap heat, sweat and moisture
Physical examination:
• Usually symmetrical external vulvar erythema, excoriation and/or lichenification
Management:
• Identify and eliminate irritant
• Local care: tub soaks/sitz baths without bath products
• Avoid soap/bubble baths on genitalia
• If soap is needed, use mild, nonscented soap or a cleanser (lauryl sulfate)
• Shampoo in shower or standing up in tub at end of bath time
• Cotton undergarments
• Avoid remaining in wet bathing suits
• Rinse chlorine from genital skin
• Application of low- to mid-potency topical steroid ointment (i.e., hydrocortisone 1–2.5%) twice daily for 1 week
• Nonsedating antihistamine in the day and sedating antihistamine nightly until symptoms improve
• Use of a barrier cream (i.e., Caloseptine®, a methol/zinc oxide/calamine/lanolin ointment) can be both soothing and protective
Box 2. Management of dysfunctional uterine bleeding.
Mild (duration of heavy bleeding <3 months/hemoglobin normal)
• Observation
• Keep menstrual calendar
• Encourage use of antiprostaglandin medications to decrease menorrhagia
Moderate (menses heavy/frequent every 1–3 weeks/mild anemia)
• If not currently bleeding:
– Initiate cyclic oral contraceptive pills, medroxyprogesterone acetate or norethindrone acetate
• If currently bleeding:
– Use taper method of monophasic OCPs to stop bleeding: (30 µg ethinyl estradiol/0.3 mg norgestrel)
– One pill every 6 h for 2 days then
– One pill every 8 h for 2 days then
– One pill every 12 h for 2 days then
– One pill daily for 3 days to complete the 21-pill pack
– Then open new 21-day pill pack and take one per day
– Provide antiemetic with this dose of estrogen to decrease nausea (i.e., promethazine 12 mg/25 mg every 4–6 h)
– Cycle patients on OCPs for 3–6 months
• If contraindication for estrogen:
– Use norethindrone acetate 5–10 mg daily
Severe (prolonged, heavy flow, anemia hemoglobin <9 g/sl)
• If hemoglobin <7 gm/dl or orthostasis present:
– Admit to hospital
– Obtain clotting studies, thyroid panel
• If hemoglobin 8–10 gm/dl:
– Can treat at home with tapering OCPs (30–50 µg ethinyl estradiol pill with norgestrel)
– Use every 4 h until bleeding slows/stops
– Then one po every 6 h for 2 days
– Then one po every 8 h for 2 days
– Then one po every 12 h to complete 21 days
– Followed by cyclic OCP use
– Need iron supplementation
– Treat for nausea as required
• If unable to tolerate oral medication:
– Use iv. premarin 25 mg every 4 h until bleeding stops then change to OCPs/or progestin
• If estrogen contraindicated:
– Norethindrone acetate 5–10 mg every 4 h followed by a similar taper
Key issues
• Isolation of the predominant organism in cases of pediatric vulvovaginitis is important for specific treatment, as organisms can be divided into nonsexually and sexually transmitted species.
• A foreign body must be considered in patients with persistent discharge or vaginal bleeding and an examination under anesthesia should be considered.
• Straddle injuries are the most common accidental genitourinary trauma, and can result in ecchymosis or hematoma formation of the vulva but rarely result in penetrative or vaginal trauma.
• The need for surgical intervention in pediatric genital trauma is low but should be considered when the site for vaginal bleeding cannot be ascertained.
• Anovulation due to immaturity of the hypothalamic–pituitary–ovarian axis is the cause of dysfunctional uterine bleeding in the majority of adolescent patients.
• Menorrhagia from coagulation disorders classically presents at menarche.
• The levonorgestrel intrauterine device is effective in decreasing menstrual loss in adolescent patients with menorrhagia and coagulation disorders.
• The use of continuous oral contraceptive pills, gonadotropin-releasing hormone agonists or progestin therapy are used to suppress proliferation of endometriosis.
• Laparoscopic surgery is indicated in patients with pelvic pain refractory to medical treatment.
References
- Farrington PF. Pediatric vulvo-vaginitis. Clin. Obstet. Gynecol.40(1), 135–140 (1997).
- Jamieson MA. Vaginal discharge and genital bleeding in childhood. In: Clinical Pediatric and Adolescent Gynecology. Sanfilippo JS, Lara-Torre E, Edmonds K, Templeman C (Eds). Informa Healthcare USA, Inc., NY, USA, 140–153 (2009).
- Koumantakis EE, Hassan EA, Deligeoroglou EK, Creatsas GK. Vulvovaginitis during childhood and adolescence. J. Pediatr. Adolesc. Gynecol.10, 39–43 (1997).
- Emans SJ. Vulvovaginal problems in the prepubertal child. In: Pediatric and Adolescent Gynecology (Fifth Edition). Emans SJ, Laufer MR, Goldstein DP (Eds). Lippincott Williams and Wilkins, PA, USA, 83–103 (2005).
- Smith YR, Berman DR, Quint EH. Premarchal vaginal discharge: findings of procedures to rule out foreign bodies. J. Pediatr. Adolesc. Gynecol.13, 227–230 (2002).
- Gerstner GJ, Grungerger W, Boschitsch E, Rotter M. Vaginal organisms in prepubertal children with and without vulvovaginitis. Arch. Gynecol.231, 247–252 (1982).
- Sanfilippo JS, Wakim NG. Bleeding and vulvovaginitis in the pediatric age group. Clin. Obstet. Gynecol.30(3), 653–661 (1987).
- Shapiro RA, Schubert CJ, Siegel RM. Neisseria gonorrhea infections in girls younger than 12 years of age evaluated for vaginitis. Pediatrics104(6), e72 (1999).
- Cuadros J, Mazon A, Martinez R et al. The aetiology of paediatric inflammatory vulvovaginitis. Eur. J. Pediatr.163, 105–107 (2004).
- Heymann W. Streptococcal vulvovaginitis. J. Am. Acad. Dermatol.61, 94–95 (2009).
- Hansen MT, Sanchez VT, Eyster K, Hansen KA. Streptococcus pyogenes pharyngeal colonization resulting in recurrent prepubertal vulvovaginitis. J. Pediatr. Gynecol.20, 315–317 (2007).
- Yerkes EB. Urologic issues in the pediatric and adolescent gynecology patient. Obstet. Gynecol. Clin. North Am.36, 69–84 (2009).
- Tibaldi C, Cappello N, Latino MA, Masuelli G, Marini S, Benedetto C. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: risk factors and rates of occurrence. Clin. Microbiol. Infect.15(7), 670–679 (2009).
- Jasper JM, Ward MA. Shigella vulvovaginitis in a prepubertal child. Pediatr. Emerg. Care22(8), 585–586 (2006).
- Caputo RV. Fungal infections in children. Dermatol. Clin.4(1), 137–149 (1986).
- Deligeoroglou E, Salakos N, Makrakis E, Chassiakos D, Hassan EA, Christopouos P. Infections of the lower female genital tract during childhood and adolescence. Clin. Exp. Obstet. Gynecol.31(3), 175–178 (2004).
- Rettig PJ, Nelson JD. Genital tract infection with Chlamydia trachomatis in prepubertal children. J. Pediatr.99(2), 206–210 (1981).
- Schachter J, Grossman M, Holt J, Sweet R, Spector S. Infections with Chlamydia trachomatis: involvement of multiple anatomic sites in neonates. J. Infect. Dis.139(2), 232–234 (1979).
- Hammerschlang MR. Chlamydial infection. J. Pediatr.114(5), 727–734 (1989).
- Black CM, Driebe EM, Howard LA et al. Multicenter study of nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in children being evaluated for sexual abuse. Pediatr. Infect. Dis. J.28(7), 608–613 (2009).
- Kellogg ND, Baillargeon JL, Lukefahr KL, Menard SW. Comparison of nucleic acid amplification tests and culture techniques in the detection of Neisseria gonorrhoeae and Chlamydia trachomatis in victims of suspected child sexual abuse. J. Pediatr. Adolesc. Gynecol.17, 331–339 (2004).
- Williams T, Callen J, Owen L. Vulvar disorders in the prepubertal female. Pediatr. Ann.15(8), 588–589 (1986).
- Yu HT, Chao A, Wang CJ et al. Integrated imaging studies and endoscopic management of purulent vaginal discharge in a 6-year-old: vaginal ectopic ureter associated with ipsilateral dysplastic kidney. Eur. J. Obstet. Gynecol. Reprod. Biol.142, 160–162 (2008).
- Hansen KA, DeWitt J. Premenarchal, recurrent vaginal discharge associated with an incomplete obstructing longitudinal vaginal septum. J. Pediatr. Adolesc. Gynecol.18, 423–426 (2005).
- Striegel AM, Myers JB, Sorensen MD, Furness PD, Koyle MA. Vaginal discharge and bleeding in girls younger than 6 years. J. Urol.176, 2632–2635 (2006).
- Paradise JE, Willis ED. Probability of vaginal foreign body in girls with genital complaints. Am. J. Dis. Child.139(5), 472–476 (1985).
- Benjamins LJ. Genital trauma in pediatric and adolescent females. J. Pediatr. Adolesc. Gynecol.22, 129–133 (2009).
- Spitzer RF, Kives S, Caccia N, Ornstein M, Goia C, Allen L. Retrospective review of unintentional female genital trauma at a pediatric referral center. Pediatr. Emerg. Care24(12), 831–835 (2008).
- Bond GR, Dowd MD, Landsman I, Rimza M. Unintentional perineal injury in prepubescent girls: a multicenter prospective report of 56 girls. Pediatrics95, 628–631 (1995).
- Gabriel NM, Clayton M, Starling SP. Vaginal laceration as a result of blunt vehicular trauma. J. Pediatr. Adolesc. Gynecol.22, e166–e168 (2009).
- Scheidler MF, Shultz BL, Schall L, Ford HR. Mechanisms of blunt perineal injury in female pediatric patients. J. Pediatr. Surg.35, 1317–1319 (2000).
- Dowd D, Fitzmaurice L, Knapp JF, Mooney D. The interpretation of urogenital findings in children with straddle injuries. J. Pediatr. Surg.29, 7–10 (1994).
- Jones JG, Worthington T. Genital and anal injuries requiring surgical repair in females less than 21 years of age. J. Pediatr. Adolesc. Gynecol.21, 207–211 (2008).
- Merritt DF. Vulvar and genital trauma in pediatric and adolescent gynecology. Curr. Opin. Obstet. Gynecol.16, 371–381 (2004).
- Mok-Lin EY, Laufer MR. Management of vulvar hematomas: use of a Word catheter. J. Pediatr. Adolesc. Gynecol.22, e156–e158 (2009).
- Emans SJ, Laufer MR, Goldstein DP. Pediatric and Adolescent Gynecology (Fifth Edition). Lippincott Williams and Wilkins, PA, USA, 950–968 (2005).
- Abou-Jaoude W, Sugarman JM, Fallat ME, Casale AJ. Indicators of genitourinary tract injury or anomaly in cases of pediatric blunt trauma. J. Pediatr. Surg.31(1), 86–90 (1996).
- McAleer IM, Kaplan GW, Scherz HC, Packer MG, Lynch FP. Genitourinary trauma in the pediatric patient. Urology42(5), 563–568 (1993).
- Golan A, Lurie S, Sagiv R, Glezerman M. Continuous-flow vaginoscopy in children and adolescents. J. Am. Assoc. Gynecol. Laparosc.7(4), 526–528 (2000).
- Habek D, Kulas T. Nonobstetrics vulvovaginal injuries: mechanism and outcome. Arch. Gynecol. Obstet.275(2), 93–97 (2007).
- Barton DJ, Sloan GM, Nichter LS, Reinisch JF. Hair-thread tourniquet syndrome. Pediatrics82, 925–928 (1988).
- Kuo JH, Smith LM, Berkowitz CD. A hair tourniquet resulting in strangulation and amputation of the clitorus. Obstet. Gynecol.99(5), 939–941 (2002).
- Pomeranz M, Schachter B, Capua T, Beyth Y. Hair-thread tourniquet syndrome of labia minor. J. Pediatr. Adolesc. Gynecol.22, 111–113 (2009).
- Bacon JL, Burgis JT. Hair thread tourniquet syndrome in adolescents: a presentation and review of the literature. J. Pediatr. Adolesc. Gynecol.18, 155–156 (2005).
- Emans SJ. Dysfunctional uterine bleeding. In: Pediatric and Adolescent Gynecology (Fifth Edition). Emans SJ, Laufer MR, Goldstein DP (Eds). Lippincott Williams and Wilkins, PA, USA, 270–283 (2005).
- Hallberg L, Hogdal AM, Nilsson L, Rybo G. Mentstrual blood loss – a population study. Variation at different ages and attempts to define normality. Acta Obstet. Gynecol. Scand.45, 320–351 (1966).
- Grover S. Bleeding disorders and heavy menses in adolescents. Curr. Opin. Obstet. Gynecol.19, 415–419 (2007).
- American College of Obstetricians and Gynecologists Committee Opinion No. 349, November 2006. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet. Gynecol.108(5), 1323–1328 (2006).
- Strickland JL, Wall JW. Abnormal uterine bleeding in adolescents. Obstet. Gynecol. Clin. North Am.30, 321–325 (2003).
- Rimsza M. Dysfunctional uterine bleeding. Pediatr. Rev.23, 227–233 (2002).
- Gidwani GP. Vaginal bleeding in adolescents. J. Reprod. Med.29(6), 417–420 (1984).
- Matytsina LA, Zoloto EV, Sinenko LV, Greydanus DE. Dysfunctional uterine bleeding in adolescents: concepts of pathophysiology and management. Prim. Care Clin. Office Pract.33, 503–515 (2006).
- Mikhail S, Varadarajan R, Kouides P. The prevalence of haemostasis in adolescents with menorrhagia referred to a haemophilia treatment centre. Haemophilia13, 627–632 (2007).
- Philipp CS, Faiz A, Dowling N et al. Age and prevalence of bleeding disorders in women with menorrhagia. Obstet. Gynecol.105, 61–66 (2005).
- James AH. Bleeding disorders in adolescents. Obstet. Gynecol. Clin. North Am.36, 153–162 (2009).
- Falcone T, Desjardins C, Bourque J, Granger L, Hemmings R, Quiros E. Dysfunctional uterine bleeding in adolescents. J. Reprod. Med.39, 761–764 (1994).
- James AH. More than menorrhagia: a review of the obsteric and gynecological manifestations of bleeding disorders. Haemophilia11, 295–307 (2005).
- Lopez JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard Soulier syndrome. Blood91(12), 4397–4418 (1998).
- George JN, Caen JP, Nurden AT. Glanzmann’s thrombasthenia: the spectrum of clinical disease. Blood75(7), 1383–1395 (1990).
- Kulp JL, Mwangi CN, Loveless M. Screening for coagulation disorders in adolescents with abnormal uterine bleeding. J. Pediatr. Adolesc. Gynecol.21, 27–30 (2008).
- ACOG Committee on Gynecologic Practice. Committee Opinion: number 451, December 2009. Von Willebrand’s disease in women. Obstet. Gynecol.1239–1241 (2009).
- Minjarez DA. Abnormal bleeding in adolescents. Semin. Reprod. Med.21(4), 363–373 (2003).
- Frishman GN. Evaluation and treatment of menorrhagia in an adolescent population. J. Minim. Invasive Gynecol.15, 682–688 (2008).
- Janssen CAH, Scholten PC, Heintz APM. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet. Gynecol.85, 977–982 (1995).
- Claessens EA, Cowell CA. Acute adolescent menorrhagia. Am. J. Obstet. Gynecol.139(3), 277–280 (1981).
- Torres A, Baszak-Radomanska E, Torres K, Paszkaowski T, Staskiewicz G, Wozniakowska E. A case of unusual course of adolescent menorrhagia: decidual cast as a side effect of treatment. Fertil. Steril.92, 1748.e5–1748.e7 (2009).
- Foster PA. The reproductive health of women with von Willebrand disease unresponsive to DDAVP: results of an international survey. Thromb. Haemost.74(2), 784–790 (1995).
- American College of Obstetricians and Gynecologists committee opinion No 392, December 2007. Intrauterine device and adolescents. Obstet. Gynecol.110, 1493–1495 (2007).
- Kingman CE, Kadir RA, Lee CA, Economides DL. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. Br. J. Obstet. Gynaecol.111(12), 1425–1428 (2004).
- Paterson H, Ashton J, Harrison-Woolrych M. A nationwide cohort study of the use of levonorgestrel intrauterine device in New Zealand adolescents. Contraception79, 433–439 (2009).
- Hall P, Maclachlan N, Thorn N, Nudd MW, Taylor CG, Garrioch DB. Control of menorrhagia by the cyclo-oxygenase inhibitors naproxen sodium and mefenamic acid. Br. J. Obstet. Gynaecol.94(6), 554–558 (1987).
- Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst. Rev.4, CD000249 (2000).
- DeVore GR, Odell O, Kase N. Use of intravenous premarin in the treatment of dysfunctional uterine bleeding – a double blind randomized control study. Obstet. Gynecol.59(3), 285–290 (1982).
- Bowkley CW, Dubel GJ, Haas RA, Soares GM, Ahn SH. Uterine artery embolization for control of life-threatening hemorrhage at menarche: brief report. J. Vasc. Interv. Radiol.18, 127–131 (2007).
- Markovitch O, Ellis M, Holzinger M, Goldberger S, Beyth Y. Severe juvenile vaginal bleeding due to Glanzmann’s thrombasthenia: case report and review of the literature. Am. Hematol.57(3), 225–227 (1998).
- Zurawin RK, Pramanik S. Endometrial balloon ablation as a therapy for intractable uterine bleeding in an adolescent. J. Pediatr. Adolesc. Gynecol.14, 119–121 (2001).
- Meirow D, Rabinovici J, Katz D, Or R, Shufaro Y, Ben-Yehuda D. Prevention of severe menorrhagia in oncology patients with treatment-induced thrombocytopenia by luteinizing hormone-releasing hormone agonist and depo-medroxyprogesterone acetate. Cancer107, 1634–1641 (2006).
- Laufer MR, Goldstein DP. Gynecologic pain: dysmenorrhea, acute and chronic pelvic pain, endometriosis and premenstrual syndrome. In: Pediatric and Adolescent Gynecology (Fifth Edition). Emans SJ, Laufer MR, Goldstein DP (Eds). Lippincott Williams and Wilkins, PA, USA, 439–460 (2005).
- Sampson JA. The development of the implantaion theory for the origin of peritoneal endometriosis. Am. J. Obstet. Gynecol.40, 549–557 (1940).
- Halme J, Becker S, Haskill S. Altered maturation and function of peritoneal macrophages: a possible role in pathogenesis of endometriosis. Am. J. Obstet. Gynecol.156, 783–789 (1987).
- Bontis JN, Vavilis DT. Etiopathology of endometriosis. Ann. N. Y. Acad. Sci.817, 305–309 (1997).
- ACOG Committee Opinion Number 310, April 2005. Endometriosis in adolescents. Obstet. Gynecol.105, 921–927 (2005).
- Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J. Pediatr. Adolesc. Gynecol.10(4), 199–202 (1997).
- Marsh EE, Laufer MR. Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly. Fertil. Steril.83, 758–760 (2005).
- Ebert AD, Fuhr N, Matthias D, Schneppel L, Papadopoulos T. Histological confirmation of endometriosis in a 9-year-old girl suffering from unexplained cyclic pelvic pain since her eighth year of life. Gynecol. Obstet. Invest.67, 158–161 (2009).
- Yamamoto K, Mitsuhashi Y, Takaike T, Takase K, Hoshiai H, Noda K. Tubal endometriosis diagnosed within one month after menarche: a case report. Tohoku J. Exp. Med.181, 385–387 (1997).
- Goldstein DP, deCholnoky C, Leventhal JM, Emans SJ. New insights into the old problem of chronic pelvic pain. J. Pediatr. Surg.14, 675–680 (1979).
- Reese KA, Reddy S, Rock JA. Endometriosis in an adolescent population: the Emory experience. J. Pediatr. Adolesc. Gynecol.9, 125–128 (1996).
- Doyle JO, Missmer SA, Laufer MR. The effect of combined surgical–medical intervention on the progression of endometriosis in an adolescent and young adult population. J. Pediatr. Adolesc. Gynecol.22, 257–263 (2009).
- Broach AN, Mansuria SM, Sanfilippo JS. Pediatric and adolescent gynecologic laparoscopy. Clin. Obstet. Gynecol.52(3), 380–389 (2009).
- Mahmood TA, Templeton A. The impact of treatment on the natural history of endometriosis. Hum. Reprod.5, 965–970 (1990).
- Howard FM. Chronic pelvic pain. Obstet. Gynecol.101(3), 594–611 (2003).
- Templeman C. Adolescent endometriosis. Obstet. Gynecol. Clin. North Am.36, 177–185 (2009).
- Harel Z. Dysmenorhea in adolescents and young adults: from pathophysiology to pharmacological treatments and management strategies. Expert Opin. Pharmacother.9(15), 2661–2672 (2008).
- Roy SN, Bhattacharya S. Benefits and risks of pharmacological agents used for the treatment of menorhagia. Drug Saf.27(2), 75–90 (2004).
- Kistner RW. Current status of the hormonal treatment of endometriosis. Clin. Obstet. Gynecol.9, 271–292 (1966).
- Edelman AB, Gallo MF, Jensen JT, Nichols MD, Schulz KF, Grimes DA. Continuous or extended cycle vs. cyclic use of combined oral contraceptives for contraception. Cochrane Database Syst. Rev.3, CD004695 (2005).
- Miller L, Notter KM. Menstrual Reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstet. Gynecol.98(5), 771–778 (2001).
- Prentice A, Deary AJ, Bland E. Progestins and anti-progestins for pain associated with endometriosis. Cochrane Database Syst. Rev. (2), CD002122 (2000).
- Sanfilippo JS, Lara-Torre E. Adolescent gynecology. Obstet. Gynecol.113(4), 935–947 (2009).
- Gold MA, Johnson LM. Intrauterine devices and adolescents. Curr. Opin. Obstet. Gynecol.20(5), 464–469 (2008).
- Yisa SB, Okenwa AA, Husemeyer RP. Treatment of pelvic endometriosis with etonogestrel subdermal implant (Implanon®). J. Fam. Plann. Reprod. Health Care31, 67–69 (2005).
- Petta CA, Ferriani RA, Abrarao MS et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum. Reprod.20, 1993–1998 (2005).
- Dlugi AM, Miller JD, Knittle J. Lupron depot (leuprolide acetate for depot suspension) in the treatment of endometriosis: a randomized, placebo-controlled, double-blind study. Fertil. Steril.54, 419–427 (1990).
- Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Obstet. Gynecol.93, 51–58 (1999).
- Dawood MY, Lewis V, Ramos J. Corical and trabecular bone mineral content in women with endometriosis: effect of gonadotropin releasing hormone agonist and danazol. Fertil. Steril.52, 21–26 (1989).
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12 month study. Lupron Add-Back Study Group. Obstet. Gynecol.91, 16–24 (1998).
- Surrey ES, Judd HL. Reduction of vasomotor symptoms and bone mineral density loss with combined norethindrone and long-acting gonadotropin-releasing hormone agonist therapy of symptomatic endometriosis: a prospective randomized trial. J. Clin. Endocrinol. Metab.75(2), 558–563 (1992).
- Kiesel L, Schweppe KW, Sillem M, Siebzehnrubl E. Should add-back therapy for endometriosis be deferred for optimal results? Br. J. Obstet. Gynaecol.103(Suppl. 14), 15–17 (1996).
- DiVasta AD, Laufer MR, Gordon CM. Bone density in adolescents treated with a GnRH agonist and add-back therapy for endometriosis. J. Pediatr. Adolesc. Gynecol.20, 293–297 (2007).
- Highfield ES, Laufer MR, Schnyer L, Kerr CE, Thomas P, Wayne PM. Adolescent endometriosis-related pelvic pain treated with acupuncture: two case reports. J. Altern. Complement. Med.12, 317–322 (2006).
- Wayne PM, Kerr CE, Schnyer RN et al. Japanese-style acupuncture for endometriosis-related pelvic pain in adolescents and young women: results of a randomized sham-controlled trial. J. Pediatr. Adolesc. Gynecol.21, 247–257 (2008).
- Walter AJ, Hentz JG, Magtibay PM, Cornella JL, Magrina JF. Endometriosis: correlation between histologic and visual findings at laparoscopy. Am. J. Obstet. Gynecol.184(7), 1407–1411 (2001).
- Stavroulis A, Saridogan E, Creighton SM, Cutner AS. Laparoscopic treatment of endometriosis in teenagers. Eur. J. Obstet. Gynecol. Reprod. Biol.125(2), 248–250 (2006).
- Laufer MR. Current approaches to optimizing the treatment of endometriosis in adolescents. Gynecol. Obstet. Invest.66, 19–27 (2008).
- Seracchioli R, Mabrouk M, Manuzzi L et al. Post-operative use of oral contraceptive pills for prevention of anatomical relapse or symptom-recurrence after conservative surgery for endometriosis. Hum. Reprod.24(11), 2729–2735 (2009).
- Al-Jefout M, Palmer J, Fraser IS. Simultaneous use of a levonorgestrel intrauterine system and an etonogestrel subdermal implant for debilitating adolescent endometriosis. Aust. N. Z. J. Obstet. Gynaecol.47, 247–249 (2007).
- Schroeder B, Sanfilippo JS, Hertweck P. Musculoskeletal pelvic pain in a pediatric and adolescent gynecology practice. J. Pediatr. Adolesc. Gynecol.13(2), 90 (2000).
- Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J. Pediatr. Adolesc. Gynecol.22, 181–185 (2009).
- Joishy M, Ashtekar CS, Jain A, Gonsalves R. Do we need to treat vulvovaginitis is prepubertal girls? Br. Med. J.330, 186–188 (2005).
- Gerschultz KL, Sucato GS, Hennon TR, Murray PJ, Gold MA. Extended cycling of combined hormonal contraceptives in adolescents: physician views and prescribing practices. J. Adolesc. Health40, 151–157 (2007).
- Revised American Society for Reproductive Medicine Classification of Endometriosis: 1996. Special Contribution. Fertil. Steril.67(5), 817–821 (1997).
Common problems in pediatric and adolescent gynecology
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to www.medscapecme.com/journal/expertob. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.com. If you are not registered on Medscape.com, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for 1.5 AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMAhhttttPRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMAt PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Which of the following best describes the difference in presentation of gynecologic problems between prepubertal and adolescent girls?
□ A No difference
□ B Cervicitis is more common in prepubertal girls
□ C Vulvovaginitis is more likely in prepubertal girls
□ D Vaginal pH is more alkaline in adolescent girls
2. Which of the following is the most likely vaginal pathogen causing vulvovaginitis in a 10-year-old girl?
□ AEscherichia coli
□ BStreptococcus pyogenes
□ CShigella
□ DHaemophilus influenzae
3. An 11-year-old girl presents with vaginal discharge, and a sexually transmitted disease from abuse is suspected. Which of the following tests is recommended as the best standard for the diagnosis of gonorrhea and Chlamydia?
□ A Urine and vaginal swtab nucleic acid amplification tests (NAATs)
□ B Vaginal and rectal cultures
□ C Serum immunoglobulin (Ig)G
□ D Immunofluorescent tests of vaginal swab
4. A 15-year-old girl has lacy, white vulvar erosion that is also seen in the oral mucosa, with hair loss and pruritus of the hands and feet. Which of the following is the most likely diagnosis?
□ A Seborrheic dermatitis
□ B Lichen sclerosus
□ C Systemic lupus erythematosus
□ D Lichen planus
5. An 8-year-old girl presents with foul-smelling, persistent vaginal discharge, and a foreign body is suspected. Which of the following is the most common vaginal foreign body found in children?
□ A Tampon
□ B Candy
□ C Toilet paper
□ D Soap
Notes
Modified from Citation[2].
iv.: Intravenous; OCP: Oral contraceptive pill; po: per os.
