Abstract
Managing poor responders in IVF is a significant challenge for fertility specialists. Several ovarian reserve tests have been developed to predict poor response. These include serum basal follicle-stimulating hormone and estradiol levels, as well as, more recently, anti-Müllerian hormone. Sonographic assessment of ovarian reserve has also been employed including ovarian volume and antral follicle count. However, the most accurate predictor of a poor response cycle is a history of poor response to gonadotropins. This review defines and details the various regimens applied to poor responders over the past several decades of assisted reproduction. In the ‘Expert commentary’, the authors review the published literature on each protocol and offer our opinion. In the ‘Five-year view’, the authors suggest the direction that the management of poor responders is heading for autologous, as well as oocyte donation. The authors hope this will be a valuable resource to our colleagues for managing infertile patients with diminished ovarian reserve.
Keywords::
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertob; (4) view/print certificate.
Release date: 1 March 2013; Expiration date: 1 March 2014
Learning objectives
Upon completion of this activity, participants will be able to:
• Analyze risk factors for a poor response to IVF therapy
• Evaluate pre-cycle adjuvant treatment among poor responders to IVF
• Evaluate adjuvant treatment at the beginning of an IVF cycle among poor responders
• Distinguish the best time for embryo transfer among poor responders to IVF
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Publisher, Future Science Group, London, UK
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Charles Vega, MD
Associate Professor and Residency Director, Department of Family Medicine, University of California-Irvine, Irvine, CA, USA
Disclosure: Charles P Vega, MD, has disclosed no relevant financial relationships.
AUTHORS AND CREDENTIALS
Martin Keltz, MD, Director, Continuum Reproductive Center, St. Luke's-Roosevelt Hospital Center, Columbia University College of Physicians, New York, NY, USA
Disclosure: Martin Keltz, MD, has disclosed no relevant financial relationships.
May-Tal Sauerbrun-Cutler, MD, Continuum Reproductive Center, St. Luke's-Roosevelt Hospital Center, Columbia University College of Physicians, New York, NY, USA
Disclosure: May-Tal Sauerbrun-Cutler has disclosed no relevant financial relationships.
Andrzej Breborowicz, MD, PhD, Continuum Reproductive Center, St. Luke's-Roosevelt Hospital Center, Columbia University College of Physicians, New York, NY, USA
Disclosure: Andrzej Breborowicz, MD, PhD, has disclosed no relevant financial relationships.
E2: Estradiol; FSH: Follicle-stimulating hormone; hCG: Human chorionic gonadotropin;
hMG: Human menopausal gonadotropin; IU: International unit; q.o.d.: Every other day.
FSH: Follicle-stimulating hormone; hCG: Human chorionic gonadotropin;
IU: International units; OCP: Oral contraceptive pill.
The introduction of IVF in 1978 was hailed as a cure for tubal factor infertility. This new technology soon expanded to other diagnostic categories. This review will focus on the application of IVF in the group of patients with unexplained infertility associated with poor ovarian reserve and advancing reproductive age. A literature search utilizing Medline searching ‘poor responders’ and ‘diminished ovarian reserve’ from the 1980s onward was used for this review.
While IVF was attempted with unexplained infertility throughout the 1980s, success rates were far below 10% live birth rates (LBRs), no better than those achieved with human menopausal gonadotropins (hMG) and intrauterine insemination. Alternatives for unexplained infertility introduced in the 1980s such as gamete intrafallopian tube transfer improved pregnancy rates to nearly 30% in young patients; however, that success rate was limited to normal responders with at least six oocytes at laparoscopic retrieval Citation[1]. One alternative introduced in the mid-1980s at the University of California, Los Angeles Medical Center (CA, USA) was oocyte donation Citation[2]. While oocyte donation has become a highly successful option with greater than 50% LBR for poor responders with recurrent IVF failure, most patients are anxious to achieve a pregnancy with their own oocytes.
The definition of poor responders has varied throughout the medical literature Citation[3–5]. One very strict definition appears in clinical gynecologic endocrinology and infertility Citation[6]. This definition limited the category of poor responders to three or fewer oocytes or follicles and an estradiol (E2) level below 500 pg/ml. In several more recent studies of poor responders undergoing IVF, poor response cycles have been defined as fewer than five oocytes retrieved in high gonadotropin dose cycles (at least 450 international units [IU] of follicle-stimulating hormone [FSH]) with a peak E2 below 1000 pg/ml Citation[6–9]. Most literature on poor responders has utilized at least 450 IU of FSH, yet several studies in the 1990s confirmed no added benefit to gonadotropin dosing above 450 IU of hMG or FSH Citation[7,10,11]. While the exact definition of a poor response cycle or poor responder has somewhat varied in the literature, the challenge of relatively low pregnancy rates has persisted.
Predicting poor response cycles has become an essential part of patient evaluation prior to IVF. While ovarian reserve testing is an extensive topic beyond the scope of this review, it is necessary to briefly mention it. The first significant serum marker for poor ovarian reserve and diminished success in IVF included the basal (day 3) FSH level Citation[12,13] and the day 10 FSH level of the clomiphene citrate (CC) challenge test first published in 1987 Citation[14]. Adding a basal E2 level to the basal FSH in the mid-1990s eliminated the many false reassuring FSH results that were suppressed by a high basal E2 level, which is a poor prognosticator in its own right Citation[15–17]. More recently, ultrasound measurements of ovarian volume and basal antral follicle count (AFC) have proven accurate in predicting poor ovarian response. The recent addition of serum anti-Müllerian hormone (AMH) levels, a protein produced by preantral follicles that can be obtained at any time in the menstrual cycle, has furthered our ability to predict poor ovarian response to gonadotropins. The currently available literature indicates that AMH may be a superior marker for predicting ovarian response over either age of the patient, day 3 FSH, E2 or inhibin B, whereas the vast majority of studies have found that AMH and AFC have similar predictive value for the poor response Citation[17]. Managing poor response cycles, however, continues to present challenges for the reproductive endocrinologist. In this review, the authors will review the available options, including adjuvants prior to cycle starts, those used at the initiation of gonadotropin therapy, the available gonadotropin regimens and doses, as well as IVF laboratory options, in handling low oocyte yield cases.
Precycle adjuvants
Dehydroepiandrosterone
The use of dehydroepiandrosterone (DHEA) supplementation in assisted reproduction was first described in a case series by Casson et al. Citation[18] and a case report by Barad and Gleicher Citation[19]. A daily dose of 80 mg for 2 months resulted in improved peak E2 levels and number of follicles in the subsequent controlled ovarian hyperstimulation (COH) cycles. Since 2005, several studies have evaluated the role of DHEA supplementation in assisted reproduction. In a few retrospective studies, DHEA supplementation, with 25 mg three-times daily for 4 months prior to IVF, improved the number of fertilized embryos and their quality Citation[20]. DHEA supplementation also decreased the cancellation rate and improved the clinical pregnancy rate Citation[21]. A retrospective study by Gleicher et al. showed decreased miscarriage rates with DHEA supplementation in a population undergoing IVF Citation[22]. Another of their retrospective studies showed an increase in serum AMH following DHEA supplementation Citation[23]. DHEA supplementation for poor responders remains controversial as the majority of available data are derived from retrospective studies with limitations such as selection bias.
In a recently published randomized controlled trial, Weiser et al. showed significantly higher LBRs and improved embryo quality in the DHEA supplemented group Citation[24]. This study included only 33 subjects; therefore, larger randomized controlled trials are needed to adequately establish the role and value of DHEA supplementation.
The mechanism by which DHEA supplementation may increase oocyte yield in poor responders is unknown. Animal models support the theory that androgens might facilitate the response of the growing follicles to FSH. In humans, this may result in higher AFC in patients with higher ovarian androgen levels such as in patients with polycystic ovary syndrome. It can also be responsible for the exaggerated ovarian response to exogenous gonadotropins in COH. A recent paper by Fanchin et al. reviewed the potential role of androgens in improving IVF outcomes and provides up-to-date data regarding various androgen supplementation agents such as DHEA and testosterone Citation[25]. Other theories include an increase of IGF-1 during gonadotropin stimulation, and improved follicular steroidogenesis Citation[26]. In some studies, higher baseline testosterone levels were found to be associated with a higher number of oocytes retrieved and improved IVF outcomes Citation[27,28]. Older patients have been shown to have diminished levels of DHEAs and may benefit from achieving normal serum levels Citation[29]. It has also been postulated that DHEA supplementation reduces follicular apoptosis, thereby increasing the pool of primordial follicles Citation[30]. Further randomized trials are required to confirm the benefit and safety of DHEA supplementation.
Simple cyst drainage prior to stimulation protocols
One of the most significant side effects of pituitary downregulation with gonadotropin-releasing hormone (GnRH) agonists in COH protocols is ovarian cyst formation. The incidence of these cysts ranges from 8 to 53% Citation[31], probably due to the different size criteria used for their diagnosis. Formation of follicular cysts may be related to the endogenous gonadotropin flare in response to mid-luteal GnRH agonists. The impact of these ovarian cysts on IVF outcomes remains controversial. There is evidence of poor IVF performance in patients who form ovarian cysts in response to GnRH agonists in both poor responders and normal responders. The worst outcomes were found with hormonally inactive cysts greater than 15 mm in a receiver operating characteristic analysis Citation[32]. Other studies, however, failed to confirm the negative prognosis of follicular cyst formation on IVF outcome Citation[33,34].
Several studies have evaluated the effect of ovarian cyst aspiration on IVF outcomes. Most of them failed to show improved outcomes with precycle aspiration Citation[35–37]. While follicular cyst formation prior to IVF is probably a poor prognosticator, further studies are required to determine whether to proceed, wait or drain the cyst prior to the cycle.
The benefits & risk of precycle oral contraceptive pill or antagonist suppression combined with estrogen priming
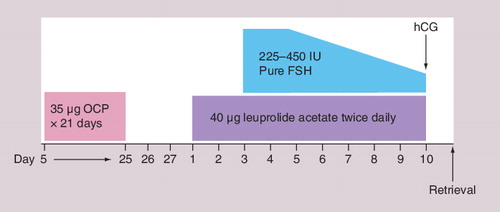
Oral contraceptive pills (OCPs) are commonly used in various IVF protocols. Patients with prior poor responses to ovarian stimulation may benefit from their use on multiple levels. OCP pretreatment in IVF protocols establishes an estrogenic environment and increases sex hormone-binding globulin levels while decreasing follicular androgen levels. The decreasing androgen levels may delay apoptosis. The progestin component of the OCP suppresses luteinizing hormone (LH) and may synchronize follicular development, leading to a more evenly sized follicular response. GnRH agonist flare cycles are commonly used in poor responders, and OCP pretreatment can prevent an early rise in progesterone by eliminating the corpus luteum. It is also believed that OCPs cause less pituitary downregulation when compared with GnRH agonists used in a long protocol.
Despite all these theoretical advantages of OCP use in IVF protocols for poor responders, numerous studies have failed to show any difference in IVF outcomes between patients pretreated with OCPs and those in whom OCPs were not used. Duvan et al. showed no difference in number of oocytes, peak E2 levels, endometrial thickness, fertilization rates and embryo quality between poor responders who underwent COH combined with microdose GnRH with or without pretreatment with OCPs Citation[38]. Kovacs et al. also failed to reveal any benefit of OCP pretreatment on IVF outcomes in patients with poor ovarian response Citation[39]. Similarly, Al-Mizyen et al. did not show any advantageous effect of pretreatment with medroxyprogesterone acetate or OCPs on IVF outcomes in GnRH flare protocol Citation[40]. In contrast to the above-mentioned studies, a study by Lindheim et al. reported that short-term pituitary suppression with OCPs in poor responders may be beneficial; however, OCPs were shown to significantly prolong stimulation and increase the dose of gonadotropins Citation[41]. Pituitary suppression with OCPs may blunt a GnRH agonist or letrozole flare response in follicular phase. The authors demonstrated, however, that while the peak FSH level following a GnRH agonist flare is lower following OCPs, the percent and total rise in FSH is not different with or without OCP pretreatment. In addition, premature elevations in progesterone were successfully blunted by the use of OCP pretreatment with a trend toward improved pregnancy rates Citation[42].
A more recent precycle adjuvant studied for poor responders is GnRH antagonists used in the late luteal phase prior to a stimulation cycle. Fanchin et al. showed that GnRH antagonists administered on day 25 of the menstrual cycle reduces size disparities among follicles Citation[43]. Several studies have confirmed an increased number of follicles and oocytes when using luteal-phase GnRH antagonists; however, these studies utilized historic controls Citation[44]. More recent studies have resulted in similar IVF outcomes when luteal E2/GnRH antagonists in GnRH antagonist protocols were compared with microdose GnRH agonist flare protocols Citation[45,46]. Several studies have found that luteal GnRH antagonists similar to OCPs increase the dose of gonadotropins required in subsequent COH cycles Citation[44,46–48]. Given the mixed results with GnRH antagonists in the luteal phase and their significantly higher cost, they are unlikely to replace precycle treatment with OCPs or E2.
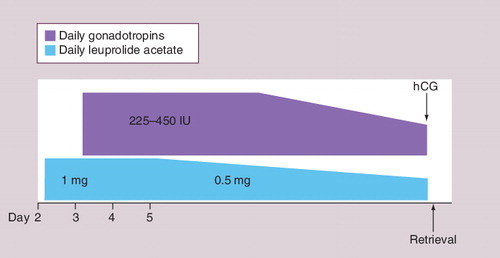
Treatment with estrogen in the luteal phase prior to COH may promote granulosa cell FSH receptor induction and suppress premature follicular development Citation[49–51]. The increased number of FSH receptors and improved response to FSH stimulation may promote oocyte maturation. Estrogen administered in the luteal phase was shown to prevent a luteal FSH rise, decreasing luteal follicular recruitment and a premature dominant follicle Citation[50,51]. Luteal estrogen administration may increase the cohort of early preantral follicles Citation[44,50,51]. Several studies have demonstrated decreased cancellation rates, and improved number of oocytes and embryos following a luteal phase E2 patch; however, improved pregnancy rates have not been confirmed Citation[48]. Concern with suppression from the precycle use of oral contraceptives along with the need to prevent an early dominant follicle in poor responder cycles has generated interest in the use of E2 in the luteal phase prior to COH cycles in IVF. Its exact role, mechanism and potential benefit still require further study.
illustrates a GnRH antagonist protocol with both antagonist and luteal E2 priming.
Adjuvants at the initiation of a cycle
GnRH analog flare protocols
The 24-h long surge in endogenous FSH and LH released when administering GnRH agonists in the early follicular phase represents one strategy to improve IVF outcomes and decrease the cancellation rate in poor responders. GnRH agonists are initiated in the follicular phase of a stimulation cycle shortly before commencing gonadotropin injections. This approach provides the advantage of a flare response to GnRH agonists, without prolonged ovarian suppression, which lowers the required dose of gonadotropins Citation[52]. Several studies evaluating GnRH agonist flare regimens in poor responders have provided conflicting results. Katayama et al. Citation[53] and Padilla et al. Citation[54] found improved cycle outcomes in poor responders with GnRH agonist flare regimens. However, Brzyski et al. raised the concern that flare regimens may increase the number of atretic follicles Citation[3]. These atretic follicles may result from the rescue of corpus luteum induced by the surge in pituitary LH in a flare cycle. The standard method of preventing corpus luteum rescue is pretreatment with OCPs; ‘delayed flare’ protocol represents a different approach, where GnRH agonist administration, which starts on day 4 of the COH cycle, provides a strategy to prevent rescue of the corpus luteum from the previous cycle. Reported clinical pregnancy rates with flare protocols in poor responders have ranged from 12.0 to 26.3% Citation[55,56]. In our retrospective study of the flare protocol in poor responders with or without OCP pretreatment Citation[57], the LBR was 32%, and our prospective trial showed similar outcomes of around 30% whether or not pretreatment with OCPs was employed Citation[42]. In summary, the success of GnRH agonist flare protocols for poor responders has led most IVF programs over the past two decades to abandon long GnRH agonist protocols in their poor responder patients .
GnRH agonist microdose flare protocols
The desire to decrease the suppressive effects of GnRH agonists during a flare protocol has prompted research on the use of the very low-dose ‘microdose’ GnRH agonist flare protocol. The ideal dose should be low enough to maintain some endogenous LH and FSH, yet high enough to generate a flare response early in the follicular phase and prevent an endogenous LH surge. It has been shown that a single dose of 25–50 µg of GnRH agonist given on cycle day 2 can increase both FSH and LH levels. The protocols utilizing minimal doses of GnRH agonists such as 20–50 µg twice daily (b.i.d.) for 2 days at the beginning of the cycle (microdose flare protocols) are usually combined with OCP pretreatment in the cycle prior to COH to prevent corpus luteum rescue Citation[58,59]. In retrospective studies, the use of microdose protocols in poor responders has shown improved cycle outcome with regards to the number of retrieved oocytes, percentage of patients undergoing embryo transfer, cancellation rates and pregnancy rates, when compared with traditional long GnRH agonist protocols Citation[5,59].
Agonist versus antagonist protocols
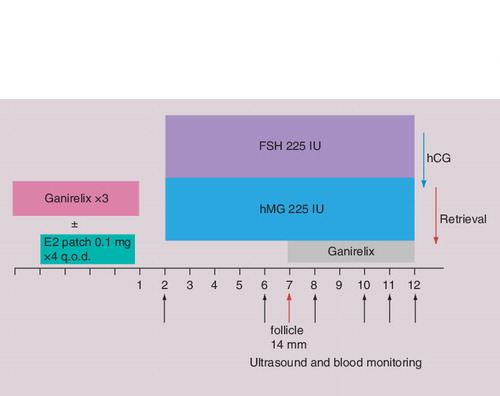
For more than 10 years GnRH antagonists have been available in IVF practice. GnRH antagonists prevent a premature LH surge without early suppression of follicular development. Most protocols commence GnRH antagonists when the mean diameter of the lead follicle reaches 14 mm and continue daily until human chorionic gonadotropin (hCG) administration. Their role in IVF protocols for poor responders has been well established. Multiple prospective randomized controlled trials compared the use of GnRH agonist flare protocols with GnRH antagonists in poor responders. A number of these trials showed no significant differences Citation[55,60,61]. One small trial displayed better outcomes when using GnRH agonist flare-up protocols in poor responders Citation[8]. In contrast, another larger randomized controlled trial on 270 subjects documented significantly higher ongoing pregnancy rates in patients treated with GnRH antagonists Citation[62]. In light of these conflicting results, a recent meta-analysis of 14 prospective randomized controlled trials by Pu et al. appears to be helpful in clinical practice Citation[63]. It documented a significant advantage of GnRH agonists over GnRH antagonists in COH for poor responders in respect to duration of stimulation. However, no significant difference was found in clinical pregnancy rates, cycle cancellation rate, number of oocytes retrieved or number of mature oocytes retrieved Citation[55,58]. The clinical issues that surround choosing an agonist flare versus an antagonist protocol include corpus luteum recovery and early rise in progesterone if a flare protocol is not pretreated with OCPs Citation[57]. For this reason, the authors generally employ flare protocols when using OCPs and antagonist protocols when not pretreating with OCPs.
Clomiphene flare protocols
A flare of endogenous gonadotropins can be also achieved with early CC administration. In these cycles, endogenous LH surge can be blocked with GnRH antagonists Citation[64]. CC flare was shown to reduce cancellation rates, increase the number of oocytes retrieved, and result in a higher implantation rate and pregnancy rates in poor responders. Similarly, another prospective study on 145 subjects showed higher E2 levels, lower cancellation rates and higher number of oocytes retrieved in the CC/GnRH antagonist protocol compared with a long GnRH agonist protocol Citation[65]. The use of a CC flare, however, raises concerns about endometrial suppression. CC is commonly and successfully used both in minimal stimulation protocols and for its flare effect in high-dose protocols in poor responders who are undergoing freeze-all cycles. Its potential for negative endometrial effects during a fresh transfer cycle necessitates further study.
Letrozole flare protocols
Letrozole is a highly selective aromatase inhibitor that has been used in assisted reproductive technology practice for at least a decade Citation[66]. By blocking estrogen synthesis and decreasing its circulating levels, letrozole exerts a flare effect on the pituitary with increased release of endogenous gonadotropins. In COH protocols, letrozole doses of 2.5–5.0 mg daily on day 3–7 of stimulation cycle have been combined with menotropins and a GnRH antagonist. A small randomized controlled trial on 38 poor responders demonstrated that the addition of letrozole in poor response cycles can decrease the total dose of FSH used when compared with a long GnRH agonist protocol Citation[9], while some retrospective studies have suggested an advantage of adding letrozole to antagonist cycles in poor responders Citation[67]. Further randomized controlled trials are therefore needed to assess its value. Letrozole flare for both high-dose and minimal-stimulation protocols has some advantages and advocates, but it is still under study.
Gonadotropins & their dosing for poor responders
Dose
A high starting gonadotropin dose in poor responders is widely practiced following a low response to a more standard dosing range of 150–300 IU of FSH. According to most authors, the starting dose for poor responders has been at least 300 IU/day, with a number of studies evaluating a daily dose of 450 IU of FSH Citation[68,69]. Retrospective studies in the mid-1990s by Hofmann et al., Manze et al. and Land et al. found no additional benefits in oocyte yield or pregnancy rates when the daily dose of FSH was greater than 450 IU Citation[7,10,70]. In a review of published prospective trials in 2003, Tarlatzis et al. found limited evidence for the efficacy of dose increases even to 450 IU of FSH Citation[69]. Studies cited included Cedrin-Durnerin et al.’s, a prospective study of 96 patients that found that starting with an initial dose of 450 IU in poor responders, stepping the dose down to 300 IU and eventually down to 150 IU was as effective as maintaining the standard maximum dose of 450 IU of FSH Citation[71]. In addition, van Hooff et al. documented that doubling the dose of FSH from 225 to 450 IU on cycle day 5 in poor-response cycles did not improve IVF pregnancy rates in a small prospective study of 46 patients Citation[72].
In their 2007 review of 29 clinical studies evaluating gonadotropin dose in IVF, Siristatidis et al. attempted to re-evaluate the maximal effective FSH dose in poor responders Citation[68]. They concluded that new prospective trials would be required to pinpoint the maximal effective dose. It remains difficult to conclude, based on the current literature, what the ideal starting and maintenance dose of FSH should be in poor responders. What appears clear from the literature is the finding that follicular recruitment occurs in the late luteal phase prior to menses and the early follicular phase. Therefore, maximal dosing, currently set at 450 IU of FSH, should be started as early in the cycle as possible. The dose may then be reduced once adequate response has been established.
hMG versus recombinant FSH
Recombinant FSH (rFSH) was introduced in the mid-1990s, and was initially touted to improve stimulation and pregnancy rates when compared with the already available equivalent dose of hMG. More recent trials have suggested that hMG may have advantages for poor responders undergoing IVF, although the data are limited Citation[73]. Mixed protocols of rFSH and hMG have become popular to provide both a high FSH dose as well as some LH activity, but there are no studies documenting any superiority of mixed protocols. In general, nearly all randomized prospective trials have documented equivalence without superiority of the myriad gonadotropins available on the market Citation[74,75].
The role of LH in stimulation protocols for poor responders
LH stimulation is available as both a recombinant LH (rLH; MD Serono, MA, USA) and in the standard urinary hMG, which contains a 1:1 ratio of FSH and LH activity. Additional LH activity during stimulation for IVF can be obtained with low doses of urinary or recombinant hCG. LH activity has been postulated to be beneficial during early follicular recruitment by increasing FSH receptors, as well as maintaining follicular development during later follicular maturation by maintaining steroid precursors. A dizzying array of articles have looked at the role of LH, both recombinant as well as mixed protocols with hMG Citation[76–80]. One reviewer has suggested that hMG, due to its LH activity, slightly increases the pregnancy rates in IVF when compared with rFSH alone Citation[73]. Most reviews and meta-analyses of the role of LH, however, have been unable to confirm any benefit or detriment of LH to IVF pregnancy or LBRs Citation[81].
The role of adding LH to rFSH stimulation in IVF is even less well studied in poor responders. With the introduction of GnRH antagonists for poor responders, it has become clear that antagonists blunt the rise in E2 during rFSH-stimulated cycles. This decreased rise in E2 can be overcome with either hMG or rLH;however, no advantage in IVF outcome has been established. However, Mochtar et al., who in their 2007 Cochrane Database review, did find no general advantage from adding rLH, they found a trend towards higher IVF pregnancy rates among a small set of poor responders when rLH was added to rFSH Citation[80]. In addition to poor responders, some authors have found a benefit to LH addition in stimulation protocols in older patients Citation[82]. Given the uncertain but widely suggested benefit of LH during stimulation with poor responders or older patients, the authors currently ride with the herd, utilizing mixed protocols of rFSH and hMG for our poor-responder patients.
Dosing intervals for poor responders
It has become common to offer b.i.d. dosing of hMG or FSH in poor responders. When using mixed protocols of hMG from vials and rFSH from a pen, the authors often recommend twice-daily injections as two injections are required regardless. Pharmacokinetic studies have suggested that a steady state is obtained after 2 days of injections, offsetting any theoretical benefit from twice-daily dosing Citation[83]. Despite no documented benefit from b.i.d. dosing, it continues to be popular, especially for mixed protocols in poor responders.
Minimal stimulation
The role of minimal stimulation protocols to produce only a few oocytes per retrieval has been suggested for both normal and poor responders. Minimal stimulation is particularly appropriate for study in poor responders, as a high dose of gonadotropins often produces no greater oocyte yield than a far less expensive cycle with oral medication or low-dose gonadotropins. Many studies have suggested that for poor responders, natural cycle IVF or IVF with minimal stimulation produces similar pregnancy rates as with full-dose gonadotropins Citation[84–87]. A significant problem with natural cycle IVF is the high cancelation rate; however, use of GnRH antagonists in the late follicular phase has shown promising results Citation[87].
In two studies including poor responders, similar pregnancy rates were found following minimal stimulation IVF when compared with full-dose gonadotropins with IVF Citation[86,87], despite a lower number of oocytes retrieved and embryos transferred. To decrease the rate of cycle cancelation and premature LH surge, one study included supplementation with GnRH antagonists and hMG, and the other administered 0.25 mg of GnRH antagonists and 75 IU rFSH daily until ovulation induction Citation[86]. For poor responders, who already have a very low oocyte yield, minimal gonadotropin stimulation is particularly attractive.
Natural, clomiphene or letrozole only IVF
There are a limited number of studies comparing natural cycle IVF with full-dose gonadotropins in poor responders. A meta-analysis of 1800 cycles of natural IVF in all types of infertility patients resulted in a 7.2% ongoing pregnancy rate per cycle and a 15.8% ongoing pregnancy rate per transfer. For normal responders, these ongoing pregnancy rates are unacceptably low. When limited to poor responders, a few studies showed promising results. One randomized trial showed that poor responders treated with natural cycles and intracytoplasmic sperm injection (ICSI) had higher implantation rates but similar clinical pregnancy rates per cycle and per transfer as patients treated with full-dose gonadotropins Citation[84]. The improved implantation rate with natural cycles may be attributed to more physiologic follicular development and recruitment improving both the quality of the oocyte and endometrium Citation[88]. Similarly, in a retrospective study, Schimberni et al. documented a 9.8% pregnancy rate per cycle, 17.1% per transfer and 16.7% per patient Citation[85]. While these rates may be comparable to pregnancy rates for very poor responders undergoing gonadotropin stimulation, our retrospective studies on poor responders found pregnancy rates close to 30% in subjects aged 40 years and under.
To increase the oocyte yield while maintaining minimal cost, several programs and investigators have utilized CC for IVF stimulation. In several randomized trials, CC stimulation alone was far more successful than natural-cycle IVF. In normal responders, CC alone increased the per cycle pregnancy rate from 0 to 4% in a natural-cycle IVF to 18% with CC Citation[89,90]. Another study found that in normal responders, CC–IVF can produce a 28% pregnancy rate and in poor responders undergoing CC–IVF, a pregnancy rate of 10% can be achieved, which is equivalent to the full-dose gonadotropins in their subjects Citation[91,92]. Given the clear advantage of CC over natural-cycle IVF demonstrated in normal responders, CC would also likely be advantageous in poor responders.
Letrozole has been studied as an adjuvant in full-dose gonadotropin IVF cycles as we have discussed earlier in this chapter. Letrozole has also been looked at as an adjuvant in minimal stimulation cycles employing a low dose of gonadotropins. Goswami et al. found that letrozole plus low-dose FSH was as effective as high-dose FSH in a prospective randomized trial of poor responders undergoing IVF Citation[9]. No studies are published, however, that assess minimal stimulation IVF using letrozole alone. Minimal stimulation protocols with and without gonadotropins continue to be an important area of clinical investigation for poor responders.
Laboratory options in poor response cycles
ICSI for low oocyte yield
Poor responders typically produce fewer than five oocytes per retrieval, which has been shown by some authors to increase the likelihood of fertilization failure Citation[88,93]. In some studies, ICSI has been successfully utilized in non-male factor infertility patients to provide higher fertilization rates than standard IVF. In a larger prospective study, despite showing no clinical advantage of ICSI in terms of implantation or pregnancy rates, the use of ICSI did affect overall fertilization rates. ICSI was associated with a higher fertilization rate per oocyte inseminated. Therefore, in couples with poor ovarian response and fertilization failure, ICSI could have a potential advantage over IVF Citation[94]. However, in the limited number of prospective studies Citation[95,96], ICSI provided similar fertilization, pregnancy and implantation rates as conventional IVF in low responder couples without a male factor. It is likely that most oocyte defects in poor responders will not be overcome by ICSI Citation[96]. Despite the lack of benefit of ICSI in limited prospective studies for poor responders, it is our practice to perform ICSI on mature-appearing oocytes when fewer than five oocytes are retrieved. If either the oocytes appear immature, or the mature-appearing oocyte is immature after stripping, the authors employ standard IVF for the remaining oocytes. An oocyte is considered immature when it is surrounded by corona radiata with a less dense cumulus oophorus.
Preimplantation genetic screening for poor responders
Couples are at an increased risk of developing chromosomally abnormal embryos after unexplained repeated IVF failures or advanced maternal age Citation[97]. Therefore, preimplantation genetic screening (PGS) utilizing FISH has been studied for patients with advanced maternal age, repeated miscarriage, repeated implantation failure and severe male factor infertility to select euploid embryos for implantation. Despite the hope that PGS would improve the LBR, clinical trials utilizing FISH technology decreased the LBRs in women of advanced reproductive age and poor responders, either due to damage to the embryos or inaccuracy of the testing. Newer methods such as comparative genomic hybridization (CGH) have been introduced with the ability to analyze the entire genome. Schoolcraft et al. performed trophectoderm biopsies utilizing CGH for chromosomal analysis with the plan to transfer only euploid embryos after vitrification Citation[98]. In comparison to a matched controlled group, there was an increased implantation rate; however, there was no increase in the delivery rates. Future randomized controlled trials are necessary to determine whether CGH can be utilized to increase delivery rates in poor responders. In addition, cleavage-stage biopsy with fresh transfer versus trophectoderm biopsy of blastocysts with future frozen embryo transfer (FET) needs to be compared. It is likely that poor responders will only be able to access cleavage-stage biopsy given their low embryo number and poor blastulation rate. The role for cleavage-stage PGS in poor responders will likely be limited to younger patients seeking single-embryo transfer.
Ideal day of transfer
The preferred day for embryo transfer has undergone a transformation in IVF over the past two decades, initially moving from day 2 to day 3 in the mid-1990s. Over the last two decades, many programs have moved to day 5, blastocyst transfer. Several randomized trials have confirmed that day 5 transfer yields similar pregnancy rates in good responders, despite transferring fewer embryos Citation[99]. The role of day 5 embryo transfer for poor responders is less clear. In fact, studies on poor responders have shown some improvement in IVF clinical outcome Citation[100,101] or no difference Citation[102,103] by moving embryo transfer back to day 2 versus day 3 postretrieval. Extending in vitro culture allows the selection of embryos with higher implantation potential. However, embryos from poor responders are prone to cleavage arrest and prolonged exposure to the deleterious effects of in vitro conditions may induce damage Citation[104].
In one prospective controlled trial, 281 women with five or fewer follicles were randomized to day 2 versus day 3 transfer. The clinical pregnancy rates per oocyte retrieval (37.2 vs 21.4%, respectively; p < 0.05) and per embryo transfer (38.9 vs 24.1%, respectively; p < 0.05) were significantly higher in the day 2 embryo transfer group compared with day 3. In addition, the results showed diminished embryo quality with embryos incubated until day 3 Citation[101]. Therefore, in poor responders where fewer oocytes and embryos are available for transfers, transferring embryos at an earlier cleavage stage appears to be beneficial. As a general rule, once the number of viable embryos is equal to the number planned to be transferred, further delay of transfer is likely to be more harmful than beneficial.
Number of embryos for transfer
There are limited prospective studies available to evaluate the optimal number of embryos to transfer in poor responders. However, Stern et al. described an algorithm to determine the maximum number of day 3 embryos to transfer in women aged 38 years and above to produce an acceptable reduction in multiple pregnancy rates without a significant reduction in LBR Citation[105]. In total, 36,103 transfers of cleavage-stage embryos were obtained from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System national database, and the analysis revealed that after age, the most important factors predictive of delivery were number of oocytes retrieved and the availability of embryos for cryopreservation. Therefore, in addition to advanced reproductive age, poor ovarian reserve and poor response may be an indication for increasing the number of embryos to transfer. We currently follow the guideline provided by American Society for Reproductive Medicine in 2009 for the number of embryos to transfer Citation[106]. These guidelines for cleavage-stage embryos are age based and suggest up to two embryos in women aged less than 35 years, up to three embryos in women aged less than 38 years, and up to four embryos in women aged less than 41 years. Even in poor responders, there is rarely an indication to supersede these guidelines.
Donor oocytes
The mainstay of management for poor responders with repeated IVF failure is oocyte donation. In 2009 reported data, the success rate of fresh oocyte donation (live births) was >50%, including all programs in the USA. It has been our policy to recommend poor responders with multiple failed IVF cycles, or those with a very poor prognosis prior to IVF, to consider oocyte donation IVF only. Our very poor prognosis group includes nearly all patients over 43 years of age, or any patient with an FSH over 15 mIU/ml, or an undetectable AMH.
Expert commentary
Managing poor responders undergoing IVF continues to be challenging, despite its increasing prevalence in IVF practice. We have reviewed the available literature addressing precycle adjuvants, early follicular-phase adjuvants, gonadotropin-stimulation protocols and finally minimal stimulation alternatives to gonadotropin-stimulation protocols intended to maximize the yield of viable oocytes. We also discuss the laboratory and transfer management of poor-response cycles. With careful management, poor responders can achieve reasonable pregnancy rates of up to 30%, for women up to 40 years of age. Given the high rate of aneuploidy in the embryos of poor responders of all ages, the use of PGS in these cases is quite appealing. The PGS could be performed in FET cycles after accumulating sufficient embryos in freeze-all embryo cycles. The problem with PGS is that it continues to be cost-prohibitive for most of our patients, as it is not generally covered by insurance. Poor-response cycles will continue to challenge the reproductive endocrinologist until a scientific breakthrough achieves the ability to replenish the ever decreasing ovarian reserve with age.
Five-year view
There are a number of newer procedures in assisted reproduction that may find a role in the management of poor responders. These include in vitro maturation (IVM), cryopreservation of oocytes or embryos from multiple retrieval cycles, as well as cryopreservation of donor oocytes allowing for commercial egg banking as we have in sperm banking. Over the next 5 years, some of the above options will become standard care in assisted reproduction, while others will no doubt be discarded when efficacy cannot be established.
IVM of human oocytes is a new procedure. Approximately 1100 cycles have been performed as of 2006 Citation[107]. Oocytes are retrieved at 8–12-mm size after either priming with a few days of FSH injections followed by hCG, or during a natural cycle followed by a hCG injection. The immature retrieved oocytes are then matured in vitro in special media. While IVM has been proposed for patients with polycystic ovary syndrome at high risk of ovarian hyperstimulation syndrome, and cancer patients who may not undergo standard stimulation, a few case reports have described success using IVM in poor responders. Liu et al. described eight patients with poor response to stimulated IVF cycles who underwent IVM as an alternative to cycle cancellation with three ongoing pregnancies Citation[108]. Li et al. has reported three successful cases of IVF for poor-responder patients averaging fewer than three oocytes per retrieval Citation[109]. While there are limited data on the use of IVM for poor responders, it may aid in cases that otherwise would have been cancelled.
The accumulation of oocytes from several ovarian-stimulation cycles or freeze-all cycles creates a similar situation as in normal responders. The freeze-all cycles are where oocytes are accumulated in multiple controlled ovarian-stimulation cycles. The patient undergoes a FET once they have reached an optimal number of oocytes. A prospective study by Cobo et al. suggested that the cumulative LBR per patient was higher in the freeze-all (36.4%) than the fresh group (23.7%) in a group of poor responders Citation[110]. The same strategy can be used for cryopreservation of embryos and FET.
The use of cryo-banked oocytes in an ovum donation program may be a realistic option in the future, significantly increasing the efficiency of the donor process. Currently, the process of synchronizing the recipient with the donor has some drawbacks, including waiting lists and coordinating recipient schedule to the timing of the donor. A recent randomized clinical trial confirmed the effectiveness of oocyte cryostorage in an ovum donation program, proving that vitrified oocytes were not inferior to fresh oocytes. The ongoing pregnancy rate per intention to treat was 43.7 and 41.7% in the vitrification and fresh groups, respectively Citation[111]. Over the next 5 years, we believe the use of cryopreservation of autologous oocytes and embryo for poor responders, as well as donor oocyte cryopreservation, will become mainstays in the management of poor responders.
Key issues
• Poor responders have significantly lower clinical and live birth rates per age group in IVF.
• Increasing the gonadotropin dose is only effective up to 450 international units of follicle-stimulating hormone.
• Avoiding luteal gonadotropin-releasing hormone agonist or protocols using oral contraceptive pill suppression in favor of gonadotropin-releasing hormone flare and antagonist protocols have become standard for poor responders.
• Early transfer of day 2–3 embryos may be advantageous for poor responders.
• Precycle adjuvants, such as dehydroepiandrosterone, estrogen priming, clomiphene and letrozole flare protocols, may be beneficial but need further study.
• Oocyte donation remains the best option for poor responders with repeat IVF failure.
References
- Asch RH, Ellsworth LR, Balmaceda JP, Wong PC. Pregnancy after translaparoscopic gamete intrafallopian transfer. Lancet 2(8410), 1034–1035 (1984).
- Buster JE, Bustillo M, Thorneycroft IH et al. Non-surgical transfer of in vivo fertilised donated ova to five infertile women: report of two pregnancies. Lancet 2(8343), 223–224 (1983).
- Brzyski RG, Muasher SJ, Droesch K, Simonetti S, Jones GS, Rosenwaks Z. Follicular atresia associated with concurrent initiation of gonadotropin-releasing hormone agonist and follicle-stimulating hormone for oocyte recruitment. Fertil. Steril. 50(6), 917–921 (1988).
- Schoolcraft W, Schlenker T, Gee M, Stevens J, Wagley L. Improved controlled ovarian hyperstimulation in poor responder in vitro fertilization patients with a microdose follicle-stimulating hormone flare, growth hormone protocol. Fertil. Steril. 67(1), 93–97 (1997).
- Surrey ES, Bower J, Hill DM, Ramsey J, Surrey MW. Clinical and endocrine effects of a microdose GnRH agonist flare regimen administered to poor responders who are undergoing in vitro fertilization. Fertil. Steril. 69(3), 419–424 (1998).
- Fritz MA, Speroff L. Clinical Gynecologic Endocrinology and Infertility. Wolters Kluwer Health/Lippincott Williams & Wilkins, PA, USA (2011).
- Land JA, Yarmolinskaya MI, Dumoulin JC, Evers JL. High-dose human menopausal gonadotropin stimulation in poor responders does not improve in vitro fertilization outcome. Fertil. Steril. 65(5), 961–965 (1996).
- Malmusi S, La Marca A, Giulini S et al. Comparison of a gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist flare-up regimen in poor responders undergoing ovarian stimulation. Fertil. Steril. 84(2), 402–406 (2005).
- Goswami SK, Das T, Chattopadhyay R et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum. Reprod. 19(9), 2031–2035 (2004).
- Manzi DL, Thornton KL, Scott LB, Nulsen JC. The value of increasing the dose of human menopausal gonadotropins in women who initially demonstrate a poor response. Fertil. Steril. 62(2), 251–256 (1994).
- Stadtmauer L, Ditkoff EC, Session D, Kelly A. High dosages of gonadotropins are associated with poor pregnancy outcomes after in vitro fertilization–embryo transfer. Fertil. Steril. 61(6), 1058–1064 (1994).
- Droesch K, Muasher SJ, Brzyski RG et al. Value of suppression with a gonadotropin-releasing hormone agonist prior to gonadotropin stimulation for in vitro fertilization. Fertil. Steril. 51(2), 292–297 (1989).
- Feldberg D, Farhi J, Ashkenazi J, Dicker D, Shalev J, Ben-Rafael Z. Minidose gonadotropin-releasing hormone agonist is the treatment of choice in poor responders with high follicle-stimulating hormone levels. Fertil. Steril. 62(2), 343–346 (1994).
- Navot D, Rosenwaks Z, Margalioth EJ. Prognostic assessment of female fecundity. Lancet 2(8560), 645–647 (1987).
- Smotrich DB, Widra EA, Gindoff PR, Levy MJ, Hall JL, Stillman RJ. Prognostic value of day 3 estradiol on in vitro fertilization outcome. Fertil. Steril. 64(6), 1136–1140 (1995).
- Evers JL, Slaats P, Land JA, Dumoulin JC, Dunselman GA. Elevated levels of basal estradiol-17β predict poor response in patients with normal basal levels of follicle-stimulating hormone undergoing in vitro fertilization. Fertil. Steril. 69(6), 1010–1014 (1998).
- Licciardi FL, Liu HC, Rosenwaks Z. Day 3 estradiol serum concentrations as prognosticators of ovarian stimulation response and pregnancy outcome in patients undergoing in vitro fertilization. Fertil. Steril. 64(5), 991–994 (1995).
- Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum. Reprod. 15(10), 2129–2132 (2000).
- Barad DH, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. Fertil. Steril. 84(3), 756 (2005).
- Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum. Reprod. 21(11), 2845–2849 (2006).
- Barad D, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J. Assist. Reprod. Genet. 24(12), 629–634 (2007).
- Gleicher N, Ryan E, Weghofer A, Blanco-Mejia S, Barad DH. Miscarriage rates after dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve: a case–control study. Reprod. Biol. Endocrinol. 7, 108 (2009).
- Gleicher N, Weghofer A, Barad DH. Improvement in diminished ovarian reserve after dehydroepiandrosterone supplementation. Reprod. Biomed. Online 21(3), 360–365 (2010).
- Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum. Reprod. 25(10), 2496–2500 (2010).
- Fanchin R, Frydman N, Even M, Berwanger da Silva AL, Grynberg M, Ayoubi JM. Androgens and poor responders: are we ready to take the plunge into clinical therapy? Fertil. Steril. 96(5), 1062–1065 (2011).
- Casson PR, Santoro N, Elkind-Hirsch K et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil. Steril. 70(1), 107–110 (1998).
- Collins JA, Wrixon W, Janes LB, Wilson EH. Treatment-independent pregnancy among infertile couples. N. Engl. J. Med. 309(20), 1201–1206 (1983).
- Karande V, Gleicher N. A rational approach to the management of low responders in in-vitro fertilization. Hum. Reprod. 14(7), 1744–1748 (1999).
- Harper AJ, Buster JE, Casson PR. Changes in adrenocortical function with aging and therapeutic implications. Semin. Reprod. Endocrinol. 17(4), 327–338 (1999).
- Krysko DV, Diez-Fraile A, Criel G, Svistunov AA, Vandenabeele P, D’Herde K. Life and death of female gametes during oogenesis and folliculogenesis. Apoptosis 13(9), 1065–1087 (2008).
- Liu HC, Lai YM, Davis O et al. Improved pregnancy outcome with gonadotropin releasing hormone agonist (GnRH-a) stimulation is due to the improvement in oocyte quantity rather than quality. J. Assist. Reprod. Genet. 9(4), 338–344 (1992).
- Keltz MD, Jones EE, Duleba AJ, Polcz T, Kennedy K, Olive DL. Baseline cyst formation after luteal phase gonadotropin-releasing hormone agonist administration is linked to poor in vitro fertilization outcome. Fertil. Steril. 64(3), 568–572 (1995).
- Herman A, Ron-El R, Golan A, Nahum H, Soffer Y, Caspi E. Follicle cysts after menstrual versus midluteal administration of gonadotropin-releasing hormone analog in in vitro fertilization. Fertil. Steril. 53(5), 854–858 (1990).
- Owj M, Ashrafi M, Baghestani AR. Ovarian cyst formation and in vitro fertilization outcome. Int. J. Gynaecol. Obstet. 87(3), 258–259 (2004).
- Qublan HS, Amarin Z, Tahat YA, Smadi AZ, Kilani M. Ovarian cyst formation following GnRH agonist administration in IVF cycles: incidence and impact. Hum. Reprod. 21(3), 640–644 (2006).
- Rizk B, Tan SL, Kingsland C, Steer C, Mason BA, Campbell S. Ovarian cyst aspiration and the outcome of in vitro fertilization. Fertil. Steril. 54(4), 661–664 (1990).
- Segal S, Shifren JL, Isaacson KB et al. Effect of a baseline ovarian cyst on the outcome of in vitro fertilization–embryo transfer. Fertil. Steril. 71(2), 274–277 (1999).
- Duvan CI, Berker B, Turhan NO, Satiroglu H. Oral contraceptive pretreatment does not improve outcome in microdose gonadotrophin-releasing hormone agonist protocol among poor responder intracytoplasmic sperm injection patients. J. Assist. Reprod. Genet. 25(2–3), 89–93 (2008).
- Kovacs P, Barg PE, Witt BR. Hypothalamic–pituitary suppression with oral contraceptive pills does not improve outcome in poor responder patients undergoing in vitro fertilization–embryo transfer cycles. J. Assist. Reprod. Genet. 18(7), 391–394 (2001).
- Al-Mizyen E, Sabatini L, Lower AM, Wilson CM, al-Shawaf T, Grudzinskas JG. Does pretreatment with progestogen or oral contraceptive pills in low responders followed by the GnRHa flare protocol improve the outcome of IVF–ET? J. Assist. Reprod. Genet. 17(3), 140–146 (2000).
- Lindheim SR, Barad DH, Witt B, Ditkoff E, Sauer MV. Short-term gonadotropin suppression with oral contraceptives benefits poor responders prior to controlled ovarian hyperstimulation. J. Assist. Reprod. Genet. 13(9), 745–747 (1996).
- Keltz MD, Gera PS, Skorupski J, Stein DE. Comparison of FSH flare with and without pretreatment with oral contraceptive pills in poor responders undergoing in vitro fertilization. Fertil. Steril. 88(2), 350–353 (2007).
- Fanchin R, Castelo Branco A, Kadoch IJ, Hosny G, Bagirova M, Frydman R. Premenstrual administration of gonadotropin-releasing hormone antagonist coordinates early antral follicle sizes and sets up the basis for an innovative concept of controlled ovarian hyperstimulation. Fertil. Steril. 81(6), 1554–1559 (2004).
- Dragisic KG, Davis OK, Fasouliotis SJ, Rosenwaks Z. Use of a luteal estradiol patch and a gonadotropin-releasing hormone antagonist suppression protocol before gonadotropin stimulation for in vitro fertilization in poor responders. Fertil. Steril. 84(4), 1023–1026 (2005).
- Ata B, Zeng X, Son WY, Holzer H, Tan SL. Follicular synchronization using transdermal estradiol patch and GnRH antagonists in the luteal phase; does it increase oocyte yield in poor responders to gonadotropin stimulation for in vitro fertilization (IVF)? A comparative study with microdose flare-up protocol. Gynecol. Endocrinol. 27(11), 876–879 (2011).
- Weitzman VN, Engmann L, DiLuigi A, Maier D, Nulsen J, Benadiva C. Comparison of luteal estradiol patch and gonadotropin-releasing hormone antagonist suppression protocol before gonadotropin stimulation versus microdose gonadotropin-releasing hormone agonist protocol for patients with a history of poor in vitro fertilization outcomes. Fertil. Steril. 92(1), 226–230 (2009).
- Elassar A, Engmann L, Nulsen J, Benadiva C. Letrozole and gonadotropins versus luteal estradiol and gonadotropin-releasing hormone antagonist protocol in women with a prior low response to ovarian stimulation. Fertil. Steril. 95(7), 2330–2334 (2011).
- Shastri SM, Barbieri E, Kligman I, Schoyer KD, Davis OK, Rosenwaks Z. Stimulation of the young poor responder: comparison of the luteal estradiol/gonadotropin-releasing hormone antagonist priming protocol versus oral contraceptive microdose leuprolide. Fertil. Steril. 95(2), 592–595 (2011).
- Clark JR, Dierschke DJ, Wolf RC. Hormonal regulation of ovarian folliculogenesis in rhesus monkeys: III. Atresia of the preovulatory follicle induced by exogenous steroids and subsequent follicular development. Biol. Reprod. 25(2), 332–341 (1981).
- Fanchin R, Cunha-Filho JS, Schonäuer LM, Kadoch IJ, Cohen-Bacri P, Frydman R. Coordination of early antral follicles by luteal estradiol administration provides a basis for alternative controlled ovarian hyperstimulation regimens. Fertil. Steril. 79(2), 316–321 (2003).
- Fanchin R, Salomon L, Castelo-Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol pre-treatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Hum. Reprod. 18(12), 2698–2703 (2003).
- Garcia JE, Padilla SL, Bayati J, Baramki TA. Follicular phase gonadotropin-releasing hormone agonist and human gonadotropins: a better alternative for ovulation induction in in vitro fertilization. Fertil. Steril. 53(2), 302–305 (1990).
- Katayama KP, Roesler M, Gunnarson C, Stehlik E, Jagusch S. Short-term use of gonadotropin-releasing hormone agonist (leuprolide) for in vitro fertilization. J. in vitro Fert. Embryo Transf. 5(6), 332–334 (1988).
- Padilla SL, Dugan K, Maruschak V, Shalika S, Smith RD. Use of the flare-up protocol with high dose human follicle stimulating hormone and human menopausal gonadotropins for in vitro fertilization in poor responders. Fertil. Steril. 65(4), 796–799 (1996).
- Akman MA, Erden HF, Tosun SB, Bayazit N, Aksoy E, Bahceci M. Comparison of agonistic flare-up-protocol and antagonistic multiple dose protocol in ovarian stimulation of poor responders: results of a prospective randomized trial. Hum. Reprod. 16(5), 868–870 (2001).
- Fasouliotis SJ, Laufer N, Sabbagh-Ehrlich S, Lewin A, Hurwitz A, Simon A. Gonadotropin-releasing hormone (GnRH)-antagonist versus GnRH-agonist in ovarian stimulation of poor responders undergoing IVF. J. Assist. Reprod. Genet. 20(11), 455–460 (2003).
- Berin I, Stein DE, Keltz MD. A comparison of gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist flare protocols for poor responders undergoing in vitro fertilization. Fertil. Steril. 93(2), 360–363 (2010).
- Kahraman K, Berker B, Atabekoglu CS et al. Microdose gonadotropin-releasing hormone agonist flare-up protocol versus multiple dose gonadotropin-releasing hormone antagonist protocol in poor responders undergoing intracytoplasmic sperm injection-embryo transfer cycle. Fertil. Steril. 91(6), 2437–2444 (2009).
- Scott RT, Navot D. Enhancement of ovarian responsiveness with microdoses of gonadotropin-releasing hormone agonist during ovulation induction for in vitro fertilization. Fertil. Steril. 61(5), 880–885 (1994).
- Olivennes F, Mannaerts B, Struijs M, Bonduelle M, Devroey P. Perinatal outcome of pregnancy after GnRH antagonist (ganirelix) treatment during ovarian stimulation for conventional IVF or ICSI: a preliminary report. Hum. Reprod. 16(8), 1588–1591 (2001).
- Schmidt DW, Bremner T, Orris JJ, Maier DB, Benadiva CA, Nulsen JC. A randomized prospective study of microdose leuprolide versus ganirelix in in vitro fertilization cycles for poor responders. Fertil. Steril. 83(5), 1568–1571 (2005).
- Lainas TG, Sfontouris IA, Papanikolaou EG et al. Flexible GnRH antagonist versus flare-up GnRH agonist protocol in poor responders treated by IVF: a randomized controlled trial. Hum. Reprod. 23(6), 1355–1358 (2008).
- Pu D, Wu J, Liu J. Comparisons of GnRH antagonist versus GnRH agonist protocol in poor ovarian responders undergoing IVF. Hum. Reprod. 26(10), 2742–2749 (2011).
- Craft I, Gorgy A, Hill J, Menon D, Podsiadly B. Will GnRH antagonists provide new hope for patients considered ‘difficult responders’ to GnRH agonist protocols? Hum. Reprod. 14(12), 2959–2962 (1999).
- D’Amato G, Caroppo E, Pasquadibisceglie A, Carone D, Vitti A, Vizziello GM. A novel protocol of ovulation induction with delayed gonadotropin-releasing hormone antagonist administration combined with high-dose recombinant follicle-stimulating hormone and clomiphene citrate for poor responders and women over 35 years. Fertil. Steril. 81(6), 1572–1577 (2004).
- Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil. Steril. 75(2), 305–309 (2001).
- Garcia-Velasco JA, Moreno L, Pacheco A et al. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil. Steril. 84(1), 82–87 (2005).
- Siristatidis CS, Hamilton MP. What should be the maximum FSH dose in IVF/ICSI in poor responders? J. Obstet. Gynaecol. 27(4), 401–405 (2007).
- Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum. Reprod. Update 9(1), 61–76 (2003).
- Hofmann GE, Toner JP, Muasher SJ, Jones GS. High-dose follicle-stimulating hormone (FSH) ovarian stimulation in low-responder patients for in vitro fertilization. J. In Vitro Fert. Embryo Transf. 6(5), 285–289 (1989).
- Cedrin-Durnerin I, Bständig B, Hervé F, Wolf J, Uzan M, Hugues J. A comparative study of high fixed-dose and decremental-dose regimens of gonadotropins in a minidose gonadotropin-releasing hormone agonist flare protocol for poor responders. Fertil. Steril. 73(5), 1055–1056 (2000).
- van Hooff MH, Alberda AT, Huisman GJ, Zeilmaker GH, Leerentveld RA. Doubling the human menopausal gonadotrophin dose in the course of an in-vitro fertilization treatment cycle in low responders: a randomized study. Hum. Reprod. 8(3), 369–373 (1993).
- Venetis CA, Kolibianakis EM, Tarlatzi TB, Tarlatzis BC. Benefits of luteinizing hormone activity in ovarian stimulation for IVF. Reprod. Biomed. Online 18(Suppl. 2), 31–36 (2009).
- Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum. Reprod. 21(12), 3217–3227 (2006).
- Al-Inany HG, Abou-Setta AM, Aboulghar MA, Mansour RT, Serour GI. Highly purified hMG achieves better pregnancy rates in IVF cycles but not ICSI cycles compared with recombinant FSH: a meta-analysis. Gynecol. Endocrinol. 25(6), 372–378 (2009).
- Barrenetxea G, Agirregoikoa JA, Jiménez MR, de Larruzea AL, Ganzabal T, Carbonero K. Ovarian response and pregnancy outcome in poor-responder women: a randomized controlled trial on the effect of luteinizing hormone supplementation on in vitro fertilization cycles. Fertil. Steril. 89(3), 546–553 (2008).
- Chung K, Krey L, Katz J, Noyes N. Evaluating the role of exogenous luteinizing hormone in poor responders undergoing in vitro fertilization with gonadotropin-releasing hormone antagonists. Fertil. Steril. 84(2), 313–318 (2005).
- De Placido G, Alviggi C, Perino A et al.; Italian Collaborative Group on Recombinant Human Luteinizing Hormone. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Hum. Reprod. 20(2), 390–396 (2005).
- Ferraretti AP, Gianaroli L, Magli MC, D’angelo A, Farfalli V, Montanaro N. Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil. Steril. 82(6), 1521–1526 (2004).
- Mochtar MH, Van der Veen, Ziech M, van Wely M. Recombinant luteinizing hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database Syst. Rev. 2, CD005070 (2007).
- Kolibianakis EM, Collins J, Tarlatzis B, Papanikolaou E, Devroey P. Are endogenous LH levels during ovarian stimulation for IVF using GnRH analogues associated with the probability of ongoing pregnancy? A systematic review. Hum. Reprod. Update 12(1), 3–12 (2006).
- Lisi F, Rinaldi L, Fishel S, Lisi R, Pepe G, Picconeri MG. Use of recombinant follicle-stimulating hormone (Gonal F) and recombinant luteinizing hormone (Luveris) for multiple follicular stimulation in patients with a suboptimal response to in vitro fertilization. Fertil. Steril. 79(4), 1037–1038 (2003).
- Seo KS, Yoon JW, Na KH et al. Evaluation of process efficiency and bioequivalence of biosimilar recombinant human chorionic gonadotropin (rhCG). BioDrugs 25(2), 115–127 (2011).
- Morgia F, Sbracia M, Schimberni M et al. A controlled trial of natural cycle versus microdose gonadotropin-releasing hormone analog flare cycles in poor responders undergoing in vitro fertilization. Fertil. Steril. 81(6), 1542–1547 (2004).
- Schimberni M, Morgia F, Colabianchi J et al. Natural-cycle in vitro fertilization in poor responder patients: a survey of 500 consecutive cycles. Fertil. Steril. 92(4), 1297–1301 (2009).
- Weghofer A, Margreiter M, Bassim S, Sevelda U, Beilhack E, Feichtinger W. Minimal stimulation using recombinant follicle-stimulating hormone and a gonadotropin-releasing hormone antagonist in women of advanced age. Fertil. Steril. 81(4), 1002–1006 (2004).
- Elizur SE, Aslan D, Shulman A, Weisz B, Bider D, Dor J. Modified natural cycle using GnRH antagonist can be an optional treatment in poor responders undergoing IVF. J. Assist. Reprod. Genet. 22(2), 75–79 (2005).
- Jenkins JM, Davies DW, Devonport H et al. Comparison of ‘poor’ responders with ‘good’ responders using a standard buserelin/human menopausal gonadotrophin regime for in-vitro fertilization. Hum. Reprod. 6(7), 918–921 (1991).
- Ingerslev HJ, Højgaard A, Hindkjaer J, Kesmodel U. A randomized study comparing IVF in the unstimulated cycle with IVF following clomiphene citrate. Hum. Reprod. 16(4), 696–702 (2001).
- MacDougall MJ, Tan SL, Hall V, Balen A, Mason BA, Jacobs HS. Comparison of natural with clomiphene citrate-stimulated cycles in in vitro fertilization: a prospective, randomized trial. Fertil. Steril. 61(6), 1052–1057 (1994).
- Branigan EF, Estes MA. Minimal stimulation IVF using clomiphene citrate and oral contraceptive pill pretreatment for LH suppression. Fertil. Steril. 73(3), 587–590 (2000).
- Awonuga AO, Nabi A. in vitro fertilization with low-dose clomiphene citrate stimulation in women who respond poorly to superovulation. J. Assist. Reprod. Genet. 14(9), 503–507 (1997).
- Hull MG, Fleming CF, Hughes AO, McDermott A. The age-related decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil. Steril. 65(4), 783–790 (1996).
- Bhattacharya S, Hamilton MP, Shaaban M et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet 357(9274), 2075–2079 (2001).
- Moreno C, Ruiz A, Simón C, Pellicer A, Remohí J. Intracytoplasmic sperm injection as a routine indication in low responder patients. Hum. Reprod. 13(8), 2126–2129 (1998).
- Gabrielsen A, Petersen K, Mikkelsen AL, Lindenberg S. Intracytoplasmic sperm injection does not overcome an oocyte defect in previous fertilization failure with conventional in-vitro fertilization and normal spermatozoa. Hum. Reprod. 11(9), 1963–1965 (1996).
- Gianaroli L, Magli MC, Ferraretti AP, Munné S. Preimplantation diagnosis for aneuploidies in patients undergoing in vitro fertilization with a poor prognosis: identification of the categories for which it should be proposed. Fertil. Steril. 72(5), 837–844 (1999).
- Schoolcraft WB, Katz-Jaffe MG, Stevens J, Rawlins M, Munne S. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil. Steril. 92(1), 157–162 (2009).
- Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst. Rev. 4, CD002118 (2007).
- Shen S, Rosen MP, Dobson AT, Fujimoto VY, McCulloch CE, Cedars MI. Day 2 transfer improves pregnancy outcome in in vitro fertilization cycles with few available embryos. Fertil. Steril. 86(1), 44–50 (2006).
- Bahceci M, Ulug U, Ciray HN, Akman MA, Erden HF. Efficiency of changing the embryo transfer time from day 3 to day 2 among women with poor ovarian response: a prospective randomized trial. Fertil. Steril. 86(1), 81–85 (2006).
- Frankfurter D, Silva CP, Mota F, Trimarchi JB, Keefe DL. The transfer point is a novel measure of embryo placement. Fertil. Steril. 79(6), 1416–1421 (2003).
- Dayal MB, Frankfurter D, Athanasiadis I, Peak D, Dubey A, Gindoff PR. Day 2 embryo transfer (ET) and day 3 ET afford similar reproductive outcomes in the poor responder. Fertil. Steril. 95(3), 1130–1132 (2011).
- Laverge H, De Sutter P, Van der Elst J, Dhont M. A prospective, randomized study comparing day 2 and day 3 embryo transfer in human IVF. Hum. Reprod. 16(3), 476–480 (2001).
- Stern JE, Goldman MB, Hatasaka H, MacKenzie TA, Surrey ES, Racowsky C; Society for Assisted Reproductive Technology Writing Group. Optimizing the number of cleavage stage embryos to transfer on day 3 in women 38 years of age and older: a Society for Assisted Reproductive Technology database study. Fertil. Steril. 91(3), 767–776 (2009).
- Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil. Steril. 92(5), 1518–1519 (2009).
- Lin YH, Hwang JL. in vitro maturation of human oocytes. Taiwan. J. Obstet. Gynecol. 45(2), 95–99 (2006).
- Liu J, Lu G, Qian Y, Mao Y, Ding W. Pregnancies and births achieved from in vitro matured oocytes retrieved from poor responders undergoing stimulation in in vitro fertilization cycles. Fertil. Steril. 80(2), 447–449 (2003).
- Li J, Xu Y, Zhou G, Guo J, Xin N. Natural cycle IVF/IVM may be more desirable for poor responder patients after failure of stimulated cycles. J. Assist. Reprod. Genet. 28(9), 791–795 (2011).
- Cobo A, Garrido N, Crespo J, José R, Pellicer A. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod. Biomed. Online 24(4), 424–432 (2012).
- Cobo A, Meseguer M, Remohí J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum. Reprod. 25(9), 2239–2246 (2010).
Managing poor resopnders in IVF
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertob. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. You are seeing a 38-year-old woman who has been receiving treatment for infertility for 18 months, including in vitro fertilization (IVF), without success. Which of the following factors is the best predictor of her risk of poor response to a cycle of IVF therapy?
□ A Basal FSH level
□ B Basal estradiol level
□ C Anti-Müllerian hormone level
□ D A history of poor response to gonadotropins
2. The patient wishes to try another cycle of IVF. What should you consider regarding pre-cycle adjuvants for her?
□ A Ovarian cyst aspiration prior to the cycle is probably the most effective means to improve her chance for treatment success
□ B Pretreatment with oral contraceptive pills (OCPs) reduces sex hormone binding-globulin levels and increases follicular androgen levels
□ C Multiple studies have failed to demonstrate a difference in outcomes among patients pretreated with OCPs
□ D Pretreatment with GnRH antagonists lowers the dose of gonadotropins necessary in subsequent cycles
3. The CT results of your patient reveal that he has a lesion consistent with LR5. This indicates that the lesion is:
□ A Probably benign
□ B Intermediate probability of HCC
□ C Probably HCC
□ D Definitely HCC
4. What should you consider regarding the adjuvant treatment during an IVF cycle?
□ A Most providers have abandoned long GnRH agonist protocols for GnRH agonist flare protocols among poor responders
□ B Microdose GnRH protocols are ineffective in improving pregnancy rates vs long GnRH agonist protocols
□ C The dose of FSH should generally exceed 450 IU
□ D rFSH has been proven to promote superior outcomes compared with hMG among poor responders