One MRM run per patient blood sample can measure many cancer biomarkers at the same time. MRM assays use less than 20 µl of blood.
AFP: α-fetoprotein; BTA: Bladder tumor antigen; CEA: Carcinoembryonic antigen; NSE: Neuron-specific enolase; PSA: Prostate-specific antigen.
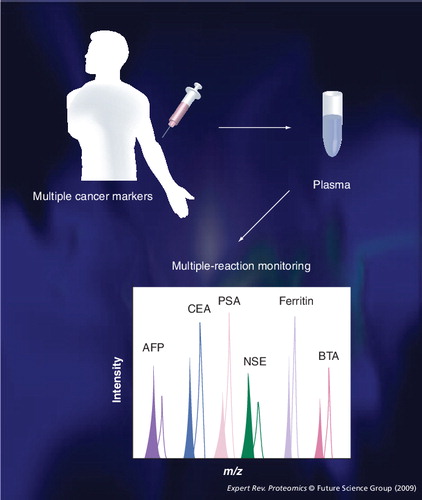
MRM: Multiple-reaction monitoring.
With recent advances in mass spectrometry (MS), the chief interest in proteomics appears to be shifting from ‘What protein is there?’ to ‘How much of the target protein is there?’ Quantification of target proteins using appropriate mass spectrometric methods is the principal goal of clinical proteomics. Thus far, multiple-reaction monitoring (MRM) has been considered to be one of the most effective tools of quantitative clinical proteomics. Regardless, there has been some skepticism regarding its applicability for clinical proteomics. To address this controversy, we discuss recent data that support the application of MRM to quantitative clinical proteomics.
Since the early use of MS, triple quadrupole liquid chromatography (LC)-MS-MS MRM has been used effectively to detect and quantify drugs and drug metabolites in pharmaceutical research. When a drug molecule is ionized, the m/z of a precursor ion is filtered in the first quadrupole (Q1). The selected precursor ion is dispersed into several fragment ions in the second quadrupole (Q2) using collision energy. Only the m/z of fragment ions of interest can pass in the third quadrupole (Q3) and, thereafter, the intensities of these ions are measured and converted to concentrations. Accordingly, for the past several years, an identical principle has been developed for target proteins in clinical proteomics.
This year’s agenda for the proteomics research group of the Association of Biomolecular Resource Facilities was ‘Relative Protein Quantification in a Clinical Matrix’ Citation[101]. Interestingly, eight out of 25 teams chose MRM or selected-reaction monitoring as their quantitation strategy to respond to the test subject. Four teams that used MRM with triple quadrupole LC-MS-MS submitted correct answers for the test measurements. MRM performance turned out to be the proportionally highest tactic among all participant teams, generating the fewest incorrect answers Citation[102].
Western blot and ELISA have been the most powerful methods for quantitative clinical proteomics and biomarker development Citation[1]; the merits of these antibody-based assays are rarely ignored. To this end, MRM can facilitate application of antibody-based assays to quantitative clinical proteomics with its own specific merits.
Since a specific antibody does not need to be developed for MRM, this technique can be quickly used to verify candidate biomarkers in case–controlled subsets of clinical samples and shorten the advance to validation stages using MRM or ELISA. The detection of low-abundance proteins, such as cytokines in the pg/ml range, is unattainable with current MRM. The concentrations of many biomarkers in the ng/ml range, however, such as prostate-specific proteins (>4 ng/ml), carcinoembryonic antigen (>2.5 ng/ml for nonsmokers) and neuron-specific enolase (>13 g/ml), may be quantifiable using MRM with appropriate sample preparations in the future Citation[103].
The throughput of MRM experiments primarily depends on the operation time of nano-LC (e.g., approximately 60 min per sample injection). It may be felt that MRM is not equipped to meet the throughput speed in clinical proteomics settings. Nevertheless, another advantage of MRM is that it processes up to 200 transitions, equivalent to approximately 50 target proteins, with an injection of a 1–2-µg peptide from less than 20 µl of blood, supplementing the lower throughput compared with ELISA. With ELISA, 96 samples can be processed within 4 h. Often, adequate antibodies are not easily developed for use in ELISA kits. By contrast, MRM is used for clinical examinations, such as neonatal screening and therapeutic drug monitoring, meeting the throughput requirements of clinicians with specificity Citation[2].
An attribute of MRM is its multiplexing capability for many target proteins per run; antibody-based assays measure a limited number of proteins simultaneously. For example, the recently developed triple quadrupole LC-MS-MS measures up to 2500 transitions in a single assay with scheduled MRM, which can be the equivalent of over 100 target proteins Citation[3]. If one wants to measure a panel of biomarkers for a single disease with one sample or monitor a panel of biomarkers for several diseases with one sample, the multiplexing measurements of MRM are suitable in either situation.
The most tedious procedure in MRM is determining the transitions for target proteins. Yet, choosing specific transitions leads to good target measurements. For each target protein, several transitions that are specific to several peptides per protein should be determined. To this end, software programs allow researchers to spend less time and effort in determining transitions: PeptideAtlas Citation[104], MRMAtlas Citation[105], GPMDB Citation[106], MRMer Citation[107] and MRMaid Citation[108] are open website resources that aid transition determinations.
For absolute quantitation using MRM, an isotope-labeled peptide must be synthesized for the target protein Citation[4,5]. An isotope-labeled peptide that consists of ten normal amino acids and two isotope-labeled valines at purities above 95% cost approximately US$600 (the estimated cost for peptide synthesis is US$4 per amino acid, and isotope-labeled valines that are labeled at five 13C and one 15N cost ∼US$550 for 250 mg, although costs vary locally).
At the initial stages of MRM quantitation, however, relative quantitation using an internal standard peptide, such as β-galactosidase, can be performed without synthesizing an isotope-labeled peptide, which saves time and cost. Nonhuman peptides, such as β-galactosidase, are distinguishable from endogenous proteins in a human sample as internal standard peptides. After eliminating crude candidates through relative quantitation, the synthesis of isotope-labeled peptides can be minimized.
Quantitation using MRM with plasma shows considerable experimental variation between runs, primarily caused by sample handling and technical instability. To reduce run-to-run variations, improvement of MRM operations should be continuously implemented. Several attempts have been applied to reduce variations. The normal concentration of plasma is approximately 70 µg/µl and, thus, minute errors in pipetting can lead to wide variation. Highly concentrated plasma should be handled in diluted concentrations to minimize sample-handling errors. The distinguishable internal-standard protein should be spiked into each sample before preparing the sample so that handling is monitored at all stages of preparation, such as high-abundance protein depletion, in-solution digestion and desalting using a C18-packed cartridge. The internal standard peptide is spiked into the final peptide mixture before peptide injection for mass spectrometry. The spiked internal standard can be used to normalize the variation of ion-spray efficiency. Consequently, a rational standard operating procedure for handling clinical samples and MRM operation should be the first steps that are considered.
Multiple-reaction monitoring can be useful in many applications of quantitative clinical proteomics, such as biomarker development. Crude lists of biomarker candidates are obtained from a representative set of clinical samples or a model cell system using comparative profiling techniques, such as 2D electrophoresis (2DE), differential labeling (e.g., isobaric tag for relative and absolute quantitation), and LC-MS-MS. The next step would be to verify the crude list of biomarkers in individual clinical samples of blood or tissue by western blot, ELISA or MRM. Depending on the availability of antibody or LC-MS-MS, the proper platform for verification is chosen. MRM would be the inevitable choice for biomarker verification when there is no appropriate antibody. Use of MRM in biomarker development also can enhance the measure of post-translational modifications, such as phosphorylation and glycosylation, which are difficult tasks for antibody-based systems Citation[6–10].
Multiple-reaction monitoring could be useful in diagnostics to detect diseases, such as cancer. For example, CEA and α-fetoprotein are cancer-specific biomarkers. Prostate-specific antigen is a tissue-specific marker for prostate cancer Citation[11], as are NSE for neuroblastoma and small cell lung cancer Citation[12], ferritin for acute-phase response Citation[13], and bladder tumor antigen for bladder cancer Citation[14,15]. These biomarkers typically are measured by ELISA of blood. If MRM methods are well established, all cancer biomarkers can be monitored as a panel in the same MRM run, which otherwise would be performed with separate ELISAs for each biomarker .
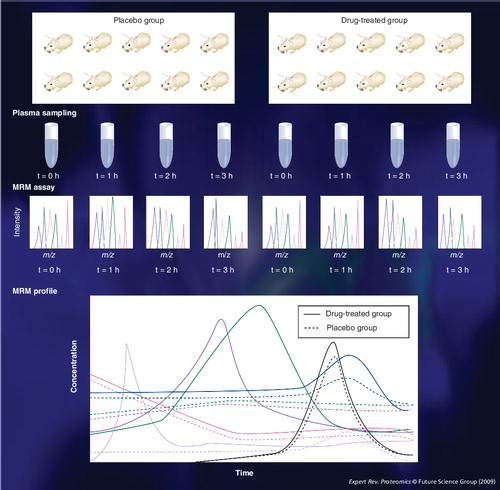
Multiple-reaction monitoring might be useful in pharmacology. Plasma samples are collected from drug-treated groups and placebo groups. MRM can be used to monitor biomarkers of efficacy or side effects over time. The effect of drug treatment can be modeled as the flux of targeted plasma proteins. If ten rats are assigned to a drug-treated group and ten to a placebo group and each plasma is collected three times every 1 h after drug administration, we can monitor the temporal profiles of tens of target proteins in the plasma of 20 rats at four time points using MRM .
In summary, the sensitivity and throughput of MRM technology does not satisfy the expectations for its use in quantitative clinical proteomics. Regardless, the potential of MRM is high with regard to applicability to clinical proteomics, since MS technology is evolving rapidly, generating new innovations in workflow, software, hardware and reagents. For example, workflow innovations include stable isotope standards and capture by antipeptide antibodies, which detect low-abundance proteins in blood Citation[4]. Novel software, such as MRM3, increases specificity to enable fewer sample workups of complex mixtures. These innovations will render the MRM assay easier and faster to use for less-trained operators Citation[16].
Current studies claim that MRM assays of biological samples can be performed at CV less than 5%, but the accuracy and precision of the entire assay, including sample preparation, should be further confirmed Citation[17]. In particular, the Human Proteome Detection and Quantitation Project has suggested that MRM with triple quadrupole LC-MS-MS should be the main platform that is used to detect and quantify the proteotypic peptides of 21,500 human proteins in blood in the ng/ml range Citation[18]. Therefore, MRM is drawing more attention from companies, and the number of companies that are developing MRM assays is increasing, including Proteome Factory AG, The Australian Proteome Analysis Facility, Nextgen Sciences, Monarch Lifesciences, Pfizer, Novartis and Eli Lilly Citation[19].
Financial & competing interests disclosure
This work was supported by the 21C Frontier Functional Proteomics Project of the Korean Ministry of Science and Technology (grant no. FPR 08-A2-110) and by Grant no. 03-2008-030 from the SNUH research fund. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Issaq HJ, Veenstra TD. Would you prefer multiple reaction monitoring or antibodies with your biomarker validation? Expert Rev. Proteomics5(6), 761–763 (2008).
- Nagy K, Takats Z, Pollreisz F, Szabo T, Vekey K. Direct tandem mass spectrometric analysis of amino acids in dried blood spots without chemical derivatization for neonatal screening. Rapid Commun. Mass Spectrom.17(9), 983–990 (2003).
- AppliedBiosystems. AB SCIEX QTRAP® 5500 LC/MS/MS System for Proteomics Applications. AppliedBiosystems, CA, USA (2009).
- Brun V, Dupuis A, Adrait A et al. Isotope-labeled protein standards: toward absolute quantitative proteomics. Mol. Cell. Proteomics6(12), 2139–2149 (2007).
- Anderson NL, Anderson NG, Haines LR et al. Mass spectrometric quantitation of peptides and proteins using stable isotope standards and capture by anti-peptide antibodies (SISCAPA). J. Proteome Res.3(2), 235–244 (2004).
- Unwin RD, Griffiths JR, Leverentz MK et al. Multiple reaction monitoring to identify sites of protein phosphorylation with high sensitivity. Mol. Cell. Proteomics4(8), 1134–1144 (2005).
- Ciccimaro E, Hevko J, Blair IA. Analysis of phosphorylation sites on focal adhesion kinase using nanospray liquid chromatography/multiple reaction monitoring mass spectrometry. Rapid Commun. Mass Spectrom.20(24), 3681–3692 (2006).
- Mayya V, Rezual K, Wu L, Fong MB, Han DK. Absolute quantification of multisite phosphorylation by selective reaction monitoring mass spectrometry: determination of inhibitory phosphorylation status of cyclin-dependent kinases. Mol. Cell. Proteomics5(6), 1146–1157 (2006).
- Hulsmeier AJ, Paesold-Burda P, Hennet T. N-glycosylation site occupancy in serum glycoproteins using multiple reaction monitoring liquid chromatography–mass spectrometry. Mol. Cell. Proteomics6(12), 2132–2138 (2007).
- Stahl-Zeng J, Lange V, Ossola R et al. High sensitivity detection of plasma proteins by multiple reaction monitoring of N-glycosites. Mol. Cell. Proteomics6(10), 1809–1817 (2007).
- Ohkura H. [Clinical usefulness of circulating tumor markers]. Gan To Kagaku Ryoho31(7), 1131–1134 (2004).
- Ishiguro Y, Kato K, Ito T et al. Nervous system-specific enolase in serum as a marker for neuroblastoma. Pediatrics72(5), 696–700 (1983).
- Beard JL, Murray-Kolb LE, Rosales FJ, Solomons NW, Angelilli ML. Interpretation of serum ferritin concentrations as indicators of total-body iron stores in survey populations: the role of biomarkers for the acute phase response. Am. J. Clin. Nutr.84(6), 1498–1505 (2006).
- Ecke TH. Focus on urinary bladder cancer markers: a review. Minerva Urol. Nefrol.60(4), 237–246 (2008).
- Budman LI, Kassouf W, Steinberg JR. Biomarkers for detection and surveillance of bladder cancer. Can. Urol. Assoc. J.2(3), 212–221 (2008).
- Niessen J, Jedlitschky G, Grube M et al. Human platelets express OATP2B1, an uptake transporter for atorvastatin. Drug Metab. Dispos.37(5), 1129–1137 (2009).
- Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics5(4), 573–588 (2006).
- Anderson NL, Anderson NG, Pearson TW et al. A human proteome detection and quantitation project: hPDQ. Mol Cell Proteomics8(5), 883–886 (2009).
- Biomarker Assay Development. Cambridge Healthtech Institute Conference. San Diego, CA, USA, 26–28 January 2009.
Websites
- Association of Biomolecular Resource Facilities www.abrf.org/index.cfm/group.show/Proteomics.34.htm#R_4
- PRG2009 Research Study. Relative protein quantification in a clinical matrixwww.auntminne.comwww.abrf.org/ResearchGroups/Proteomics/Studies/PRG2009_presentation.pdf
- University of Iowa Health Center. Tumor marker testswww.uihealthcare.com/topics/medicaldepartments/cancercenter/tumormarker/index.html
- PeptideAtlas www.peptideatlas.org
- MRMAtlas www.mrmatlas.org/index.php
- GPM Database http://gpmdb.thegpm.org
- MRMerhttp://proteomics.fhcrc.org/CPL/MRMer.html
- MRMaid www.mrmaid.info