Abstract
The five main subtypes of ovarian surface epithelial carcinoma (high-grade serous, low-grade serous, endometrioid, clear cell and mucinous) are different diseases, with differences in genetic and environmental risk factors, precursor lesions, molecular events during oncogenesis, patterns of spread and response to treatment. With recent advances in surgical pathology, it is possible to reproducibly diagnose these subtypes in routine surgical pathology practice. This review examines these subtypes of ovarian carcinoma, focusing on differential diagnosis, molecular features and current treatment strategies. The increasing understanding of the molecular abnormalities associated with each subtype is leading to exploration and introduction of more subtype-specific treatment of ovarian carcinoma.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertob; (4) view/print certificate.
Release date: 24 December 2012; Expiration date: 24 December 2013
Learning objectives
Upon completion of this activity, participants will be able to:
• Analyze the epidemiology and prognosis of ovarian cancer
• Assess characteristics of high-grade serous carcinomas
• Assess characteristics of low-grade serous carcinomas
• Evaluate other subtypes of ovarian carcinoma
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Publisher, Future Science Group, London, UK.
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Charles P Vega, MD
Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine, CA, USA.
Disclosure: Charles P Vega, MD, has disclosed no relevant financial relationships.
AUTHORS AND CREDENTIALS
Chris MJ Conklin, MD
Department of Pathology and Laboratory Medicine, Vancouver General Hospital and University of British Columbia, Vancouver, BC, Canada.
Disclosure: Chris MJ Conklin, MD, has disclosed no relevant financial relationships.
C Blake Gilks, MD, FRCPC
Department of Pathology and Laboratory Medicine, Vancouver General Hospital and University of British Columbia, Vancouver, BC, Canada.
Disclosure: C Blake Gilks, MD, FRCPC, has disclosed no relevant financial relationships.
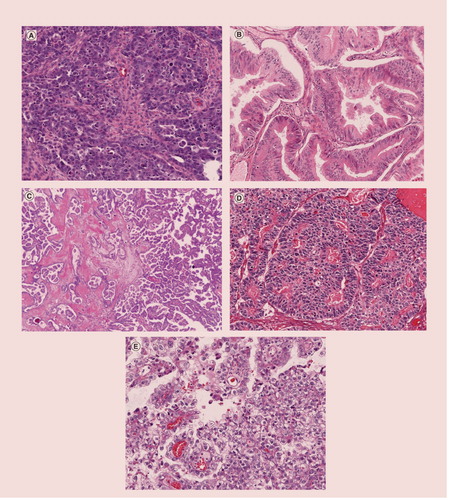
(A) High-grade serous carcinoma, (B) mucinous carcinoma, (C) low-grade serous carcinoma, (D) endometrioid carcinoma and (E) clear cell carcinoma.
Approximately 96% of ovarian carcinomas can be diagnosed as one of these five subtypes (HGSC [71% of cases], MC [3.2%], EC [8.3%], CCC [9.5%], LGSC [4.1%]), which have distinct molecular abnormalities and behaviors. These frequencies are based on data from British Columbia, Canada.
CCC: Clear cell carcinoma; EC: Endometrioid carcinoma; HGSC: High-grade serous carcinoma; LGSC: Low-grade serous carcinoma; MC: Mucinous carcinoma.
Data taken from Citation[36].
![Figure 2. Incidence of ovarian carcinomas by subtype.Approximately 96% of ovarian carcinomas can be diagnosed as one of these five subtypes (HGSC [71% of cases], MC [3.2%], EC [8.3%], CCC [9.5%], LGSC [4.1%]), which have distinct molecular abnormalities and behaviors. These frequencies are based on data from British Columbia, Canada.CCC: Clear cell carcinoma; EC: Endometrioid carcinoma; HGSC: High-grade serous carcinoma; LGSC: Low-grade serous carcinoma; MC: Mucinous carcinoma.Data taken from Citation[36].](/cms/asset/b7e06ce4-d5c4-4119-804f-7c122a707214/ierb_a_11205318_f0002_b.jpg)
Ovarian cancer is the seventh most commonly diagnosed female cancer worldwide, accounting for almost 4% of all female cancers Citation[1,2]. Furthermore, the proportion of ovarian cancer is increasing due to effective Papanicolaou smear screening programs, leading to reduced incidence of cervical cancer Citation[3]. Ovarian surface epithelial carcinomas are the most common malignant ovarian tumors, accounting for 90% of cases, and are the most lethal gynecological malignancies Citation[4,5].
The majority of ovarian carcinomas are detected at advanced stage. Since the introduction of platinum/taxane-based therapy, the only progress in therapy has been the introduction of intraperitoneal chemotherapy and bevacizumab. Although the former is associated with prolonged survival, it is accompanied by serious adverse side effects, such that treatment cannot be completed as frequently as planned Citation[6,7]. The latter treatment regimen is associated with a modest increase in progression-free survival, but not with overall survival Citation[8,9]. Thus, there are unresolved issues around both of these new treatments and neither has entered widespread, routine use in ovarian cancer management. Currently, all histological subtypes of ovarian carcinoma are treated in a similar manner, with surgery and chemotherapy based on stage at diagnosis. Whereas patient prognosis has improved for many other solid cancers, the 5-year survival of women with ovarian cancer in developed countries has remained stable at 30–40% Citation[1].
Recent advances have forced the medical community to change the way ovarian carcinoma is viewed. Historically, ovarian surface epithelial tumors were thought to arise from the ovarian surface mesothelial cells, and that subsequent metaplastic change led to the development of the four main ovarian carcinoma cell types (serous, endometrioid, mucinous and clear cell) Citation[10]. However, it is now understood that different histological subtypes of ovarian carcinomas arise from distinct precursor lesions, which are not necessarily ovarian in origin. The majority of high-grade serous carcinomas (HGSCs) are now believed to arise from distal fallopian tube epithelium as serous tubal intraepithelial carcinoma Citation[11–14]. Ovarian endometrioid carcinomas (ECs) and clear cell carcinomas (CCCs) are associated with endometriosis, the presumed precursor lesion, in 23–42% of cases Citation[15,16]. A precursor lesion for primary ovarian mucinous carcinoma (MC) has not yet been identified.
Molecular genetic analyses have shown that different morphological subtypes of ovarian carcinoma have distinct mutation profiles. For example, low-grade serous carcinomas (LGSCs) and HGSCs were once thought to be part of a continuum of serous neoplasia; however, the majority of LGSCs have KRAS and BRAF mutations and are genomically stable Citation[17], whereas HGSCs have abnormalities of BRCA1 or BRCA2 and TP53, and show chromosomal instability Citation[18]. Furthermore, the response to treatment varies considerably across the ovarian carcinoma subtypes Citation[19], which has led to recent subtype-specific treatment trials Citation[20], emphasizing the importance of accurate subtype diagnosis.
Historically, diagnosis of ovarian carcinoma subtype has been only modestly reproducible Citation[21–24]; however, recent studies have shown very high reproducibility in diagnostic subtyping of ovarian surface epithelial carcinomas Citation[25,26]. A number of advances in diagnostic pathology have underpinned this progress. For example, the recognition that glandular lesions showing serous differentiation are best classified as HGSCs rather than ECs or mixed serous/endometrioid has increased diagnostic accuracy and is supported by molecular genetic analysis Citation[26]. More than 98% of ovarian surface epithelial carcinomas can be assigned to one of the five major subtypes, HGSC, CCC, EC, MC and LGSC Citation[25], based on routine pathological assessment . In this review, the authors outline the recent advances in histopathology, molecular genetics and immunohistochemistry, and therapy of these five main ovarian surface epithelial carcinoma subtypes.
Serous carcinomas
One of the most important advances in the understanding of ovarian carcinoma over the last 10 years is the recognition that HGSCs and LGSCs are distinct disease entities Citation[10,27–29]. This discovery was initially based upon molecular differences; most LGSCs have mutations in KRAS and BRAF Citation[29], alterations shared with serous borderline tumors (SBTs) Citation[30], which are thought to be a precursor of LGSCs. Most HGSCs, by contrast, have somatic mutations in TP53 Citation[31], and approximately half of all cases have abnormalities of BRCA1 or BRCA2 Citation[32,33]. Furthermore, HGSCs are not related to SBTs, and are believed to originate from the distal fallopian tube epithelium Citation[11,12,14]. Based solely on histological criteria, LGSCs and HGSCs can be reliably distinguished from one another. Because of these differences, HGSCs and LGSCs will be discussed separately.
High-grade serous carcinoma
HGSCs account for approximately 70% of malignant ovarian surface epithelial carcinomas in North America and in Europe, although this subtype is less common in other parts of the world Citation[34,35]. Almost 90% of HGSCs present with advanced-stage (stage III or IV) disease Citation[36,37]. Most HGSCs have spread beyond the pelvis at the time of diagnosis, accounting for low median survival times.
It is now believed that most HGSCs arise from the distal, fimbriated end of the fallopian tube, a finding supported by the observation that both familial or sporadic cases of HGSCs have synchronous tubal intraepithelial carcinoma in most cases and that these lesions share TP53 mutations and immunoreactivity for PAX8, a transcription factor expressed in secretory tubal epithelium; examination of telomere length in tubal intraepithelial carcinomas also supports the tubal lesions being precursors rather than metastases Citation[35]. The propensity of HGSCs to spread transcelomically, with bulky intraperitoneal disease, makes it challenging to determine the primary site of the serous carcinoma (ovarian, peritoneal, fallopian tube, among others) in an individual case. The designation ‘pelvic HGSC’ has been suggested for such cases, to avoid speculation about the primary site. While the primary site (i.e., fallopian tube vs ovary) has profound implications for screening or prevention strategies, it does not impact on the management for advanced-stage HGSCs, making this a reasonable approach in clinical practice.
Morphology of HGSCs
Macroscopically, HGSCs of the ovary are usually large, bilateral and demonstrate a mix of solid, cystic and papillary growth. The solid regions are tan-white, and typically contain regions of necrosis and hemorrhage. The carcinoma often invades through the capsule and grows on the surface of the ovary. The fallopian tube may be overgrown and obliterated; however, sometimes a polypoid tumor growth is seen at the fimbriated end. The omentum often shows diffuse involvement with multiple discrete and coalescing tumor nodules (referred to as ‘omental cake’), and the peritoneal surface may be studded with metastatic carcinoma.
Microscopically, HGSC is characterized by a wide variety of architectural patterns, which may coexist within the same tumor and in the same tissue section. The most common pattern is ‘papillary’, consisting not of well-formed fibrovascular cores in most cases, but instead of highly stratified epithelium with a fenestrated, tufted, or slit-like architecture. Less common patterns include solid, glandular and transitional like. All growth patterns share the same cytological features; the tumor cells are usually intermediate to large in size, with prominent nucleoli visible at low magnification. The nuclei are distinctly pleomorphic, showing more than a threefold variation in size; the primary diagnostic criterion in distinguishing HGSCs from LGSCs. Sometimes, bizarre mononuclear giant cells are seen. High mitotic rate and abundant apoptotic bodies are characteristic of HGSCs. In cases where the nuclear pleomorphism is equivocal in establishing a diagnosis of HGSC versus LGSC, a mitotic rate of greater than 12/10 high-power field supports a diagnosis of HGSC Citation[34,38].
Molecular features of HGSCs
Approximately half of all patients with ovarian HGSCs have either hereditary (germline) or somatic mutations in BRCA1 or BRCA2, or loss of BRCA1 expression in tumor cells as a result of methylation of its promoter (BRCA2 is not inactivated by promoter methylation). The prevalence of germline mutations varies between populations studied (16–26%) Citation[32,33,39], with mutations in BRCA1 consistently being more common than BRCA2 mutations. BRCA1/2 mutations are almost exclusively seen in HGSC subtype of ovarian carcinoma. Given the high frequency of BRCA1/2 mutations in patients with HGSC, and the lack of sensitivity of family history in identifying these patients, it is believed that all such patients should be referred for genetic counseling and testing Citation[40]. For those patients with mutations, there can then be counseling regarding breast cancer screening and risk-reducing surgery. Loss of BRCA1/2 is lethal to normal cells; however, 95% of HGSCs have TP53 mutations early in oncogenesis, permitting cells to survive subsequent loss of BRCA1/2 Citation[37]. These two changes result in loss of ability to repair dsDNA breaks, resulting in chromosomal instability Citation[31]. As a result, HGSCs are typically aneuploid with complex karyotypes. The landmark Cancer Genome Atlas study of HGSCs showed many somatic copy number alterations, which is a characteristic feature of this cancer subtype, but that recurrent mutations (apart from BRCA1, BRCA2 and TP53) are uncommon Citation[41].
Among immunohistochemical markers, immunoreactivity to WT-1 is particularly useful in the distinction of serous ovarian carcinomas from other subtypes. Approximately 70% of LGSCs and 80% of HGSCs are positive for WT-1, compared with less than 5% positivity of other ovarian subtypes Citation[42,43]. Estrogen receptor (ER) is positive in more than two-thirds of serous carcinomas, and is also expressed in ECs, but is negative in almost all CCCs and MCs Citation[44]. With respect to the differential diagnosis between HGSCs and LGSCs, abnormal p53 staining (i.e., either strong diffuse staining or complete absence of staining) and a high Ki-67 index are supportive of a diagnosis of HGSC.
HGSC can be confused with LGSC, EC and CCC. The differential diagnosis with LGSC has been discussed previously, which is based primarily on identification of at least threefold nuclear variation in HGSCs . The distinction between HGSCs and LGSCs is not usually problematic when multiple sections are available for review; however, with a very small sample, the differential diagnosis can be very problematic. p53 staining and Ki-67 index have been suggested in this circumstance, but a cutoff point for Ki-67 index to distinguish between HGSCs and LGSCs has not been established. The differential diagnosis between HGSCs and ECs has historically been very problematic, with considerable variability in practice in different centers Citation[19,26,45]. As a result, a variable proportion of HGSCs with glandular pattern were erroneously diagnosed as EC in the past. The key morphological feature for distinction is the identification of high-grade nuclear atypia in HGSCs as well as coexistence with other HGSC patterns. In addition, squamous differentiation, when present, supports a diagnosis of EC. In morphologically challenging cases, WT-1 is immensely useful; it is positive in a large majority of HGSCs and negative in most ECs Citation[26,45]. The distinction between HGSCs and CCCs can be challenging, especially in cases of HGSCs with clear cell changes Citation[46]. The presence of more typical HGSC is strong evidence in support of a diagnosis of HGSC with clear cell change. A high mitotic rate favors HGSC Citation[46]. Moreover, an immunohistochemical panel of three markers, ER, WT-1 and HNF-1b, a transcription factor related to glycogen metabolism, can be helpful, with negative staining for ER and WT-1, and positive staining for HNF-1b indicative of CCC Citation[47].
Therapy
The initial therapeutic approach for HGSCs is usually surgical tumor debulking followed by chemotherapy. However, a recent randomized clinical trial has shown that for some patients with advanced-stage HGSC, equivalent outcomes can be obtained if they first receive three to four cycles of chemotherapy, followed by interval debulking Citation[47]. With either strategy, optimal debulking, with no macroscopic residual disease, is the most important prognostic indicator. Most HGSCs (80%) respond well to platinum/taxane therapy initially, with drug resistance emerging during subsequent treatment cycles. A minority of cases of HGSCs (20%) are refractory to platinum-based chemotherapy from the time of presentation, but the basis for this drug resistance is not known. Poly (ADP-ribose) polymerase (PARP) inhibitors represent a possible therapeutic intervention. PARP is a key enzyme involved in ssDNA repair, and its inhibition can be used to exploit the loss of DNA double-strand break repair in HGSCs. PARP inhibitors cause the death of cells also lacking double-strand break repair capability, while normal cells are unaffected. Initial studies have shown that the PARP inhibitor, olaparib, extends survival in a BRCA2-mutated ovarian cancer xenograft model Citation[48], and the results of an initial clinical trial show activity against HGSC in patients with BRCA mutations and also in patients without such mutations Citation[49,50]. Unfortunately, routine use of PARP inhibitors remains some time in the future, with additional trial data needed (but not anticipated in the near future, based on currently active trials). As noted previously, targeting angiogenesis with bevacizumab has been used Citation[8,9], but the improvements in outcome have been modest and there is a need for predictive biomarkers to identify those patients who stand to benefit from this therapy.
Low-grade serous carcinoma
LGSCs are uncommon, accounting for approximately 3% of ovarian surface epithelial carcinomas, and the average age at diagnosis is lower than for HGSCs Citation[34]. When confined to the ovary (stage Ia), the prognosis is greater than 95%, achieved with surgical intervention alone; however, the majority of LGSCs present at an advanced stage, and although the disease is relatively indolent (mean survival: 4.2 years) Citation[34,51], the long-term survival is similar to HGSC.
Morphology of LGSCs
Macroscopically, LGSCs are often bilateral, exhibiting fine papillary growths, which are often indistinguishable from SBTs. Compared with HGSCs, there is less necrosis and hemorrhage. Often there are firm extraovarian implants that have a gritty texture caused by abundant psammoma-body formation.
Microscopically, LGSCs grow in a well-developed papillary pattern with fibrovascular cores. Numerous psammoma bodies are evident, and nuclear uniformity is the key feature in the distinction from HGSC, with less than threefold variation in nuclear size. Nucleoli may be prominent; however, this is not a criterion used in diagnostic subtyping. By definition, the tumor cells are invasive, either in nests or in single cells. If the invasive foci measure less than 10 mm2, the tumor is considered to be microinvasive Citation[52]. Although rare, LGSCs can progress to high-grade carcinoma Citation[53,54], although the relationship of these carcinomas to usual HGSCs is doubtful.
Molecular features of LGSCs
The molecular alterations of LGSCs are distinct from HGSCs. Instead, LGSCs share molecular changes with SBT Citation[27], suggesting a continuum of disease from SBT to LGSC. Both LGSCs and SBTs lack TP53 mutations Citation[27], and neither are associated with BRCA germline mutations nor other hereditary ovarian cancer syndromes. In addition, the majority of LGSCs and SBTs harbor somatic, activating mutations in KRAS and BRAF, resulting in constitutive activation of the MAP-kinase pathway, believed to be central to their pathogenesis Citation[29,55–57]. Interestingly, recurrences of SBTs can present as LGSCs Citation[58], and more than half of LGSCs are seen in association with SBT Citation[34]. Furthermore, LGSCs are diploid or near diploid, do not have chromosomal instability and lack the complex mutations seen in HGSCs.
As previously stated, the majority of LGSCs and HGSCs are positive for WT-1, ER and progesterone receptors, and negative for HNF-1b Citation[42,44]. The main differential is between LGSCs and SBTs. The distinction is based solely on histological evidence of invasion, and there are no immunohistochemical stains that can assist. The term ‘SBT with microinvasion’ is reserved for SBT with small invasive foci of less than 10 mm2.
Therapy
It is generally believed that LGSCs do not respond to conventional platinum-based chemotherapy Citation[58]; however, studies on the therapeutic response of this tumor are limited, as LGSC has only recently been recognized as a distinct ovarian carcinoma subtype Citation[19]. Since the discovery of MAP-kinase mutations in LGSCs, there have been trials that have investigated targeted therapies. Cabozantinib, a potent MAP-kinase inhibitor, has shown positive treatment results in advanced ovarian cancer, irrespective of platinum-based chemotherapy response Citation[59]. In addition, there is an ongoing Phase II study performed by the Gynecological Oncology Group investigating the effectiveness of AZD6244 (AstraZeneca), another MAP-kinase inhibitor Citation[60].
Ovarian MCs
Ovarian mucinous tumors account for 10–15% of primary ovarian tumors; however, approximately 80% are benign mucinous tumors (cystadenomas or cystadenofibromas) Citation[61], and most of the remainder are mucinous borderline tumors. The diagnosis of primary ovarian MCs is challenging and involves consideration of both clinical and pathological information, as metastatic gastrointestinal adenocarcinomas originating from the appendix, stomach, pancreas or colon enter the differential diagnosis Citation[62]. Once metastases to the ovary are excluded, primary ovarian MCs comprise between 2 and 8% of ovarian surface epithelial carcinomas in North America Citation[34,35,63,64].
More than 90% of MCs are low-grade tumors (grade 1 or 2), and approximately 80% are diagnosed at stage I or II Citation[36]. The mortality associated with MCs is relatively low, as MCs, regardless of stage, have a 90% 5-year survival Citation[62]. However, when diagnosed at an advanced stage, the outcome is poor compared with HGSCs due to its poor response rate to standard platinum-based chemotherapy Citation[65,66].
Morphology of MCs
Macroscopically, MCs are usually large (15–20 cm), multilocular, cystic tumors Citation[67,68]. Solid regions of firm, fleshy, white or tan tissue may be present, and in larger tumors, foci of hemorrhage or necrosis are often seen. More than 90% of MCs are unilateral without surface growth Citation[69]. However, rupture is common because of the large size and mucin content of this tumor. Bilateral and small tumors (less than 10 cm) are likely to be metastatic, while large unilateral tumors are more commonly primary Citation[63].
Microscopically, most MCs are intestinal type. The cells are columnar with eosinophilic cytoplasm and tend to stratify into two or more layers, and sometimes goblet cells are present. The nuclei are enlarged, vesicular and have coarse chromatin with prominent nucleoli. The frequency of mitotic figures ranges from few to many, and often atypical mitotic figures are present. The growth pattern is glandular and cystic, and often the glands are crowded and complex with irregular infoldings and protrusions into the surrounding stroma. Two patterns of invasion are described, and have potential clinical implications. The first is expansile type, characterized by confluent, back-to-back complex malignant glands with minimal to no intervening stroma, and exceeding 10mm2 in area. This pattern of invasion is almost always associated with stage I disease and predicts an excellent prognosis Citation[64]. The second pattern is termed infiltrative type and shows malignant glands, clusters or individual cells infiltrating the stroma, associated with a desmoplastic stromal response – a pattern associated with a worse prognosis Citation[70].
Some MCs lack intestinal features, and instead have endocervical-like cells with columnar cells and prominent mucinous cytoplasm. The tumor cells line glands, cysts and papillae. Although endocervical-like mucinous borderline tumors are relatively common, MCs are almost exclusively of intestinal type. An important feature of MCs is intratumoral heterogeneity. Benign, borderline and intraepithelial carcinoma frequently coexist within a tumor, thus the need for adequate sampling. A minimum of one section per centimeter of tumor is required, focusing on the more solid regions. Mucinous borderline tumor with intraepithelial carcinoma is defined as tumors with malignant cytological features of the epithelium, but lacking invasive carcinoma, as defined previously. Although considered an in situ form of MCs, such tumors rarely recur (less than 5%), and the recurrence usually has the morphology of a high-grade MC and behaves in an aggressive fashion, with metastases to bone, lungs and other organs Citation[64]. An unusual histological feature, apparently unique to MCs, is the occasional finding of mural nodules composed of ‘sarcoma-like’ reactive stromal proliferation, sarcoma or anaplastic carcinoma. If the mural nodules are localized to the wall of an unruptured cyst, the prognosis is more favorable Citation[71,72]; however, despite complete surgical removal, some of these tumors recur, and when they do, the anaplastic component predominates.
Molecular features of MCs
The most common mutation in MC is the activation of KRAS, which occurs early in tumorigenesis, is present in up to 75% of cases Citation[73,74] and is a molecular alteration shared with LGSC Citation[56]. However, unlike LGSCs, MCs do not have BRAF mutations. Interestingly, KRAS mutations are also seen in mucinous cystadenomas and borderline mucinous tumors, supporting a stepwise progression from borderline tumors to MCs Citation[73]. More than 50% of colorectal and more than 90% of pancreatic adenocarcinomas have KRAS mutations, so KRAS cannot be used to distinguish MCs from metastatic gastrointestinal carcinomas. Moreover, recent studies have shown that HER2 is amplified in 15–20% of MCs, representing an alternative means of activating the MAPK pathway Citation[75–77]. Not surprisingly, given that the mutations in KRAS and HER2 target the same pathway, they are almost mutually exclusive Citation[78].
Immunohistochemical staining has an important role in distinguishing primary ovarian MCs from metastatic MCs, and with current staining techniques, such a distinction is possible in a large majority of cases; where uncertainty remains after immunostaining, clinical investigations such as endoscopy or imaging can be undertaken. However, when interpreting the result, it is important to consider the nature of staining and not just whether the stain is positive or negative, as well as considering the clinical history. Almost all primary ovarian MCs are CK7-positive, compared with colorectal adenocarcinomas, which are typically CK7 negative Citation[79]. However, the immunoreaction for CK7 is usually weak and focal, and staining for CDX-2 can be similar between ovarian MCs and colorectal adenocarcinomas Citation[80]. By contrast, CK20 staining is often relatively weak and focal in ovarian MC, while appendiceal or colonic metastases typically show strong diffuse CK20 positivity. Metastatic pancreatic adenocarcinomas can be distinguished from primary ovarian MCs in some cases based on Dpc4 immunohistochemistry; Dpc4 staining is negative in 50% of pancreatic adenocarcinomas and is typically focally or diffusely positive in primary ovarian MCs Citation[81]. Metastatic cervical adenocarcinomas in the ovaries can be distinguished by their strong, diffuse positivity for p16, and demonstration of human papilloma virus DNA Citation[82]. Higher-grade MCs may show mucin depletion, and serous and ECs of the ovaries enter the differential diagnosis. MCs are typically negative for ER and WT-1, compared with ECs (ER-positive) and serous carcinomas (ER- and WT-1-positive) Citation[42].
Therapy
Combined surgery and chemotherapy is currently the only approved treatment for advanced-stage ovarian MC Citation[65]. It is difficult to determine the response rate of MCs to adjuvant chemotherapy because older studies invariably include a mix of metastatic and primary MCs. However, recent studies have shown a significantly lower response rate to platinum-based chemotherapy (<40%) compared with HGSCs Citation[83], prompting a search for other therapies. Trastuzumab seems like an obvious treatment option in patients whose tumors show HER2 amplification and overexpression. However, to date, there are limited data on efficacy, consisting of individual cases; one patient with platinum-resistant disease had a complete remission with Herceptin® (Genentech, CA, USA) treatment in combination with platinum-based chemotherapy Citation[76]. Given that primary ovarian MCs and gastrointestinal adenocarcinomas share some molecular alterations, chemotherapeutic regimens traditionally used for gastrointestinal carcinomas have been tried for primary ovarian MCs. Oxaliplatin and 5-fluorouracil chemotherapy have shown activity in ovarian MC experimental models Citation[84], and a Phase II trial involving patients with ovarian MCs treated with irinotecan and mitomycin-C showed a response rate of 52% with five complete responses Citation[85]. The current standard of care for MCs, however, is platinum/taxane chemotherapy.
Ovarian ECs
The prevalence of ovarian ECs has apparently decreased recently, and it currently accounts for 10% of ovarian surface epithelial carcinomas Citation[37]. The reduction in incidence is attributable to inappropriate classification in the past, where ovarian carcinomas with glandular morphology were frequently diagnosed as high-grade ECs. However, a significant proportion of these carcinomas were found to express WT-1 Citation[26,42,86], be chromosomally unstable and have TP53 mutations; that is, they are molecularly indistinguishable from HGSCs, and thus are best diagnosed as HGSCs Citation[87]. ECs represent the majority of low-grade ovarian carcinomas, and usually present with low-stage disease (International Federation of Gynecology and Obstetrics [FIGO] stage I or II) Citation[37]. Most ECs are diagnosed in women during the perimenopausal or postmenopausal period Citation[19]. Interestingly, there is a strong association with endometriosis, with the condition present in 20–40% of ECs. In some such cases, the ECs arise in an endometriotic cyst. 15–20% of cases of ovarian ECs are associated with endometrioid adenocarcinomas of the endometrium Citation[88,89]. Although there is strong evidence linking endometriosis to ECs (and CCCs), there are no tools available at present to identify those patients with endometriosis who are more likely to develop carcinoma.
Morphology of ECs
Macroscopically, ECs are variably cystic and solid, and generally have smooth outer surfaces Citation[90,91]. Regions of hemorrhage, necrosis and residual endometriosis are common. Between 80 and 90% of cases are unilateral Citation[88,92].
Microscopically, the majority of ECs have a glandular or papillary architecture and resemble endometrioid adenocarcinomas of the uterus. The epithelium is composed of stratified, nonmucinous glandular epithelial cells; the nuclei may contain nucleoli. Squamous differentiation, in the form of morules, occurs in approximately 50% of cases. Clear cells sometimes occur in ECs and may be either glandular (secretory type) or squamous. Distinction from CCCs is based on the lower nuclear grade features of the ‘clear cells’ in ECs, and the architectural features of CCCs. The degree of atypia, amount of nuclear stratification and extent to which the glands coalesce to form solid foci increase as the grade increases. ECs can be graded using the same criteria as for endometrial adenocarcinomas of endometrioid type Citation[93]. In addition to glandular architecture, ECs may have a villoglandular growth pattern. A rare variant of ECs can mimic sex cord-stromal tumors. Some tumors exhibit a microglandular growth pattern, characterized by round or small rosette-like glands, and can be mistaken for granulosa cell tumor Citation[94]. Sertoliform ECs have regions characterized by long, branching, tubular glands or trabeculae. This variant can mimic a Sertoli or Sertoli–Leydig cell tumor, especially when the stroma is abundant and fibrous, and luteinized cells are present Citation[94,95]. The oxyphilic variant of EC has prominent, large polygonal tumor cells with eosinophilic cytoplasm and round central nuclei with prominent nucleoli Citation[96]. The spindle cell variant contains bland spindle cells in lobulated nests, admixed with ribbons and cords of tumor cells. The clinical, microscopic and immunohistochemical features all serve to distinguish these ovarian ECs from sex cord-stromal tumors. Ovarian ECs usually occur in post- or peri-menopausal women, and usually lack steroid hormone productions.
Molecular features of ECs
ECs are typically chromosomally stable. The most common genetic abnormalities detected in ovarian ECs are somatic mutations of CTNNB1 (b-catenin) and PTEN genes Citation[97,98]. The incidence of CTNNB1 mutations in ovarian ECs ranges from 38 to 50%. Common locations for the mutations in CTNNB1 are phosphorylation sites of serine–threonine residues coded in exon 3, targeted by glycogen synthase kinase 3-B. These mutations probably render cellular b-catenin insensitive to adenomatous polyposis coli-mediated downregulation, resulting in increased nuclear and cytoplasmic levels of b-catenin. The end result is aberrant function of the Wnt-signaling pathway, which ultimately has antiapoptosis effects. Normal CTNNB1 results in membranous staining by immunohistochemistry. CTNNB1 mutations result in focal nuclear and cytoplasmic staining. This altered staining pattern is seen in up to 85% of ovarian ECs with squamous differentiation Citation[99]. ECs of the ovary have similar frequencies of microsatellite instability compared with endometrial adenocarcinomas Citation[97].
The prevalence of Lynch syndrome, with microsatellite instability, in patients with ovarian ECs is approximately 3%, making ECs, along with CCCs, the most common ovarian carcinomas in this patient population. This prevalence of microsatellite instability is similar to adenocarcinomas of the colon and endometrium Citation[100], suggesting a need for mismatch repair testing on certain patients diagnosed with ovarian EC.
The coexistence of endometrial adenocarcinomas and ovarian ECs is well documented. These tumors behave as if they are independent synchronous low-stage primary tumors of ovary and endometrium, with a favorable prognosis. Almost all ovarian ECs express ER. The same is true for endometrial adenocarcinomas, suggesting a potential role of hormonal environment in the genesis of these two tumors, given the well-established role of unopposed estrogen stimulation as a risk factor for endometrioid endometrial adenocarcinoma.
Common differential diagnoses include HGSCs of the ovary, ovarian sex-cord tumors, and metastatic colon, endometrial and endocervical adenocarcinomas . HGSCs of the ovaries can usually be distinguished based upon greater nuclear variability and high mitotic rate, and absence of squamous differentiation. However, high-grade nuclear atypia can occur in ovarian ECs; and in these cases, especially when there is no low-grade ECs present, WT-1 is a useful immunohistochemical marker, being positive in HGSCs and negative in ovarian ECs. Ovarian sex cord-stromal tumors are positive for WT-1, inhibin and calretinin, compared with negative staining in ovarian ECs Citation[101–103]. Metastatic colonic adenocarcinoma is one of the most common metastatic carcinomas affecting the ovary. Immunohistochemically, colon carcinoma is positive for CDX-2 and generally negative for CK7. By contrast, ovarian ECs are positive for CK7 (97%) and rarely positive for CK20 (13%). Rare cases of ovarian ECs with extensive mucinous differentiation are positive for CDX-2 Citation[104]. Endocervical adenocarcinomas sometimes metastasize to the ovaries. CK7 and CK20 staining pattern is similar between endocervical adenocarcinoma and ovarian ECs. However, p16 can be used, being diffusely positive in a large majority of endocervical adenocarcinomas and only focally positive in ovarian EC Citation[105].
Therapy
Patients with ovarian ECs typically present at an earlier stage, with few presentations with ascites compared with other ovarian carcinoma subtypes Citation[106] and a comparatively favorable prognosis. Stage I ovarian ECs have a greater than 90% 10-year disease-specific survival, compared with 70% for CCCs and 40% for ovarian serous carcinomas Citation[26]. The grade of ovarian ECs does not appear to influence prognosis Citation[26], however there is little data on grade 3 EC, which is rare. The standard of therapy for high-risk ovarian ECs is debulking surgery followed by platinum- and taxane-based chemotherapy, which has shown to have a better response rate than single agent or other platinum combinations Citation[106].
The molecular profile of ovarian ECs has led to investigations regarding targeted therapy. Ovarian carcinoma, like breast and endometrial carcinoma, is considered to be estrogen responsive. Antiestrogenic effect, with either tamoxifen or progesterone therapy, has a disease stabilizing effect on endometrioid endometrial adenocarcinoma, and selective ER modulators may have a similar effect on ER-positive ovarian carcinomas, such as ECs. However, studies have shown mixed results. While some clinical trials have demonstrated that tamoxifen has a small but favorable effect on recurrent ovarian carcinoma Citation[105,106], bazedoxifene, a selective estrogen receptor modulator, slowed invasion and growth of ovarian cancer cells in a mouse model, but showed no effect on tumor burden, metastatic nodule formation and ascites Citation[99]. Aromatase is a major source of estrogen synthesis, converting androgens to estrogens Citation[106]. Clinical studies have shown that aromatase inhibitors produce a clinical response in up to 35% of estrogen-sensitive ovarian carcinomas and stable disease rates of 20–42% in recurrent ovarian carcinoma cases Citation[107–109].
Finally, histone deacetylation and acetylation act as epigenetic controls of gene expression and promoter functions. Alterations in histone deacytelases have been reported in several tumor entities, including ovarian ECs Citation[110]. Preclinical studies using drugs that act as histone deacytelase inhibitors have shown increased induction of apoptosis in ovarian EC cell lines and reduction in tumor size in mouse models Citation[111].
CCCs of the ovary
In North America, CCCs of the ovary is the second or third most common ovarian carcinoma, depending on the population studied, accounting for 5–10% of all ovarian tumors Citation[37]. However, CCC is more common in East Asia, and especially Japan, at least relative to other ovarian carcinoma subtypes Citation[112]. Similar to ovarian EC, CCC commonly presents at an early stage, with most CCCs presenting with FIGO stage I/II disease Citation[36], and relatively few cases with peritoneal or nodal metastases Citation[113,114]. Although survival rates of low-stage CCC are relatively favorable, stage-for-stage, CCC is considered an unfavorable histological subtype, with a poor response to platinum-based chemotherapy Citation[112]. Paraneoplastic syndromes occur in women with CCC, including hypercalcemia Citation[115] and thromboembolic events, such as deep venous thrombosis and pulmonary emboli Citation[116].
At least 50% of CCCs are associated with endometriosis, especially atypical endometriosis Citation[117,118]. Atypical endometriosis refers to a heterogeneous group of lesions, including endometriosis with atypical hyperplasia and endometriosis with hobnail metaplasia and nuclear atypia Citation[119].
Morphology of ovarian CCCs
The most characteristic gross appearance of CCC is a solid and cystic tumor that may be accompanied by endometriosis. When endometriosis is present, the CCC component may take the form of mural nodules of papillary tumor protruding into the lumen of an endometriotic cyst. CCCs can show an adenofibromatous architecture with innumerable small cysts separated by fibrous stroma. Many CCCs are associated with surface adhesions due to chronic endometriosis. Tumors that are confined to the ovaries (FIGO stage I) are usually unilateral. However, when all stages of CCCs are considered, approximately 30% are bilateral.
The characteristic microscopic features of CCC include: multiple complex papillae, dense hyaline basement membrane material that expands the papillary cores, and hyaline bodies. In addition, tubules lined by cuboidal cells with clear cytoplasm and filled with eosinophilic secretions are particularly characteristic. A variety of cell types are present, including clear cells, cells with granular eosinophilic cytoplasm and hobnail cells with clear or eosinophilic cytoplasm. Usually a mixture of cell types is present. The clear cells are low columnar, cuboidal or polygonal and have abundant clear cytoplasm, central nuclei and prominent nucleoli. The cells contain glycogen and stain with periodic acid-Schiff stain. Mitotic activity is generally lower in CCCs compared with other ovarian carcinoma subtypes (with the exception of LGSCs), and the low rate has been proposed as a possible explanation for the poor response to chemotherapy Citation[120]. In a study, a mitotic rate of 6 or greater per 10 high-power field was an adverse prognostic factor in CCC Citation[120]. Grading is not of proven significance in CCCs and, in practice, all are considered high grade (grade 3) Citation[26].
There are a number of differential diagnoses to be considered with CCCs . The papillary architecture of CCCs can be confused with SBTs, especially at frozen section; however, the unilateral nature of CCCs and higher-grade cytological features should allow the correct diagnosis to be made Citation[121]. In HGSCs, any clear cell has the same immunophenotype as the serous component, and usually have greater than threefold variability in nuclear size. In addition, these tumors with serous and clear cell components usually have a high mitotic rate, supporting the diagnosis of HGSCs with clear cell change, instead of mixed serous/CCCs or CCCs Citation[46]. Finally, ovarian ECs with clear cell change do not have the high nuclear atypia observed in CCCs Citation[122].
Molecular features of ovarian CCCs
CCCs are characterized by a low level of chromosomal instability and lack the complex karyotypes of HGSCs. In addition, CCCs are not associated with BRCA abnormalities Citation[32]. Relatively little is known regarding the genetic alterations of CCCs. KRAS Citation[123] and PTEN Citation[123] mutations are reported in a minority of CCCs. In addition, microsatellite instability is present in some CCCs Citation[124,125]. The most consistently demonstrated abnormality in CCC is mutation of the oncogene PIK3CA, which is reported to occur in up to 33% of cases Citation[126]. This mutation activates the PI3K/AKT pathway, promoting increased cell proliferation, invasion and decreased apoptosis. Lynch syndrome is characterized by a germline mutation in mismatch repair proteins and is associated with increased incidence of tumors, including ovarian carcinomas. A recent study has shown that 17% of CCC cases occurring in women younger than 50 years of age had mismatch repair defects, making CCCs, along with ECs, the most common ovarian subtypes associated with Lynch syndrome Citation[124]. In addition, ARID1A, a tumor suppressor gene, has recently been shown to be mutated in 46% of CCCs Citation[127].
Immunohistochemistry is rarely required for the diagnosis of CCC. CCC is positive for CK7 and negative for CK20, whereas renal CCC is negative for both CK7 and CK20. CCC is negative for ER and WT-1 in more than 95% of cases Citation[53]. TP53 staining can occur in CCC, but diffuse, string nuclear staining (as seen in HGSC) is distinctly uncommon Citation[44,46]. HNF-1b is highly sensitive (82–100%) and a specific marker for CCC Citation[128], with only rare focal positivity reported in ovarian EC, serous and MCs.
While both EC and CCC are thought to arise from endometriosis, the molecular abnormalities suggest different oncogenic pathways, with EC arising in a hormonally dependent manner and CCC arising through mechanisms independent of hormonal signaling, with HNF-1b playing a central role, analogous to type II endometrial carcinomas Citation[129].
Therapy
CCCs do not respond as well to standard platinum-based chemotherapy, compared with HGSCs Citation[112,130–132]. The reported differences in response rate (15–45%) may be due in part to the more genomically stable and lower mitotic rate of CCCs compared with HGSCs. Currently, there are no superior alternatives to platinum-based chemotherapy; however, a study showed that postoperative, whole abdominal radiotherapy was effective in improving disease-free survival and overall-survival in patients with stage Ic–III CCC compared with platinum-based chemotherapy alone Citation[133]. Another retrospective study demonstrated improved outcomes for patients with CCC who received radiotherapy and chemotherapy, compared with patients who received chemotherapy alone Citation[134], and inhibitors to VEGF signaling have shown promise in a preclinical model Citation[133]. CCCs have the greatest frequency of PIK3CA mutations among ovarian carcinoma subtypes. However, attempts at targeted therapy have largely been unsuccessful due to the significant toxicity associated with PIK3CA inhibitors Citation[135].
Conclusion
The advances in molecular genetics and immunohistochemistry have contributed significantly to our current situation, where it is possible to accurately and reproducibly subclassify ovarian surface epithelial carcinomas into five main subtypes: HGSC, CCC, EC, MC and LGSC. These subtypes show distinct genetic alterations, natural history and response to chemotherapy, and are best considered to be distinct diseases . Accurate diagnosis will serve as the foundation as we progress towards subtype-specific therapy for ovarian carcinoma.
Expert commentary
There has been a dramatic shift in our understanding of ovarian carcinoma over the last 5 years, as we have moved away from the traditional ‘one disease, one treatment’ approach. This shift is underpinned by recognition that ovarian carcinoma is five different diseases, with differences in risk factors, patterns of spread, response to therapy and outcomes. These five ovarian carcinoma subtypes also have characteristic molecular abnormalities that are just starting to be documented, as subtype-specific research into ovarian carcinoma pathogenesis is now becoming the norm. Furthermore, in the past 5 years, there has been significant progress in diagnostic surgical pathology, such that it is now possible for ovarian carcinoma subtypes to be accurately and reproducibly diagnosed in routine surgical pathology practice.
Five-year view
Over the next 5 years, we will see a dramatic surge forward in our understanding of the molecular basis of the less-common subtypes of ovarian carcinoma (LGSC, EC, MC and CCC), which together account for 30% of cases. There will be increasing efforts to offer subtype-specific treatment for ovarian carcinoma, based on our improved understanding of the different biologies of these subtypes.
Table 1. High-grade serous carcinoma versus low-grade serous carcinoma.
Table 2. Differential diagnosis of ovarian mucinous carcinoma.
Table 3. Differential diagnosis of ovarian endometrioid carcinoma.
Table 4. Differential diagnosis of ovarian clear cell carcinoma.
Table 5. Clinical and molecular differences between the main histological subtypes of ovarian carcinoma.
Key issues
• Ovarian surface epithelial carcinomas are the most common malignant ovarian tumors and the most lethal gynecological malignancies.
• Advances in immunohistochemistry and molecular analyses have dramatically increased the diagnostic accuracy of ovarian surface epithelial carcinoma subtype diagnosis.
• More than 98% of ovarian surface epithelial carcinomas can be assigned to one of five major subtypes: high-grade serous carcinoma, clear cell carcinoma, endometrioid carcinoma, mucinous carcinoma and low-grade serous carcinoma.
• The five main subtypes have distinct molecular abnormalities and treatment responses, and are best regarded as distinct diseases.
• New subtype-specific treatment strategies are being developed, targeting molecular abnormalities specific for each subtype.
• Poly (ADP-ribose) polymerase P inhibitors have shown promise in the treatment of high-grade serous carcinoma, through exploitation of inherent double-strand break–repair defects.
• MAPK inhibitors have been tested in low-grade serous carcinoma, PIK3CA inhibitors in clear cell carcinoma, tamoxifen in endometrioid carcinoma and Herceptin® in mucinous carcinoma, with varying results.
• Further subtype-specific therapeutic trials are required to improve outcomes for patients with ovarian carcinoma.
References
- Coleman MP, Forman D, Bryant H et al.; ICBP Module 1 Working Group. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 377(9760), 127–138 (2011).
- Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract. Res. Clin. Obstet. Gynaecol. 20(2), 207–225 (2006).
- Anttila A, Ronco G, Clifford G et al. Cervical cancer screening programmes and policies in 18 European countries. Br. J. Cancer 91(5), 935–941 (2004).
- Prat J. Pathology of the Ovary. WB Saunders Co., PA, USA (2004).
- Scully RE, Young RH, Clement PB. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Int. J. Gynecol. Pathol. 18(3), 288 (1999).
- Walker JL. Intraperitoneal chemotherapy for epithelial ovarian cancer. Presented at: 2009 ASCO Meeting. FL, USA, 29 May – 2 June 2009.
- Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst. Rev. 1, CD005340 (2006).
- Perren TJ, Swart AM, Pfisterer JA et al.; ICON7 Investigators. A Phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 365(26), 2484–2496 (2011).
- Burger RA, Brady MF, Bookman MA et al.; Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 365(26), 2473–2483 (2011).
- Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am. J. Surg. Pathol. 34(3), 433–443 (2010).
- Folkins AK, Jarboe EA, Saleemuddin A et al. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol. Oncol. 109(2), 168–173 (2008).
- Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin. Med. Res. 5(1), 35–44 (2007).
- Folkins AK, Jarboe EA, Roh MH, Crum CP. Precursors to pelvic serous carcinoma and their clinical implications. Gynecol. Oncol. 113(3), 391–396 (2009).
- Piek JM, van Diest PJ, Zweemer RP et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J. Pathol. 195(4), 451–456 (2001).
- McMeekin DS, Burger RA, Manetta A, DiSaia P, Berman ML. Endometrioid adenocarcinoma of the ovary and its relationship to endometriosis. Gynecol. Oncol. 59(1), 81–86 (1995).
- Modesitt SC, Tortolero-Luna G, Robinson JB, Gershenson DM, Wolf JK. Ovarian and extraovarian endometriosis-associated cancer. Obstet. Gynecol. 100(4), 788–795 (2002).
- Schmeler KM, Gershenson DM. Low-grade serous ovarian cancer: a unique disease. Curr. Oncol. Rep. 10(6), 519–523 (2008).
- Köbel M, Huntsman D, Gilks CB. Critical molecular abnormalities in high-grade serous carcinoma of the ovary. Expert Rev. Mol. Med. 10, e22 (2008).
- Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum. Pathol. 40(9), 1213–1223 (2009).
- Trimble EL, Fountain J, Birrer MJ. Recommendations of the 2005 ovarian cancer state of the science meeting. Gynecologic Oncol. 103(2), S26 (2006).
- Cramer SF, Roth LM, Ulbright TM et al. Evaluation of the reproducibility of the World Health Organization classification of common ovarian cancers. With emphasis on methodology. Arch. Pathol. Lab. Med. 111(9), 819–829 (1987).
- Sakamoto A, Sasaki H, Furusato M et al. Observer disagreement in histological classification of ovarian tumors in Japan. Gynecol. Oncol. 54(1), 54–58 (1994).
- Lund B, Thomsen HK, Olsen J. Reproducibility of histopathological evaluation in epithelial ovarian carcinoma. Clinical implications. APMIS 99(4), 353–358 (1991).
- Hernandez E, Bhagavan BS, Parmley TH, Rosenshein NB. Interobserver variability in the interpretation of epithelial ovarian cancer. Gynecol. Oncol. 17(1), 117–123 (1984).
- Köbel M, Kalloger SE, Baker PM et al. Diagnosis of ovarian carcinoma cell type is highly reproducible: a transcanadian study. Am. J. Surg. Pathol. 34(7), 984–993 (2010).
- Gilks CB, Ionescu DN, Kalloger SE et al.; Cheryl Brown Ovarian Cancer Outcomes Unit of the British Columbia Cancer Agency. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum. Pathol. 39(8), 1239–1251 (2008).
- Singer G, Kurman RJ, Chang HW, Cho SK, Shih IeM. Diverse tumorigenic pathways in ovarian serous carcinoma. Am. J. Pathol. 160(4), 1223–1228 (2002).
- Vang R, Shih IeM, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 16(5), 267–282 (2009).
- Singer G, Oldt R 3rd, Cohen Y et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl. Cancer Inst. 95(6), 484–486 (2003).
- Singer G, Shih IeM, Truskinovsky A, Umudum H, Kurman RJ. Mutational analysis of KRAS segregates ovarian serous carcinomas into two types: invasive MPSC (low-grade tumor) and conventional serous carcinoma (high-grade tumor). Int. J. Gynecol. Pathol. 22(1), 37–41 (2003).
- Ho CL, Kurman RJ, Dehari R, Wang TL, Shih IeM. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res. 64(19), 6915–6918 (2004).
- Ahmed AA, Etemadmoghadam D, Temple J et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 221(1), 49–56 (2010).
- Press JZ, De Luca A, Boyd N et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer 8, 17 (2008).
- Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int. J. Gynecol. Pathol. 23(1), 41–44 (2004).
- Malpica A, Deavers MT, Lu K et al. Grading ovarian serous carcinoma using a two-tier system. Am. J. Surg. Pathol. 28(4), 496–504 (2004).
- Risch HA, McLaughlin JR, Cole DE et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am. J. Hum. Genet. 68(3), 700–710 (2001).
- Köbel M, Kalloger SE, Huntsman DG et al.; Cheryl Brown Ovarian Cancer Outcomes Unit of the British Columbia Cancer Agency, Vancouver BC. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int. J. Gynecol. Pathol. 29(3), 203–211 (2010).
- Kuhn E, Meeker A, Wang TL, Sehdev AS, Kurman RJ, Shih IeM. Shortened telomeres in serous tubal intraepithelial carcinoma: an early event in ovarian high-grade serous carcinogenesis. Am. J. Surg. Pathol. 34(6), 829–836 (2010).
- Malpica A, Deavers MT, Tornos C et al. Interobserver and intraobserver variability of a two-tier system for grading ovarian serous carcinoma. Am. J. Surg. Pathol. 31(8), 1168–1174 (2007).
- Schrader KA, Hurlburt J, Kalloger SE et al. Germline BRCA1 and BRCA2 mutations in ovarian cancer: utility of a histology-based referral strategy. Obstet. Gynecol. 120(2 Pt 1), 235–240 (2012).
- Shaw PA, McLaughlin JR, Zweemer RP et al. Histopathologic features of genetically determined ovarian cancer. Int. J. Gynecol. Pathol. 21(4), 407–411 (2002).
- Bell D, Berchuck A, Birrer M et al. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
- Köbel M, Kalloger SE, Boyd N et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 5(12), e232 (2008).
- Al‐Hussaini M, Stockman A, Foster H, McCluggage WG. WT‐1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology 44(2), 109–115 (2004).
- Al-Hussaini M, Stockman A, Foster H, McCluggage WG. WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology 44(2), 109–115 (2004).
- Han G, Gilks CB, Leung S et al. Mixed ovarian epithelial carcinomas with clear cell and serous components are variants of high-grade serous carcinoma: an interobserver correlative and immunohistochemical study of 32 cases. Am. J. Surg. Pathol. 32(7), 955–964 (2008).
- Köbel M, Kalloger SE, Carrick J et al. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am. J. Surg. Pathol. 33(1), 14–21 (2009).
- Vergote I, Tropé CG, Amant F et al.; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 363(10), 943–953 (2010).
- Kortmann U, McAlpine JN, Xue H et al. Tumor growth inhibition by olaparib in BRCA2 germline-mutated patient-derived ovarian cancer tissue xenografts. Clin. Cancer Res. 17(4), 783–791 (2011).
- Gershenson DM, Sun CC, Lu KH et al. Clinical behavior of stage II–IV low-grade serous carcinoma of the ovary. Obstet. Gynecol. 108(2), 361–368 (2006).
- Oza AM, Cibula D, Oakn1in A et al. Olaparib plus paclitaxel plus carboplatin (P/C) followed by olaparib maintenance treatment in patients (pts) with platinum-sensitive recurrent serous ovarian cancer (PSR SOC): a randomized, open-label Phase II study. J. Clin. Oncol. 30(Suppl.) Abstract 5001 (2012).
- Bell DA, Longacre TA, Prat J et al. Serous borderline (low malignant potential, atypical proliferative) ovarian tumors: workshop perspectives. Hum. Pathol. 35(8), 934–948 (2004).
- Parker RL, Clement PB, Chercover DJ, Sornarajah T, Gilks CB. Early recurrence of ovarian serous borderline tumor as high-grade carcinoma: a report of two cases. Int. J. Gynecol. Pathol. 23(3), 265–272 (2004).
- Dehari R, Kurman RJ, Logani S, Shih IeM. The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am. J. Surg. Pathol. 31(7), 1007–1012 (2007).
- Armes JE, Lourie R, de Silva M et al. Abnormalities of the RB1 pathway in ovarian serous papillary carcinoma as determined by overexpression of the p16(INK4A) protein. Int. J. Gynecol. Pathol. 24(4), 363–368 (2005).
- Mayr D, Hirschmann A, Löhrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol. Oncol. 103(3), 883–887 (2006).
- Sieben NL, Macropoulos P, Roemen GM et al. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J. Pathol. 202(3), 336–340 (2004).
- Crispens MA, Bodurka D, Deavers M, Lu K, Silva EG, Gershenson DM. Response and survival in patients with progressive or recurrent serous ovarian tumors of low malignant potential. Obstet. Gynecol. 99(1), 3–10 (2002).
- Buckanovich RJ, Berger R, Sella A, Sikic BI, Shen X, Ramies DA. Activity of cabozantinib (XL184) in advanced ovarian cancer patients (pts): results from a Phase II randomized discontinuation trial (RDT). J. Clin. Oncol. 29, S15 (2011).
- Jones S, Wang TL, Kurman RJ et al. Low-grade serous carcinomas of the ovary contain very few point mutations. J. Pathol. 226(3), 413–420 (2012).
- Koonings PP, Campbell K, Mishell DR Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet. Gynecol. 74(6), 921–926 (1989).
- Naik JD, Seligmann J, Perren TJ. Mucinous tumours of the ovary. J. Clin. Pathol. 65(7), 580–584 (2012).
- Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am. J. Surg. Pathol. 27(7), 985–993 (2003).
- Ludwick C, Gilks CB, Miller D, Yaziji H, Clement PB. Aggressive behavior of stage I ovarian mucinous tumors lacking extensive infiltrative invasion: a report of four cases and review of the literature. Int. J. Gynecol. Pathol. 24(3), 205–217 (2005).
- Shimada M, Kigawa J, Ohishi Y et al. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol. Oncol. 113(3), 331–334 (2009).
- Hess V, A’Hern R, Nasiri N et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J. Clin. Oncol. 22(6), 1040–1044 (2004).
- Hart WR, Norris HJ. Borderline and malignant mucinous tumors of the ovary. Histologic criteria and clinical behavior. Cancer 31(5), 1031–1045 (1973).
- Watkin W, Silva EG, Gershenson DM. Mucinous carcinoma of the ovary. Pathologic prognostic factors. Cancer 69(1), 208–212 (1992).
- de Nictolis M, Montironi R, Tommasoni S et al. Benign, borderline, and well-differentiated malignant intestinal mucinous tumors of the ovary: a clinicopathologic, histochemical, immunohistochemical, and nuclear quantitative study of 57 cases. Int. J. Gynecol. Pathol. 13(1), 10–21 (1994).
- Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am. J. Surg. Pathol. 24(11), 1447–1464 (2000).
- Rodríguez IM, Prat J. Mucinous tumors of the ovary: a clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am. J. Surg. Pathol. 26(2), 139–152 (2002).
- Provenza C, Young RH, Prat J. Anaplastic carcinoma in mucinous ovarian tumors: a clinicopathologic study of 34 cases emphasizing the crucial impact of stage on prognosis, their histologic spectrum, and overlap with sarcomalike mural nodules. Am. J. Surg. Pathol. 32(3), 383–389 (2008).
- Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. KRAS mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer 79(8), 1581–1586 (1997).
- Gemignani ML, Schlaerth AC, Bogomolniy F et al. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol. Oncol. 90(2), 378–381 (2003).
- McCaughan H, Um I, Langdon SP, Harrison DJ, Faratian D. HER2 expression in ovarian carcinoma: caution and complexity in biomarker analysis. J. Clin. Pathol. 65(7), 670–671; author reply 671 (2012).
- McAlpine JN, Wiegand KC, Vang R et al. HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer 9, 433 (2009).
- Lin WL, Kuo WH, Chen FL et al. Identification of the coexisting HER2 gene amplification and novel mutations in the HER2 protein-overexpressed mucinous epithelial ovarian cancer. Ann. Surg. Oncol. 18(8), 2388–2394 (2011).
- Anglesio M, Kommoss S, Tolcher M et al. Molecular characterization of mucinous ovarian tumors supports a stratified treatment approach with HER2 targeting in 18% of carcinomas. J. Pathol. doi:10.1002/path.4088 (2012) (Epub ahead of print).
- Park SY, Kim HS, Hong EK, Kim WH. Expression of cytokeratins 7 and 20 in primary carcinomas of the stomach and colorectum and their value in the differential diagnosis of metastatic carcinomas to the ovary. Hum. Pathol. 33(11), 1078–1085 (2002).
- Vang R, Gown AM, Wu LS et al. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Mod. Pathol. 19(11), 1421–1428 (2006).
- Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int. J. Gynecol. Pathol. 21(4), 391–400 (2002).
- Ronnett BM, Yemelyanova AV, Vang R et al. Endocervical adenocarcinomas with ovarian metastases: analysis of 29 cases with emphasis on minimally invasive cervical tumors and the ability of the metastases to simulate primary ovarian neoplasms. Am. J. Surg. Pathol. 32(12), 1835–1853 (2008).
- Pectasides D, Fountzilas G, Aravantinos G et al. Advanced stage mucinous epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol. Oncol. 97(2), 436–441 (2005).
- Sundar S, Symonds RP, Decatris MP et al. Phase II trial of oxaliplatin and 5-fluorouracil/leucovorin combination in epithelial ovarian carcinoma relapsing within 2 years of platinum-based therapy. Gynecol. Oncol. 94(2), 502–508 (2004).
- Shimizu Y, Umezawa S, Hasumi K. A Phase II study of combined CPT-11 and mitomycin-C in platinum refractory clear cell and mucinous ovarian carcinoma. Ann. Acad. Med. Singap. 27(5), 650–656 (1998).
- Leitao MM Jr, Boyd J, Hummer A et al. Clinicopathologic analysis of early-stage sporadic ovarian carcinoma. Am. J. Surg. Pathol. 28(2), 147–159 (2004).
- Schwartz DR, Kardia SL, Shedden KA et al. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res. 62(16), 4722–4729 (2002).
- Falkenberry SS, Steinhoff MM, Gordinier M, Rappoport S, Gajewski W, Granai CO. Synchronous endometrioid tumors of the ovary and endometrium. A clinicopathologic study of 22 cases. J. Reprod. Med. 41(10), 713–718 (1996).
- Irving JA, Catasús L, Gallardo A et al. Synchronous endometrioid carcinomas of the uterine corpus and ovary: alterations in the beta-catenin (CTNNB1) pathway are associated with independent primary tumors and favorable prognosis. Hum. Pathol. 36(6), 605–619 (2005).
- Brescia RJ, Dubin N, Demopoulos RI. Endometrioid and clear cell carcinoma of the ovary. Factors affecting survival. Int. J. Gynecol. Pathol. 8(2), 132–138 (1989).
- Kline RC, Wharton JT, Atkinson EN, Burke TW, Gershenson DM, Edwards CL. Endometrioid carcinoma of the ovary: retrospective review of 145 cases. Gynecol. Oncol. 39(3), 337–346 (1990).
- Russell P. The pathological assessment of ovarian neoplasms. II: the proliferating ‘epithelial’ tumours. Pathology 11(2), 251–282 (1979).
- Zaino RJ, Kurman RJ, Diana KL, Morrow CP. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer 75(1), 81–86 (1995).
- Young RH, Prat J, Scully RE. Ovarian endometrioid carcinomas resembling sex cord-stromal tumors. A clinicopathological analysis of 13 cases. Am. J. Surg. Pathol. 6(6), 513–522 (1982).
- Roth LM, Liban E, Czernobilsky B. Ovarian endometrioid tumors mimicking Sertoli and Sertoli–Leydig cell tumors: sertoliform variant of endometrioid carcinoma. Cancer 50(7), 1322–1331 (1982).
- Pitman MB, Young RH, Clement PB, Dickersin GR, Scully RE. Endometrioid carcinoma of the ovary and endometrium, oxyphilic cell type: a report of nine cases. Int. J. Gynecol. Pathol. 13(4), 290–301 (1994).
- Catasús L, Bussaglia E, Rodrguez I et al. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum. Pathol. 35(11), 1360–1368 (2004).
- Palacios J, Gamallo C. Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 58(7), 1344–1347 (1998).
- Romero IL, Lee W, Mitra AK et al. The effects of 17b-estradiol and a selective estrogen receptor modulator, bazedoxifene, on ovarian carcinogenesis. Gynecol. Oncol. 124(1), 134–141 (2012).
- Hampel H, Stephens JA, Pukkala E et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology 129(2), 415–421 (2005).
- Deavers MT, Malpica A, Liu J, Broaddus R, Silva EG. Ovarian sex cord-stromal tumors: an immunohistochemical study including a comparison of calretinin and inhibin. Mod. Pathol. 16(6), 584–590 (2003).
- Matias-Guiu X, Pons C, Prat J. Müllerian inhibiting substance, alpha-inhibin, and CD99 expression in sex cord-stromal tumors and endometrioid ovarian carcinomas resembling sex cord-stromal tumors. Hum. Pathol. 29(8), 840–845 (1998).
- Aguirre P, Thor AD, Scully RE. Ovarian endometrioid carcinomas resembling sex cord-stromal tumors. An immunohistochemical study. Int. J. Gynecol. Pathol. 8(4), 364–373 (1989).
- Groisman GM, Meir A, Sabo E. The value of CDX2 immunostaining in differentiating primary ovarian carcinomas from colonic carcinomas metastatic to the ovaries. Int. J. Gynecol. Pathol. 23(1), 52–57 (2004).
- Vang R, Gown AM, Farinola M et al. p16 expression in primary ovarian mucinous and endometrioid tumors and metastatic adenocarcinomas in the ovary: utility for identification of metastatic HPV-related endocervical adenocarcinomas. Am. J. Surg. Pathol. 31(5), 653 (2007).
- Storey DJ, Rush R, Stewart M et al. Endometrioid epithelial ovarian cancer: 20 years of prospectively collected data from a single center. Cancer 112(10), 2211–2220 (2008).
- Bowman A, Gabra H, Langdon SP et al. CA125 response is associated with estrogen receptor expression in a Phase II trial of letrozole in ovarian cancer. Clin. Cancer Res. 8(7), 2233–2239 (2002).
- Li YF, Hu W, Fu SQ, Li JD, Liu JH, Kavanagh JJ. Aromatase inhibitors in ovarian cancer: is there a role? Int. J. Gynecol. Cancer 18(4), 600–614 (2008).
- Smyth JF, Gourley C, Walker G et al. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen receptor-positive patients. Clin. Cancer Res. 13(12), 3617–3622 (2007).
- Weichert W, Denkert C, Noske A et al. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia 10(9), 1021–1027 (2008).
- Takai N, Narahara H. Human endometrial and ovarian cancer cells: histone deacetylase inhibitors exhibit antiproliferative activity, potently induce cell cycle arrest, and stimulate apoptosis. Curr. Med. Chem. 14(24), 2548–2553 (2007).
- Sugiyama T, Kamura T, Kigawa J et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88(11), 2584–2589 (2000).
- Mizuno M, Kikkawa F, Shibata K et al. Long-term follow-up and prognostic factor analysis in clear cell adenocarcinoma of the ovary. J. Surg. Oncol. 94(2), 138–143 (2006).
- Behbakht K, Randall TC, Benjamin I, Morgan MA, King S, Rubin SC. Clinical characteristics of clear cell carcinoma of the ovary. Gynecol. Oncol. 70(2), 255–258 (1998).
- Tsunematsu R, Saito T, Iguchi H, Fukuda T, Tsukamoto N. Hypercalcemia due to parathyroid hormone-related protein produced by primary ovarian clear cell adenocarcinoma: case report. Gynecol. Oncol. 76(2), 218–222 (2000).
- Recio FO, Piver MS, Hempling RE, Driscoll DL. Lack of improved survival plus increase in thromboembolic complications in patients with clear cell carcinoma of the ovary treated with platinum versus nonplatinum-based chemotherapy. Cancer 78(10), 2157–2163 (1996).
- Mandai M, Yamaguchi K, Matsumura N, Baba T, Konishi I. Ovarian cancer in endometriosis: molecular biology, pathology, and clinical management. Int. J. Clin. Oncol. 14(5), 383–391 (2009).
- Kobayashi H. Ovarian cancer in endometriosis: epidemiology, natural history, and clinical diagnosis. Int. J. Clin. Oncol. 14(5), 378–382 (2009).
- Ballouk F, Ross JS, Wolf BC. Ovarian endometriotic cysts. An analysis of cytologic atypia and DNA ploidy patterns. Am. J. Clin. Pathol. 102(4), 415–419 (1994).
- Itamochi H, Kigawa J, Sugiyama T, Kikuchi Y, Suzuki M, Terakawa N. Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstet. Gynecol. 100(2), 281–287 (2002).
- Sangoi AR, Soslow RA, Teng NN, Longacre TA. Ovarian clear cell carcinoma with papillary features: a potential mimic of serous tumor of low malignant potential. Am. J. Surg. Pathol. 32(2), 269–274 (2008).
- Silva EG, Young RH. Endometrioid neoplasms with clear cells: a report of 21 cases in which the alteration is not of typical secretory type. Am. J. Surg. Pathol. 31(8), 1203–1208 (2007).
- Otsuka J, Okuda T, Sekizawa A et al. KRAS mutation may promote carcinogenesis of endometriosis leading to ovarian clear cell carcinoma. Med. Electron Microsc. 37(3), 188–192 (2004).
- Jensen KC, Mariappan MR, Putcha GV et al. Microsatellite instability and mismatch repair protein defects in ovarian epithelial neoplasms in patients 50 years of age and younger. Am. J. Surg. Pathol. 32(7), 1029–1037 (2008).
- Cai KQ, Albarracin C, Rosen D et al. Microsatellite instability and alteration of the expression of hMLH1 and hMSH2 in ovarian clear cell carcinoma. Hum. Pathol. 35(5), 552–559 (2004).
- Kuo KT, Mao TL, Jones S et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am. J. Pathol. 174(5), 1597–1601 (2009).
- Wiegand KC, Shah SP, Al-Agha OM et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 363(16), 1532–1543 (2010).
- Kato N, Sasou S, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1b) in clear cell tumors and endometriosis of the ovary. Mod. Pathol. 19(1), 83–89 (2006).
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 15(1), 10–17 (1983).
- Pectasides D, Fountzilas G, Aravantinos G et al. Advanced stage clear-cell epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol. Oncol. 102(2), 285–291 (2006).
- Shimizu M, Toki T, Takagi Y, Konishi I, Fujii S. Immunohistochemical detection of the Wilms’ tumor gene (WT-1) in epithelial ovarian tumors. Int. J. Gynecol. Pathol. 19(2), 158 (2000).
- Goff BA, Sainz de la Cuesta R, Muntz HG et al. Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol. Oncol. 60(3), 412–417 (1996).
- Nagai Y, Inamine M, Hirakawa M et al. Postoperative whole abdominal radiotherapy in clear cell adenocarcinoma of the ovary. Gynecol. Oncol. 107(3), 469–473 (2007).
- Hoskins PJ, Le N, Gilks B et al. Low-stage ovarian clear cell carcinoma: population-based outcomes in British Columbia, Canada, with evidence for a survival benefit as a result of irradiation. J. Clin. Oncol. 30(14), 1656–1662 (2012).
- Molckovsky A, Siu LL. First-in-class, first-in-human Phase I results of targeted agents: highlights of the 2008 American society of clinical oncology meeting. J. Hematol. Oncol. 1, 20 (2008).
Differential diagnosis and clinical relevance of ovarian carcinoma subtypes
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertob. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Which of the following statements regarding the epidemiology and prognosis of ovarian cancer is most accurate?
□ A It is the second most common female cancer worldwide
□ B Ovarian surface epithelial carcinomas are the most common form of ovarian cancer
□ C Bevacizumab improves cancer-related and overall mortality outcomes
□ D There is clear differentiation of treatment based on the type of ovarian cancer
2. Which of the following statements regarding the features and treatment of high-grade serous carcinoma (HGSC) is most accurate?
□ A Half of cases have abnormalities of BRCA1 or BRCA2
□ B The most common histologic pattern is glandular
□ C HGSC can be differentiated from other subtypes by a lack of reactivity to WT-1
□ D Initial response to platinum/taxane therapy is usually poor
3. What should you consider in managing patients with low-grade serous carcinomas (LGSCs)?
□ A Surgery alone is effective for approximately 35% of patients with LGSC limited to the ovary
□ B There is greater variability in nuclear size compared with HGSC
□ C LGSCs share molecular changes associated with serous borderline tumors (SBT)
□ D LGSC readily responds to platinum-based chemotherapy
4. Which of the following statements regarding ovarian carcinoma is most accurate?
□ A Most mucinous carcinomas are high-grade tumors
□ B The most common mutation in mucinous carcinomas is PIK3CA
□ C Endometrioid carcinoma is always bilateral
□ D At least 50% of cases of clear cell carcinoma are related to endometriosis