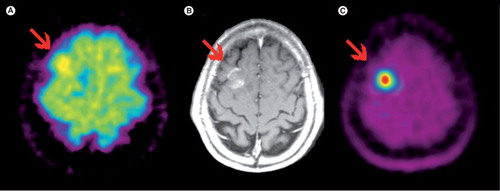
Figure 1. (A)18F-FDG; (B) contrast-enhanced MRI; (C) 11C-MET PET. Glioblastoma in the right frontal lobe, which is hard to delineate in the 18F-FDG PET. However, amino-acid PET with 11C-MET clearly shows the lesion with excellent tumor to background contrast.
Approximately 1–2% of all tumors are brain tumors. Gliomas represent 45% of all brain tumors (50% being highly malignant), followed by meningiomas (27%), with an incidence of 2–16/100,000 Citation[1]. The American Cancer Society estimated that, in 2005, primary brain tumors were the cause of death of approximately 12,760 women and men Citation[2,3]. Precise tumor localization and exact delineation from surrounding healthy brain tissue is of the utmost importance for planning tumor resection and/or radiation therapy, as well as for assessment of tumor recurrence after primary therapy. Moreover, novel targeted therapeutics mainly used after primary resection and radiation therapy lead to an increasing demand for functional and molecular imaging techniques for assessment of therapeutic response. MRI, at present, is still the gold standard in the primary diagnosis of brain tumors. Conventional morphological MRI provides information with respect to size and localization as well as additional insights on secondary phenomena, that is edema and bleeding, which cannot be supplied in the same quality by PET Citation[4]. However, the specificity for the differentiation of vital tumor tissue versus inflammatory processes, edema and reactive changes, is limited. Moreover, tumor borders determined by the use of contrast-enhanced MRI often under- or over-estimate the true extent of the tumor Citation[5,6]. It is our firm belief, that in this respect the molecular information of PET using novel tracers provides vital information in combination with classical neuroimaging techniques, such as MRI and computed tomography (CT). In addition, functional MRI techniques including diffusion-weighted imaging (DWI), magnetic resonance spectroscopy (MRS), diffusion-tensor imaging (DTI), dynamic contrast-enhanced imaging (DCE-MRI) and dynamic susceptibility imaging (DSC MRI) are in this context, in our opinion, not competing but complementary modalities to PET.
Amino acid-based PET tracers for brain tumor imaging
While PET and PET/CT using the tracer 18F-fluorodeoxyglucose (18F-FDG) has become a success story for many tumors outside the CNS, the unfavorable characteristics of 18F-FDG in the CNS have hampered its success for imaging of brain tumors. While brain tumors, like other tumors, may show an increased glucose metabolism, which can be visualized and quantified with 18F-FDG, the major disadvantage of 18F-FDG for the diagnosis of a brain tumor is the high background uptake caused by the high glucose metabolism of the brain itself, resulting in a low signal–background ratio. To improve PET imaging in the CNS, radioactively labeled amino acids have been synthesized with many fewer limitations compared with 18F-FDG, that is 11C-methionine (MET), 11C-tyrosine, 11C-leucine and amino acid derivatives, such as 18F-fluorethyltyrosine (FET) . Most primary gliomas show a high uptake of amino acids in comparison with normal brain tissues resulting in an excellent signal–background ratio. The enhanced uptake of amino acids into brain tumor tissue seems to be mainly attributable not to an increase of transport systems, the L or L-type amino acid transporters (LAT) Citation[7–9]. The advantage of the 18F-labeled tracer 18F-FET compared with 11C-labeled amino acids is the longer half-life of 18F (110 min) compared with 11C (20 min). Therefore, 18F-FET can be distributed to centers without an on-site cyclotron in a satellite concept comparable with 18F-FDG. Comparative studies of 11C-MET and 18F-FET with respect to uptake and image contrast revealed similar results for both tracers Citation[10–13]. The following main indications for PET neuroimaging with amino-acid tracers have evolved:
• Pretreatment definition of tumor extent for preoperative staging, for radiation therapy planning and for selecting the most appropriate biopsy site Citation[14–17];
• Detection of tumor recurrence and differentiation of recurrence and radiation necrosis Citation[18–20];
• (Early) therapy response assessment Citation[17,21–23].
Primary diagnosis & work-up of brain tumors
Owing to the low uptake of 18F-FET in normal brain tissue (gray and white matter) compared with the high uptake in many primary brain tumors, amino acid tracers allow for the delineation of tumor boundaries with high accuracy Citation[24]. There is a significantly higher uptake in malignant lesions compared with chronic and subacute nontumor lesions, which may demonstrate similar contrast enhancement patterns such as malignant lesions in CT or MRI Citation[25]. Even low-grade tumors show a significant tracer uptake in many cases Citation[26]. A threshold has been established for the definition of a ‘pathological’ tracer uptake: a ratio of tumor uptake to the uptake of the contralateral hemisphere of 1.6 or 1.5 or larger for FET and MET, respectively, is considered typical for vital tumor tissue Citation[25,27]. Using these thresholds, malignant lesions can be differentiated from benign lesions with a sensitivity and specificity of approximately 80%. However, 25% of low-grade gliomas do not take up amino acid tracers, limiting the value of amino acid tracers to rule out low-grade gliomas. Clinically speaking, a high 18F-FET uptake is highly suggestive for a primary brain tumor, however, it is not possible to differentiate between low- and high-grade tumors. No uptake virtually excludes high-grade gliomas but does not rule out low-grade tumors, which can be PET negative.
MRI-guided stereotactic biopsy might not always result in a correct tumor tissue biopsy: in some cases the changes in the MRI signal may exceed the true tumor boundaries or tumor may be present in an area with no contrast enhancement in case of higher-grade tumor areas Citation[12,24,27,28]. By contrast, biopsy studies revealed true positive amino acid tracer uptake even in non-solid tumor parts, resulting in a better detection of infiltrative growth of gliomas Citation[29,30]. Moreover, this leads to a better differentiation between tumor tissue and reactive changes, such as edema, compared with MRI or CT Citation[16,31]. Additionally it improves the detection rate of residual tumor tissue after resection and results in a better definition of target volumes before radiation therapy Citation[5,6,32].
Detection of tumor recurrence
Another limitation of MRI is the detection of recurrent disease. Often, residual tumor tissue cannot be accurately distinguished from postoperative changes or radiation necrosis Citation[13]. By contrast, 18F-FET-PET and other amino acid tracers can verify tumor recurrence with a high sensitivity and specificity and also differentiate between post-therapeutic changes and tumor tissue. A sensitivity of 100% and a specificity of 93% has been reported for this indication Citation[20].
Response assessment
Concerning evaluation of therapy response, several studies demonstrated promising results for 18F-FET-PET. Examinations during chemotherapy and radioimmunotherapy demonstrated a high correlation of 18F-FET-PET uptake and response to treatment Citation[33,34]. Functional changes can be visualized earlier in 18F-FET-PET than morphological changes in MRI Citation[21]. On the other hand, 18F-FDG has proven to be of limited value for early therapy response assessment Citation[35,36].
Grading & prognosis
The value of amino acid tracers as imaging markers for prognosis is still controversial. Whereas some studies only see a limited value Citation[37] others propose a high value, particularly for grade II gliomas Citation[38–40]. Furthermore, recently published data showed a potential correlation of dynamic 18F-FET-PET imaging and clinical outcome of the patients Citation[41]. The value of amino acid tracers for grading so far is considered to be rather limited owing to the fact that even low-grade gliomas eventually show high tracer uptake. However, some recent studies have suggested dynamic acquisition and evaluation of uptake kinetics may lead to better estimation of tumor grading Citation[42,43].
The assessment of grading and prognosis may represent one of the few remaining indications for 18F-FDG. Glucose metabolism can be used to characterize the aggressiveness of brain tumors and may supplement pathological grading Citation[44,45]. However, a differentiation between WHO grade III and IV tumors is not possible owing to the comparable high uptake. Studies comparing 18F-FDG uptake and patient survival demonstrated a high correlation in patients with primary gliomas and recurrence of gliomas Citation[44,46,47].
Imaging of specific biological processes of brain tumors with novel PET tracers
While diagnosis of brain tumors and tumor recurrence is mainly the domain of amino acid tracers, several tracers may show a high potential to evaluate specific biological features of tumors outside and within the CNS but still require further validation.
Hypoxia tracers, such as 18F-FAZA or 18F-FMISO, can be used to evaluate radiation sensitivity of brain tumors. This is of major relevance as hypoxic tumor areas do not respond well to radiation therapy Citation[1,48]. However, limited permeability of the blood–brain barrier may hamper their application in CNS.
For imaging of proliferative activity, 18F-FLT has shown promising results. 18F-FLT is metabolized by thymidine kinase 1 and, thus, is a marker for DNA synthesis. Its uptake correlates better with Ki-67 proliferation index compared with 18F-FDG Citation[49]. In recurrent disease 18F-FLT performed better compared with 18F-FDG in detecting tumor tissue and was a better predictor for disease progression and survival. Moreover, 18F-FLT holds promise as an imaging biomarker for therapy response assessment Citation[50,51].
Concerning molecular imaging of angiogenesis, imaging specific biological targets of the angiogenic cascade is promising Citation[52]. In this respect the integrin αvβ3 is an interesting target, as it is expressed on activated endothelial cells and is known to be overexpressed in gliomas Citation[53]. The feasibility of imaging αvβ3 expression in patients with tumors outside the CNS and in gliomas has been demonstrated with the tracer 18F-Galacto-RGD Citation[54–57]. Future studies will have to show if the concept of molecular imaging of angiogenesis is of clinical relevance, for example for response assessment of antiangiogenic therapy.
Conclusions & perspective
While 18F-FDG-PET still plays a role for assessment of tumor grade and dedifferentiation of gliomas over time, amino acid tracers such as 18F-FET are vastly superior to 18F-FDG for most indications (e.g., tumor delineation, assessment of recurrence and therapy response). Interestingly the regional use of amino acid PET varies substantially. In Europe, especially in Germany, 18F-FET-PET is increasingly used for glioma imaging in centers where PET is available. By contrast, the USA amino acid PET is not yet frequently performed and 18F-FDG is still the standard tracer for primary brain tumors. Thus PET imaging of gliomas with amino acid tracers is a ‘sleeping beauty’ in many parts of the world, waiting to be awakened by a kiss! Hopefully, the combined use of PET using novel tracers and advanced MRI such as DCE-MRI, DWI and MRS will increase the benefit for patients in the future. In this respect, great hope lies in combined MR-PET imaging, already realized with the brain PET MR-insert Citation[58]. With fully integrated MR-PET systems currently being developed, a one-stop-shop approach for a complete assessment of brain tumor biology and morphology within the concept of individualized medicine seems feasible in the not too distant future.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Roelcke U, Leenders KL. PET in neuro-oncology. J. Cancer Res. Clin. Oncol.127(1), 2–8 (2001).
- Jemal A, Murray T, Ward E et al. Cancer statistics, 2005. CA Cancer J. Clin.55(1), 10–30 (2005).
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol.64(6), 479–489 (2005).
- Benard F, Romsa J, Hustinx R. Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin. Nucl. Med.33(2), 148–162 (2003).
- Miwa K, Shinoda J, Yano H et al. Discrepancy between lesion distributions on methionine PET and MR images in patients with glioblastoma multiforme: insight from a PET and MR fusion image study. J. Neurol. Neurosurg. Psychiatry75(10), 1457–1462 (2004).
- Grosu AL, Weber WA, Astner ST et al.11C-methionine PET improves the target volume delineation of meningiomas treated with stereotactic fractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys.66(2), 339–344 (2006).
- Kanai Y, Segawa H, Miyamoto K et al. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem.273(37), 23629–23632 (1998).
- Langen KJ, Broer S. Molecular transport mechanisms of radiolabeled amino acids for PET and SPECT. J. Nucl. Med.45(9), 1435–1436 (2004).
- Langen KJ, Muhlensiepen H, Holschbach M et al. Transport mechanisms of 3-[123I]iodo-α-methyl-L-tyrosine in a human glioma cell line: comparison with [3H]methyl-L-methionine. J. Nucl. Med.41(7), 1250–1255 (2000).
- Weber WA, Wester HJ, Grosu AL et al. O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur. J. Nucl. Med.27(5), 542–549 (2000).
- Langen KJ, Jarosch M, Muhlensiepen H et al. Comparison of fluorotyrosines and methionine uptake in F98 rat gliomas. Nucl. Med. Biol.30(5), 501–508 (2003).
- Pauleit D, Floeth F, Tellmann L et al. Comparison of O-(2–18F-fluoroethyl)-L-tyrosine PET and 3–123I-iodo-a-methyl-L-tyrosine SPECT in brain tumors. J. Nucl. Med.45(3), 374–381 (2004).
- Popperl G, Gotz C, Rachinger W et al. Value of O-(2-[18F]fluoroethyl)- L-tyrosine PET for the diagnosis of recurrent glioma. Eur. J. Nucl. Med. Mol. Imaging31(11), 1464–1470 (2004).
- Goldman S, Levivier M, Pirotte B et al. Regional methionine and glucose uptake in high-grade gliomas: a comparative study on PET-guided stereotactic biopsy. J. Nucl. Med.38(9), 1459–1462 (1997).
- Pirotte B, Goldman S, David P et al. Stereotactic brain biopsy guided by positron emission tomography (PET) with [F-18]fluorodeoxyglucose and [C-11]methionine. Acta Neurochir. Suppl.68, 133–138 (1997).
- Pirotte B, Goldman S, Dewitte O et al. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J. Neurosurg.104(2), 238–253 (2006).
- Voges J, Herholz K, Holzer T et al.11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact. Funct. Neurosurg.69(1–4 Pt 2), 129–135 (1997).
- Tsuyuguchi N, Takami T, Sunada I et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery – in malignant glioma. Ann. Nucl. Med.18(4), 291–296 (2004).
- Van Laere K, Ceyssens S, Van Calenbergh F et al. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur. J. Nucl. Med. Mol. Imaging32(1), 39–51 (2005).
- Rachinger W, Goetz C, Popperl G et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery57(3), 505–511 (2005).
- Nariai T, Tanaka Y, Wakimoto H et al. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J. Neurosurg.103(3), 498–507 (2005).
- Wurker M, Herholz K, Voges J et al. Glucose consumption and methionine uptake in low-grade gliomas after iodine-125 brachytherapy. Eur. J. Nucl. Med.23(5), 583–586 (1996).
- Wyss M, Hofer S, Bruehlmeier M et al. Early metabolic responses in temozolomide treated low-grade glioma patients. J. Neurooncol.95(1), 87–93 (2009).
- Pauleit D, Floeth F, Hamacher K et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain128(Pt 3), 678–687 (2005).
- Herholz K, Holzer T, Bauer B et al.11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology50(5), 1316–1322 (1998).
- Jacobs AH, Kracht LW, Gossmann A et al. Imaging in neurooncology. NeuroRx2(2), 333–347 (2005).
- Floeth FW, Pauleit D, Wittsack H-J et al. Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-L-tyrosine and magnetic resonance spectroscopy. J. Neurosurg.102(2), 318–327 (2005).
- Levivier M, Goldman S, Pirotte B et al. Diagnostic yield of stereotactic brain biopsy guided by positron emission tomography with [18F]fluorodeoxyglucose. J. Neurosurg.82(3), 445–452 (1995).
- Kracht LW, Miletic H, Busch S et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin. Cancer Res.10(21), 7163–7170 (2004).
- Pirotte B, Goldman S, Massager N et al. Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J. Nucl. Med.45(8), 1293–1298 (2004).
- Ogawa T, Shishido F, Kanno I et al. Cerebral glioma: evaluation with methionine PET. Radiology186(1), 45–53 (1993).
- Nuutinen J, Sonninen P, Lehikoinen P et al. Radiotherapy treatment planning and long-term follow-up with [11C]methionine PET in patients with low-grade astrocytoma. Int. J. Radiat. Oncol. Biol. Phys.48(1), 43–52 (2000).
- Popperl G, Goldbrunner R, Gildehaus FJ et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging32(9), 1018–1025 (2005).
- Popperl G, Gotz C, Rachinger W et al. Serial O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur. J. Nucl. Med. Mol. Imaging33(7), 792–800 (2006).
- Brock CS, Young H, O’Reilly SM et al. Early evaluation of tumour metabolic response using [18F]fluorodeoxyglucose and positron emission tomography: a pilot study following the Phase II chemotherapy schedule for temozolomide in recurrent high-grade gliomas. Br. J. Cancer82(3), 608–615 (2000).
- De Witte O, Levivier M, Violon P et al. Prognostic value positron emission tomography with [18F]fluoro-2-deoxy-D-glucose in the low-grade glioma. Neurosurgery39(3), 470–476 (1996).
- Ceyssens S, Van Laere K, de Groot T et al. [11C]methionine PET, histopathology, and survival in primary brain tumors and recurrence. AJNR Am. J. Neuroradiol.27(7), 1432–1437 (2006).
- De Witte O, Goldberg I, Wikler D et al. Positron emission tomography with injection of methionine as a prognostic factor in glioma. J. Neurosurg.95(5), 746–750 (2001).
- Ribom D, Smits A. Baseline 11C-methionine PET reflects the natural course of grade 2 oligodendrogliomas. Neurol. Res.27(5), 516–521 (2005).
- Smits A, Westerberg E, Ribom D. Adding 11C-methionine PET to the EORTC prognostic factors in grade 2 gliomas. Eur. J. Nucl. Med. Mol. Imaging35(1), 65–71 (2008).
- Thiele F, Ehmer J, Piroth MD et al. The quantification of dynamic FET PET imaging and correlation with the clinical outcome in patients with glioblastoma. Phys. Med. Biol.54(18), 5525–5539 (2009).
- Popperl G, Kreth FW, Herms J et al. Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J. Nucl. Med.47(3), 393–403 (2006).
- Popperl G, Kreth FW, Mehrkens JH et al. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. Imaging34(12), 1933–1942 (2007).
- Alavi JB, Alavi A, Chawluk J et al. Positron emission tomography in patients with glioma. A predictor of prognosis. Cancer62(6), 1074–1078 (1988).
- Kim CK, Alavi JB, Alavi A, Reivich M. New grading system of cerebral gliomas using positron emission tomography with F-18 fluorodeoxyglucose. J. Neurooncol.10(1), 85–91 (1991).
- Barker FG, 2nd, Chang SM, Valk PE, Pounds TR, Prados MD. 18-Fluorodeoxyglucose uptake and survival of patients with suspected recurrent malignant glioma. Cancer79(1), 115–126 (1997).
- Padma MV, Said S, Jacobs M et al. Prediction of pathology and survival by FDG PET in gliomas. J. Neurooncol.64(3), 227–237 (2003).
- Bruehlmeier M, Roelcke U, Schubiger PA, Ametamey SM. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. J. Nucl. Med.45(11), 1851–1859 (2004).
- Choi SJ, Kim JS, Kim JH et al. [18F]3´-deoxy-3´-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur. J. Nucl. Med. Mol. Imaging32(6), 653–659 (2005).
- Chen W, Cloughesy T, Kamdar N et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J. Nucl. Med.46(6), 945–952 (2005).
- Chen W, Delaloye S, Silverman DH et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J. Clin. Oncol.25(30), 4714–4721 (2007).
- Beer AJ, Schwaiger M. Imaging of integrin αvβ3 expression. Cancer Metastasis Rev.27(4), 631–644 (2008).
- Schnell O, Krebs B, Wagner E et al. Expression of integrin αvβ3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol.18(3), 378–386 (2008).
- Beer AJ, Haubner R, Sarbia M et al. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin αvβ3 expression in man. Clin. Cancer Res.12(13), 3942–3949 (2006).
- Beer AJ, Grosu AL, Carlsen J et al. [18F]galacto-RGD positron emission tomography for imaging of αvβ3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res.13(22 Pt 1), 6610–6616 (2007).
- Haubner R, Weber WA, Beer AJ et al. Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F]galacto-RGD. PLoS Med.2(3), e70 (2005).
- Schnell O, Krebs B, Carlsen J et al. Imaging of integrin αvβ3 expression in patients with malignant glioma by [18F] galacto-RGD positron emission tomography. Neuro. Oncol.11(6), 861–870 (2009).
- Judenhofer MS, Wehrl HF, Newport DF et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat. Med.14(4), 459–465 (2008).