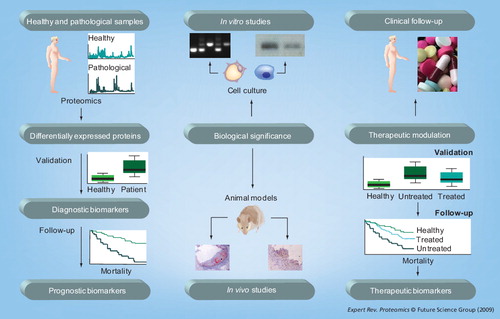
Translational medicine is aimed at completing a feedback loop, beginning from the bedside to the bench, with observations made in human studies Citation[1]. Application of ’omics technologies to the identification of novel diagnostic/prognostic biomarkers of atherothrombosis may result in defining novel mechanisms of pathological remodeling of the arterial wall, in order to go back again to address their role as therapeutic targets in humans .
In this article, we will focus on describing recent findings obtained applying this translational approach to human vascular samples by using traditional proteomic technologies, along with novel technologies that are useful for discovery-driven studies. We will highlight two aspects in the potential use of proteomics to biomarker discovery (thoroughly reviewed in Citation[2]): the importance of separation of both discovery and validation phases, and the interest of biological fluids from organs (e.g., secretome) to identify disease-associated biomarkers.
Identification of biomarkers of atherosclerosis
With the aim of identifying novel biomarkers of atherosclerosis, proteins differentially secreted by normal versus pathological vessels were analyzed using 2DE and mass spectrometry (MS) Citation[3]. In the discovery phase, decreased heat-shock protein (HSP)27 levels were found in atherosclerotic arteries compared with healthy arteries Citation[4]. In the validation phase, circulating concentrations of HSP27 were decreased in the plasma of patients with atherosclerosis compared with healthy subjects, indicating that HSP27 could be a novel diagnostic biomarker of atherosclerosis. By contrast, its potential role as a prognostic biomarker has only been tested in the Women’s Health Study Citation[5]. Plasma concentrations of HSP27 were determined at baseline among initially healthy women who subsequently either developed or did not develop cardiovascular events during a 6-year follow-up. In this prospective study, baseline HSP27 plasma concentration was not associated with incident cardiovascular events, although confirmations with other prospective studies are warranted.
Besides the use of identified proteins as surrogate diagnostic/prognostic markers of cardiovascular pathologies, the following step in this translational approach is the exploration of their biological significance. Extracellularly, decreased HSP27 levels could reflect the existence of proteolytic activities in pathological arterial walls. Intracellularly, diminished HSP27 expression is linked to increased vascular smooth muscle cells apoptosis, a determinant of plaque instability Citation[6]. Interestingly, diminished HSP27 expression has been recently found in unstable versus stable plaques by 2D analysis Citation[7]. Finally, in an experimental model of atherosclerosis, HSP27 overexpression reduced lesion progression, suggesting its potential role as a therapeutic target Citation[8].
Following a similar approach but using SELDI-TOF MS technology, soluble form of TNF weak inducer of apoptosis (sTWEAK) was identified as a protein released in lower amounts by atherosclerotic plaques in culture than nonatherosclerotic arteries Citation[9]. Accordingly, patients with carotid stenosis had lower plasma sTWEAK levels than control subjects. Further validation studies in asymptomatic subjects demonstrated that sTWEAK plasma concentrations are negatively associated with carotid intima media thickness (a surrogate marker of atherosclerosis). Additionally, lower sTWEAK plasma levels are also present in subjects with chronic kidney disease or Type 2 diabetes, conditions associated with a high risk for atherosclerotic disease Citation[10]. Moreover, sTWEAK plasma concentrations are a good predictor biomarker for total and cardiovascular mortality in patients undergoing hemodialysis, even more so when combined with inflammatory status markers Citation[11]. These data indicate that sTWEAK could be a novel diagnostic and prognostic biomarker of atherosclerosis.
The biological consequence of sTWEAK diminution in cardiovascular diseases is actually unknown. In this context, the scavenger receptor CD163 uptakes haptoglobin–hemoglobin (Hp–Hb) complexes for the removal and metabolism of the potent oxidant Hb. In addition to Hp–Hb, monocyte CD163 can also bind TWEAK, which may serve to sequester TWEAK from its primary receptor Fn14 Citation[12]. The presence of CD163 could have beneficial consequences through removing of TWEAK and diminishing the deleterious effects of TWEAK/Fn14 interaction. Finally, conventional treatment with hypolipemic drugs, such as statins, can diminish the expression of Fn14 Citation[13], indicating that TWEAK/Fn14 could be novel potential therapeutic targets in atherothrombosis.
Identification of biomarkers of abdominal aortic aneurysm
In ongoing studies, different layers (luminal/abluminal) of the intraluminal thrombus of abdominal aortic aneurysm (AAA) were incubated, and the released proteins were analyzed by 2D DIGE. Several differentially released proteins that are involved in different mechanisms, such as proteolysis and redox balance, have been identified [Martinez-Pinna et al., Unpublished Data]. Among them, α1 antitrypsin (ATT) release was increased in the luminal layer of AAA compared with the abluminal layer. Interestingly, circulating ATT levels were significantly increased in the serum of patients with AAA relative to healthy subjects. Moreover, a positive correlation between ATT and AAA growth in the previous 12 months was observed Citation[14]. In a population study of 6075 healthy men followed for 19 years, increased ATT levels – in conjunction with other biomarkers – were associated with a higher incidence of fatal or repaired AAA Citation[15]. The biological role of ATT as an inhibitor of elastase in AAA is well established Citation[16]. Interestingly, recent evidences suggest a new potential antiatherothrombotic property of high-density lipoprotein attributable to ATT Citation[17].
Novel potential approaches to vascular proteomics
New approaches to quantitatively studying low-abundance proteins and their functional analysis into specific protein networks, along with use of improved sampling methods for the analysis of specific subproteomes, will be of crucial importance in the post-genomic era Citation[18].
The use of surface plasmon resonance (SPR) techniques coupled to MS to discover protein interaction partners will be a demanding approach. A molecule (the ligand) is immobilized on a sensor chip and then exposed to protein extracts injected through the surface of the chip by a microfluidic system. The interactions between the immobilized ligand and the proteins in the extract are monitored in real time; proteins interacting with the ligand could be recovered, digested with trypsin and identified by MS. Considering the SPR sensor surface as a chromatographic matrix, SPR-MS is similar to the conventional affinity chromatographic purification-step-preceding MS in protein discovery, but with real-time quantification of the capturing and elution process. With the subsequent increasing of the detection limits in SPR-MS, this approach will enter into the field of high-throughput protein-interaction discovery, multiprotein analysis and protein-complex delineation in atherothrombotic research.
Similarly, other proteomic approaches will rely on the identification of specific protein classes or the study of their active fraction. There are several methods that use specific ‘capture compound’ (CC) molecules to fractionate complex samples for fishing specific functional subproteomes Citation[19,20]. CCs interact with proteins through a selectivity function; a reactivity function forms a covalent bond with the interacting proteins under UV light, and a pull-out function (biotin moiety) allows isolation of the captured proteins directly from cell lysates to reducing their complexity Citation[20]. Then, these selected proteins are identified and quantified by high-sensitivity mass spectrometer equipments, such as the Orbitrap system. There are several CCs available for different protein subsets based on the affinity to the selectivity group. An example of commercially available CCs is cyclic nucleotide monophosphates, which are important mediators for the activation of several signaling cascades. Virtually any small molecule will be amenable to be attached, as new selectivity functions into a new CC, increasing the coverage for specific proteins playing a role in the atherothrombotic process.
The qualitative and quantitative analysis is a challenging task, even with the state-of-the-art separation and analysis capabilities of current proteomic technologies. Multiple reaction monitoring (MRM) or selected reaction monitoring (SRM) are highly selective and sensitive MS scan models for the quantification of low-abundance proteins in protein mixtures and for the characterization of post-translational modifications. MRM is a powerful method exploiting the capabilities of triple quadrupole mass spectrometers to filter for a selected group of precursor peptides from a few interesting proteins (the potential biomarkers) within a protein mixture. In a MRM assay, the so-called transition filter is monitored with high selectivity: the mass of the precursor peptide of interest is set in the first mass analyzer (Q1), while a specific diagnostic peptide fragment is predefined in the third mass analyzer (Q3). Several of these unique transition filters can be monitored over time quantitatively with high selectivity and sensitivity Citation[21].
Conclusion & future outlook
In the last few years, proteomics has been applied to many diseases, identifying hundreds of proteins in thousands of papers published worldwide. However, these never-ending lists of proteins must be functionally interpreted with the aim to translate the novel findings into clinical applications. For that purpose, collaborative efforts between clinicians, scientists, bioinformatics, enterprises, such as that developed in the Fighting Aneurysmal Disease project, are warranted Citation[101].
We have discussed various examples of novel atherothrombotic biomarkers identified by proteomic analysis. However, at present, no biomarker or biomarker profile has been translated into clinical practice (although they have been identified in the last 5 years and the proposed time for any biomarker reaching the clinical scenario is ~10 years). While proteomics is playing a major role in the discovery phase of potential biomarkers, validation has traditionally been conducted using antibody-based methods, such as western blotting and ELISA. However, MS-targeted methods will become a major contributor for quantification of these potential biomarkers and their modifications in the validation phases Citation[22]. In any case, even when a biomarker does not meet all the criteria to be translated into clinical practice (e.g., specificity and sensibility), it could be useful to understand the pathological mechanisms of the disease and may potentially be used as imaging and therapeutic targets.
Financial & competing interests disclosure
The papers of the authors cited in this editorial have been made in collaboration with Olivier Meilhac and Jean-Baptiste Michel and have been supported from the Spanish Ministerio de Ciencia y Tecnología (SAF2007/63648), Fundacion Ramon Areces, CAM (S2006/GEN-0247), Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, Redes RECAVA (RD06/0014/0035) European Network (HEALTH F2–2008–200647) and EUS2008–03565. The CNIC is supported by the Ministerio de Ciencia e Innovación and the Fundación Pro CNIC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Mankoff SP, Brander C, Ferrone S, Marincola FM. Lost in translation: obstacles to translational medicine. J. Transl. Med.2(1), 14 (2004).
- Lescuyer P, Hochstrasser D, Rabilloud T. How shall we use the proteomics toolbox for biomarker discovery? J. Proteome Res.6(9), 3371–3376 (2007).
- Durán MC, Martín-Ventura JL, Mas S et al. Characterization of the human atheroma plaque secretome by proteomic analysis. Methods Mol. Biol.357, 141–150 (2007).
- Martin-Ventura JL, Duran MC, Blanco-Colio LM et al. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation110(15), 2216–2219 (2004).
- Kardys I, Rifai N, Meilhac O et al. Plasma concentration of heat shock protein 27 and risk of cardiovascular disease: a prospective, nested case-control study. Clin. Chem.54(1), 139–146 (2008).
- Martín-Ventura JL, Nicolas V, Houard X et al. Biological significance of decreased HSP27 in human atherosclerosis. Arterioscler. Thromb. Vasc. Biol.26(6), 1337–1343 (2006).
- Lepedda AJ, Cigliano A, Cherchi GM et al. A proteomic approach to differentiate histologically classified stable and unstable plaques from human carotid arteries. Atherosclerosis203(1), 112–118 (2009).
- Rayner K, Chen YX, McNulty M et al. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ. Res.103(2), 133–141 (2008).
- Blanco-Colio LM, Martín-Ventura JL, Muñoz-García B et al. Identification of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) as a possible biomarker of subclinical atherosclerosis. Arterioscler. Thromb. Vasc. Biol.27(4), 916–922 (2007).
- Kralisch S, Ziegelmeier M, Bachmann A et al. Serum levels of the atherosclerosis biomarker sTWEAK are decreased in Type 2 diabetes and end-stage renal disease. Atherosclerosis199(2), 440–444 (2008).
- Carrero JJ, Ortiz A, Qureshi AR et al. Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol.4(1), 110–118 (2009).
- Moreno JA, Muñoz-García B, Martín-Ventura JL et al. The CD163-expressing macrophages recognize and internalize TWEAK potential consequences in atherosclerosis. Atherosclerosis (2009) (In Press).
- Muñoz-García B, Martín-Ventura JL, Martínez E et al. Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: modulation by atorvastatin. Stroke37(8), 2044–2053 (2006).
- Vega de Ceniga M, Esteban M, Quintana JM et al. Search for serum biomarkers associated with abdominal aortic aneurysm growth--a pilot study. Eur. J. Vasc. Endovasc. Surg.37(3), 297–299 (2009).
- Engström G, Börner G, Lindblad B, Janzon L, Lindgärde F. Incidence of fatal or repaired abdominal aortic aneurysm in relation to inflammation-sensitive plasma proteins. Arterioscler. Thromb. Vasc. Biol.24(2), 337–341 (2004).
- Fontaine V, Touat Z, Mtairag el M et al. Role of leukocyte elastase in preventing cellular re-colonization of the mural thrombus. Am. J. Pathol.164(6), 2077–2087 (2004).
- Ortiz-Muñoz G, Houard X, Martín-Ventura JL et al. HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB J. (2009) (In Press).
- Calvo E, Camafeita E, Diaz JF, Lopez JA. Mass spectrometry for studying the interaction between small molecules and proteins. Curr. Proteomics5(1), 20–34 (2008).
- Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem.77, 383–414 (2008).
- Köster H, Little DP, Luan P et al. Capture compound mass spectrometry: a technology for the investigation of small molecule protein interactions. Assay Drug Develop. Technol.5, 381–390 (2007).
- Kim K, Kim Y. Preparing multiple-reaction monitoring for quantitative clinical proteomics. Expert Rev. Proteomics6(3), 225–229 (2009).
- Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat. Biotechnol.24(8), 971–983 (2006).
Website
- Fighting Aneurysmal Disease Project. www.fighting-aneurysm.org