Abstract
In the past decade there have been many changes in the field of vitreoretinal surgery. Transconjunctival sutureless systems have revolutionized some of the approaches to pars plana vitrectomy as the introduction of smallgauge trocar cannula systems allows entry into the vitreous cavity without dissecting conjunctiva and requires no suture to close routinely at the end of surgery. Smaller ports and sutureless surgery have a number of benefits for the patient and surgeon and hence have been widely adopted. However, there are some concerns that smaller gauge or sutureless vitrectomy surgery could increase the risk of endophthalmitis – a devastating infectious complication of intraocular surgery. The aim of this review is to present the changes that have occurred in vitreoretinal surgery in recent years and to summarize the evidence relating to endophthalmitis following vitrectomy.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/expertop; (4) view/print certificate.
Release date: 31st May 2012; Expiration date: 31st May 2013
Learning objectives
Upon completion of this activity, participants should be able to:
• Evaluate the risk of endophthalmitis after vitrectomy
• Distinguish means to reduce the risk of endophthalmitis after vitrectomy
• Perform adequate interventions for patients with suspected endophthalmitis after vitrectomy
• Assess the microbiology of post-vitrectomy endophthalmitis
Financial & competing interests disclosure
PUBLISHER
Elisa Manzotti,Publisher, Future Science Group, London, UK
Disclosure:Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Charles P Vega, MD,Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine, CA, USA
Disclosure:Charles P. Vega, MD, has disclosed no relevant financial relationships.
AUTHORS
Jonathan C Park,Ophthalmic Specialist Training Registrar, West of England Eye Unit, Royal Devon and Exeter NHS Foundation Trust, Devon, UK
Disclosure:Jonathan C Park has disclosed no relevant financial relationships.
Balasubramanian Ramasamy,Ophthalmic Specialist Training Registrar, Wirral University Teaching Hospital NHS Foundation Trust, Arrowe Park Hospital, Wirral, Merseyside, UK
Disclosure:Balasubramanian Ramasamy has disclosed no relevant financial relationships.
Roland H Ling,Consultant Ophthalmologist, West of England Eye Unit, Royal Devon and Exeter NHS Foundation Trust, Devon, UK
Disclosure:Roland H Ling received sponsorships from Novartis and Allergan for attending conferences.
Som Prasad,MS, FRCSEd, FRCOphth, FACS, Consultant Ophthalmologist, Wirral University Teaching Hospital NHS Foundation Trust, Arrowe Park Hospital, Wirral, Merseyside, UK
Disclosure:Som Prasad, MS, FRCSEd, FRCOphth, FACS, has disclosed no relevant financial relationships.
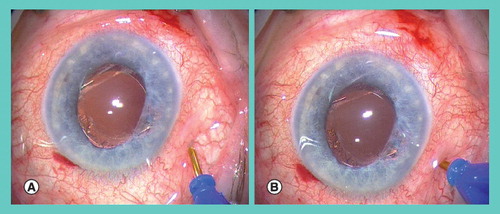
An initial oblique step (A) is followed by a perpendicular step (B). Prior to the first step the conjunctiva is displaced to ensure the conjunctival incision does not overly the scleral incision. After surgery the wound is typically self-sealing and no sutures are required.
Provided courtesy of S Prasad (Consultant Ophthalmic Surgeon, Wirral University Hospitals, UK).
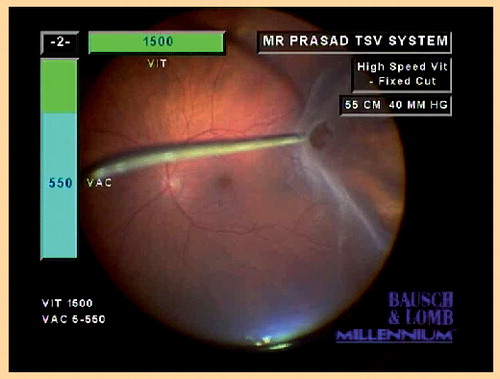
Here the right hand is being used to indent while the left hand controls the ocutome.
Provided courtesy of S Prasad (Consultant Ophthalmic Surgeon, Wirral University Hospitals, UK).
Overview
Vitrectomy, removal of the vitreous gel, is a commonly performed intraocular surgery. As with all intraocular surgeries, vitrectomy carries a risk of endophthalmitis. Endophthalmitis is a devastating intraocular infection that poses a high risk of severe visual loss or loss of the eye itself.
In the past few years, there has been a large shift in practice from the traditional 20-gauge vitrectomy (where both the sclera and conjunctiva are sutured at the end of surgery) to 20-gauge vitrectomy with sutureless self-sealing sclerotomies or to either 23-gauge or 25-gauge vitrectomy (smaller port surgery, which routinely does not require any sutures to close). Various benefits for both the patient and surgeon have been demonstrated for these smaller gauge sutureless vitrectomy systems. This change is not that dissimilar to the parallel historically seen in cataract surgery, where there was a transition from sutured extra-capsular wounds to sutureless clear corneal incisions. However, there is some concern that small-gauge sutureless vitrectomy surgery increases the risk of endophthalmitis and this will be explored in this review.
The aim of this review is to present the changes that have occurred in vitreoretinal surgery in recent years, and to summarize the evidence relating to endophthalmitis following vitrectomy. To achieve this, it is necessary to consider recent vitreoretinal surgical advances, and then to explore the evidence relating to endophthalmitis incidence, risks, presenting clinical features, current management strategies and outcomes.
Recent vitreoretinal surgical advances
Vitreoretinal surgical techniques have continued to advance ever since Jules Gonin, an ophthalmologist from Lausanne, Switzerland, reported his first successful cases of repair in 1925.
Various external approaches to treat retinal detachment such as scleral buckles and cryotherapy soon followed, and in 1968 the first internal approach by Kasner was documented by performing a vitrectomy with an anterior approach for amyloidosis Citation[1]. Unsurprisingly, this, ‘open sky’ approach was fraught with difficulties.
Robert Machemer, in the early 1970s, devised, developed and implemented a technique that would form the foundation of all future posterior, pars plana vitrectomies Citation[2,3]. Machemer’s approach was via the pars plana using a 17-gauge infusion and vitreous infusion suction cutter (VISC) and therefore left the anterior segment relatively intact.
In 1975, O’Malley and Heintz refined this technique by separating the functions of Machemer’s VISC, which allowed a reduction in port size to 20-gauge – one port for the cutter/suction and one port for the infusion Citation[4]. With the introduction of the light pipe (endoillumination) through a third port, the traditional, 20-gauge, three-port pars plana vitrectomy (PPV) procedure became established.
Since then, further attempts to minimize trauma to the eye and shorten surgical time have been dominated by reducing instrumentation size further. There have been other technical advances in retinal surgery, but the key change in practice that relates to the concern of possibly increasing the risk of endophthalmitis is the development of smaller port and sutureless surgery.
In 1990, the first attempt to employ smaller ports (25-gauge, 0.51-mm port diameter) was made by de Juan and Hickingbotham, but it was not commercialized at this time since the equipment available was too flexible Citation[5]. In 1996, Chen first described sutureless sclerotomies whilst using 20-gauge tunnels Citation[6,7]. Fujii, working with de Juan in 2002 then developed a transconjunctival sutureless vitrectomy (TSV) 25-gauge system that has been successfully commercialized Citation[8]. Similarly, in 2005, Eckardt developed a TSV 23-gauge system (0.61-mm port diameter), which has also been widely and successfully commercialized Citation[9]. Even smaller instruments continue to appear, as shown by trials with 27-gauge surgery, but these have not yet been widely adopted Citation[10].
In the past 5 years, the switch in use from 20-gauge, sutured vitrectomies to 20-gauge self-sealing sutureless vitrectomies Citation[6,7] and 23- or 25-gauge TSV represents a landmark in the evolution of vitreoretinal surgery. There is an increasing trend across the globe to use smaller gauge systems, exemplified by the Preferences and Trends survey conducted by the American Society of Retina Specialists finding that in 2004, 48% had never tried small-gauge vitrectomy but by 2007, 80% used it for most of their cases Citation[11]. At the British and Eire Association of Vitreo Retinal Surgeons annual meeting in 2011 approximately a third of surgeons reported using small-gauge vitrectomy for most of their cases Citation[12].
This major shift towards sutureless surgery reflects the acceptance of retinal surgeons that the TSV approach provides equivalent or better primary outcomes than 20-gauge sutured surgery and offers other additional benefits. Various studies have shown that the primary outcomes of the surgery are similar for various surgical indications, and visual outcomes after surgery for TSV has been found to be equivalent or better for rhegmatogenous retinal detachment, macular hole, epiretinal membrane, tractional macular edema and diabetic vitreous hemorrhage Citation[13–21]. Benefits for the patient with TSV relate to there being less surgical trauma resulting in decreased postoperative inflammation Citation[22], reduced postoperative discomfort Citation[23] and reduced surgically induced astigmatism Citation[24,25]. While these benefits are welcomed, it should be remembered that most patients having vitrectomy with the standard 20-gauge surgery do not suffer particularly significant postoperative pain anyway Citation[22], and the alteration in astigmatism tends to return to baseline after 3 months Citation[23].
Another proposed benefit demonstrated by some studies is that TSV reduces the operating time, which increases theater efficiency and also reduces the intraoperative time for the patient Citation[8,16–18]. However, although the time to open/close the ports is reduced, due to the reduced flow through the smaller instruments, the time while operating within the vitreous cavity can be increased, leading to no substantial difference overall in time of procedure Citation[26,27]. The introduction of newer systems with cut rates of up to 5000/min have improved flow rates through small-gauge systems, so the potential for an overall saving in time is finally being delivered.
In addition to TSV systems, other vitreoretinal advances include the blade-shaped trocars for better oblique entry and introduction of improved illumination systems such as the chandelier light, which permits bimanual surgery that is particularly useful for peripheral indentation to search for retinal breaks at the vitreous base (a vital step in all vitrectomy surgery) (see ).
Complications relating to TSV relative to 20-gauge vitrectomy are harder to investigate than the primary surgical outcomes and benefits as described above. There is a concern that TSV may predispose, via wound leak and hypotony, to endophthalmitis and this issue is explored in the next section.
Endophthalmitis following vitrectomy: incidence
The incidence of complications following TSV relative to 20-gauge surgery is more difficult to evaluate, relative to establishing the primary outcome of the surgery, since serious complications are relatively rare and hence large numbers are required to confirm or refute any difference. Furthermore, the lack of long-term follow-up and nonstandardization of technique precluding inter-study comparison complicates interpretation of risk with TSV. A recent evaluation by the American Academy of Ophthalmology concluded that on the basis of level II and level III evidence, the overall safety profile of TSV is similar to that established for conventional 20-gauge vitrectomy and provides comparable visual acuity Citation[28]. The report also highlights the need for continued close surveillance to further assess complications such as endophthalmitis.
It is important to put endophthalmitis following vitrectomy in context with the endophthalmitis rates for other intraocular surgical procedures. A 10-year retrospective study by Aaberg et al. in 1998 was a landmark study that compared endophthalmitis rates for different types of ophthalmic surgery Citation[29]. Overall, 54 patients from 58,123 operations had endophthalmitis, giving an overall rate of approximately one in 1100. The rates were highest for secondary intraocular lens placement (one in 273) and lowest for vitrectomy (one in 2186), which was found to be almost twice as rare as that for cataract surgery (one in 1225) – see . It should be noted, however, that this incidence for endophthalmitis following vitrectomy was based on only three cases from 6557 vitrectomies.
More recently, in 2010 Wykoff et al. reported the endophthalmitis rates for different types of ophthalmic surgery Citation[30]. This study showed that the 8-year frequency of acute-onset postoperative endophthalmitis was 0.025% (14 of 56,672 intraocular surgeries). The rate was 0.028% (eight out of 28,568) for cataract surgery and 0.011% (two out of 18,492) for PPV. Both PPV endophthalmitis cases followed 20-gauge surgery and no cases followed small-gauge, transconjunctival PPV (n = 2262).
Other studies have yielded more cases of endophthalmitis following vitrectomy since larger numbers of operations were retrospectively reviewed Citation[31–36]. Despite several years of observation, only a handful of cases are observed, not only owing to the rarity of the complication, but also owing to the study area being relatively small and the concern that with retrospective review cases are missed. Retrospective studies estimating the rate of endophthalmitis following 20-gauge sutured vitrectomy, prior to the mainstream introduction of TSV, are summarized in .
The first case of endophthalmitis following TSV was following 25-gauge vitrectomy, reported by Taylor and Aylward in 2005 Citation[37]. For 25-gauge TSV, several retrospective studies have followed this and these are summarized in Citation[38–44].
In 2007, Shaikh et al. had two cases of endophthalmitis following 129 consecutive 25-gauge TSV’s Citation[38]. This alarming rate of one in 65 is perhaps explained by the nature of the two patients involved. The first was an eye-rubber who was noncompliant with drops. The second was also noncompliant with drops and following the surgery drank alcohol to excess and passed out to awake with grease on his hands. Kunimoto and Kaiser found the rate to be 12.5-times higher for 25-gauge TSV relative to 20-gauge in their same series Citation[39]. Seven of their 3103 TSV patients developed endophthalmitis (incidence one in 443), relative to only one in 5498 for 20-gauge surgery. The authors highlight that the relatively high rate could represent the tendency of rare events such as endophthalmitis to occur in clusters (cluster bias) and demonstrate a need for a study that covers a greater geographical area over a longer period. The authors suggested sclerotomy modification to reduce the risk of infection, first by the use of bevelled rather than straight wounds and also to first displace the conjunctiva from the sclera so the entry holes are not aligned and thus improving wound integrity. Chen et al. found a seven-times increased risk of endophthalmitis with 25-gauge TSV (one per 431) relative to 20-gauge (one per 3046) but their series was too small for this trend to reach statistical significance Citation[40]. In 2008, Scott et al. also found an increased rate of endophthalmitis following 25-gauge TSV (11 per 1307 giving an incidence of one in 119) relative to 20-gauge vitrectomy (two per 6375 giving an incidence of one in 3188) – a 27-times increased rate of infection Citation[41]. Of these 11 cases, eight had straight incisions. In 2011, Scott et al. provided an update on their incidence of endophthalmitis after TSV after switching to angled rather than straight incisions and also displacing the conjunctiva – relative to their prior series the rate was lower for 25-gauge (one in 789), and no difference was observed between 20-gauge (one in 4403) versus 23-gauge (one in 3362) Citation[42].
The concept that angled relative to straight incisions are protective against infection is further supported by Shimada et al. who were the first to find no difference in endophthalmitis rates between 20-gauge (one per 3592) and 25-gauge TSV (one per 3343) Citation[43]. Although subset analysis for straight versus angled incision for 25-gauge TSV failed to show a statistically significant result, there was a trend that angled incision was protective, given that of the 2801 eyes that had 25-gauge angled incisions no patients had infection, whereas for straight incisions the endophthalmitis rate was one in 542. Other operative steps implemented to reduce infection risk highlighted by the authors were irrigation of the conjunctiva, rigorous checking of ports at end of surgery and completing a peripheral vitrectomy to prevent harboring pathogens in a peripheral vitreous skirt and to reduce risk of a vitreous wick.
Hu et al. subsequently also found no increased rate of endophthalmitis in their series of 25-gauge TSV (one per 1424) versus 20-gauge (none per 1948) Citation[44]. The authors propose that the relatively low rate of endophthalmitis in their series could be attributed to two surgical steps. First, at the end of the procedure, subconjunctival antibiotic steroid is injected specifically over the sclerotomy ports to both deliver antibiotics to the eye and also reposit any vitreous wick that may have prolapsed. Secondly, air or gas is liberally used to provide an endotamponade to oppose hypotony and wound leak (even if the case otherwise would not require such tamponade). This, to some degree, is supported by the observation that all cases in Kunimoto and Kaiser’s series had no endotamponade Citation[39]. However, in the latter series of three endophthalmitis cases reported by Scott et al. in 2011 Citation[42], two of these cases had an endotamponade. It is therefore not convincing that endotamponade is an effective way to reduce the risk of endophthalmitis.
There are fewer studies available that have examined endophthalmitis following 23-gauge TSV. Fine et al., 2007 while reporting on initial experiences with 23-gauge TSV disclosed a case of presumed infectious endophthalmitis (one per 77 cases) Citation[45]. Somewhat reassuringly, Parolini et al. had no cases of endophthalmitis following 23-gauge TSV in 943 consecutive operations Citation[46] and Scott et al. only had one case in 3362 operations for 23-gauge Citation[42]. By contrast, Haas et al. had one case of endophthalmitis following 23-gauge TSV in only 64 consecutive operations Citation[47].
Endophthalmitis following vitrectomy: risk factors
There are both theoretical and epidemiological concerns that TSV may increase the risk of endophthalmitis. Theoretical concerns that TSV could predispose to endophthalmitis are highlighted in Citation[48]. Laboratory studies on human cadaveric eyes and rabbit eyes have demonstrated that sutured 20-gauge sclerotomies are the most secure, and that sutureless sclerotomy leakage for 23- and 25-gauge can be minimized by angled (oblique) incision rather than straight incision Citation[49–51].
Epidemiological concerns that TSV may increase the risk of endophthalmitis have arisen due to the lack of consistency with the patterns noted in observational studies. The studies available have made valiant efforts to attempt to establish the incidence and risk factors for endophthalmitis following vitrectomy. They are, however, limited by their retrospective nature, relatively small numbers and coverage of only a small geographical area. Inter-study comparison is limited owing to the lack of standardization of endophthalmitis diagnostic criteria (such as culture status) and inclusion criteria (ideally confounders such as open globe trauma, intraocular foreign body and dropped nuclear fragment should be excluded).
It has been postulated that postoperative hypotony can be a marker for subclinical wound leak (hypotony in the presence of a secure scleral and conjunctival wound) and therefore potentially increase the risk of endophthalmitis. It has been demonstrated in a real clinical setting with postoperative optical coherence tomographic analysis of the sutureless sclerotomy wounds, that postoperative intraocular pressure is higher with good wound closure Citation[52]. Clinicians should therefore be wary of postoperative hypotony since this could be a marker of wound leak. It may be necessary to return to theater to reassess the wound and close more securely with sutures. Intraoperatively, it remains crucial to assess wound integrity, and if any doubt of wound leak is present then the sclerotomy should be sutured. Moreover, the changing concept regarding the remnant vitreous skirt in sutureless small-gauge vitrectomy leading to vitreous incarceration in the wound can help in preventing hypotony but can potentially be a risk factor for infection Citation[48].
Also of note is the fact that in 20-gauge vitrectomy the opening of the wound is 20-gauge for 20-gauge instruments, but in 23- or in 25-gauge vitrectomy the scleral wound is larger, as this depends on the trocars.
The safest way to close a leaking TSV sclerotomy is unknown. One option is to simply pass one suture through both conjunctiva and sclera. Alternatively, a conjunctival peritomy can be performed and then the scleral and conjunctival openings are closed in separate layers. The former avoids conjunctival opening, but with this single suture technique there is a suture exposed on the ocular surface that is linked to a partial thickness scleral tract and this could potentially provide a pathway for pathogens.
Use of new forceps both intraoperatively and also to remove trocars at the end of surgery have been reported by Bartz-Schmidt et al. in April 2011, which appears to aid better wound closure after 23-gauge surgery Citation[53].
Evidence for the role of intraocular triamcinolone during vitrectomy as a risk factor for postoperative endophthalmitis has been conflicting with case reports of Staphylococcus epidermidis-associated endophthalmitis, which are challenged by a multicenter study by Sakamoto et al. in 2004 showing a very low incidence of one in 1886 (0.053%) Citation[54–56].
In addition to the above factors being proposed as a possible risk factor for endophthalmitis following vitrectomy, there is concern that diabetes mellitus increases the risk of infection following vitrectomy. Theoretically this is possible for two reasons. Firstly, those with diabetes mellitus are systemically immunocompromised and it is well accepted for other infections (such as cellulitis or wound infections) that diabetes increases the risk. Secondly, if the operation is for proliferative diabetic retinopathy requiring challenging procedures such as delamination, the operation is likely to be longer with more instruments passing in and out of the eye and therefore locally increasing the risk of infection directly. There is a recurrent trend in several studies where the proportion of patients with endophthalmitis following vitrectomy are more likely to have diabetes than the proportion of the study sample itself – see Citation[31–36,39–41,43]. However, in the same study set, these cases are not compared with controls (patients having vitrectomy but not getting endophthalmitis), so no clear statistically significant link between diabetes and endophthalmitis has been demonstrated.
By contrast, other studies have found no relationship between endophthalmitis following vitrectomy and diabetes mellitus. None of the three cases from Scott et al., 2011 Citation[42] had diabetes and neither of the two cases reported by Shaikh et al., 2007 Citation[38] had diabetes. Interestingly, for cataract surgery diabetes mellitus has not been shown to increase risk the risk of endophthalmitis, but other forms of immunosuppression (such as malignancy or blood dyscrasia) do – as established by a large scale, nationwide prospective study Citation[57]. However, it is likely that the severity of diabetes is greater in patients undergoing vitrectomy relative to cataract surgery, since those patients having vitrectomy for diabetic eye complications have advanced diabetes. Overall, the evidence suggests diabetes is probably a risk factor for endophthalmitis following vitrectomy – but to what extent, and whether or not extra precautions should be taken, remains unknown.
Endophthalmitis following vitrectomy: clinical features & microbiology data
It is well accepted that high-risk cases for subsequent infection following vitrectomy include trauma (if open globe), intraocular foreign body and endophthalmitis itself. In these cases, intraocular contamination by pathogens has already occurred prior to the vitrectomy. Operating for these cases is, however, relatively rare. The main indications for vitrectomy are retinal detachment, macular hole, epi-retinal membrane and proliferative diabetic retinopathy such as vitreous hemorrhage. For these procedures, like other intraocular surgery, it is likely that contamination of the eye with pathogens occurs at the time of surgery Citation[58]. Tominaga et al. in 2010 showed that transconjunctival 25-gauge microincision vitrectomy surgery had a significantly (p = 0.007) higher incidence of vitreous contamination at the beginning of surgery compared with conventional 20-gauge PPV and identified Propionibacterium acnes as the most common isolate Citation[59]. Clearly, the origin of the pathogens is likely to be the conjunctiva and lashes, hence the importance of flooding the fornix with povidone-iodine and subsequent conjunctival irrigation to reduce the bacterial load. Complicated surgery is more likely to increase the risk of infection due to prolonged operating time and due to the need for more manipulation of the eye thereby, increasing the risk of bacterial contamination at the time of surgery. It has been demonstrated in rabbit eyes that inoculation of the vitreous with only five bacteria (Pseudomonas aeruginosa) results in endophthalmitis, whereas inoculation of the anterior chamber with 50 million bacteria does not Citation[60,61]. Therefore, contamination of the vitreous is more likely to result in endophthalmitis than contamination of the anterior chamber, probably because the vitreous is avascular and relatively isolated from humoral immunity mechanisms Citation[60].
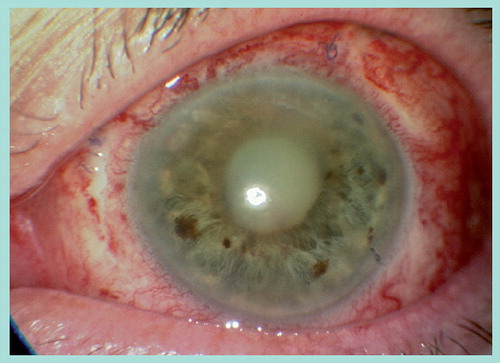
It therefore follows that one can expect endophthalmitis following vitrectomy to occur within the first few days of surgery, given that contamination of the intraocular structures probably occurs at the time of surgery. This does appear to be the case, as with the majority of cases reported from the studies discussed above. Rather than having a mildly sore eye that gradually settles, patients experience acutely progressive eye redness, pain and blurring. Blurring may be less prominent with vitrectomy surgery relative to cataract surgery, because if an endotamponade has been used the vision is already blurred. Typically patients have a red anterior segment with a hypopyon and it is not uncommon to have an absent red reflex due to vitreous opacities (see ).
It remains uncertain whether or not patients with sutureless vitrectomy present later with endophthalmitis, since in eyes with hypotony and wound leak the pathogenesis may be early postoperative intraocular contamination with pathogens rather than at the time of surgery. The clinician should therefore have a very high index of concern for endophthalmitis following vitrectomy in patients presenting within the first few weeks with progressive redness, pain and blurring associated with a red eye and hypopyon. It is safer to treat as presumed infectious endophthalmitis and treat with immediate intravitreal antibiotics, rather than assuming an over-zealous sterile inflammation and treating with steroids.
Various studies have documented the microbiological profile of organisms implicated in causing endophthalmitis following vitrectomy. Elfrig et al. in 2004 in their 20-year study interval, showed that the overall incidence rate of post vitrectomy endophthalmitis was 0.039% (six out of 15,326) Citation[34]. Cultured organisms were S. aureus (n = 3), Proteus mirabilus (n = 1), and Staphylococcus epidermidis/P. aeruginosa (n = 1) Citation[34]. Other organisms which have been implicated are Enterococcus and Bacillus cereus. Review of the current evidence shows that S. aureus (coagulase negative) is the most common organism isolated from cases of postvitrectomy endophthalmitis Citation[62–66].
Endophthalmitis following vitrectomy: management & outcome
There are various treatment options available to the clinician faced with the devastating complication of endophthalmitis following vitrectomy. Unfortunately, despite this, the outcome is typically poor (see ) and worse than endophthalmitis following cataract surgery Citation[29,57]. Studies looking at visual outcomes show that final visual acuity in patients with post-vitrectomy endophthalmitis is variable with Scott et al. in 2008 reporting eight patients out of 13 had final visual acuity of ≥20/400, and four had visual acuity of ≥20/63 Citation[41]. On the other hand, Elfrig et al. in 2004 reporting visual acuity after treatment for endophthalmitis, which ranged from 2/200 to no light perception, with a final vision of light perception or no light perception in four of six (67%) eyes Citation[34]. The reason for the worse outcome in eyes undergoing vitrectomy relative to cataract surgery is firstly due to the eyes having less visual potential (limited by conditions such as diabetic maculopathy or prior retinal detachment) but also since the observed intraocular infection is more severe, perhaps reflecting posterior compartment contamination rather than intracameral compartment contamination.
Perhaps the most important point is to not assume sterile inflammation, but rather to recognize and immediately treat the patient for presumed infectious endophthalmitis. Therefore, if the patient did have endophthalmitis the eye may be salvaged, and if it was a sterile inflammation then it is unlikely that significant harm would have been endured by treating with immediate intra-vitreal antibiotics. Conversely, delayed treatment of endophthalmitis due to the presumption that the patient has sterile inflammation is catastrophic.
The studies discussed above report the different treatment options for endophthalmitis following vitrectomy. Firstly, and most importantly, prompt intra-vitreal antibiotics should be given. These antibiotics should cover both Gram-positive and Gram-negative bacteria, and ideally be prepared by the pharmacy in sterile conditions and readily available to the clinician. At the same time, prior to antibiotic injection a vitreous cavity sample and anterior chamber sample should be sent safely for immediate microscopy, culture, sensitivity and PCR (for the likely pathogens). It may not always be possible to obtain a vitreous cavity sample – for example if the cavity is filled with silicone oil, but attempts should be made to do so, and failing that a sample from the anterior chamber can always be taken. A subsequent negative culture does not preclude a diagnosis of infectious endophthalmitis, since the samples are challenging to obtain and process and can therefore yield no positive result despite there clearly being an infectious process clinically. The proportion of culture-positive cases in previous studies is approximately 70% with a range of 0 to 100% – see Citation[29,32,34–36,38,40–43,62].
Other treatment options reported in the above studies include topical antibiotics, systemic antibiotics, revision vitrectomy and systemic steroids. Given the rarity of cases it is not possible to know the importance of these extra measures, and their risks and benefits must be considered for each unique case – a challenging situation for the clinician.
Expert commentary
It is an exciting time for the vitreoretinal surgeon, given the recent explosion of new equipment that has become available following technological advances. Although the principles are unchanged, the style of vitrectomy surgery has advanced dramatically in the past few years.
Surgeons should be wary that with these new techniques come new complications, and altered risk for pre-existing complications. With respect to endophthalmitis, it is likely that with the introduction of sutureless surgery the rate has remained acceptably low, but surgeons should discuss with their patients that the evidence currently available is conflicting and limited. Given that endophthalmitis is a disastrous complication, and given that vitrectomy is a commonly performed procedure (approximately 20,000 per year in England, UK, according to Department of Health’s Hospital Episode Statistics data), it would only take a small increased risk of this complication to result in significant visual morbidity. This current uncertainty should be balanced against the benefits of TSV and incorporated into patient consent for vitrectomy surgery. This should not preclude the use of TSV, but rather take the appropriate steps to minimize infection and actively engage in the reporting of adverse events to the vitreoretinal community.
The authors of this review are currently conducting a large-scale prospective study to further investigate endophthalmitis following vitrectomy. Two years of prospective surveillance across the UK in association with the British Ophthalmic Surveillance Unit (BOSU) is almost complete. The BOSU provides an excellent infrastructure to prospectively investigate rare problems. All independent Ophthalmologists in the UK are sent a, ‘Gold card’, which contains a few rare conditions under surveillance, which, if observed, should be reported to the Royal College of Ophthalmologists who then pass this report information to the relevant study team. Over the past 10 years, BOSU has helped evaluate numerous rare conditions, including endophthalmitis following cataract surgery Citation[51], and maintains an excellent reporting rate of approximately 70% established with each study having validation centers where the external reporting rate is compared with in-house documentation of these rare events. Such surveillance will clarify the clinical presentation, incidence, current management and outcome of endophthalmitis following vitrectomy. In addition, these cases of endophthalmitis following vitrectomy are to be compared with control patients randomly selected from across the UK in order to establish risk factors for endophthalmitis following vitrectomy and in particular whether or not TSV surgery and diabetes are significant risk factors for this devastating complication. Such a nationwide and prospective style of study design will hopefully significantly improve the evidence base for this subject.
Five-year view
Hopefully the outcome of the nationwide, 2-year prospective surveillance of endophthalmitis following vitrectomy in the UK will provide a more reliable evidence base for this disastrous complication. A desired finding will be that the study shows that sutureless vitrectomy does not significantly increase the risk of endophthalmitis, since the other benefits that this technique provides are welcomed by both surgeons and patients. If it does not, then the degree of increased risk will have to be balanced with the other risks and benefits that such surgery can provide. The study may also clarify to what degree diabetes increases the risk of endophthalmitis. This is important to aid informed consent for patients with diabetes receiving vitrectomy surgery and also raises questions as to whether or not additional steps should be taken to minimize the risk of infection in patients with diabetes. For example, this may involve stricter glycemic control during the perioperative period, which could possibly necessitate preoperative admission and hospital physician led control of the diabetes.
In a few years’ time some of the pending questions will no doubt be answered, but many more will be then be asked, and the evidence base to deal with them will no doubt remain lacking. For example, it is unlikely that epidemiological evidence will be available to advise upon the technique in which a leaking transconjunctival port should be sutured intraoperative (single pass through conjunctiva and sclera vs conjunctival peritomy and closure of sclera and conjunctiva in separate layers). Similarly, it is unlikely that the ‘safe’ limit of glycemic control required prior to vitrectomy in patients with diabetes will be universally established.
As vitreoretinal techniques evolve and outcomes continue to improve, the threshold for vitrectomy will continue to fall. However, this trend should not go unchecked, since severe visual loss due to infection will unfortunately always be a possibility.
Table 1. Endophthalmitis rates following different types of intraocular surgery.
Table 2. Retrospective studies estimating the incidence of endophthalmitis following 20-gauge sutured vitrectomy, prior to the mainstream introduction of transconjunctival sutureless vitrectomy.
Table 3. Retrospective studies estimating the incidence of endophthalmitis following 25-gauge, transconjunctival sutureless vitrectomy, and the relative risk when compared with 20-gauge vitrectomy incidence within the same study.
Table 4. Theoretical concerns relating to how transconjunctival sutureless surgery may predispose to endophthalmitis.
Table 5. Trends suggesting diabetes mellitus is a risk factor for endophthalmitis following vitrectomy.
Table 6. Proportion of eyes with positive cultures and final visual outcome following endophthalmitis following vitrectomy.
Key issues
• In the past few years there has been a vitreoretinal revolution: a major shift towards the use of smaller-gauge surgery and transconjunctival sutureless vitrectomy (TSV) has occurred.
• TSV provides a number of benefits such as reduced postoperative inflammation, discomfort and astigmatism, and possibly reduced operating time.
• TSV has been found by some studies, in both a laboratory and clinical epidemiological setting, to increase the risk of endophthalmitis. This conflicts with other epidemiological studies that have found no increased risk relative to the standard 20-gauge sutured vitrectomy.
• It is likely that povidone-iodine, conjunctival irrigation, conjunctival displacement, stepped or angled (not straight) sclerotomy incisions and strict intraoperative checking for wound leak reduce the risk of endophthalmitis following vitrectomy. Using gas or air endotamponade to reduce wound leak and hypotony is controversial. The safest way to close a leaking TSV port is unknown.
• It is likely that diabetes mellitus is a risk factor for endophthalmitis following vitrectomy since this trend has been observed, but this remains to be established by a prospective case–control study.
• If diabetes is a risk factor for endophthalmitis following vitrectomy, then it may be necessary in the future to take additional steps to reduce this risk.
• All patients should be aware that vitrectomy surgery carries a small risk of endophthalmitis and unfortunately the outcome following this devastating complication is typically poor.
• The awaited 2-year prospective, nationwide (UK) surveillance and case–control study in association with the British Ophthalmic Surveillance Unit should clarify the clinical presentation, incidence, risk factors, current management and outcome of endophthalmitis following vitrectomy.
References
- Kasner D, Miller GR, Taylor WH, Sever RJ, Norton W. Surgical treatment of amyloidosis of the vitreous. Trans. Am. Acad. Ophthalmol. Otolaryngol.72, 410–418 (1968).
- Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans. Am. Acad. Ophthalmol. Otolaryngol.75, 813–820 (1971).
- Machemer R, Parel JM, Norton EW. Vitrectomy: a pars plana approach – technical improvements and further results. Trans. Am. Acad. Ophthalmol. Otolaryngol.76, 462–466 (1972).
- O’Malley C, Heintz RM. Vitrectomy with an alternative instrument system. Ann. Ophthalmol.7, 585–589 (1975).
- de Juan E Jr, Hickingbotham D. Refinements in microinstrumentation for vitreous surgery. Am. J. Ophthalmol.109, 218–220 (1990).
- Chen JC. Sutureless pars plana vitrectomy through self-sealing sclerotomies. Arch. Ophthalmol.114, 1273–1275 (1996).
- Schmidt J, Nietgen GW, Brieden S. Self-sealing, sutureless sclerotomy in pars plana vitrectomy. Klin. Monatsbl. Augenheilkd.215(4), 247–251 (1999).
- Fujii GY, de Juan E Jr, Humayun MS et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology109, 1807–1813 (2002).
- Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina25, 208–211 (2005).
- Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y. A 27 gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology117(1), 93–102 (2010).
- Mittra RS, Pollack JS. Preferences and Trends Survey. Poster presented at: 25th Annual American Society of Retina Specialists Meeting. Indian Wells, CA, USA, 1–5 December 2007.
- Prasad S, Soni M. BEAVRS 2011 Survey. Poster presented at: The British and Eire Association of Vitreo-Retinal Surgeons Annual Meeting. Canterbury, UK, 10–11 November 2011.
- Sayed KM, Naito T, Farouk MM et al. Twenty five-gauge sutureless vitrectomy versus 20-gauge vitrectomy in epiretinal membrane surgery. J. Med. Invest.59(1–2), 69–78 (2012).
- Ibarra MS, Hermel M, Prenner JL, Hassan TS. Longer-term outcomes of transconjunctival sutureless 25-gauge vitrectomy. Am. J. Ophthalmol.139, 831–836 (2005).
- Patelii F, Radice P, Zumbo G, Frisone G, Fasolino G. 25-gauge macular surgery: results and complications. Retina27(6), 750–754 (2007).
- Lakhanpal RR, Humayun MS, de Juan E Jr et al. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology112, 817–824 (2005).
- Hikichi T, Matsumoto N, Ohtsuka H et al. Comparison of one-year outcomes between 23- and 20-gauge vitrectomy for preretinal membrane. Am. J. Ophthalmol.147, 639–643 (2009).
- Misra A, Ho-Yen G, Burton RL. 23-gauge sutureless vitrectomy and 20-gauge vitrectomy: a case series comparison. Eye23, 1187–1191 (2009).
- Tewari A, Shah GK, Fang A. Visual outcomes with 23-gauge transconjunctival sutureless vitrectomy. Retina28, 258–262 (2008).
- Tsang CW, Cheung BT, Lam RF et al. Primary 23-gauge transconjunctival sutureless vitrectomy for rhegmatogenous retinal detachment. Retina28, 1075–1081 (2008).
- Von Fricken MA, Kunjukunju N, Weber C, Ko G. 25-Gauge sutureless vitrectomy versus 20-gauge vitrectomy for the repair of primary rhegmatogenous retinal detachment. Retina29, 444–450 (2009).
- Inoue Y, Kadonosono K, Yamakawa T et al. Surgically induced inflammation with 20-, 23-, 25-gauge vitrectomy systems: an experimental study. Retina29, 477–480 (2009).
- Wickham L, Bunce C, Kwan AS, Bainbridge J, Aylward GW. A Pilot randomised controlled trial comparing the post-operative pain experience following vitrectomy with a 20-gauge system and the 25-gauge transconjunctival system. Br. J. Ophthalmol.94(1), 36–40 (2010).
- Citirik M, Batman C, Bicer T, Zilelioglu O. Keratometric alterations following the 25-gauge transconjunctival sutureless pars plana vitrectomy versus the conventional pars plana vitrectomy. Clin. Exp. Optom.92, 416–420 (2009).
- Galway G, Drury B, Cronin BG, Bourke RD. A comparison of induced astigmatism in 20- vs 25-gauge vitrectomy procedures. Eye24, 315–317 (2010).
- Kellner L, Wimpissinger B, Stolba U, Brannath W, Binder S. 25-gauge vs 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br. J. Ophthalmol.91, 945–948 (2007).
- Wimpissinger B, Kellner L, Brannath W et al. 23-gauge versus 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br. J. Ophthalmol.92, 1483–1487 (2008).
- Recchia FM, Scott IU, Brown GC et al. Ophthalmic technology assessment. Small gauge pars plana vitrectomy. A report by the American Academy of Ophthalmology. Ophthalmology117, 1851–1857 (2010).
- Aaberg TM Jr, Flynn HW Jr, Schiffman J, Newton J. Nosocomial acute-onset postoperative endophthalmitis survey. Ophthalmology105(6), 1004–1010 (1998).
- Wykoff CC, Parrott MB, Flynn HW Jr et al. Nosocomial acute-onset postoperative endophthalmitis at a university teaching hospital (2002–2009). Am. J. Ophthalmol.150(3), 392–398 (2010).
- Ho PC, Tolentino FI. Bacterial endophthalmitis after closed vitrectomy. Arch. Ophthalmol.102(2), 207–210 (1984).
- Cohen SM, Flynn HW Jr, Murray TG, Smiddy WE. Endophthalmitis after pars plana vitrectomy. The post vitrectomy endophthalmitis study group. Ophthalmology102(5), 702–712 (1995).
- Zhang S, Ding X, Hu J, Gao R. Clinical features of endophthalmitis after vitreoretinal surgery. Yan Ke Xue Bao19, 39–43 (2003).
- Eifrig CW, Scott IU, Flynn HW Jr, Smiddy WE, Newton J. Endophthalmitis after pars plana vitrectomy: incidence, causative organisms, and visual acuity outcomes. Am. J. Ophthalmol.138(5), 799–802 (2004).
- Joondeph BC, Blanc JP, Polkinghorne PJ. Endophthalmitis after pars plana vitrectomy: a New Zealand experience. Retina25(5), 587–589 (2005).
- Mollan SP, Mollan AJ, Konstantinos C, Durrani OM, Butler L. Incidence of endophthalmitis following vitreoretinal surgery. Int. Ophthalmol.29, 203–205 (2009).
- Taylor SR, Aylward GW. Endophthalmitis following 25-gauge vitrectomy. Eye (Lond.)19(11), 1228–1229 (2005).
- Shaikh S, Ho S, Richmond PP, Olson JC, Barnes CD. Untoward outcomes in 25-gauge versus 20-gauge vitreoretinal surgery. Retina27, 1048–1053 (2007).
- Kunimoto DY, Kaiser RS. Incidence of endophthalmitis after 20 and 25 gauge vitrectomy. Ophthalmology112(12), 2133–2137 (2007).
- Chen JK, Khurana RN, Nguyen QD, Do DV. The incidence of endophthalmitis following transconjunctival sutureless 25 vs 20 gauge vitrectomy. Eye23(4), 780–784 (2009).
- Scott IU, Flynn HW, Dev S et al. Endophthalmitis after 25 gauge and 20 gauge pars plana vitrectomy: incidence and outcomes. Retina28(1), 138–142 (2008).
- Scott IU, Flynn Jr HW, Acar N et al. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol.249, 377–380 (2011).
- Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Incidence of endophthalmitis after 20 and 25 gauge vitrectomy: causes and prevention. Ophthalmology115(12), 2215–2220 (2008).
- Hu AYH, Bourges JL, Shah SP et al. Endophthalmitis after pars plana vitrectomy. Ophthalmology116, 1360–1365 (2009).
- Fine HF, Iranmanesh R, Iturralde D, Spaide RF. Outcomes of 77 consecutive cases of 23 gauge transconjunctival vitrectomy surgery for posterior segment disease. Ophthalmology114, 1197–1200 (2007).
- Parolini B, Romanelli F, Prigione G, Pertile G. Incidence of endophthalmitis in a large series of 23-gauge and 20-gauge transconjunctival pars plana vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol.247, 895–898 (2009).
- Haas A, Seidel G, Steinbrugger I et al. Twenty-three-gauge and 20-gauge vitrectomy in epiretinal membrane surgery. Retina30, 112–116 (2010).
- Aylward GW. Sutureless vitrectomy. Ophthalmologica225, 67–75 (2011).
- Gupta OP, Maguire JI, Eagle RC Jr, Garg SJ, Gonye GE. The competency of pars plana vitrectomy incisions: a comparative histologic and spectrophotometric analysis. Am. J. Ophthalmol.147(2), 243–250 (2009).
- Taban M, Ventura AA, Sharma S, Kaiser PK. Dynamic evaluation of sutureless vitrectomy wounds: an OCT and histopathology study. Ophthalmology115, 2221–2228 (2008).
- Singh RP, Bando H, Brasil OF, Williams DR, Kaiser PK. Evaluation of wound closure using different incision techniques with 23-gauge and 25-gauge microincision vitrectomy systems. Retina28, 242–248 (2008).
- Chen D, Lian Y, Cui L, Lu F, Ke Z, Song Z. Sutureless vitrectomy incision architecture in the immediate post-operative period evaluated in vivo using optical coherence tomography. Ophthalmology117(10), 2003–2009 (2010).
- Leitritz MA, Spitzer MS, Bartz-Schmidt KU. New forceps for 1-step valve-free 23-gauge vitrectomy. Retina.31(4), 810–811 (2011).
- Sakamoto T, Enaida H, Kubota T et al. Incidence of acute endophthalmitis after triamcinolone-assisted pars plana vitrectomy. Am. J. Ophthalmol.138(1), 137–138 (2004).
- Yamashita T, Doi N, Sakamoto T. Weak symptoms of bacterial endophthalmitis after a triamcinolone acetonide-assisted pars plana vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol.242(8), 679–681 (2004).
- Mitamura Y, Miyano N, Ohtsuka K. Bacterial endophthalmitis after triamcinolone acetonide-assisted pars plana vitrectomy. Jpn J. Ophthalmol.49(6), 538–539 (2005).
- Kamalarajah S, Ling R, Silvestri G et al. Presumed infectious endophthalmitis following cataract surgery in the UK: a case–control study of risk factors. Eye21, 580–586 (2007).
- Leong JK, Shah R, McCluskey PJ, Benn RA, Taylor RF. Bacterial contamination of the anterior chamber during phacoemulsification cataract surgery. J. Cataract Refract. Surg.28, 826–833 (2002).
- Tominaga A, Oshima Y, Wakabayashi T et al. Bacterial contamination of the vitreous cavity associated with transconjunctival 25-gauge microincision vitrectomy surgery. Ophthalmology117(4), 811–817 (2010).
- Hatano H. Experimental Pseudomonas endophthalmitis in rabbits: intracameral inoculation of two pseudomonal strains. Nippon Ganka Gakkai Zasshi86, 839–845 (1982).
- Hatano H, Sasaki T, Tanaka N. Pseudomonas endophthalmitis in rabbits – intravitreal inoculation of two pseudomonal strains. Nippon Ganka Gakkai Zasshi92, 1758–1764 (1988).
- Abi-Ayad N, Gambrelle J, Duquesne N, Fleury J, Grange JD, Kodjikian L. [Endophthalmitis after plana vitrectomy: incidence, microbiology and visual outcomes]. J. Fr. Ophthalmol.30(4), 397–402 (2007).
- Wu L, Berrocal MH, Arévalo JF, Carpentier C et al. Endophthalmitis after pars plana vitrectomy: results of the Pan American Collaborative Retina Study Group. Retina31(4), 673–678 (2011).
- Oshima Y, Kadonosono K, Yamaji H et al. Multicenter survey with a systematic overview of acute-onset endophthalmitis after transconjunctival microincision vitrectomy surgery. Japan Microincision Vitrectomy Surgery Study Group. Am. J. Ophthalmol.150(5), 716–725, e1 (2010).
- Wani VB, Al Sabti K, Kumar N et al. Endophthalmitis after vitrectomy and vitrectomy combined with phacoemulsification: incidence and visual outcomes. Eur. J. Ophthalmol.19(6), 1044–1049 (2009).
- Taban M, Ufret-Vincenty RL, Sears JE. Endophthalmitis after 25-gauge transconjunctival sutureless vitrectomy. Retina26(7), 830–831 (2006).
A review of endophthalmitis following vitrectomy
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/expertop. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. You are seeing a 62-year-old woman referred to your clinic for possible vitrectomy for proliferative diabetic retinopathy. You explain the risk of complications associated with vitrectomy, including endophthalmitis. Overall, what can you tell her regarding the risk of endophthalmitis associated with vitrectomy?
□ A Endophthalmitis occurs more commonly with vitrectomy compared with secondary lens placement
□ B Endophthalmitis occurs more commonly with vitrectomy than any other ocular surgery
□ C Nearly all cases of endophthalmitis associated with vitrectomy resolve without any long-term effects
□ D Endophthalmitis associated with vitrectomy is associated with worse outcomes than endophthalmitis associated with cataract surgery
2. Which of the following statements regarding interventions to reduce this patient’s risk of endophthalmitis following vitrectomy is most accurate?
□ A Conjunctival displacement should reduce the risk of endophthalmitis
□ B Intraocular triamcinolone is now a standard of care during vitrectomy to prevent endophthalmitis
□ C Endotamponade should reduce the risk of endophthalmitis
□ D Postoperative hypotony is associated with a lower risk of endophthalmitis
3. The patient undergoes vitrectomy but then returns 2 days later with significant redness and pain in the eye. What intervention is appropriate at this time?
□ A Treatment with topical antibiotics only
□ B No intervention given the high probability of sterile inflammation
□ C Treatment with intravitreal antibiotics
□ D Treatment with systemic steroids
4. You perform a vitreous cavity sample in this patient. What is the most common organism isolated from patients with endophthalmitis after vitrectomy?
□ ACorynebacterium diphtheriae
□ BStaphylococcus epidermidis
□ CStaphylococcus aureus
□ DPseudomonas aeruginosa