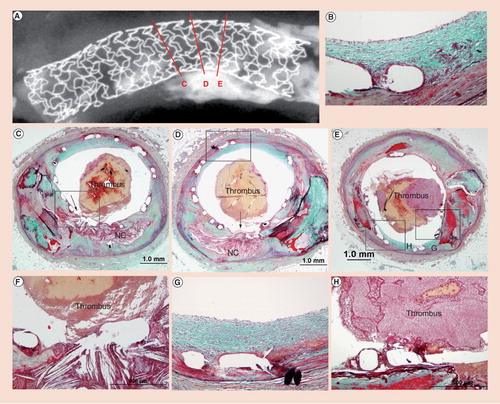
An 81-year-old male died suddenly 2 months after Taxus™ stent implantation in the left anterior descending artery for acute myocardial infaction. (A) Radiograph showing the area of plaque rupture (C, D & E) with three histologic sections. (B & D) Low-power sections of site of plaque rupture with (F) corresponding high powers. Note absence of fibrous cap and lack of stent coverage with overlay platelet-rich thrombus mixed with red cells. (D & E) Nonculprit sites with neointimal coverage of stent struts (boxed areas) and (G & B) persistant fibrin deposition. (E & H) Low and high powers of adjoining area of calcification at the site of plaque rupture (arrow). (H) Note fibrin coverage of stent struts, which are overlying an area of calcification with luminal platelet rich thrombus.
(B & E) High-power images of strut regions (H & E) showing presence of fibrin at 1 month and absence at 36 months. Note the strut outline is barely visible at 36 months. α actin-positive smooth muscle cells are observed in the neointima and media at (C) 1 month and (F) 36 months. (G) Complete degradation of the polymer strut with surrounding basophilic deposition of calcium (H & E). (H) Alcian blue positive proteoglycan infiltrated the matrix of the Bioabsorbable Vascular Solutions stent stent strut. (I) Calcification is seen around the degraded stent strut (von Kossa).
Modified and reproduced with permission from Citation[18].
![Figure 2. Representive histological sections of Bioabsorbable Vascular Solutions stent in pig coronary arteries removed at (A) 1 month and (D) 36 months (EVG staining).(B & E) High-power images of strut regions (H & E) showing presence of fibrin at 1 month and absence at 36 months. Note the strut outline is barely visible at 36 months. α actin-positive smooth muscle cells are observed in the neointima and media at (C) 1 month and (F) 36 months. (G) Complete degradation of the polymer strut with surrounding basophilic deposition of calcium (H & E). (H) Alcian blue positive proteoglycan infiltrated the matrix of the Bioabsorbable Vascular Solutions stent stent strut. (I) Calcification is seen around the degraded stent strut (von Kossa).Modified and reproduced with permission from Citation[18].](/cms/asset/b1913664-24e0-4625-b20e-88ff4c22a663/ierk_a_11210643_f0002_b.jpg)
Intracoronary stents became the standard of care for the treatment of obstructive coronary artery disease following their approval by the US FDA in 1994. Stenting was especially successful for the treatment of early complications, such as sudden collapse of the artery and dissections, which were common following the use of balloon angioplasty on its own. Although there has been an improvement, this technology still had its limitations, namely a high incidence of recurrent luminal obstruction (restenosis) in at least 30–40% of individuals. Preclinical and clinical studies documented the importance of strut thickness and design of stents. However, none of the modifications appreciably reduced the incidence of in-stent restenosis. Many systemic and local drug therapies were successful at reducing intimal formation in animals but failed to show benefit in humans. There followed a period of a decade of a ‘love affair’ with intravascular pharmacotherapy and brachytherapy, with success in the latter but late stent thrombosis and restenosis occurring as a complication. Then, in 2004, came the current era of drug-eluting stents (DESs), involving stent-based delivery of antiproliferative agents, which reduced restenosis rates dramatically in clinical trials without significant adverse effects and became the standard of care for the percutaneous treatment of coronary artery disease. However, the acceptance proved to be overly enthusiastic after several case reports raised concerns about an increased risk of late stent thrombosis (LST). In Barcelona (Spain) in 2006, a presentation at the European Society of Cardiology (ESC) by Camenzind ignited a ‘firestorm’ with report of increased late stent thrombosis in the Cypher™ (Cordis Johnson & Johnson Corp., FL, USA) clinical program, which reported an increase in all-cause death versus bare-metal stents (BMS), whereas the Taxus™ (Boston Scientific, MA, USA) program only reported adjudicated cardiac death. Statistical differences were observed between DESs and BMSs for myocardial infarction and death but could only be documented in the Cypher and not for the Taxus program. Again in Barcelona, 3 years later (2009) at another ESC meeting, another session was organized to discuss ‘Is it time to turn the page on Barcelona 2006?’ The speakers included Camazind, Kastrati and James. Camazind presented a study demonstrating an enduring risk of LST of 0.6 per year and accumulative incidence of 3.3% at 4-year follow-up, whereas Kastrati emphasized that all DESs are effective in reducing restenosis with reductions in repeat revascularization and no increase in LST. James from Uppsala Clinical Research Center, Sweden, on the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) declared that ‘SCAAR scare’ is no more; however, he stated that the Achilles heel of DESs remains stent thrombosis. Therefore, controversies remain and we need to look into the future and determine how can we improve DESs and what steps have already been taken by the industry and what new technology can be applied for the long-term safety of DESs.
What are the problems with current DESs (first generation)?
Preclinical and autopsy studies have documented delayed arterial healing in DESs, as evidenced by the persistence of fibrin, incomplete re-endothelialization and sparse smooth muscle cell coverage, compared with BMSs implanted for the same duration Citation[1]. Subsequently, we showed that poor endothelial cell coverage of the stent struts was the single best correlate of late stent thrombosis, irrespective of duration of implant. Within DESs, arterial healing is heterogeneous and is dependent on underlying plaque morphology and also on the location of the stent. For example, stent thrombosis is higher in patients receiving DESs for acute myocardial infarction (AMI; plaque rupture) compared with stable lesions. Furthermore there is heterogeneity of healing in AMI at culprit sites (location of plaque rupture), with a further delay of healing at these sites compared with nonculprit sites (i.e., away from the plaque rupture site) . Similarly stent thrombosis at bifurcation sites is significantly greater in DESs than with BMSs. Flow disturbances at bifurcation have been well described, but with the use of DESs, which themselves induce delayed healing, there remains a continued risk of LST. With better imaging modalities, such as optical coherence tomography and angioscopy, which allow the assessment of strut coverage by neointimal tissue in living patients, it may be possible to predict future events in high-risk patients. A recent clinical study by Kubo et al. reported that the lack of neointimal strut coverage was more frequent in patients with unstable angina compared with stable angina Citation[2], consistent with autopsy findings.
Another distinct feature of the first generation of DES pathology is the observation of localized hypersensitivity reaction in the surrounding stented artery. We have observed hypersensitivity reaction in five cases and all had thrombotic occlusions with a high prevalence of positive remodeling and stent malapposition. Since late-acquired malapposition of DESs is reported in 10% of patients receiving these devices, it is important that clinicians be aware of the likelihood of the risk of thrombosis in such patients secondary to a hypersensitivity reaction. Hypersensitivity reaction has been observed exclusively with the Cypher stent, which could be either from hypersensitivity to the drug sirolimus or to the polymer (although sirolimus is less likely to be the cause as very few cases have been reported when was used to treat patients with kidney transplant). The most likely culprits are the polymers (i.e., the polyethylene co-vinyl acetate [PEVA] and poly n-butyl methacrylate [PBMA]), which have both been associated with hypersensitivity reactions in humans and animals when they are used in nonvascular locations Citation[3,4].
Second-generation DESs
Recently, second-generation DESs with zotarolimus (Endeavor™, Medtronic Vascular, CA, USA) and everolimus (Xience™, Abbott Vascular, CA, USA) have been approved by the US FDA. These second-generation DESs contain improvements in three different components: stent platform, total loaded dose of drug/release kinetics and type of polymer Citation[5]. In both, stainless steel has been replaced by cobalt chromium, which has resulted in greater radio-opacity and reduced strut thickness, which alone promotes more-rapid re-endothelialization. A newer analog of the drug sirolimus, known as everolimus, is used in lower concentrations on the Xience V stent but demonstrates similar release kinetics as the Cypher stents. On the other hand, Endeavor contains a higher concentration of zotarolimus, another analogue of sirolimus, but demonstrated more rapid release kinetics. The biological responses in the swine model at 28 days to Endeavor resulted in a decrease in neointimal growth, as well as less inflammation compared with Cypher, and less fibrin deposition compared with Taxus and Cypher stents. A comparison of the re-endothelialization in the rabbit model also showed greater endothelialization in the second-generation compared with the first-generation DESs Citation[6]. Polymers remain nonerodible with fluoropolymer on Xience V and phosphorylcholine on the Endeavor stent; however, the total polymer thickness is far less. Indeed, second-generation Xience V stent has shown significant reduction in target-vessel failure compared with the Taxus stent in randomized clinical trials (p = 0.04) Citation[7] and less myocardial infarction and all-cause death in the Xience at 2 years. Whereas the Endeavor stent, up to 4 years, showed similar incidence of target vessel revascularization as Cypher (7.7 vs 6.5%, respectively), long-term follow-up showed a significantly lower cumulative rate of death and myocardial infaction at 5 years for Endeavor versus Driver (5.6 vs 8.4%), and significantly less Academic Research Consortium definition of definite and probable myocardial infaction compared with historic Cypher and Taxus published data Citation[8].
Biodegradable polymers: are they better than nonerodible polymers?
The safety as well as efficacy of DESs should not be based solely on the degree of neointimal suppression but rather on the arterial healing and lack of excessive inflammation. A major problem with first-generation DESs, expecially Cypher, is polymer-induced inflammation. Since polymers employed in DESs do not degrade, the potential exists for long-term inflammation and, possibly, adverse cardiac events, as discussed previously. In addition to the improvements with the second-generation DESs, newer devices will employ biodegradable polymers. The bioerodible polymers include polyesters, polyorthoesters, polyanhydrides, polyphosphazens and polyurethanes. The earliest and the most commonly used biodegradable polymers are the polyesters, which included poly(lactide), poly(glycolide) and poly(glyco-co-lactic acid). The most important property, at least from pathologists point of view, is that they should not evoke an inflammatory/toxic response and should metabolize in the body once it has fulfilled its purpose. Of course, it should have a long shelflife and be sterilizable. The biodegradable polymer-coated polylactic acid (PLA) Biolimus A9 Nabori™(Terumo, Belgium) and BioMatrix® (Biosensors International, Singapore) have been approved recently in Europe and significantly reduced angiographic restenosis success in the first-in-man Stent Eluting A9 Biolimus Trial in Humans (STEALTH) Citation[9] and Limus Eluted From A Durable Versus Erodible Stent Coating (LEADERS) Citation[10] trials. The studies in pigs and rabbits also showed significant reduction in neointimal growth and better endothelialization than the nonerodible Cypher and Taxus stents. Similarly, significantly less inflammation was observed for the Nabori stent in the rabbit model, and better neointimal coverage than historic first-generation DESs. Cypher and Taxus have been associated with impaired local endothelium-dependent vasomotion at adjacent stent segments Citation[11]. Similarly, Hamilos et al. recently showed preservation of endothelial function of adjacent biolimus-A9 eluting Nabori stent in man Citation[12], thus suggesting that it is probably the characteristic of bioerodible polymer that is different in the two stents and not the drug, since both drugs bind to the FK binding protein 12 and, subsequently, inhibit the mTOR (preventing the degradation of p27kip1, a cyclin-dependent kinase inhibitor that plays an important role in regulating vascular smooth muscle cell migration and proliferation). In conclusion, we believe that biodegradable polymers are safer than nonerodible polymers because polymer degradation and the resulting inflammatory response is a transient phenomenon – if it does occur – leaving a BMS behind.
Future directions in DESs
Another attractive alternative is to replace the metal stent with completely bioabsorbable stents. This is conceptually appealing, as even BMSs induce inflammation and are associated with thrombosis and late restenosis. In addition, permanent metal stents make surgical intervention difficult owing to an inability to graft to these areas. From animal and human studies it has been demonstrated that, because the erodible stent disappears with time, this leaves the vessel in its native state with intact vasoreactivity. Moreover, the stent can be imaged noninvasively over its lifetime. Tamai and Igaki, 9 years ago, demonstrated the concept of using a PLA polymer stent successfully in man Citation[13]. More recently, Biotronik (Berlin, Germany) introduced the first biodegradable magnesium AMS stent composed of 93% magnesium and 7% other Earth metals with thromboresistant properties. However, the structural integrity was only maintained for 2 months and was replaced by inorganic salts (calcium). Initial clinical trials carried out in the peripheral and coronary arteries have been disappointing, resulting in higher restenosis than BMS Citation[14].
An alternative approach by Abbott Vascular (CA, USA) used a drug-eluting polymer on a bioabsorable stent (BVS). The BVS has a backbone of poly-L-lactic acid (PLLA) that provides the support and a coating of poly-D,L-lactic (PDLLA) contains and controls the antiproliferative agent everolimus . A Bioabsorbable Everolimus-Eluting Coronary Stent Trial (ABSORB I) showed promising results in 30 patients with single de novo coronary artery lesion with 2-year follow-up Citation[15]. At 2 years, there were no cardiac deaths or ischemia-driven revascularization or stent thrombosis, and only one myocardial infarction (non-Q wave). By optical coherence tomography, there was 19 ± 9% mean diameter stenosis, and by angiography at 2 years there was a 0.48 ± 0.28-mm increase in neointimal tissue, which was no different from 6 months. However, by intravascular ultrasound, lumen area enlarged between 6 months and 2 years, and by optical coherence tomography, there was a decrease in echogenicity in 34.5% of struts early, and the rest were well apposed to the vessel wall with preservation of vasomotion Citation[16]. These data support the concept behind this technology as an attractive alternative to metal stents coated with drugs and polymers that inhibit neointimal formation. Some hurdles, including the size of the struts and ability to use this technology in all-comer cases with severe multivessel disease, still need to be overcome.
Prohealing technology
Another alternative could be a technology that utilizes a prohealing approach, such as anti-CD34 coating on DESs to improve re-endothelialization by attracting circulating progenitor endothelial cells or applying drugs on the abluminal surface and, therefore, allowing re-endothelialization on the luminal surface Citation[17]. Some have suggested a rough surface of metal stents also allow more rapid and greater endothelialization, along with the use of Arg–Gly–Asp peptide coating Citation[18]. These newer technologies will have to undergo preclinical testing and, finally, clinical trials to prove their efficacy, both of which represent considerable hurdles.
Summary
There is no doubt that the introduction of coronary artery stents has been a successful story. Impressive efforts in basic research and pathologic studies have lead to a better understanding and improvement in the stent device. However, the question needs to be asked: will the new generation of DESs be better than the previous generation of stents? To improve upon the second generation of stents will not only require a greater basic understanding of drug-release kinetics in atherosclerotic vessels but also, better stent surface technology that will allow drugs to be released in a similar way to current DESs but without the inhibition of re-endothelialization. Any modification of DESs will need large, randomized, clinical trials to show superiority and safety in long-term clinical studies. However, such trials may be beyond affordability and we will, therefore, have to rely on better animal models. We also need to fully understand the role of underlying atherosclerosis in stented arteries, as well as the induction of new atherosclerotic disease formation within the neointima, in order to prevent untoward effects of DESs that have been reported.
It is incontrovertible that DESs have been beneficial for the vast majority of patients, allowing them to avoid unwanted secondary procedures, such as repeat revasculation. This advance has come at the price of continuing long-term dual antiplatelet therapy – with its increased bleeding risk – in order to mitigate against the possibility of late stent thrombosis secondary to delayed healing. However, there is still more progress that needs to be made in this field.
Financial & competing interests disclosure
Aloke V Finn has received research funding from Medtronic AVE. Renu Virmani is receiving company-sponsored research support from Medtronic AVE; Abbott Vascular; Conor Medsystems; OrbusNeich Medical; Terumo Corporation; Cordis Corporation; BioSensors International; Prescient Medical; Biotronik; and Alchimedics. Virmani is also a consultant for Medtronic AVE; Abbott Vascular; Prescient Medical; and Biotronik. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Nakazawa G, Finn AV, Ladich E et al. Drug-eluting stent safety: findings from preclinical studies. Expert Rev. Cardiovasc. Ther.6(10), 1379–1391 (2008).
- Kubo T, Imanishi T, Kitabata H et al. Comparison of vascular response after sirolimus-eluting stent implantation between patients with unstable and stable angina pectoris a serial optical coherence tomography study. JACC: Cardiovasc. Imag.1(4), 475–484 (2008).
- Ahmed DD, Sobczak SC, Yunginger JW. Occupational allergies caused by latex. Immunol. Allergy Clin. N. Am.23(2), 205–219 (2003).
- Leggat PA, Kedjarune U. Toxicity of methyl methacrylate in dentistry. Int. Dental J.53(3), 126–131 (2003).
- Nakazawa G, Finn AV, Kolodgie FD, Virmani R. A review of current devices and a look at new technology: drug-eluting stents. Expert Rev. Med. Devices6(1), 33–42 (2009).
- Joner M, Nakazawa G, Finn AV et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J. Am. Coll. Cardiol.52(5), 333–342 (2008).
- Stone GW, Midei M, Newman W et al. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trial. Circulation119(5), 680–686 (2009).
- Meredith IT, Ormiston J, Whitbourn R et al. Four-year clinical follow-up after implantation of the endeavor zotarolimus-eluting stent: ENDEAVOR I, the first-in-human study. Am. J. Cardiol.100(8B), 56M-61M (2007).
- Grube E, Hauptmann K, Buellesfeld L, Lim V, Abizaid A. Six-month results of a randomized study to evaluate safety and efficacy of a biolimus A9 eluting stent with a biogradable polymer coating. Eurointervention1, 53–57 (2005).
- Windecker S, Serruys PW, Wandel S et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS), a randomised non-inferiority trial. Lancet.372(9644), 1163–1173 (2008).
- Togni M, Windecker S, Cocchia R et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J. Am. Coll. Cardiol.46(2), 231–236 (2005).
- Hamilos MI, Ostojic M, Beleslin B et al. Differential effects of drug-eluting stents on local endothelium-dependent coronary vasomotion. J. Am. Coll. Cardiol.51(22), 2123–2129 (2008).
- Tamai H, Igaki K, Kyo E et al. Initial and 6-month results of biodegradable poly-L-lactic acid coronary stents in humans. Circulation102(4), 399–404 (2000).
- Erbel R, Di Mario C, Bartunek J et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet369(9576), 1869–1875 (2007).
- Ormiston JA, Serruys PW, Regar E et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB), a prospective open-label trial. Lancet371(9616), 899–907 (2008).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB), 2-year outcomes and results from multiple imaging methods. Lancet373(9667), 897–910 (2009).
- Nakazawa G, Granada JF, Alviar C et al. Anti-CD34 antibodies immobilized on the surface of sirolimus eluting stents enhance stent endothelialization. J. Am. Coll. Cardiol. Intervent. (2009) (In Press).
- Srivatsa SS, Edwards WD, Boos CM et al. Histologic correlates of angiographic chronic total coronary artery occlusions: influence of occlusion duration on neovascular channel patterns and intimal plaque composition. J. Am. Coll. Cardiol.29(5), 955–963 (1997).
- Vorpahl M, Finn AF, Nakano M, Virmani R. The bioabsorption process: tissue and cellular mechanisms and outcomes. EuroIntervention (2010) (In Press).