In countries with adequate tertiary sewage treatment plants, the contamination from hospitals into the environment is largely negated, blocking the bacterial gene carriage into nosocomial settings. However, widespread contamination of the environment results in high carriage of New Delhi metallo-β-lactamase-1 as normal flora, which significantly impacts on importation into hospital settings.
Promiscuous plasmids and genetic fluidity aside, the unbridled and indiscriminate use of antibiotics, lack of infection control policies and an inadequate national surveillance system have added to the spread of New Delhi metallo-β-lactamase-1 (NDM-1). Notwithstanding, as NDM-1 bacteria are mainly spread via the fecal–oral route, the dissemination of NDM-1 in the Indian community is highly likely and, therefore, inadequate sewage systems, which are often present in these countries, only further exacerbates the problem. Accordingly, the solution requires holistic actions and a change of social priorities which, if invoked, will almost certainly be too late to save one of medicine’s precious and long-standing resources: antibiotics.
In 2005, we co-wrote a review on metallo-β-lactamases (MBLs) entitled ‘MBLs: the quiet before the storm?’ Citation[1–4]. The title reflected the review which speculated that MBLs would become more prolific and that the key example would be the clinical challenge of Verona imipenemase (VIM)-2 in Pseudomonas aeruginosa. Such prophetic notions came to pass for countries such as Russia, which currently has a national endemic of VIM-2-positive P. aeruginosa causing enormous therapeutic challenges throughout its vast country. However, the authors of the review did not foresee the emergence of a new and far more insidious MBL: NDM-1. The first public disclosure of NDM-1 occurred in 2008 at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) in the USA, followed by a subsequent publication reporting its genetic context and kinetics in December 2009 Citation[5]. In March 2010, Deshpande et al. published a study examining the incidence of NDM-1-positive bacteria from their local hospital in Mumbai and found that 22 out of 24 carbapenem-resistant Enterobacteriaceae carried NDM-1 Citation[6]. Interestingly, NDM-1-positive strains included Klebsiella pneumoniae, Escherichia coli and Enterobacter spp. This study was eulogized by a lively correspondence by Ghafur in the same journal forewarning against the perils of NDM-1 in Indian hospitals, blaming the general apathy of Indian clinicians and predicting the end of treatable infections Citation[7]. Despite these sometimes controversial publications, very little media interest if any was generated – this was the ‘quiet before the storm’. On 8 August 2010, prior to our publication, a news release was disseminated to national and international press agencies which heralded the start of an extraordinary episode that would influence international trade and cause a mini-run on stock markets in China Citation[101]. Much of this interest was the general notion that this new ‘superbug’ had emerged in the UK; however, this was largely through importation from Southern Asia – probably India. Thus, overnight, the Indian subcontinent became the focal point of global interest on antibiotic resistance. In response to this media interest, a systematic, energetic and sometime vitriolic reaction came from Indian ministers, officials and surgeons criticizing the study and the authors – often inaccurately. So the question prevails, why this overreaction from the Indian authorities when the name and the origin of the resistance was published in December 2009?
The reaction received through the Indian media was often irrational and directed at the sentences: ‘It is disturbing, in context, to read calls in the popular press for UK patients to opt for corrective surgery in India with the aim of saving the NHS money. As our data show, such a proposal might ultimately cost the NHS substantially more than the short-term saving and we would strongly advise against such proposals’ Citation[102].
The key point of these remarks is that it was directed at UK patients being paid by the British tax payer and as the main authors of the said article are, directly or indirectly, supported by the British tax payer we felt we had a moral obligation to forewarn of the possible dangers of being exposed to NDM-1-positive bacteria. Most interestingly, the corresponding protests in India mainly came from surgeons who work at large private hospitals (Mumbai, Kolkatta, Delhi and Chennai) and are themselves involved in ‘added value travel’, or as more commonly known in the West as ‘medical tourism’. This trade currently caters for 450,000 people per year usually for elective surgery but increasingly for corrective surgery. This industry is worth approximately US$2 billion per year and is forecast to increase by 30% per annum.
At this stage of the saga, the Indian microbiologists were largely silent about the study. More disturbingly, the Indian authors of the article became under immense pressure from the Indian media and denied either the data and/or the conclusions of the study despite signing a declaration that they had agreed with the study and content of the said article immediately prior to publication. Moreover, an inquest was initiated about the transport of strains outside of India which was ironic given the international spread of NDM-1 via patients undergoing surgery in India. It was clear from the comments raised by ministers and surgeons alike that they know very little regarding the molecular biology of antibiotic resistance.
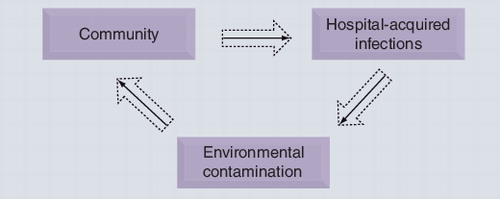
What was clearly unfolding was a systematic campaign of self denial of the immense problem currently facing India/Pakistan and, ultimately, the WHO. Unfortunately for the Indian authorities, subsequent reports universally support our original findings and indeed the recent study by the REACT group assessing multidrug-resistant (MDR) strains showed that seven out of eight tourists returning from India were colonized by extended-spectrum β-lactamase (ESBL)-positive bacteria having not carried these prior to departure Citation[8]. Moreover, in monitoring the ESBL-positive bacteria, the rates of ESBL carriage in Indian hospitals is 79%, the exact amount carried in the community (79%), which is incidentally the highest in the world Citation[9]. Although the data on NDM-1 are currently few, there is supportive evidence that NDM-1-positive bacteria are widespread in the community [Walsh TR, Toleman MA, Unpublished Data]. A recent case of NDM-1-positive bacteria was a Taiwanese cameraman who was shot in the stomach outside a mosque in Delhi and became subsequently endogenously infected with a NDM-1-positive K. pneumoniae (NDM-1-positive bacteria is present as normal gut flora). Such oral–fecal transmission would not be an issue but in India it is a colossal problem as highlighted by a UN report claiming that 650 million Indians do not have proper sanitation and probably even more do not have access to clean water. Disturbingly, the sewage system in the capital cannot even cater for the city’s population – indeed, it operates at 60% capacity Citation[10]. Therefore, the demarcation between ‘clean’ and ‘dirty’ are often blurred, which breaches the fundamental barrier to containing the spread of MDR pathogens. As depicted in , the cycle from hospital resistance to the community is normally arrested at the hospital importation stage; in India (and probably Pakistan and Bangladesh) this cycle is maintained and its momentum is unchecked.
In 2001, the WHO launched a very worthy document articulating various action points to curb antibiotic resistance. Among the numerous proposals, some of the key points were the following:
• Ban the use of nonprescribed antibiotics and self-medication
• Initiate prudent antibiotic formularies in hospitals
• Implement stringent infection control policies
• Establish national surveillance programs
• Openly collaborate with international groups to further our understanding of antibiotic resistance
Sadly, at a national level, India has not only failed to implement these, in many instances it has failed to even acknowledge them.
Some have argued that NDM-1 is nothing new and that other countries have problems with carbapenemases (e.g., VIM, K. pneumoniae carbapenemase [KPC]) such as Greece, Israel and the USA. However, such proponents are ill-informed as to why NDM-1 is unique and why it is highly likely to become the CTX-M-15 of the 21st Century. The ease of transition in India from environment to hospitals and visa versa aside, blaNDM-1 is located on remarkably plastic plasmids – genetic structures that can move with ease from one bacterium to another – and it is now very clear that blaNDM-1 plasmids are highly promiscuous. For example, KPC-1 and -2 were isolated from patients in 1997 and 1998, respectively, yet only recently has it been reported in Acinetobacter spp. (not Acinetobacter baumannii) and Pseudomonas aeruginosa. By contrast, the NDM-1 SENTRY study clearly shows that NDM-1 was present in 2006 and has subsequently been found in nearly all clinical species of Enterobacteriaceae, A. baumannii and Pseudomonas spp. Citation[11,12] and thus appears to transcend the genus/family barrier with ease.
Sequencing of blaNDM-1 plasmids have revealed they contain up to 14 other antibiotic resistance genes [Walsh TR, Toleman MA, Unpublished Data] such that the recipient bacterium is resistant to all antibiotics apart from tigecycline and colistin – this is very different to other mobile carbapenemase resistance genes. These plasmids contain numerous insertion sequence common region elements which explain why, in some strains, blaNDM-1 can be seen on the chromosome and also carried on numerous different plasmids in the same strain. Such genetic fluidity can be influenced by activation of the S.O.S response which can, in turn, be triggered by the sub-MIC presence of fluoroquinolones Citation[13–15]. Unfortunately, in India, the massive and unchecked use of antibiotics has resulted in detectable and significant levels of ciprofloxacin in Indian waste and water which will, in turn, render the bacteria more receptive to either receiving DNA and, once it’s established, rearranging it Citation[12].
The occurrence of NDM-1 can, with few exceptions, be directly linked to either India or Pakistan. Moreover, in addition to the SENTRY report, showing the presence of NDM-1 in India in 2006, many of the cases reported elsewhere around the world can be directly traced back to India or Pakistan. Among the 29 UK patients, 17 (59%) had prior travel to the subcontinent, with 14 (48%) hospitalized there Citation[16]. Given that the UK reference laboratory has actively sought carbapenem-resistant Enterobacteriaceae since 1998 and did not find any since 2007, it almost certainly was not present prior to this date. It has been argued that the same clonal lineages could not be found in both countries, questioning the link Citation[17–20]. However, those that raise this argument must appreciate the rapid dissemination and subsequent plasticity of labile blaNDM-1 plasmids among bacterial populations. Those countries reporting NDM-1 bacteria with direct links to India include Sweden, Norway, The Netherlands, France, Germany, Austria, Singapore, Japan, Hong Kong, Oman, the USA, Australia, Canada, Taiwan and Israel as well as the UK and European CDC Citation[16,21–27]. Other cases such as the fatality in Belgium had undergone an operation in Pakistan.
NDM-1 is a product of the global economy. It would appear that the emergence of NDM-1-positive bacteria is as much a response of human behavior as it is the bacteria becoming ‘smarter’. Did Fleming not warn us in his 1945 lecture that once these miracle drugs are out in the public domain and subsequently abused, resistance will ensue? So what went wrong? It appears that our insatiable appetite for profit and our global lack of sagacity has had its consequences and has, ultimately, resulted in this unenviable position that we, globally, currently find ourselves in. The stock exchanges are a sign of consumer confidence and the hall mark of recovery from periodic recessions; however, many of these are linked to activities that further spread antibiotic resistance such as airlines where the human propensity to continually travel appears ravenous. Everywhere an individual goes, he/she carries 100 trillion bacteria with him/her with the ultimate consequences of passing these bacteria on – ultimately we are vehicles of MDR bacteria, not to mention contracting pathogenic bacteria from polluted environments as witnessed at the recent Commonwealth games Citation[28].
The lack of basic hygiene facilities in many countries has substantially exacerbated this problem – the simple concept of keeping clean from dirty is still desperately lacking in many countries yet did the Greeks not teach us this over 2500 years ago? Of course, there is little profit to be had in building sanitation works or, indeed, building public toilet facilities which the poor can use. Such basic facts dictate why India has one of the highest death rates of young children in the world Citation[29,30].
Additionally, the quick, unregulated sale of antibiotics has resulted in massive unchecked consumption Citation[31,32] where detectable levels of antibiotics are present in human sewage, providing an ever-present selective pressure and triggering bacterial SOS systems – ultimately breeding ‘superbugs’ Citation[33–35] . The use of antibiotics in farming is no better – antibiotics mean fatter animals and better profit margins regardless of breeding MDR bacteria Citation[36].
Sadly, which many fail to grasp, antibiotic resistance is a global phenomena and thus merits an unfailing global response. In 2011, we have DNA arrays, high-throughput screening, genomic sequences and more, yet we do not have a publicly funded global surveillance system, which is both shocking and shameful. Such a network must operate with absolute transparency and accountability, and not be undermined by national scheming or political intrigue. There seems little point in one country taking to heart the recommendations of the Copenhagen Summit and the WHO report, while others blatantly ignore it. People are global – our normal flora is global and thus so is MDR bacteria Citation[8,37,38]. Unbridled capitalism has lured us into a throw-away society where longevity is sacrificed for quick profit and thus long-term programs that will protect future generations from the scourge of MDR, and now pandrug-resistant bacteria, are also sacrificed on the alter of modernity. Politicians come and go in 4–5 year cycles as do their policies. The WHO must step up and, in assuming global responsibility, ensure that all countries are committed to implement long-term holistic measures to fully engage local and national MDR issues – the time for politically correct susurrations has long since expired.
Fleming’s warning has fallen on ears deafened by the sound of falling money. It seems inevitable that future generations will not only bemoan the erratic weather patterns we will leave them due to global warming, but they will also lament that, for those who knew better, we created superbugs with little regard to the long-term consequences. There is coming a time when our ‘magic bullets’ are no longer ‘magic’ or ‘bullets’ Citation[39–42] and they will conclude that, despite knowing what to do, the political appetite to act nobly and honorably, was singularly lacking. In the case of India, the global scientific spotlight has forced changes that 6 months ago were not thought humanly possible, and such changes must be fully embraced. However, there is a lingering, nagging fear that it is too little and far too late.
Five-year view
There is little doubt that, by 2016, NDM-1 will have eclipsed KPC as the most common carbapenemase and will start to emulate CTX-M in its global presence – its unique genetic scaffold and mankind’s lack of sagacity dictate that this will unfold as foretold. Whether we will witness changes in national government policies or a global communal effort to engage MDR and pandrug-resistant bacteria championed by the WHO remains to be seen – we can but hope!
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev.18, 306–325 (2005).
- Patzer JA, Walsh TR, Weeks J, Dzierzanowska D, Toleman MA. Emergence and persistence of integron structures harbouring VIM genes in the Children’s Memorial Health Institute, Warsaw, Poland, 1998–2006. J. Antimicrob. Chemother.63, 269–273 (2009).
- Quinones-Falconi F, Galicia-Velasco M, Marchiaro P et al. Emergence of Pseudomonas aeruginosa strains producing metallo-β-lactamases of the IMP-15 and VIM-2 types in Mexico. Clin. Microbiol. Infect.16(2), 126–131 (2009).
- Yakupogullari Y, Poirel L, Bernabeu S, Kizirgil A, Nordmann P. Multidrug-resistant Pseudomonas aeruginosa isolate co-expressing extended-spectrum β-lactamase PER-1 and metallo-β-lactamase VIM-2 from Turkey. J. Antimicrob. Chemother.61, 221–222 (2008).
- Yong D, Toleman MA, Giske CG et al. Characterization of a new metallo-β-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother.53, 5046–5054 (2009).
- Deshpande P, Rodrigues C, Shetty A, Kapadia F, Hedge A, Soman R. New Delhi metallo-β-lactamase (NDM-1) in Enterobacteriaceae: treatment options with carbapenems compromised. J. Assoc. Physicians India58, 147–149 (2010).
- Ghafur AK. An obituary on the death of antibiotics! J. Assoc. Physicians India58, 143–144 (2010).
- Tangden T, Cars O, Melhus A, Lowdin, E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum β-lactamases: a prospective study with Swedish volunteers. Antimicrob. Agents Chemother.54, 3564–3568 (2010).
- Hawser SP, Bouchillon SK, Hoban DJ et al. Emergence of high levels of extended-spectrum-β-lactamase-producing Gram-negative bacilli in the Asia–Pacific region: data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program, 2007. Antimicrob. Agents Chemother.53, 3280–3284 (2009).
- Jamwal P, Mittal AK, Mouchel JM. Efficiency evaluation of sewage treatment plants with different technologies in Delhi (India). Environ. Monit. Assess.153, 293–305 (2009).
- Castanheira M, Deshpande LM, Mathai D, Bell JM, Jones RN, Mendes RE. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY antimicrobial surveillance program (2006–2007). Antimicrob Agents Chemother. DOI: 10.1128/AAC.01497-10 (2010) (Epub ahead of print).
- Diwan V, Tamhankar AJ, Khandal RK et al. Antibiotics and antibiotic-resistant bacteria in waters associated with a hospital in Ujjain, India. BMC Public Health10, 414 (2010).
- Dorr T, Vulic M, Lewis K. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol.8, e1000317 (2010).
- Lopez E, Blazquez, J. Effect of subinhibitory concentrations of antibiotics on intrachromosomal homologous recombination in Escherichia coli.Antimicrob. Agents Chemother.53, 3411–3415 (2009).
- Beaber JW, Hochhut B, Waldor MK. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature427, 72–74 (2004).
- Kumarasamy KK, Toleman MA, Walsh TR et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis.10(9), 597–602 (2010).
- Goel N, Wattal C. New Delhi metallo-β-lactamase 1. Lancet Infect. Dis.10, 751; author reply 752–754 (2010).
- New Delhi metallo-β-lactamase 1. Lancet Infect. Dis.10, 749–750; author reply 752–754 (2010).
- Sirohi B. New Delhi metallo-β-lactamase 1. Lancet Infect. Dis.10, 750; author reply 752–754 (2010).
- Tempe DK. New Delhi metallo-β-lactamase 1. Lancet Infect. Dis.10, 750–751; author reply 752–754 (2010).
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-β-lactamase – United States, 2010. MMWR59, 750 (2010).
- Hsueh PR. New Delhi metallo-β-lactamase-1 (NDM-1): an emerging threat among Enterobacteriaceae. J. Formos. Med. Assoc.109, 685–687 (2010).
- Rolain JM, Parola P, Cornaglia G. New Delhi metallo-β-lactamase (NDM-1): towards a new pandemia? Clin. Microbiol. Infect.16, 1699–1701 (2010).
- Leverstein-van Hall MA, Stuart JC, Voets GM et al. Carbapenem-resistant Klebsiella pneumoniae following foreign travel. Ned. Tijdschr. Geneeskd154, A2013 (2010).
- Bruhn C. NDM-1-producing bacteria-danger due to ‘super germs’? Dtsch Med. Wochenschr.135, p38 (2010).
- Poirel L, Ros A, Carricajo A et al. Extremely drug-resistant Citrobacter freundii identified in a patient returning from India and producing NDM-1 and other carbapenemases. Antimicrob. Agents Chemother.55(1), 447–448 (2010).
- Poirel L, Lagrutta E, Taylor P, Pham J, Nordmann P. Emergence of metallo-β-lactamase NDM-1-producing multidrug-resistant Escherichia coli in Australia. Antimicrob. Agents Chemother.54, 4914–4916 (2010).
- Shaw MT, Leggat PA, Chatterjee S. Travelling to India for the Delhi XIX Commonwealth Games 2010. Travel. Med. Infect. Dis.8, 129–138 (2010).
- Ensink JH, Blumenthal UJ, Brooker S. Wastewater quality and the risk of intestinal nematode infection in sewage farming families in hyderabad, India. Am. J. Trop. Med. Hyg.79, 561–567 (2008).
- Million Death Study Collaborators. Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet376(9755), 1853–1860 (2010).
- Sarma JB, Ahmed GU. Prevalence and risk factors for colonisation with extended spectrum β-lactamase producing enterobacteriacae vis-a-vis usage of antimicrobials. Indian. J. Med. Microbiol.28, 217–220 (2010).
- Pandey AA, Thakre SB, Bhatkule PR. Prescription analysis of pediatric outpatient practice in Nagpur city. Indian J. Community Med.35, 70–73 (2010).
- Sengupta N, Alam SI, Kumar RB, Singh L. Diversity and antibiotic susceptibility pattern of cultivable anaerobic bacteria from soil and sewage samples of India. Infect. Genet. Evol.11(1), 64–77 (2010).
- Nagshetty K, Channappa ST, Gaddad SM. Antimicrobial susceptibility of Salmonella Typhi in India. J. Infect. Dev. Ctries4, 70–73 (2010).
- Shakibaie MR, Jalilzadeh KA, Yamakanamardi SM. Horizontal transfer of antibiotic resistance genes among Gram negative bacteria in sewage and lake water and influence of some physico–chemical parameters of water on conjugation process. J. Environ. Biol.30, 45–49 (2009).
- Bhandare S, Paturkar AM, Waskar VS, Zende RJ. Prevalence of microorganisms of hygienic interest in an organized abattoir in Mumbai, India. J. Infect. Dev. Ctries4, 454–458 (2010).
- Webster PC. Global action urged in response to new breed of drug-resistant bacteria. CMAJ182, 1602–1603 (2010).
- MacPherson DW, Gushulak BD, Baine WB et al. Population mobility, globalization, and antimicrobial drug resistance. Emerging Infect. Dis.15, 1727–1732 (2009).
- Boucher HW, Talbot GH, Bradley JS et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis.48, 1–12 (2009).
- Rice LB. Emerging issues in the management of infections caused by multidrug-resistant Gram-negative bacteria. Cleve. Clin. J. Med.74(Suppl. 4), S12–S20 (2007).
- Rice LB. Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin. Infect. Dis.43(Suppl. 2), S100–S105 (2006).
- Rice LB. Unmet medical needs in antibacterial therapy. Biochem. Pharmacol.71, 991–995, (2006).
Websites
- International travel increasing spread of new drug-resistant bacteria: is this the end of antibiotics? www.physorg.com/news200670948.html
- Lakhani N. NHS ‘could save millions’ by flying patients to India. The Independent www.independent.co.uk/life-style/healthand-families/health-news/nhs-could-save-millions-by-flyingpatients-to-india-1870215.html