Abstract
Dry eye disease is a very common multifactorial disease of the lacrimal functional unit that results in tear film instability, hyperosmolarity, chronic irritation and inflammation of the ocular surface. Diagnostic tools have been developing rapidly, however, both classic dry eye tests (Schirmer I test, fluorescein staining of the surface epithelium and tear film break-up time) and noninvasive imaging techniques are essential for an exact diagnosis. The management of dry eye syndrome can be either conservative or invasive based on the severity of the disease. The basic aim of treatment is to improve quality of life and reduce subjective complaints and objective ocular surface alterations in dry eye patients. The first line of treatment is tear substitution with artificial tear drops, gels and ointments. In moderate cases preservative-free tear supplementation, topical anti-inflammatory therapy and retinol treatment is recommended. Temporary or permanent punctal plug occlusion, therapeutic contact lenses or moisture chamber use constitute other options. In severe cases the application of topical autologous serum, systemic anti-inflammatory therapy, androgen substitution, secretagogues and surgical intervention can be effective. In the future, noninvasive diagnostic tools and instruments such as screening methods are likely to be developed. In addition, causal therapy of the dry eye will play a greater role, including cyclosporine therapy, secretion stimulation, growth factor-containing artificial tears, as well as secratogogues, immunomodulants and androgenic complexes for severe forms of the disease.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at www.medscape.org/journal/expertophth; (4) view/print certificate.
Release date: February 14, 2011; Expiration date: February 14, 2012
Learning objectives
• Describe the symptoms and underlying pathophysiology of dry eye disease
• Describe the diagnostic workup for dry eye disease
• Describe treatment for dry eye disease
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Editorial Director, Future Science Group, London, UK.
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay, MD
Freelance writer and reviewer, Medscape, LLC.
Disclosure: Laurie Barclay has disclosed no relevant financial relationships.
AUTHORS AND CREDENTIALS
László Módis Jr, MD
Department of Ophthalmology, Medical and Health Science Center, University of Debrecen, Nagyerdei krt. 98, Debrecen 4012, Hungary.
Disclosure: László Módis Jr has disclosed no relevant financial relationships.
Eszter Szalai, MD
Department of Ophthalmology, Medical and Health Science Center, University of Debrecen, Nagyerdei krt. 98, Debrecen 4012, Hungary.
Disclosure: Eszter Szalai has disclosed no relevant financial relationships.
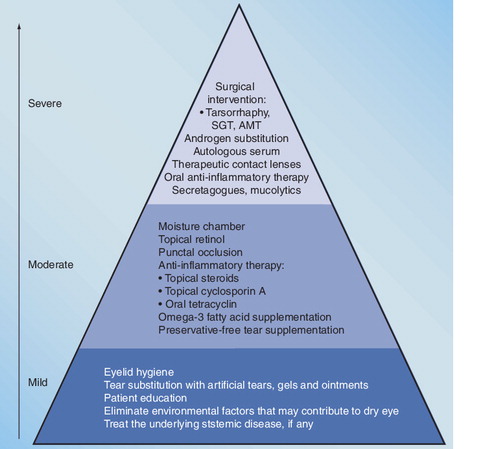
Patients who fail to respond to a particular treatment should be stepped up to the next level.
AMT: Amniotic membrane transplantation; SGT: Salivary gland transplantation.
Dry eye disease (also known as keratoconjunctivitis sicca) is characterized by precorneal tear film instability (reduced tear break-up time [BUT]) and damage of the exposed surface epithelium (fluorescein or Bengal rose staining of the corneal and conjunctival epithelia), which cause chronic irritation of the ocular surface. In most cases, dry eye symptoms are associated with decreased lacrimal gland secretion. However, the disease can be related to quantitatively normal lacrimal production and glandular hypersecretion. Reduced tear volume is a common cause of ocular manifestations; nevertheless, lacrimal hyposecretion evaluated by the Schirmer test is not essential in the diagnosis of dry eye disease. At the 2007 International Dry Eye Workshop, the definition of the disease was reviewed and rephrased as follows:
“Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.” Citation[1].
Dry eye disease is a very common condition that affects 5–30% of the population Citation[2,3].
Anatomical & physiological overview
The ocular surface is composed of the cornea, bulbar and tarsal conjunctiva and covered by three layers of the continuously renewing precorneal tear film. The lacrimal glands, the ocular surface and the connecting sensory and motor nerves together are referred to as the lacrimal functional unit Citation[4]. The air–tear film interface is the first refractive surface of the eye with a refractive index of 1.337 Citation[5]. The normal tear film volume is 6–7 µl and the pH of the precorneal tear film is 7.6 Citation[6–8]. The outermost layer of the tear film is the lipid layer secreted principally by the meibomian glands of the eyelid. Polar (phospholipids and glycolipids) and nonpolar lipid components (cholesterol and cholesterol esters) of the lipid film play the main role in preventing evaporation between blinks. The middle aqueous layer comprises of 98% of the tear film and is produced predominately by the lacrimal gland in the superotemporal region of the orbit and by the accessory lacrimal glands. In addition to the high water content, electrolytes, glucose, amino acids, urea, enzymes and proteins (immunoglobulin, lysozyme, lactoferrin, albumin and prealbumin) can also be found dissolved in the aqueous phase of the tear Citation[9,10]. It provides moisture to the ocular surface, facilitates the transport of oxygen and nutrients to the cornea, and maintains protection against microbial infections. The innermost mucin layer is formed by the secretion of conjunctival goblet cells and stratified ocular surface epithelium Citation[11]. Secreted (MUC5AC) and membrane-associated mucins (MUC1 and MUC4) are essential for spreading the tear film and lubricating the ocular surface Citation[12]. Membrane-associated mucins that form the glycocalix are attached to the microvilli (cornea) and microplicae (conjunctiva) of the epithelial cells. Limbal stem cells are responsible for corneal epithelial renewal and regeneration Citation[13,14].
Pathogenesis
The spontaneous blinking rate (5–10 blinks/s) is regulated by certain brain structures and ensures the optical quality of the eye by spreading the tear film over the ocular surface. Afferent impulses of reflex blinking originating from the eye surface and nasal mucosa travel in the trigeminal nerve to its sensory nucleus at the pons. The trigeminal afferents convey signals to the medulla and make synaptic connections with the spinal trigeminal tract and nucleus. This connects with the facial nerve nuclei placed at the pontine level of the brainstem. The impairment of this reflex pathway can result in dry eye disease; however, the exact mechanism remains unclear. One hypothesis is that minor trauma and infrequent or incomplete blinking can cause damage to the ocular surface. The inflammation theory of dry eye disease is supported by several scientific evidences Citation[15,16]. The secretory immune system of the ocular surface (tear film IgA and IgA transporter secretory component) is regulated by androgens Citation[17]. In experimental animal models, decreased androgen levels have been proven to generate apoptosis of interstitial cells and necrosis of acinar cells Citation[18]. Moreover, androgen therapy reduced the inflammation in both salivary and lacrimal glands Citation[19]. Furthermore, tissue-specific androgens suppress IL-1β-mediated nitric oxide production in acinar cells of the lacrimal gland Citation[20]. Androgen hormones are responsible for the regulation of lacrimal glands, thus androgen insufficiency (e.g., menopause, aging and antiandrogen therapy) can lead to aqueous tear-deficient dry eye Citation[21]. Androgens also contribute to the formation of the lipid tear layer, therefore androgen deficiency is responsible for meibomian gland dysfunction (MGD) and evaporative dry eye Citation[22]. However, ocular surface inflammation is considered as a secondary consequence Citation[1].
Classification
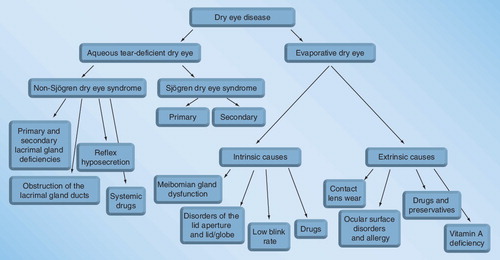
According to the International Dry Eye Workshop Report, dry eye disease comprises two major etiopathogenic groups: aqueous tear-deficient dry eye and evaporative dry eye Citation[1].
Aqueous tear-deficient dry eye
Tear-deficient dry eye can be subdivided into Sjögren syndrome and non-Sjögren syndrome dry eye groups. The latter form of dry eye has several primary causes, including the lack of a lacrimal gland (congenital alacrima) and impairment or dysfunction of the lacrimal gland. Non-Sjögren syndrome dry eye can also occur secondary to a variety of conditions as listed in Box 1.
Sjögren syndrome dry eye is associated with autoimmune inflammation in the lacrimal glands. The disease is classified as primary or secondary. Primary Sjögren syndrome is characterized by keratoconjunctivitis sicca, xerostomia and other glandular and extraglandular symptoms. Secondary Sjögren syndrome is associated with other systemic autoimmune disorders, such as rheumatoid arthritis, systemic lupus erythematosus, Wegener’s granulomatosis, scleroderma, polymyositis, dermatomyositis, polyartheritis, Hashimoto’s thyroiditis and Raynaud syndrome.
Evaporative dry eye
Hyperevaporative dry eye caused by increased evaporation rate, increased evaporative tear loss and unstable tear film comprises of the following types of dry eyes:
• Changes in tear composition (lack of lipid content; primary type: lack of glands and distichiasis; secondary type: blepharitis and MGD);
• Abnormalities of eyelids;
• Reduced blinking rate or incomplete blinking (office workers, Parkinsonism and schizophrenia);
• Ocular surface irregularities;
• Contact lens wear.
Since obstructive MGD is the most common cause of evaporative dry eye and the prevalence of MGD varies between 30.5 and 71.7% Citation[1,23,24], this etiopathogenic subgroup is considered to be more prevalent than aqueous tear deficiency Citation[25]. However, altered meibomian gland function can be associated with Sjögren syndrome dry eye, indicating the mixed occurrence of the two subtypes Citation[26,27].
Subjective symptoms
The most common symptoms are burning, itching, scratching, foreign body sensation, photophobia and red eye. Dry eye patients typically complain of a gritty or sandy feeling in the eye. Reviewing past and present medical history can be an important step in the diagnosis process before the physical examination. Recently, patient-reported experiences of dry eye symptoms have been recorded via questionnaires. The Dry Eye Questionnaire elicits the presence and severity of ocular surface symptoms and captures information regarding age, gender, work ability, computer usage, ophthalmic and systemic therapy, presence of allergy and self-assessment Citation[28]. Other questionnaires such as the National Eye Institute Visual Function Questionnaire and the Ocular Surface Disease Index© questionnaire evaluate the frequency and/or severity of individual dry eye symptoms, and their impact on the quality of the patient’s life Citation[29,30].
Objective clinical signs & diagnosis
Classical tests for the diagnosis & differential diagnosis of dry eye disease
• Slit-lamp examination (measuring the upper and lower tear menisci, lid-parallel conjunctival folds and lid-parallel conjunctival fold [LIPCOF]);
• Aqueous tear production (Schirmer test);
• Staining of the ocular surface (fluorescein, rose Bengal and lissamine green);
• Tear film stability (tear film BUT).
Commonly used laboratory tests
• Mucus ferning test;
• Conjunctival impression cytology;
• Tear film interferometry.
Other laboratory measurements
• Tear film osmolarity;
• Tear lysozyme level;
• Tear lactoferrin level;
• Tear protein electrophoresis;
• Tear glycoprotein content;
• Tear immunoglobulin content.
Clinical examination
Slit-lamp examination
In moderate-to-severe cases, dry eye disease can be diagnosed based on subjective symptoms and slit-lamp findings. Tear meniscus characteristics (meniscus height, depth and radius of curvature) have been reported to be useful in identifying patients with aqueous-deficient dry eye Citation[31].
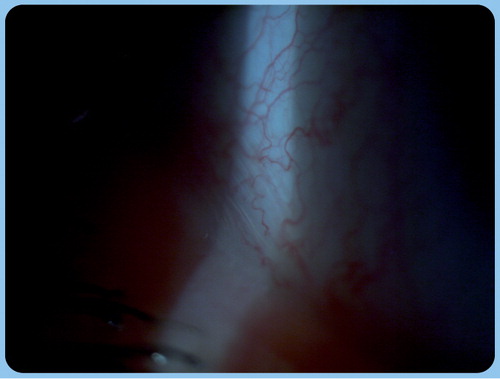
In severe aqueous tear-deficient dry eye, corneal damage due to exsiccation may lead to filament formation, which is composed of desquamated epithelial cells from the ocular surface (cornea and conjunctiva) and excessive mucus formation (filamentary keratitis) Citation[32]. Presence and grade of LIPCOF (conjunctivochalasis) can also be evaluated by slit-lamp examination . Conjunctival folds can be found in all four quadrants (upper temporal, upper nasal, lower nasal and lower temporal) and they form parallel to the posterior eyelid margin. However, LIPCOF can be observed generally in the lower temporal quadrant. Conjunctival folds are related to the increased frictional force between eyelids and the conjunctiva rather than to aging. Increased friction is considered to be the sum of intrinsic and extrinsic effects leading to dry eye. Presence of conjunctivochalasis is a sensitive indicator of dry eye disease since the test demonstrates a high positive predictive value for dry eye of more than 93% Citation[33]. It should be noted that conjunctival folds can be the cause or consequence of dry eye disease Citation[34,35]. The following grading system is based on slit-lamp examination of conjunctival folds in the lower temporal quadrant:
• LIPCOF grade 0: no permanently present fold – normal;
• LIPCOF grade 1: single, small fold; smaller than the normal tear meniscus – mild intensitiy of dry eye disease;
• LIPCOF grade 2: fold of up to the height of the normal tear meniscus, multiple folds – moderate intensity of dry eye disease;
• LIPCOF grade 3: fold being higher than the normal tear meniscus, multiple folds – severe intensity of dry eye disease Citation[33].
The Schirmer test, that is, the measurement of tear production (mainly the aqueous phase of tear), can be performed with (basal tear secretion: Schirmer I) or without topical anesthesia (basal tear secretion: Schirmer I; reflex tear secretion: Schirmer II with nasal stimulation). For the Schirmer I test, a standardized filter paper strip is inserted into the lower conjunctival sac, and after 5 min, the amount of wetting can be measured in millimeter units. The most commonly used threshold value is 10 mm or less within 5 min for screening dry eye (with a sensitivity of 83.6% and specificity of 69.8%), and 5 mm or less within 5 min for confirmation of the diagnosis (with a sensitivity of 76.9% and specificity of 72.4%) Citation[36].
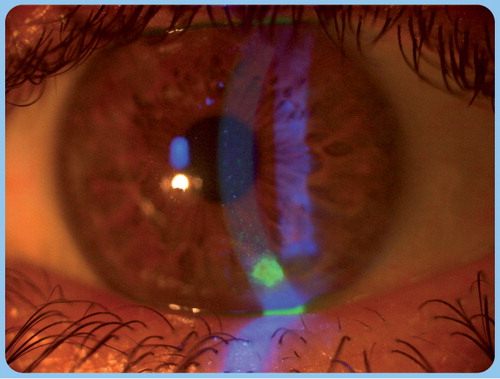
Vital staining is a widely used method for studying the integrity and viability of corneal and conjunctival epithelial cells. After instillation of a drop of fluorescein solution, damaged epithelial cells with disrupted intercellular connections stain bright green with fluorescein under cobalt blue-filtered light. The conjunctiva and cornea is divided into six and five zones, and fluorescein staining can be scored on a 0–3 scale in each zone Citation[37].
Tear film BUT is the time interval measured between the last blink and the appearance of the first dry spot Citation[38]. Fluorescein BUT is a widely used method for determining the stability of precorneal tear film and identifying patients with evaporative dry eye. For slit-lamp examination, a fluorescein strip is applied in the lower conjunctival sac and after blinking, a break in the tear film is visible as a dark spot under cobalt blue-filtered light. A BUT of more than 10 s is considered a normal value Citation[39]. Since fluorescein BUT is an invasive technique that induces reflex tearing, noninvasive BUT (NBUT) is a measurement of the time that elapsed between the last blink and the first appearance of specular reflex distortion Citation[40]. Videokeratoscopy and wavefront aberrometry are also applied techniques for the measurement of tear film stability Citation[41,42].
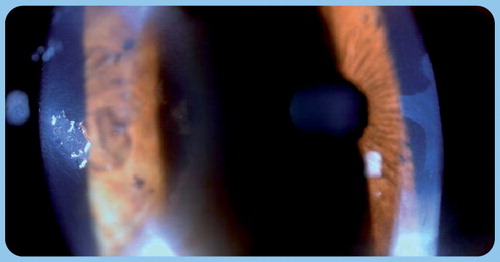
Since classical dry eye tests have considerable limitations, for the accurate diagnosis and monitoring of treatment, conjunctival impression cytology has become more widespread. After topical anesthesia, cytology samples are collected from the temporal and superior conjunctival regions with a sterile cellulose acetate filter strip, and the specimens are studied after Periodic acid-Schiff and Papanicolau staining. The evaluation is performed on a standardized scale ranging from 0 to 3, based on the number of goblet cells, morphology of epithelial cells and nucleocytoplasmic ratio Citation[43]. The specificity of the method is fairly high (93.9–100%), and it is also highly sensitive (87.5–100%) Citation[43,44].
Elevated tear osmolarity is thought to play a significant role in ocular surface inflammation and corneal epithelial damage Citation[1,45–47]. Tear osmolarity measurement has been considered to be a reliable method for detecting dry eye Citation[48,49]. Contrary to the laboratory procedures (electrical conductivity, freezing point depression and vapor pressure evaluation), a recently introduced noninvasive tear osmolarity assessment system is capable of quantifying tear osmolarity using a tear sample of 50 nl. A result of 316 mOsms/l or more has been suggested for diagnosing patients with dry eye disease Citation[49].
Apart from slit-lamp examination, tear meniscus characteristics can be obtained with reflective meniscometry Citation[50], interferometry Citation[51,52], strip meniscometry Citation[53] and, recently, with anterior segment optical coherence tomography Citation[54]. Semiquantitative tear film interferometry is of value in differentiating aqueous tear-deficient dry eye and lipid tear deficiency. Interference patterns arise from the reflection of incident light from the anterior and posterior surface of the lipid tear layer Citation[51].
Several studies reported poor correlation between subjective dry eye symptoms and objective signs Citation[2,55–62]. This finding can be attributed to the low repeatability and poor reliability of the conventional tear film tests Citation[63–66]. By contrast, recent papers demonstrated a significant relationship between increased tear osmolarity and disease severity, which indicated the importance of evaluating tear osmolarity for the diagnosis of dry eye disease Citation[67,68]. Moreover, some of the standard tests are specific for one etiopathogenic subgroup of the disease, for example, the Schirmer test evaluates the aqueous phase secretion, whereas altered meibomian gland status can be characteristic of evaporative dry eye. Tear film hyperosmolarity is considered a key pathophysiological factor, both in aqueous tear-deficient and in evaporative dry eye disease Citation[69]. The lack of efficacy of Schirmer, BUT, and corneal and conjunctival staining was demonstrated in identifying mild-to-moderate disease and only tear osmolarity was shown to have a linear relationship to a composite severity index over the entire range of severity Citation[67].
The dry eye severity scale proposed by the Delphi Panel Report has proved to be a practical method of grading the severity of the disease (mild, moderate and severe), treatment planning and control of the therapy Citation[70]. However, a continuous severity scale has been recently developed considering both objective clinical signs and subjective complaints, including the Schirmer test, BUT, vital staining of the ocular surface, Ocular Surface Disease Index, meibomian grading score and tear osmolarity results Citation[67].
Management
General considerations
Based on the pathogenesis of the disease it is important to emphasize that dry eye is treatable; however, it cannot be completely eliminated. Therefore, proper patient education and psychological therapy is also essential in dry eye patients. Physicians should encourage and motivate the patient to implement lifestyle and behavioral changes by reducing monitor use, maintaining a high humidity environment, avoiding windy conditions and consuming essential fatty acids.
Cooperation with fellow specialists is also important, especially when dry eye occurs in association with systemic diseases (e.g., autoimmune disorders). In such cases, the ophthalmologist is advised to have a consultation with the internist, rheumatologist, gynecologist and dentist. Systemic diseases have a fluctuating course and ophthalmic complaints may differ accordingly, in spite of the treatment.
Local and systemic allergic reactions and inflammation usually worsen the dry eye conditions. Moreover, the patient’s microenvironment should be taken into consideration. Cold and hot temperature, dry vicinity, excessive monitor usage, dusty or smoky surroundings, air-conditioning, spicy dishes and alcohol consumption have a negative influence on the course of the disease.
Generally applied medications such as antihypertensive drugs (e.g., β-blockers, reserpine, thiazides and diuretics), analgesic drugs, antidepressants, neuroleptics, psychopharmacological drugs, antihistamines, anti-Parkinson drugs, antimigraine medicines, cytostatic agents, estrogen derivatives and oral contraceptives diminish tear production. The locally used antiglaucoma eye drops (carboanhydrase inhibitors and β-blockers) are capable of decreasing the number of goblet cells.
The treatment of dry eye disease can be either conservative or invasive based on the severity of the disease.
Conservative treatment
Tear substitution
In all forms of dry eye primary tear supplementation is recommended by differently acting artificial teardrops and ophthalmic ointments (Boxes 2 & 3). Based on subjective complaints and clinical symptoms together with systemic diseases, each patient must be treated individually. The basic aim of the treatment is to reduce subjective complaints and objective ocular surface alterations in dry eye patients and finally to restore quality of life.
For substituting the aqueous tear phase (decreased Schirmer value), products with high sodium chloride and polyvinyl alcohol content are mostly used. The tear film-stabilizing effect of the alcoholic drugs is also important. Products with povidone are similar to mucin and also decrease the tear’s surface tension. The therapeutic effect can be extended (decreased tear film BUT) if the medicine stays longer on the ocular surface by certain drops containing hypromellose and carboxymethylcellulose. Ophthalmic ointments containing petrolatum, paraffin and carbomer also have a long-lasting and protective effect. These are usually applied complementarily with the artificial tear supplement during the day, but owing to their substance, they may cause temporary visual disturbance, therefore they are suggested for night-time use.
In the case of ocular surface staining (conjunctiva and cornea), drugs containing dextran and retinol are suggested because they protect and regenerate the epithelium Citation[71]. Further epithelization can be achieved by using hemoderivatum and dexpanthenol. The increased electrolyte content of tear substitutes such as sodium, calcium, chloride and bicarbonate ions also has a beneficial effect on the corneal epithelial layer Citation[72].
Preservatives in artificial tears also have a bacteriostatic/bactericidal effect. Consequently, although the edge of the bottle reaches the eyelid or the eye surface when dropping, the whole fluid will not become infected. However, long-lasting application of drugs with preservatives may cause disruption of the epithelial cell–cell contacts, allergic reaction, decreased goblet cell density or inflammation Citation[25]. Benzalkonium chloride, chlorobutanol and cetrimide are the most common preservatives in artificial tears that after a prolonged application induce toxic epitheliopathy. Therefore, in advanced dry eye, changing to preservative-free artificial tears is reasonable and recommended Citation[25,72]. Their advantage is that most preservative-free tear drops may be used together with contact lenses.
Generally, when the patient drops more than four times a day, preservative-free tears are recommended Citation[71,72]. However, artificial tear products with hydroxypropyl guar also contain preservatives, that, when dropped in the eye, cause the the fluid to turn into a gel substance similar to the superficial glycocalix system. Therefore, a long-lasting protective layer forms on the ocular surface, enabling a natural regeneration process Citation[73].
Anti-inflammatory agents
In cases of advanced and severe dry eye owing to the inflammatory pathomechanism, local and systemic immunosuppressive therapy can be administered, usually as supplementary therapy.
Corticosteroids usually improve both the subjective and objective signs and symptoms of the disease. Local corticosteroids can be applied when chronic conjunctival redness is present, usually with punctate keratitis (corneal fluorescein staining) Citation[74]. Local corticosteroid eye drops (0.1% fluorometholone two to four times/day for 2–4 weeks) is also suggested in cases after refractive surgery when temporary dry eye disease occurs Citation[75].
Systemic administration is recommended in patients with autoimmune disorders (e.g., Sjögren syndrome) in a short pulse (40 mg on alternate days) under the supervision of an autoimmune specialist Citation[76]. Both local and systemic application may cause cataract and glaucoma formation, therefore regular check-up is mandatory.
The application of topical cyclosporine A (0.05%) also has a beneficial effect on dry eye conditions and is capable of improving the Schirmer value, corneal fluorescein staining and goblet cell density Citation[77].
Eyelid therapy
Patients with altered eyelid status (MGD, meibomianitis and blepharitis) should take care of lid hygiene. Several warming methods are proposed to be effective, such as infrared or broad-spectrum warm compresses Citation[78,79], warm moist eye devices Citation[80] and disposable eye warming devices Citation[81,82]. Tetracycline with its antibacterial, anti-inflammatory and antiangiogenic activities can be used systematically Citation[83,84]. The dosing of oral tetracycline varies between 20 and 100 mg/day and usually lasts for 3 months in an intermittent modality Citation[85]. Azithromycin 1.0% ophthalmic solution has been suggested as an adequate treatment for moderate-to-severe blepharitis owing to its antibiotic and anti-inflammatory effects Citation[86–89].
Autologous serum
Autologous serum is prepared from the patients’ blood and can be used in different solutions (20–100%). It should be prepared under sterile conditions in a certified laboratory unit. It contains several growth factors, vitamin A and fibronectin, and also has an anti-inflammatory effect; therefore it has been beneficial in advanced and severe cases of the disease Citation[90].
Secretagogues
Increased tear secretion can be achieved with the application of local and systemic secretagogues. Systemic cholinergic agonists can be used in patients with Sjögren syndrome. However, these drugs may produce serious side effects, such as sweating and nausea. Most of these secretagogues are under investigation and they do not apply routinely in every day practice Citation[71].
Eye & ocular surface protection
Different types of contact lenses (both soft and rigid gas permeable) can be used in the management of dry eye patients with filamentary keratitis or epitheliopathy Citation[72]. The protection of the ocular surface can be achieved with contact lenses if at least minimal tear production is present. Spectacle shield and moisture chamber goggles can be applied to keep a certain level of humidity around the eyes Citation[72].
Fatty acid supplements
Omega-3 fatty acids (eicosapentaenoic acid, docosahexaenoic acid and α-linolenic acid) are especially beneficial in patients with systemic autoimmune inflammatory disorders associated with dry eye disease Citation[91]. The American Dietetic Association and the Dietitians of Canada recommended a 500 mg/day intake of omega-3 fatty acids Citation[92]. However, twice-daily usage of oral linolenic acid (28.5 mg) has been proven to reduce ocular surface inflammation and symptoms Citation[93].
Hormonal therapy
Androgen hormones
Experimental and clinical research results suggest that substitutive hormonal therapy with androgens can be an effective and safe treatment option both in aqueous tear-deficient and in hyperevaporative dry eye diseases Citation[94,95,201].
Semi-invasive therapy
Punctal plugs
Punctal occlusions can be applied in temporary and permanent ways. Temporary punctual occlusion can be achieved with the help of absorbable and nonabsorbable plugs. A recently introduced hydrophilic acrylic polymer with thermodynamic properties is capable of taking an individual formation after implantation into the patients’ canaliculus. Permanent occlusion can be performed either by cautery or by laser applications. Punctal occlusion is advised in advanced cases of the disease with significant corneal staining and prolonged tear film BUT Citation[96], as well as in toxic epitheliopathy, which can occur in patients with tear hyperosmolarity Citation[97].
Invasive (surgical) therapy
In severe cases, usually in patients with systemic and different autoimmune diseases, ocular inserts can be placed in the lower fornix. These inserts are capable of providing a moisturizing effect for hours and maintaining humidity on the ocular surface Citation[98].
In severe dry eye disease, permanent lacrimal punctual occlusion may be beneficial. Corneal epithelial defects and ulcerations can be covered by amniotic or mucous membrane transplantation Citation[99,100].
Partial or total tarsorrhaphy is useful in patients with delayed epithelial wound healing and who are refractory to the aforementioned treatments Citation[101]. Salivary gland transplantation may be successful in patients with advanced lacrimal gland dysfunction Citation[102].
Expert commentary
Dry eye disease is an inflammatory disease with tear film instability, hyperosmolarity and chronic irritation of the ocular surface. The diagnosis of dry eye is based on symptoms, clinical examination and diagnostic tests. The Schirmer I test, fluorescein staining of the surface epithelium and tear film BUT assessment are the classic dry eye tests that are commonly used in diagnosis of the disease. However, several noninvasive methods have been introduced into clinical practice for screening dry eye and confirming the diagnosis, such as lid-parallel conjuntival folds, NBUT, tear osmolarity measurement, tear meniscometry and interferometry techniques. Several studies reported a poor correlation between subjective dry eye symptoms and objective signs.
The preferred therapy should be decided on according to the patient’s subjective complaints and the objective symptoms. It is important to emphasize that treating the underlying systemic disease and eliminating causative factors that may contribute to dry eye are essential. The first line of treatment is tear substitution with eye drops, gels and ointments. Highly viscous solutions and gels can avoid the frequent use of aqueous solutions and further intoxication of the ocular surface. In cases when the epithelium is severely damaged (toxic epitheliopathy) preservative-free artificial tears should be applied. High osmolarity measured in the tear sample renders osmoprotective artificial tears containing osmolytes (e.g., L-carnitine, erythritol, glycerin and polyols) necessary to protect the ocular surface from osmotic stress. If the conjunctival impression cytology confirms squamous metaplasia, a drop containing vitamin A is recommended. If mucus filaments are present on the ocular surface, artificial tears with mucolytic agents are advisable. In moderate and severe cases local and systemic immunosuppressive therapy and the application of autologous serum are usually efficient. Stimulating tear secretion can only be effective if the lacrimal gland is at least partially functional.
When the artificial tear supplementation and anti-inflammatory therapy fail to be effective, temporary or permanent punctal plug occlusion, therapeutic contact lenses or moisture chamber can be necessary.
In severe cases, surgical treatment can also be applied when the cornea is covered with amniotic membrane. At the end stage of dry eye, tarsorrhaphy is a possible solution.
The therapeutic pyramid based on the objective alterations and the severity of the disease is summarized in .
Five-year view
A wide range of instruments that are capable of creating high-resolution images of the whole anterior segment of the eye globe are already available. Some of these machines, such as high-speed videokeratoscopy and wavefront aberrometry, can precisely analyze the whole cornea and also the precorneal tear film, and play an important role in the proper diagnosis of the dry eye with touching the ocular surface. In addition, recently introduced noncontact machines can evaluate different aspects of the tear film, such as stability (NBUT) and osmolarimetry.
In the future, the causal therapy of the dry eye will play a greater role. Local cyclosporine therapy has already achieved promising results Citation[77]. Secretion stimulation, mucin stabilizing and mucolytic agents and experiments with local androgenic complexes are also in an advanced stage Citation[71,95]. Systemic application of essential fatty acids such as omega-3 appears to have a promising effect with regards to reducing ocular surface irritation.
The most exciting and promising therapeutic options are the application of different growth factors, neural factors and related molecules in tear substitution. In severe cases systemic immunomodulation may have a putative role in the treatment of dry eye. Cyclosporine A 0.05% ophthalmic emulsion has been widely applied to moderate and severe ocular surface inflammations. Furthermore, topical anti-CD4 monoclonal antibody can also be beneficial by suppressing the activation of CD4+ T cells Citation[103]. Oral immunosuppressive therapy should be considered in severe dry eye patients with systemic autoimmune disorders Citation[104].
Box 1. Systemic conditions that may contribute to non-Sjögren dry eye disease.
• HIV positivity
• Sarcoidosis
• Lymphoma
• Hemochromatosis
• Amyloidosis
• Graft-versus-host disease
• Endocrine ophthalmopathy
• Injuries, surgical interventions and radiation therapy
• Obstruction of the lacrimal gland ducts (burn, chemical injury, severe infection, autoimmune inflammation, radiation, pemphigoid, trachoma, erythema multiforme, Steven–Johnson syndrome and Lyell syndrome)
• Corneal sensory loss (contact lens wear, neuroparalytic keratitis, facial palsy, keratoplasty and excimer laser intervention)
• Neurogen dysfunction of lacrimal secretion (Riley–Day syndrome)
Box 2. The applied artificial tears and other tear substitutes can be grouped according to the chemical composition of their agents.
• Inorganic salts
• Lipids (paraffin, lanoline and lecithin) and waxes
• Monosaccharides and disaccharides
• Alcohols
• Polysaccharides (cellulose derivatives, dextran and mucopolysaccharides)
• Gelatins
• Synthetic polymers
• Biological materials (autologous serum and several mucins)
Box 3. In dry eye therapy the following basic medications can be applied.
• Water-based artificial tear (demulcent drugs)
• Ophthalmic ointments (ocular lubricants and emollient drugs)
• Ophthalmic gels
• Viscoelastic materials
• Ocular inserts
Key issues
• Dry eye is a multifactorial disease that causes subjective complaints and tear film alteration, which lead to damage of the ocular surface.
• The diagnosis of dry eye is based on the subjective symptoms and certain clinical tests, such as fluorescein staining of the surface epithelium, the Schirmer I test, and tear film break-up time. Several noninvasive screening methods can confirm the diagnosis, such as lid-parallel conjuntival folds, noninvasive break-up time and tear osmolarity measurement.
• In mild cases, tear substitution consists of artificial tear drops, gels and ointments. It is necessary to treat the underlying systemic disease, if any, and eliminate causative factors that may contribute to dry eye.
• In moderate dry eye preservative-free tear supplementation, topical anti-inflammatory therapy and retinol treatment are recommended. When the conservative therapy fails to be effective, temporary or permanent punctal plug occlusion, therapeutic contact lenses or moisture chamber usage can be necessary.
• In severe cases topical autologous serum, systemic anti-inflammatory therapy, androgen substitution, secretagogues and surgical intervention may be necessary.
References
- No authors listed. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf.5(2), 75–92 (2007).
- McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology105(6), 1114–1119 (1998).
- Shimmura S, Shimazaki J, Tsubota K. Results of a population-based questionnaire on the symptoms and lifestyles associated with dry eye. Cornea18(4), 408–411 (1999).
- Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res.78(3), 409–416 (2004).
- Patel S, Boyd KE, Burns J. Age, stability of the precorneal tear film and the refractive index of tears. Cont. Lens Anterior Eye23(2), 44–47 (2000).
- Mishima S, Gasset A, Klyce SD, Baum JL. Determination of tear volume and tear flow. Invest. Ophthalmol.5(3), 264–276 (1966).
- Fischer FH, Wiederholt M. Human precorneal tear film pH measured by microelectrodes. Graefes Arch. Clin. Exp. Ophthalmol.218(3), 168–170 (1982).
- Yamada M, Kawai M, Mochizuki H, Hata Y, Mashima Y. Fluorophotometric measurement of the buffering action of human tears in vivo.Curr. Eye Res.17(10), 1005–1009 (1998).
- Sariri R, Ghafoori H. Tear proteins in health, disease, and contact lens wear. Biochemistry (Mosc.)73(4), 381–392 (2008).
- Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clin. Chim. Acta369(1), 17–28 (2006).
- Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest. Ophthalmol. Vis. Sci.36(9), 1818–1827 (1995).
- Watanabe H. Significance of mucin on the ocular surface. Cornea21(2 Suppl. 1), S17–S22 (2002).
- Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv. Ophthalmol.44(5), 415–425 (2000).
- Sun TT, Lavker RM. Corneal epithelial stem cells: past, present, and future. J. Investig. Dermatol. Symp. Proc.9(3), 202–207 (2004).
- Wilson SE. Inflammation: a unifying theory for the origin of dry eye syndrome. Manag. Care12(12 Suppl.), 14–19 (2003).
- Porola P, Laine M, Virkki L, Poduval P, Konttinen YT. The influence of sex steroids on Sjögren’s syndrome. Ann. NY Acad. Sci.1108, 426–432 (2007).
- Sullivan DA, Allansmith MR. Hormonal influence on the secretory immune system of the eye: androgen modulation IgA levels in tears of rats. J. Immunol.134(5), 2978–2982 (1985).
- Azzarolo AM, Wood RL, Mircheff AK et al. Androgen influence on lacrimal gland apoptosis, necrosis, and lymphocytic infiltration. Invest. Ophthalmol. Vis. Sci.40(3), 592–602 (1999).
- Sato EH, Sullivan DA. Comparative influence of steroid hormones and immunosuppressive agents on autoimmune expression in lacrimal glands of a female mouse model of Sjogren’s syndrome. Invest. Ophthalmol. Vis. Sci.35(5), 2632–2642 (1994).
- Beauregard C, Brandt P. Down regulation of interleukin-1β-induced nitric oxide production in lacrimal gland acinar cells by sex steroids. Curr. Eye Res.29(1), 59–66 (2004).
- Sullivan DA, Wickham LA, Rocha EM, Kelleher RS, Silveira LA, Toda I. Influence of gender, sex steroid hormones and the hypothalamic–pituitary axis on the structure and function of the lacrimal gland. Adv. Exp. Med. Biol.438, 11–42 (1998).
- Sullivan DA, Sullivan BD, Evans JE et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann. NY Acad. Sci.966, 211–222 (2002).
- Molinari JF, Stanek S. Meibomian gland status comparison between active duty personnel and U.S. veterans. Mil. Med.165(8), 591–593 (2000).
- Viso E, Gude F, Rodríguez-Ares MT. The association of meibomian gland dysfunction and other common ocular diseases with dry eye: a population-based study in Spain. Cornea30(1), 1–6 (2011).
- Albietz JM, Bruce AS. The conjunctival epithelium in dry eye subtypes: effect of preserved and non-preserved topical treatments. Curr. Eye Res.22(1), 8–18 (2001).
- Shimazaki J, Goto E, Ono M, Shimmura S, Tsubota K. Meibomian gland dysfunction in patients with Sjögren syndrome. Ophthalmology105(8), 1485–1488 (1998).
- Sullivan DA, Schaumberg DA, Suzuki T et al. Sex steroids, meibomian gland dysfunction and evaporative dry eye in Sjögren’s syndrome. Lupus11(10), 667 (2002).
- McMonnies C, Ho A, Wakefield D. Optimum dry eye classification using questionnaire responses. Adv. Exp. Med. Biol.438, 835–838 (1998).
- Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). Arch. Ophthalmol.116(11), 1496–1504 (1998).
- Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch. Ophthalmol.118(5), 615–621 (2000).
- Mainstone JC, Bruce AS, Golding TR. Tear meniscus measurement in the diagnosis of dry eye. Curr. Eye Res.15(6), 653–661 (1996).
- Tanioka H, Yokoi N, Komuro A et al. Investigation of the corneal filament in filamentary keratitis. Invest. Ophthalmol. Vis. Sci.50(8), 3696–3702 (2009).
- Höh H, Schirra F, Kienecker C, Ruprecht KW. [Lid-parallel conjunctival folds are a sure diagnostic sign of dry eye]. Ophthalmologe92(6), 802–808 (1995).
- Yokoi N, Komuro A, Maruyama K, Tsuzuki M, Miyajima S, Kinoshita S. New surgical treatment for superior limbic keratoconjunctivitis and its association with conjunctivochalasis. Am. J. Ophthalmol.135(3), 303–308 (2003).
- Wang Y, Dogru M, Matsumoto Y et al. The impact of nasal conjunctivochalasis on tear functions and ocular surface findings. Am. J. Ophthalmol.144(6), 930–937 (2007).
- Vitali C, Moutsopoulos HM, Bombardieri S. The European Community Study Group on diagnostic criteria for Sjogren’s syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjogren’s syndrome. Ann. Rheum. Dis.53(10), 637–647 (1994).
- Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J.21(4), 221–232 (1995).
- Gulati A, Dana R. Keratoconjunctivitis sicca: clinical aspects. In: The cornea (Scientific Foundations and Clinical Practice). Foster CS, Azar DT, Dohlman CS (Eds). Lippincott Williams & Wilkins, PA, USA, 603–627 (2005).
- Lemp MA, Hamill JR. Factors affecting tear film breakup in normal eyes. Arch. Ophthalmol.89(2), 103–105 (1973).
- Mengher LS, Bron AJ, Tonge SR, Gilbert DJ. A non-invasive instrument for clinical assessment of the pre-corneal tear film stability. Curr. Eye Res.4(1), 1–7 (1985).
- Németh J, Erdélyi B, Csákány B et al. High-speed videotopographic measurement of tear film build-up time. Invest. Ophthalmol. Vis. Sci.43(6), 1783–1790 (2002).
- Montés-Micó R, Alió JL, Muñoz G, Charman WN. Temporal changes in optical quality of air–tear film interface at anterior cornea after blink. Invest. Ophthalmol. Vis. Sci.45(6), 1752–1757 (2004).
- Nelson JD, Havener VR, Cameron JD. Cellulose acetate impressions of the ocular surface. Dry eye states. Arch. Ophthalmol.101(12), 1869–1872 (1983).
- Rivas L, Oroza MA, Perez-Esteban A, Murube-del-Castillo J. Morphological changes in ocular surface in dry eyes and other disorders by impression cytology. Graefes Arch. Clin. Exp. Ophthalmol.230(4), 329–334 (1992).
- Gilbard JP, Carter JB, Sang DN, Refojo MF, Hanninen LA, Kenyon KR. Morphologic effect of hyperosmolarity on rabbit corneal epithelium. Ophthalmology91(10), 1205–1212 (1984).
- Gilbard JP, Rossi SR, Gray KL. A new rabbit model for keratoconjunctivitis sicca. Invest. Ophthalmol. Vis. Sci.28(2), 225–228 (1987).
- Gilbard JP, Rossi SR, Gray KL, Hanninen LA, Kenyon KR. Tear film osmolarity and ocular surface disease in two rabbit models for keratoconjunctivitis sicca. Invest. Ophthalmol. Vis. Sci.29(3), 374–378 (1988).
- Farris RL. Tear osmolarity – a new gold standard? Adv. Exp. Med. Biol.350, 495–503 (1994).
- Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: determination of a referent value for dry eye diagnosis. Invest. Ophthalmol. Vis. Sci.47(10), 4309–4315 (2006).
- Yokoi N, Bron A, Tiffany J, Brown N, Hsuan J, Fowler C. Reflective meniscometry: a non-invasive method to measure tear meniscus curvature. Br. J. Ophthalmol.83(1), 92–97 (1999).
- Goto E, Dogru M, Kojima T, Tsubota K. Computer-synthesis of an interference color chart of human tear lipid layer by a colorimetric approach. Invest. Ophthalmol. Vis. Sci.44(11), 4693–4697 (2003).
- Uchida A, Uchino M, Goto E et al. Noninvasive interference tear meniscometry in dry eye patients with Sjogren syndrome. Am. J. Ophthalmol.144(2), 232–237 (2007).
- Dogru M, Ishida K, Matsumoto Y et al. Strip meniscometry: a new and simple method of tear meniscus evaluation. Invest. Ophthalmol. Vis. Sci.47(5), 1895–1901 (2006).
- Ibrahim OM, Dogru M, Takano Y et al. Application of visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology117(10), 1923–1929 (2010).
- Schein OD, Tielsch JM, Munõz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly: a population-based perspective. Ophthalmology104(9), 1395–1401 (1997).
- Baudouin C. The pathology of dry eye. Surv. Ophthalmol.45(Suppl. 2), S211–S220 (2001).
- Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv. Ophthalmol.45(Suppl. 2), S199–S202 (2001).
- Mathers WD, Dolney AM, Kraemer D. The effect of hormone replacement therapy on the symptoms and physiologic parameters of dry eye. Adv. Exp. Med. Biol.506(Pt B), 1017–1022 (2002).
- Begley CG, Chalmers RL, Abetz L et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest. Ophthalmol. Vis. Sci.44(11), 4753–4761 (2003).
- Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea23(8), 762–770 (2004).
- Bourcier T, Acosta MC, Borderie V et al. Decreased corneal sensitivity in patients with dry eye. Invest. Ophthalmol. Vis. Sci.46(7), 2341–2315 (2005).
- Lemp MA. Advances in understanding and managing dry eye disease. Am. J. Ophthalmol.146(3), 350–356 (2008).
- Lee JH, Hyun PM. The reproducibility of the Schirmer test. Korean J. Ophthalmol.2(1), 5–8 (1988).
- Cho P, Brown B, Chan I et al. Reliability of the tear break-up time technique of assessing tear stability and the locations of the tear break-up in Hong Kong Chinese. Optom. Vis. Sci.69(11), 879–885 (1992).
- Cho P, Yap M. Schirmer test. II. A clinical study of its repeatability. Optom. Vis. Sci.70(2), 157–159 (1993).
- Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea23(3), 272–285 (2004).
- Sullivan BD, Whitmer D, Nichols KK et al. An objective approach to dry eye disease severity. Invest. Ophthalmol. Vis. Sci.51(12), 6125–6130 (2010).
- Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr. Eye Res.35(7), 553–564 (2010).
- Berta A, Higazy MT, Petricek I et al. Red eye: differential diagnosis & management. Internat. Ophthalmol.28(Suppl. 1), 1–64 (2008).
- Behrens A, Doyle JJ, Stern L et al. Dysfunctional tear syndrome. A Delphi approach to treatment recommendations. Cornea25(8), 900–907 (2006).
- Lemp MA. Management of dry eye disease. Am. J. Manag. Care14(3 Suppl.), S88–S101 (2008).
- No authors listed. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf.5(2), 163–178 (2007).
- Petricek I, Berta A, Higazy MT, Németh J, Prost ME. Hydroxypropyl-guar gellable lubricant eye drops for dry eye treatment. Expert Opin. Pharmacother.9(8), 1431–1436 (2008).
- Pflugfelder SC. Antiinflammatory therapy for dry eye. Am. J. Ophthalmol.137(2), 337–342 (2004).
- Pepose JS, Mujtaba AQ. Refractive surgery and dry eye disease. In: Dry Eye Disease: The Clinician’s Guide to Diagnosis and Treatment. Asbell PA, Lemp MA (Eds). Thieme Medical Publishers, NY, USA, 132–140 (2006).
- Tabbara KF, Frayha RA. Alternate-day steroid therapy for patients with primary Sjögren’s syndrome. Ann. Ophthalmol.15(4), 358–361 (1983).
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology107(4), 631–639 (2000).
- Mori A, Oguchi Y, Goto E et al. Efficacy and safety of infrared warming of the eyelids. Cornea18(2), 188–193 (1999).
- Goto E, Monden Y, Takano Y et al. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br. J. Ophthalmol.86(12), 1403–1407 (2002).
- Matsumoto Y, Dogru M, Goto E et al. Efficacy of a new warm moist air device on tear functions of patients with simple meibomian gland dysfunction. Cornea25(6), 644–650 (2006).
- Mori A, Shimazaki J, Shimmura S, Fujishima H, Oguchi Y, Tsubota K. Disposable eyelid-warming device for the treatment of meibomian gland dysfunction. Jpn J. Ophthalmol.47(6), 578–586 (2003).
- Di Pascuale MA, Liu TS, Trattler W, Tseng SC. Lipid tear deficiency in persistent dry eye after laser in situ keratomileusis and treatment results of new eye-warming device. J. Cataract Refract. Surg.31(9), 1741–1749 (2005).
- Frucht-Pery J, Sagi E, Hemo I, Ever-Hadani P. Efficacy of doxycycline and tetracycline in ocular rosacea. Am. J. Ophthalmol.116(1), 88–92 (1993).
- Zengin N, Tol H, Gunduz K, Okudan S, Balevi S, Endogru H. Meibomian gland dysfunction and tear film abnormalities in rosacea. Cornea14(2), 144–146 (1995).
- Alikhan A, Kurek L, Feldman SR. The role of tetracyclines in rosacea. Am. J. Clin. Dermatol.11(2), 79–87 (2010).
- Akpek EK, Vittitow J, Verhoeven RS et al. Ocular surface distribution and pharmacokinetics of a novel ophthalmic 1% azithromycin formulation. J. Ocul. Pharmacol. Ther.25(5), 433–439 (2009).
- Luchs J. Azithromycin in DuraSite for the treatment of blepharitis. Clin. Ophthalmol.4, 681–688 (2010).
- Foulks GN, Borchman D, Yappert M et al. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea29(7), 781–788 (2010).
- Haque RM, Torkildsen GL, Brubaker K et al. Multicenter open-label study evaluating the efficacy of azithromycin ophthalmic solution 1% on the signs and symptoms of subjects with blepharitis. Cornea29(8), 871–877 (2010).
- Kojima T, Ishida R, Dogru M et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: a prospective randomized case–control study. Am. J. Ophthalmol.139(2), 242–246 (2005).
- Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocul. Surf.8(1), 18–28 (2010).
- Harris W. You are what you eat applies to fish too. J. Am. Diet Assoc.108(7), 1131–1133 (2008).
- Barabino S, Rolando M, Camicione P et al. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea22(2), 97–101 (2003).
- Sullivan DA, Krenzer KL, Sullivan BD, Tolls DB, Toda I, Dana MR. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest. Ophthalmol. Vis. Sci.40(6), 1261–1265 (1999).
- Worda C, Nepp J, Huber JC, Sator MO. Treatment of keratoconjunctivitis sicca with topical androgen. Maturitas37(3), 209–212 (2001).
- Taban M, Chen B, Perry JD. Update on punctal plugs. Compr. Ophthalmol. Update7(5), 205–212 (2006).
- Tai MC, Cosar CB, Cohen EJ, Rapuano CJ, Laibson PR. The clinical efficacy of silicone punctal plug therapy. Cornea21(2), 135–139 (2002).
- McDonald M, D’Aversa G, Perry HD, Wittpenn JR, Donnenfeld ED, Nelinson DS. Hydroxypropyl cellulose ophthalmic inserts (lacrisert) reduce the signs and symptoms of dry eye syndrome and improve patient quality of life. Trans. Am. Ophthalmol. Soc.107, 214–221 (2009).
- Kruse FE, Cursiefen C. Surgery of the cornea: corneal, limbal stem cell and amniotic membrane transplantation. Dev. Ophthalmol.41, 159–170 (2008).
- Henderson HW, Collin JR. Mucous membrane grafting. Dev. Ophthalmol.41, 230–242 (2008).
- Cosar CB, Cohen EJ, Rapuano CJ et al. Tarsorrhaphy: clinical experience from a cornea practice. Cornea20(8), 787–791 (2001).
- Geerling G, Sieg P. Transplantation of the major salivary glands. Dev. Ophthalmol.41, 255–268 (2008).
- Hayashi Y, Ishimaru N, Arakaki R et al. Effective treatment of a mouse model of Sjögren’s syndrome with eyedrop administration of anti-CD4 monoclonal antibody. Arthritis Rheum.50(9), 2903–2910 (2004).
- Cordero-Coma M, Anzaar F, Sobrin L, Foster CS. Systemic immunomodulatory therapy in severe dry eye secondary to inflammation. Ocul. Immunol. Inflamm.15(2), 99–104 (2007).
Website
- Connor CG. Treatment of keratoconjunctivitis sicca with topical androgen. Invest. Ophthalmol. Vis. Sci. 44, (2003) (E-Abstract 2450) http://abstracts.iovs.org/cgi/content/abstract/44/5/2450 (Accessed 16 December 2010)
Dry eye diagnosis and management
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertophth. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Based on the above review by Drs. Módis and Szalai, which of the following statements about the symptoms and underlying pathophysiology of dry eye disease is most likely correct?
□ A Dry eye disease is a rare condition with a single known cause
□ B Symptoms of dry eye disease may include burning, itching, scratching, foreign body sensation, photophobia, and red eye
□ C The pathophysiology of dry eye disease includes tear film instability, hypo-osmolarity, chronic irritation, and inflammation of the ocular surface
□ D Sjögren syndrome is a form of evaporative dry eye
2. Your patient is a 45 year-old white woman complaining of burning and a gritty sensation like sand in the eyes. Based on the above review, which of the following statements is most likely to apply to her diagnostic workup?
□ A Schirmer test measures lid-parallel conjunctival fold (LIPCOF)
□ B Slit-lamp measures aqueous tear production
□ C Staining of the ocular surface can be tested with fluorescein, bengal green, and/or lissamine rose
□ D Noninvasive break-up time and tear osmolarity measurement can help confirm the diagnosis of dry eye
3. Diagnostic workup of the patient in question 2 confirmed the presence of dry eye. Based on the above review, which of the following statements is most likely to apply to her management?
□ A The first line of treatment is topical anti-inflammatory therapy
□ B For moderate dry eye, androgen substitution is needed
□ C Severe dry eye is usually treated successfully with retinol treatment
□ D Underlying systemic disease, if any, should be treated and causative factors eliminated