Venous thrombosis holds a special fear in the hearts of the worried, well-traveling population. Reports of ‘economy-class syndrome’ caused by deep-vein thrombosis (DVT) and pulmonary embolism (PE) have led to a mushrooming industry in over-the-counter antithrombosis stockings, foot compressors and onboard aircraft pilates videos. While there is little doubt that immobility can result in clot formation in venous circulation, the question of why some people experience venous clots compared to others given all other factors being equal is debated. There is no doubt that venous thrombosis is multifactorial, resulting from an interaction between environmental and genetic risk factors, with the former causing blood stasis and the latter an increase in coagulability or a deficiency of fibrinolysis Citation[1]. Although environmental risk factors, such as economy-class syndrome, have captured the public’s imagination, the influence of inherited determinants has made considerable head-way in recent times. Data from twin- and family-based studies suggest that over 60% of the variation in risk could be attributed to genetic factors Citation[2,3].
Reports on families with an increased predisposition to venous thrombosis, resulting from single-gene defects, emerged as early 1906 Citation[4]. Subsequently, hundreds of mutations of different genes have been identified, and form part of the wider picture of the etiology of DVT. However, monogenic disorders are relatively rare and do not account for a large-population effect. Advances in understanding the genetics of polygenic disorders has led to an appreciation of the genetic complexity likely to be involved in blood coagulability or deficiency of fibrinolysis. The case–control allelic-association model has been the most used, with numerous studies having been performed using multiple, single-nucleotide polymorphisms in a large number of candidate genes.
Abnormalities of naturally occurring anticoagulants, such as protein C, protein S and antithrombin, have been found to be rather uncommon risk factors for venous thrombosis, whereas, by comparison, gain-of-function mutations of the coagulation Factor V (Factor V Leiden) and Factor II (prothrombin G20210A) have been associated with a larger proportion of venous thromboembolism cases Citation[5]. Factor V Leiden and prothrombin G20210A are two of the more common variants studied that have been strongly associated with an increased risk of DVT but explain only a small proportion of DVT events. The discovery of Factor V Leiden caused an increase in interest in the genetics of DVT and other cardiovascular diseases. The prevalence of this mutation – up to 20% of idiopathic DVT cases – provided a driver for further research into novel mutation identification technology Citation[6]. However, many of the candidate-gene-based studies have provided conflicting results, often because they have been underpowered.
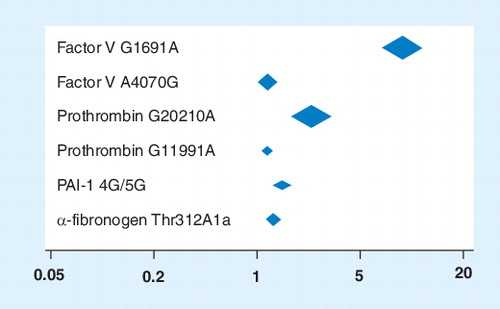
One established way of increasing the power of such undertakings and providing tighter confidence intervals is to perform a high-quality, comprehensive meta-analysis. Our group has undertaken such a meta-analysis; to our knowledge, the largest of its kind Citation[7]. A total of 173 publications were identified, addressing 28 polymorphisms in 21 genes in 310,593 subjects (126,525 cases and 184,068 controls), resulting in 45 meta-analyses conducted under both recessive and dominant models. Of the 45 meta-analyses, 20 had more than 1000 cases while four had over 10,000 cases. Statistically significant at-risk associations with venous thromboembolism were identified for four genes (six polymorphisms) in Caucasians: Factor V G1691A (odds ratio [OR]: 9.45; 95% CI: 6.72–13.30; p < 0.0001), Factor V A4070G (OR: 1.24; 95% CI: 1.02–1.52; p = 0.03), Prothrombin G20210A (OR: 3.17; 95% CI: 2.19–3.46; p < 0.00001), Prothrombin G11991A (OR: 1.17; 95% CI: 1.07–1.27; p = 0.0007), PAI-1 4G/5G (OR: 1.62; 95% CI: 1.22–2.16; p = 0.0008) and a-fibrinogen Thr312Ala (OR: 1.37; 95% CI: 1.14–1.64; p = 0.0008) . Analyses for MTHFR/C677T in Chinese/Thai populations (OR: 1.57; 95% CI: 1.23–2.00; p = 0.0003) and ACE I/D in African–American populations (OR: 1.5; 95% CI: 1.03–2.18; p = 0.03) also found significantly at-risk associations with venous thromboembolism. Factor XIII Val34Leu (OR: 0.80; 95% CI: 0.68–0.94; p = 0.007) and β-fibrinogen 455 G/A (OR: 0.84; 95% CI: 0.72–0.97; p = 0.02) both showed significant protective effects Citation[7].
As the number of subjects studied was very large, attributable risks could be determined with a high degree of robustness and with tight confidence intervals. The population attributable risk – a broad measure of genetic accountability to the clinical phenotype – was 17.0% for Factor V G1691A and up to 30.1% for PAI-1 4G/5G polymorphism in Caucasian populations. Although not entirely reliable, these population attributable risks suggest that a genetic contribution could account for as many as 200,000 deaths per year from venous thromboembolic-related events in the USA alone.
Notwithstanding the advantages of such large-scale enterprises (and acknowledging all their limitations), these results are not the beginning of the end but rather the end of the beginning. Molecular technology is moving at a frantic pace and the arrival of successful large-scale genome-wide association (GWA) studies will potentially allow us to learn much more about thrombotic etiology without being confined to a priori hypotheses. Despite the undoubted advantages of GWA studies, they come at a considerable price, which can be measured by the size of samples required (several thousands) and associated DNA repository and laboratory costs (millions of dollars). However, we do not believe even this will be the beginning of the end. That is only likely to even be considered once deep sequencing (base-by-base) of affected DNA repositories is undertaken. Such technology is currently available Citation[101] but the cost is prohibitive. A word of caution, however, should be sounded to those clinicians who have tirelessly recruited DNA samples and allowed them to be used in case–control studies and, latterly, GWA studies. Quantities of extracted DNA are not infinite and the ‘wise man’ should ensure that they have enough stock to be able to participate in the next deep-sequencing race that is likely to occur once cost is reduced to acceptable levels. Failing to save for such a day may leave investigators who have spent many years in clinical phenotyping and collecting DNA samples participating in the early stages of the genetic revolution but failing to be able to compete at the final and most important hurdle.
So, where do we go from here, except wait for technology to become cheaper? Robust risk estimates have been established Citation[7] but does this now mean the economy-class syndrome-worried public ought to have genetic screening? Such screening, no doubt, could be bought over the internet without explanation of the result, further heightening fears or, more worryingly, implying a false sense of security. However, we cannot make the case for such screening in complex disorders yet, as the susceptibility risk at an individual level remains low, even if the population risk is high. Indeed, most subjects with thrombophilic variants will not develop venous thrombosis Citation[8]. However, multifactorial diseases by their very nature imply that, given the appropriate environmental situation (e.g., embarking on a long-haul flight, taking the contraceptive pill Citation[9], pregnancy and the puerperium Citation[10]), knowledge of a pre-existing genetic risk could provide a prophylactic advantage, which, it could be argued, the ‘consumer’ ought to be given the opportunity to be ‘armed’ with.
What is obviously lamentable in all our findings is the paucity of data relating to those of non-European descent Citation[11]. Investigators in complex disorders should not be too disheartened, as the initial and entirely reasonable natural response is to study the local, readily available population, which is often Caucasian, located around international institutions of excellence. However, complacency should not be allowed to be established with either those investigators or the funding bodies. A large proportion of the healthcare budget is disproportionately used for the poorer population who are often from ethnic minorities. They too deserve our attention; indeed, comparative genetics between Caucasians and other ethnic groups may also provide important information about etiological mechanisms, as well as novel therapeutic targets.
The end of the beginning may have approached but the ultimate end is a very long way off indeed.
Financial & competing interests disclosure
Pankaj Sharma has received honoraria for lecturing in industry-sponsored meetings and has received industry funding for attending national and international meetings. He has received research grants from pharmaceutical companies and has been a paid consultant to the biotech industry and been a member of industry advisory boards. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript
References
- Oguzulgen IK, Ekim N, Erkekol FO, Altinok B, Akar N. Is tissue-plasminogen activator gene polymorphism a risk factor for venous thromboembolism in every population? J. Thromb. Thrombolysis19(1), 61–63 (2005).
- Souto JC, Almasy L, Borrell M et al. Genetic susceptibility to thrombosis and its relationship to physiological risk factors: the GAIT study. Genetic Analysis of Idiopathic Thrombophilia. Am. J. Hum. Genet.67(6), 1452–1459 (2000).
- Larsen TB, Sorensen HT, Skytthe A, Johnsen SP, Vaupel JW, Christensen K. Major genetic susceptibility for venous thromboembolism in men: a study of Danish twins. Epidemiology14(3), 328–332(2003).
- Briggs JB. Recurrent phlebitis of obscure origin. Bull. John Hopkins Hosp.16, 228–233 (1905).
- Colaizzo D, Amitrano L, Iannaccone L et al. Gain-of-function gene mutations and venous thromboembolism: distinct roles in different clinical settings. J. Med. Genet.44(6), 412–416 (2007).
- Hooper WC, De SC. Venous thromboembolism: implications for gene-based diagnosis and technology development. Expert Rev. Mol. Diagn.2(6), 576–586 (2002).
- Gohil R, Peck G, Sharma P. The genetics of venous thromboembolism. A meta-analysis involving approximately 120,000 cases and 180,000 controls. Thromb. Haemost.102(2), 360–370 (2009).
- Vossen CY, Conard J, Fontcuberta J et al. Familial thrombophilia and lifetime risk of venous thrombosis. J. Thromb. Haemost.2(9), 1526–1532 (2004).
- Vandenbroucke JP, Koster T, Briet E, Reitsma PH, Bertina RM, Rosendaal FR. Increased risk of venous thrombosis in oral-contraceptive users who are carriers of Factor V Leiden mutation. Lancet344(8935), 1453–1457 (1994).
- Lindhoff-Last E, Luxembourg B. Evidence-based indications for thrombophilia screening. Vasa37(1), 19–30 (2008).
- Ariyaratnam R, Casas JP, Whittaker J, Smeeth L, Hingorani AD, Sharma P. Genetics of ischaemic stroke among persons of non-European descent: a meta-analysis of eight genes involving approximately 32,500 individuals. PLoS Med.4(4), e131 (2007).
Website
- 1000 Genomes. A deep catalog of human genetic variation. www.1000genomes.org