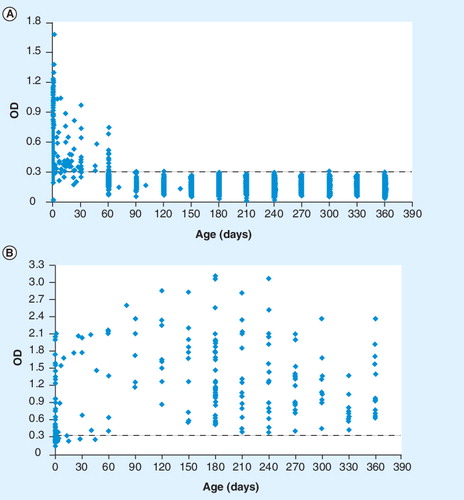
(A) Anti-shed acute-phase antigen IgG antibodies of 2074 noninfected infants. (B) Anti-shed acute-phase antigen IgG antibodies of 209 congenitally T. cruzi-infected infants.
OD: Optical density.
Chagas’ disease, or American trypanosomiasis, which is caused by the protozoan parasite Trypanosoma cruzi, is the most common cause of heart disease in Latin America, and approximately 13,000–45,000 chagasic patients die each year. Owing to the increasing control of the transmission routes mediated by the vector, blood transfusion and organ transplant, vertical transmission has become more relevant in terms of public health Citation[1]. It is a consensus that congenital T. cruzi infection will be a pressing public health problem for at least the next 20 years, when the pool of infected women of child-bearing age will decrease to insignificant levels. Congenital transmission, estimated to cause 15,000 cases per year, is seen as a continuous source of infection for newborns in endemic countries as well as in nonendemic countries with the immigration of infected women Citation[101].
Congenital transmission cannot be prevented and etiological treatment of infected women is not recommended during pregnancy. However, early detection of the congenitally infected infant ensures therapeutic success. Unfortunately, in most countries endemic with Chagas’ disease, neither pregnant women nor newborns are routinely tested for T. cruzi infection. Congenital cases are frequently asymptomatic, thereby passing unnoticed unless specific tests are carried out.
Currently, only direct parasite detection tests are able to confirm infection at birth. Parasite detection tests are difficult to perform routinely on a large-scale basis. Owing to parasite lysis, sensitivity decreases over time, which is a problem when transporting tests from rural areas to specialized centers. The tests also show low detection rates when used in routine laboratory conditions by inexperienced parasitologists. Conventional IgG serology allows the diagnosis of congenital infection in infants only over 9 months of age, following the disappearance of maternal antibodies. In noninfected infants, the conventional serology at 6, 7 and 8 months of age, is still positive due to maternal IgG (false positives) in 7, 4.3 and 1.6% of the cases, respectively Citation[2,102]. Thus, the long-term follow-up period that is required to confirm whether infants are infected is a major constraint in developing countries. Previous experiences in Argentina and Paraguay have shown that the loss to follow-up of these children is approximately 70–80% Citation[2,3].
The most sensitive technique (higher than 90%) for early detection is PCR, but it is not recommended for T. cruzi-infection diagnosis at the public health level in endemic countries, it is not available at primary healthcare settings in developing countries and it has to be reserved for specialized centers Citation[102].
Shed acute-phase antigen (SAPA) was described with main expression during the acute phase of T. cruzi infection associated with the infective stage of tripoamastigote Citation[4]. The use of SAPA for the diagnosis of congenital Chagas’ infection Citation[2,5] and infection in children residing in endemic areas has been described previously Citation[6].
An ELISA system Citation[7] with the recombinant protein SAPA was developed in 1998 in Paraguay Citation[2,5,103], and it is currently used in the public health system for the detection of IgG antibodies anti-SAPA in serum and in total blood with filter paper. The cut-off optical density of the ELISA-SAPA is 0.300 Citation[2,5], with good discrimination between positive and negative infected infants aged 3–12 months. Our results, based on a large-scale study on serial screening of serum samples (n = 2283) from infants born to T. cruzi-infected mothers who were between 1 day and 1 year of age by an ELISA-SAPA assay Citation[2,103], indicate that this is the best serological test allowing the earliest possible diagnosis of congenital T. cruzi infection (see ). This experience shows the feasibility of controlling the incidence of congenitally acquired T. cruzi infections on a wide scale by means of a specific screening program at the primary healthcare level. The ELISA-SAPA is performed with the components of a commercial kit developed by the Departamento de Bioquímica y Producción of the Instituto de Investigaciones en Ciencias de la Salud-Universidad Nacional de Asunción (IICS-UNA) named Chagas/IICS Citation[6]. The system was standardized by the Departamento de Biología Molecular y Genética of the IICS-UNA and the antigen used was a recombinant SAPA of plasmid pGex obtained by genetic engineering techniques. The insert used was released by Carlos Frasch (Instituto de Investigaciones Biotecnológicas, Universidad Nacional de San Martín, Argentina) in 1998.
We recommend the serological test using the recombinant protein SAPA in combination with conventional serology, since it allows the identification of congenital cases among those infants infected by T. cruzi and not detected by conventional serology, the detection of false-positive infected infants due to maternal IgG antibodies, and the assessment of congenital transmission at 3 months of age with the detection of IgG antibodies against SAPA, since maternally transmitted SAPA-specific antibodies disappear in babies earlier than conventional antibodies.
The detection of anti-SAPA antibodies for a timely diagnosis and a routine confirmation of congenital infection must be validated in different regions of the Americas since validated serological diagnosis tests that are able to detect specific antibodies in infants with congenital T. cruzi infection at birth are not available.
Acknowledgments
The authors thank the staff of the Regiones Sanitarias III and IX of the Ministry of Health of Paraguay.
Financial & competing interests disclosure
This study was supported by the United Nations Development Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases and the Universidad Nacional de Asunción. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- World Health Organization. Report of the Expert Committee on the Control of Chagas’ Disease. World Health Organization, Geneva, Switzerland 115 (2002).
- Russomando G, Almiron M, Candia N et al. [Implementation and evaluation of a locally sustainable system of prenatal diagnosis to detect cases of congenital Chagas disease in endemic areas of Paraguay]. Rev. Soc. Bras. Med. Trop.38(2), 49–54 (2005).
- Sosa-Estani S. Congenital transmission of Trypanosoma cruzi infection in Argentina. Rev. Soc. Bras. Med. Trop.38(2), 29–32 (2005).
- Affranchino JL, Ibanez CF, Luquetti AO et al. Identification of a Trypanosoma cruzi antigen that is shed during the acute phase of Chagas’ disease. Mol. Biochem. Parasitol.34, 221–228 (1989).
- Mallimaci MC, Sosa-Estani S, Russomando G et al. Early diagnosis of congenital Trypanosoma cruzi infection, using shed acute phase antigen, in Ushuaia, Tierra del Fuego, Argentina. Am. J. Trop. Med. Hyg.82(1), 55–59 (2010).
- Breniere SF, Yaksic N, Telleria J, Bosseno MF et al. Immune response to Trypanosoma cruzi shed acute phase antigen in children from an endemic area for Chagas’ disease in Bolivia. Mem. Inst. Oswaldo Cruz.92(4), 503–507 (1997).
- Otani MM, Vinelli E, Kirchhoff LV et al. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion49(6), 1076–1082 (2009).
Websites
- Pan-American Health Organization. PAHO/HDM/CD/476/07. Report of the technical consultation on information, education and communication (IEC) on congenital Chagas’ disease (2007) www.paho.org/English/AD/DPC/CD/dch-congenita-iec-07.doc
- Carlier Y, Torrico F. Congenital infection with Trypanosoma cruzi: from mechanisms of transmission to strategies for diagnosis and control. Conclusions of round tables and synopsis of an International Colloquium. Cochabamba, Bolivia, 6–8 November 2002 www.scielo.br/pdf/rsbmt/v36n6/a24v36n6.pdf
- Russomando G. Assessment of a locally sustainable system for Chagas’ disease diagnosis in two endemic regions of Paraguay. TDR News 16 www.who.int/tdrold/research/finalreps/pdf/fr16.pdf