Abstract
Reversible cerebral vasoconstriction syndromes (RCVS) are characterized by recurrent acute severe headaches, namely thunderclap headaches, and multifocal segmental vasoconstrictions. Interest has arisen in the definitions, clinical presentations, differential diagnoses, risk factors and complications of RCVS. This article will comprehensively review the milestone monographs and the latest research work addressing these issues. Studies that have focused on the relationship between RCVS and thunderclap headache will be detailed. We will also discuss research on the enigmatic pathophysiology and potential therapeutic approaches. Up-to-date information and challenges, undergoing studies and future research directions will be deeply probed.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/expertneurothera; (4) view/print certificate.
Release date: August 24, 2011; Expiration date: August 24, 2012
Learning objectives
Upon completion of this activity, participants should be able to:
• Describe the clinical presentation and diagnosis of RCVS
• Describe the underlying pathophysiology and complications of RCVS
• Describe management and treatment strategies for RCVS
Financial & competing interests disclosure
EDITOR
Elisa Manzotti,Editorial Director, Future Science Group, London, UK
Disclosure:Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay, MD,Freelance writer and reviewer, Medscape, LLC
Disclosure:Laurie Barclay, MD, has disclosed no relevant financial relationships.
AUTHORS
Shih-Pin Chen, MD, PhD,Department of Neurology, Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan; and Faculty of Medicine, National Yang-Ming University School of Medicine, Taipei, Taiwan
Disclosure:Shih-Pin Chen, MD, PhD, has disclosed no relevant financial relationships.
Jong-Ling Fuh, MD,Department of Neurology, Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan; and Faculty of Medicine, National Yang-Ming University School of Medicine, Taipei, Taiwan
Disclosure:Jong-Ling Fuh, MD, has disclosed no relevant financial relationships.
Shuu-Jiun Wang, MD,Department of Neurology, Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan; and Faculty of Medicine, National Yang-Ming University School of Medicine, Taipei, Taiwan
Disclosure:Shuu-Jiun Wang, MD, has disclosed no relevant financial relationships.
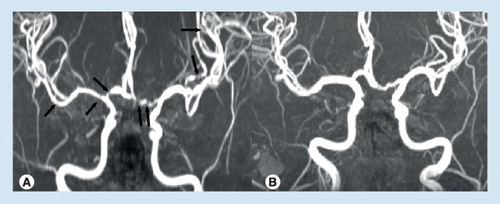
(A) Multi-focal segmental vasoconstrictions and (B) their normalization in a patient with reversible cerebral vasoconstriction syndrome (vasoconstrictions are indicated by black arrows).
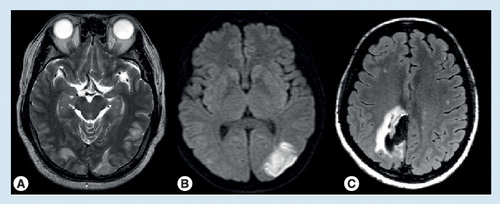
(A) Posterior reversible encephalopathy syndrome; (B) cerebral infarction; (C) intracerebral hemorrhage.
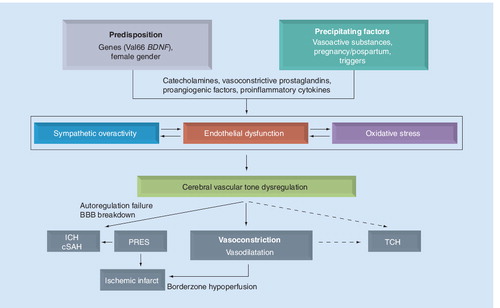
cSAH: Cortical subarachnoid hemorrhage, ICH: Intracerebral hemorrhage; PRES: Posterior reversible encephalopathy syndrome; TCH: Thunderclap headache.
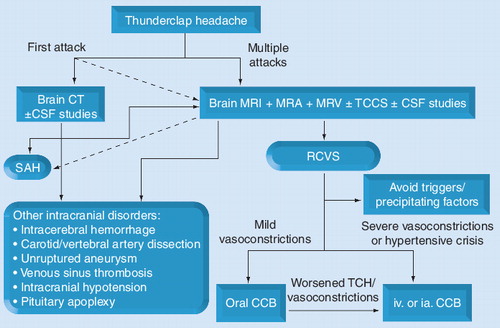
CCB: Calcium-channel blockers; CSF: Cerebrospinal fluid; CT: Computed tomography; ia.: Intra-arterial; iv.: Intravenous; MRA: Magnetic resonance angiography; MRV: Magnetic resonance venography; RCVS: Reversible cerebral vasoconstriction syndromes; SAH: Subarachnoid hemorrhage; TCCS: Transcranial color-coded sonography; TCH: Thunderclap headache.
Reversible cerebral vasoconstriction syndromes (RCVS) are characterized by acute-onset severe headaches, namely thunderclap headaches, and reversible segmental cerebral vasoconstriction Citation[1]. This clinico–radiological syndrome was previously known as Call–Fleming syndrome Citation[2], thunderclap headache (TCH) with reversible vasospasm Citation[3,4], benign angiopathy of the CNS (BACNS) Citation[5], postpartum angiopathy Citation[6,7], migrainous vasospasm or migraine angiitis Citation[8], or drug-induced cerebral arteritis or angiopathy Citation[9,10], depending on whether patients present to specialists in stroke, headache, rheumatology or obstetrics. As a unifying term for a constellation of disorders Citation[1], RCVS has attracted much more attention in recent years. It has been recognized that RCVS is not uncommon and still underdiagnosed Citation[11]. With accumulating data, especially from several large series Citation[12–17], the clinical features and temporal evolution of RCVS has become much clearer. However, several issues remain unresolved; for example, the perplexing relationship between RCVS and TCH, the appropriateness of including primary and secondary RCVS in the same diagnostic entity, the lack of gold-standard criteria and definition of typical and atypical cases, the ethnic differences in complication rates, or the therapeutic efficacy of calcium-channel blockers and so on. Besides, studies specifically focusing on the pathophysiology of RCVS remain scarce Citation[18]. It is still uncertain what constitutes the pathophysiological network of RCVS. To address these issues, this article offers a comprehensive review of studies to date and how they may help in better understanding this enigmatic disorder. In addition to a summary of the best available evidence, we will provide a commentary and 5-year view of the needs for future research and treatment efforts for RCVS.
The relationship between RCVS & thunderclap headache
The term TCH is used to describe an unanticipated, severe headache reaching peak intensity within 1 min. TCH is traditionally linked to aneurysmal subarachnoid hemorrhage (SAH); however, an increasing numbers of secondary causes of TCH have been identified Citation[19]. When a patient reports having the worst headache that he/she has ever experienced, an exhaustive investigations should be carried out to exclude all possible intracranial lesions. If no underlying cause is discovered, the TCH is considered primary. Primary TCH was adopted as a diagnostic entity in the International Classification of Headache Disorders, 2nd Edition (ICHD-II; code 4.6) (Box 1)Citation[20], but controversies exist regarding this diagnostic entity. For example, TCHs elicited by triggers such as coughing, exertion or sexual activity could be coded separately in the ICHD-II (code 4.2–4.4) Citation[20]. However, up to 80% of patients with recurrent TCHs have identifiable triggers, such as Valsalva-like maneuvers (exertion, defecation, sexual activity or cough), bathing or emotional disturbance Citation[12,15,21]. In addition, it is often the case that one patient can have multiple TCHs elicited by several different triggers Citation[12]. It is illogical to code multiple headache diagnoses within one patient when the characteristics of the headaches are exactly the same. Furthermore, some patients with these TCH variants without identifiable causes exhibited angiographic findings of reversible cerebral vasoconstriction Citation[3,12,22,23]. A prospective study found that patients with idiopathic recurrent TCHs with or without vasoconstriction (coded separately as BACNS [code 6.7.3] or primary TCH according to the ICHD-II) (Box 1) exhibited the same clinical characteristics Citation[12]. It is plausible that primary TCH and RCVS are spectra of the same disorder Citation[12]. As vasoconstrictions might not be evident during the early stage of RCVS and some imaging modalities might not be sensitive enough to detect vasoconstrictions in distal arterioles, it is likely that some patients are diagnosed with primary TCH because they do not receive serial angiographic follow-ups.
It has been demonstrated that TCH is usually the initial symptom of RCVS, and up to 82–100% of patients with RCVS had repeated attacks of TCHs during the clinical course Citation[12,15,17]. In our experience, when a patient experiences multiple TCHs within 1–2 weeks, RCVS is the most probable diagnosis. On occasion, patients with RCVS cannot be sure whether their headaches reaches the maximal intensity from the onset within 1 min, but these headaches are ubiquitously acute and severe. Sentinel headaches, presumably due to ‘warning leak,’ stretching or dissection in the weakened walls of an aneurysm, can present as TCHs preceding the hemorrhage in up to 10–43% of patients with aneurysmal SAH Citation[24]. The numbers of sentinel headaches tend to be one or two. However, as it is difficult for patients with SAH to report their prior headache history exactly, whether it is possible that one can have ‘multiple’ sentinel headaches prior to SAH is uncertain. If a patient presents with TCH and is also found to have both an unruptured aneurysm and diffuse multisegmental cerebral vasoconstriction Citation[25], it is still controversial whether the aneurysm should be treated as a first priority, even though the diagnosis is preferably RCVS and the aneurysm may be a coincidental finding.
Some overlapping syndromes or comorbidities may provide the link between TCH and RCVS. Posterior reversible encephalopathy syndrome (PRES) is the leading one, which will be addressed in detail in the pathophysiology section. Arterial dissection is a recently identified one. It has been a well-known secondary cause of TCH, supposedly attributable to a distension of the artery by the mural hematoma stimulating pain-sensitive receptors Citation[26]. However, it was recently identified that arterial dissection, either extracranial or intracranial, could be a comorbid condition of RCVS Citation[14–16,27,28]. It is uncertain whether there is a causal relationship between arterial dissection and RCVS, or whether both conditions could possibly be attributed to an underlying constitutional arteriopathy. Nonetheless, arterial dissection should be carefully sought in patients with suspected RCVS, and prospective studies are required to investigate the prevalence of reversible cerebral vasoconstrictions in patients with arterial dissection.
Primary versus secondary RCVS
Reversible cerebral vasoconstriction syndromes can be either primary Citation[12,13] or secondary to various factors Citation[15]. An expanding list of possible etiologies of RCVS has been identified Citation[1,12,15,17,29]. Primary RCVS is more common than previously thought. It is possible that some of these patients could have certain etiologies that have remained unknown, and some could have certain unidentified genetic predispositions that make them more susceptible. In this article, ‘primary RCVS’ is temporarily taken to cover all of the cryptogenic and idiopathic syndromes. In a prospective study of RCVS conducted in a hospital-based headache clinic in Taiwan, almost all of the patients have primary RCVS Citation[12]. In another large-scale study from France, RCVS was primary in 37% of the study cohort Citation[15]. Vasoactive substances as a group is the most common incriminated secondary cause of RCVS, which accounts for half of the patients in both French and American studies Citation[16,17]. Hence, a history of drug exposure should be sought in patients with RCVS. The three most common classes of vasoactive drugs are illicit drugs (such as cannabis, ecstasy, cocaine or amphetamine), selective serotonin-reuptake inhibitors and over-the-counter agents (nasal decongestants or diet pills) Citation[16,17]. Postpartum state is the second common cause, which accounts for approximately 9% of the cases Citation[16,17]. Immunosuppressants or cytotoxic agents are occasionally the culprits, and the diagnosis of RCVS should be kept in mind for patients who experience a sudden severe headache and are being treated with these medications.
Despite etiological heterogeneity, the clinical features of primary or the various secondary RCVS are distinctively similar. Female predominance is a universal phenomenon among different ethnic groups Citation[12,15,17]. Most patients are middle-aged (mean age ∼40–50 years), with the female patients being 10 years older than the male patients Citation[12,15,17]. Pediatric patients are occasionally seen Citation[17,30,31]. Headache characteristics were nearly identical in different studies Citation[12,15,17]. Blood pressure surges (systolic blood pressure >160 mmHg) accompanying headache attacks were observed in more than a third of patients Citation[12,14,15]. Despite great clinical resemblance, there are some debatable issues. It is uncertain whether those without any inciting factors have exactly the same ‘disease’ as those with known causes. It is reasonable to hypothesize that there are some shared common pathogenic pathways contributing to the vasoconstrictions or TCH between those with primary and secondary ones. However, it is hard to believe that there are no specific biological effects exerted by these exposed substances Citation[32] or humoral factors associated with puerperium Citation[33,34] that distinguish the secondary ones from the primary ones. As knowledge evolves, we might be able to redefine or subclassify this ‘syndrome’ with mechanism- or pathophysiology-specific perspectives.
Complications of RCVS & ethnic differences
Reversible cerebral vasoconstriction syndrome is not always benign. Patients with RCVS are complicated by transient ischemic attacks (up to 16%), PRES (9–14%), brain edema (38%), ischemic strokes (4–54%), cortical SAH (up to 34%), intracerebral hemorrhage (ICH; up to 20%) or subdural hemorrhage (2%) Citation[1,12,15–17]. illustrates some of the complications. Patients with a mean flow velocity of greater than 120 m/s in the middle cerebral artery and a Lindegaard index of greater than 3 are associated with higher risks of PRES or ischemic stroke Citation[13]. Female gender (odds ratio: 4.05) and history of migraine (odds ratio: 2.34) were two independent risk factors for hemorrhagic complications Citation[16]. Seizures, either generalized or focal, are noted in up to 21% of the patients Citation[1,12,15,17]. Focal neurological deficits are found in 9–63% of the patients Citation[1,12,15,17]. Permanent deficits were noted in 3–9% of patients in prospective series Citation[14,16], and 9–29% in retrospective studies Citation[17,35]. Mortality had been noted in a few patients Citation[17]. Intracranial hemorrhage Citation[16] or infarction Citation[17] had been linked to poor outcome.
There are substantial proportional differences of complications between different series. One major factor that accounts for the differences could be the variance of patient population between institutions. Patients in the Taiwanese study were mostly recruited from headache clinics, and they rarely had secondary causes. The French cohort was recruited from an emergency headache center and a stroke unit. Whereas, the patients in the American study were mostly referrals and inpatients that were more likely to harbor brain lesions. Ethnic predisposition could be another important issue. A recent study provided circumferential support. It was found that patients carrying the Val allele of BDNF Val66Met polymorphism were more likely to have more severe vasoconstrictions than Met homozygotes Citation[18]. The frequency of the Met66 allele is considerably low in African or Caucasian populations, but higher in Asian populations Citation[36]. Although the BDNF gene could not represent all of the genetic variance that might influent severity of RCVS Citation[18], the results of this study might indicate that patients in Western countries are more vulnerable to severe vasoconstrictions, and therefore more complications, than Asian patients. Nonetheless, prospective studies are required to investigate whether Caucasian or African individuals are more likely to have hemorrhagic complications than Asian individuals after stringently controlling variables such as demographics, extent and duration of exposure to precipitating factors, disease duration and therapeutic interventions.
Diagnostic work-up & criteria for RCVS
Brain magnetic resonance (MR) images, including angiography and venography, are methods of choice for studies of RCVS Citation[14]. For differential diagnosis and the evaluation of complications, the MR sequences should include T1, T2, fluid-attenuated inversion-recovery imaging, gradient-echo (T2*) imaging, diffusion-weighted imaging and apparent diffusion coefficient mapping Citation[11]. Cervical MRI, including T1 fat-saturation sequence with contrast, should be considered if cervical artery dissection is suspected. Transcranial color-coded sonography can be used to monitor the hemodynamic changes and predict risks of ischemic complications in patients with RCVS Citation[13]. Cerebrospinal fluid (CSF) study is appropriate to exclude SAH, infection or inflammation of the CNS, but in our experience, it seldom increases diagnostic yield when a patient has experienced multiple TCHs and magnetic resonance angiography (MRA) has demonstrated multifocal segmental vasoconstrictions in the absence of neck stiffness.
An angiographic study to demonstrate cerebral vasoconstrictions and their reversibility is the essential component for diagnosing RCVS. Vasoconstrictions in RCVS are usually pervasive and have outlasted headache resolution Citation[13–16], so that regular follow-up beyond remission of TCH is required. Centripetal progression of vasoconstriction was proposed Citation[15], but we observed this phenomenon in some, but not all, patients Citation[14]. Conventional angiography is the gold standard Citation[1]; however, it is invasive and not feasible for frequent follow-ups Citation[37]. In one large series, up to 9% of patients experienced transient neurological deficits after catheter angiography Citation[15]. Hence, we suggest that catheter angiography should only be reserved for those with diagnostic difficulties. By contrast, MRA is noninvasive and valid in studying vasoconstrictions in RCVS Citation[14]. Computed tomography angiography (CTA) could also be a useful tool in evaluating vasoconstrictions in RCVS. However, radiation exposure and potential effects of contrast medium prevent patients from undergoing frequent follow-ups.
A major radiological differential diagnosis of RCVS is primary angiitis of the CNS (PACNS). As RCVS and PACNS could both involve small and large intracranial vessels Citation[14,38], it is difficult to differentiate these two syndromes solely by angiographic findings. The best clinical indicator to differentiate the two is that repetitive TCHs have never been reported in PACNS. Complications including PRES, brain infarcts and hemorrhages are typically located in watershed zones Citation[4,14,17]. On the other hand, when seeing a patient with intracranial hemorrhage distributed over the watershed zones, physicians should keep the diagnosis of RCVS in mind. The occurrence of these complications could outlast headache resolution Citation[13,14,16]. Cortical SAH, presumably resulting from minor leaks or rupture of surface vessels, is usually minimal, overlying a few cortical sulci, with disproportionate widespread short-segmental vasoconstriction Citation[15]. By contrast, the delayed vasospasm in aneurismal SAH is usually long-segmental and has a close spatial relationship with the bleeding site Citation[1]. Linear, serpentine or dot-like distal hyperintense vessels over the cortical sulci on fluid-attenuated inversion-recovery imaging should be differentiated from cortical SAH. These hyperintense vessels could be noted in 22% of patients in our experience and up to 70% in an American cohort Citation[17].
Prior to the proposal of RCVS being the unifying term, the ICHD-II proposed diagnostic criteria for BACNS (code 6.7.3) (Box 1)Citation[20]. Calabrese et al. also summarized the critical elements for the diagnosis of RCVS (Box 2)Citation[1]. Most cases with RCVS could fulfill these criteria. However, such criteria are not yet satisfactory and inclusive enough. Based on our experiences, we proposed that CSF studies might not be a requisite element if the patient has experienced multiple TCHs and had characteristic angiographic findings. In addition, the duration criterion of reversibility needs to be modified. Vasoconstrictions in most patients normalized within 3 months; however, we have noticed that some patients ran a more protracted course. If the vasoconstrictions had improved greatly by 3 months, even though not completely normalized, ‘reversibility’ could still be claimed. There are also some patients with atypical presentation, such as those without recallable ‘acute severe headaches’ Citation[17] or with relapse during follow-up Citation[14,39]. Further elaboration to cover these cases is necessary when revising the criteria in the future.
Pathophysiology of RCVS
The exact pathophysiology of RCVS remains unknown. As the etiologies of RCVS are heterogeneous, the underlying mechanisms are probably multifactorial. With accumulating data, there are some proposed pathophysiological mechanisms (briefly summarized in ).
Dysfunctional regulation of cerebral vascular tone might be the central element in the pathogenesis of RCVS. A sudden alteration of central vascular tone may lead to segmental vasoconstriction and vasodilatation in small vessels and trigger TCH by abruptly stretching vessel walls at the initial stage of RCVS Citation[16]. Aberrant sympathetic response of cerebral vasculature is a preferred hypothesis Citation[37]. Blood pressure surge Citation[12,15], triggers with elevated sympathetic tone Citation[12,15], ingestion of sympathomimetic vasoactive substances Citation[10,14,15,17], pheochromocytoma Citation[40–42] and acute hypertensive crises Citation[43] all support the role of sympathetic overactivity. In addition, it is hypothesized that some of the immunologic and biochemical factors known to regulate vascular tone in the delayed vasospasm in SAH might also be important in the pathophysiology of vasoconstriction in RCVS Citation[44–46], although direct evidences are lacking. Recently, the BDNF Val66Met polymorphism has been linked to vasoconstriction in patients with RCVS Citation[18]. In animal studies, BDNF is found to cause perivascular inflammation and vasoconstriction under circumstances of sympathetic overactivity Citation[47]. BDNF could also upregulate neuropeptide Y, a vasoconstrictive sympathetic cotransmitter Citation[48]. Furthermore, we have recently noticed that urine levels of 8-iso-PGF2α were higher in patients with RCVS than patients with other severe headache or normal control Citation[49]. A role of oxidative stress and endothelial dysfunction in the pathogenesis of vascular tone regulation in RCVS is highly plausible.
Posterior reversible encephalopathy syndrome is found in a substantial proportion of patients with RCVS Citation[4,13,14,16,17], and up to 87% of patients with PRES were found to have diffuse vasoconstriction, focal vasculopathy or vessel pruning Citation[50]. Besides, acute severe headaches are not uncommon in patients with PRES. Immunosuppressive or cytotoxic agents that might cause RCVS are frequently reported in PRES Citation[51]. A shared pathophysiology between these two overlapping syndromes is highly probable. Blood pressure surge is observed in nearly half of patients with RCVS Citation[14], which might result from underlying sympathetic overactivity or a stress response to the excruciating headaches. As hypertensive encephalopathy constitutes a substantial proportion of PRES Citation[51], it is also possible that the abrupt blood pressure elevation could be an active player in the pathogenesis of PRES in RCVS. It is hypothesized that in PRES, the autoregulation is impaired. The endothelial control of arteriolar vascular tone is overwhelmed, which leads to a vicious cycle of homeostatic failure and progressive increase in vascular resistance. Increased vascular permeability ensues, which contributes to the vasogenic edema in PRES Citation[50,51]. The progressive increase of vascular resistance results in diffuse vasoconstriction. When vasoconstrictions become severe and involve more proximal vessels, irreversible ischemic change over the watershed zones develops Citation[4,50]. In line with this concept, we recently found that severe vasoconstrictions in the M1 segment of middle cerebral artery and P2 segment of posterior cerebral artery were associated with a higher risk of PRES and ischemic stroke in patients with RCVS Citation[14].
Postpartum angiopathy, one of the secondary RCVS, has many overlapping clinical, laboratory and radiographical features with eclampsia and preeclampsia. These disorders might belong to the same disease spectrum and have some shared pathophysiological mechanisms Citation[29,52,53]. It was demonstrated that placental growth factor (PlGF), soluble PlGF receptor (sFlt-1) Citation[33,34] and a soluble TGF-β1 receptor (soluble endoglin) Citation[54] correlate with the presence of eclampsia. The ratio of sFlt-1 to PLGF could also be used to predict the occurrence of preeclampsia Citation[34]. A recent case report suggested that the balance of these antiangiogenic and proangiogenic factors could also play a role in the pathogenesis of postpartum angiopathy Citation[55]. However, these mechanisms maynot be applicable to those who are not pregnant or being in the puerperium.
A recent retrospective study reported that up to 17% of the studied patients received open-brain biopsy or full autopsy Citation[17]. Extensive histological studies demonstrated no evidence of arterial inflammation or infection. One case report that detailed both histological and electron-microscopical examinations showed that major cerebral arteries were normal except for a patch of subendothelial thickening in the posterior cerebral artery Citation[55]. No specific morphologic abnormalities were found to correlate with the vasoconstrictions.
Therapeutic strategies
Reversible cerebral vasoconstriction syndromes should be treated as an emergent condition. Neuroimaging studies or lumbar puncture should be carried out as soon as possible. Withdrawal of secondary causes and avoidance of triggers are the first things to enact. No pharmacological treatment has gained enough evidence. Glucocorticoids were recently shown to be independent predictors of a poor outcome Citation[17], and thus their use is not recommended. A recent case report demonstrated that indomethacin might cause reversible cerebral vasoconstriction phenomena, and should be used with caution in acute treatment of headache Citation[56]. The calcium-channel blocker nimodipine has been shown to be effective in aborting the headaches in 64–83% of patients in open-label series Citation[12,15,57]. A recent retrospective study demonstrated that monotherapy with calcium-channel blockers is associated with good outcome Citation[17]. In our clinical practice, most patients were given oral nimodipine (30–60 mg every 4 h) or intravenous nimodipine (0.5–2 mg/h), based on the effectiveness of headache abortion, severity or progression of vasoconstrictions, or presence of PRES or ischemic stroke. A suggested diagnostic and therapeutic workflow is illustrated in . However, it should be stressed that not every patient can benefit from nimodipine and dose escalation may worsen the condition in some patients Citation[15]. In our practice, we monitored blood pressure every 2 to 4 h when parenteral treatment was required. If hypotension (systolic blood pressure <100 mmHg) occurred, it should be corrected immediately by dose tapering or normal saline hydration. None of our patients had experienced complications from this practice.
Other calcium-channel blockers, such as nicardipine Citation[31] or verapmil Citation[58], have been shown effective in case reports. Magnesium sulfate has been attempted in a small subset of patients with postpartum angiography, with acceptable outcomes Citation[59]. Intravenous prostacyclin is effective in one case report Citation[60]. Intra-arterial nimodipine Citation[61–63], verapamil Citation[64] or the phosphodiesterase inhibitor milrinone Citation[58] had been employed in some cases with refractory vasoconstrictions, with satisfactory outcomes. However, risk of reperfusion injury after intra-arterial intervention should be considered Citation[55]. It is uncertain how long the therapy should be maintained. As the risks of ischemic stroke or PRES outlast headache resolution Citation[13,14], maintenance therapy beyond headache resolution is warranted. In our practice, we continue to treat the patient until complete or nearly complete normalization of vasoconstrictions were observed. To explore the actual efficacy of these drugs, prospective randomized placebo-controlled trials are required. Study efficacy end points may reasonably include the number and frequency of thunderclap headaches, severity of vasoconstrictions (by flow velocity or MRA vasoconstriction scores) and incidences of ischemic and hemorrhagic complications.
Conclusion
Reversible cerebral vasoconstriction syndrome is a potentially devastating syndrome that has been increasingly recognized in recent years. The clinical features and angiographic findings of this clinical emergency are distinctive. When encountering a patient with the clinical hallmark of ‘recurrent TCHs’, looking for its counterpart ‘reversible cerebral vasoconstrictions’ is mandatory. Primary TCH and RCVS are considered spectra of the same disorder; even if there is no initial vasoconstriction, a follow-up angiographic study should be performed. Vasoconstrictions tend to outlast headache resolutions, and contribute to risks of ischemic complications. Complications including PRES, ischemic strokes or intracranial hemorrhage are highly prevalent and associated with poor prognosis. The diagnostic criteria are not yet satisfactory and require further refinement. Possible pathophysiology includes sympathetic overactivity, oxidative stress, endothelial dysfunction and imbalance of proangiogenic and antiangiogenic factors. Nimodipine might be effective for TCHs in RCVS, but randomized placebo-controlled trials are still required to establish optimal treatment strategies.
Expert commentary
It was only less than 5 years since the proposal of RCVS as a unifying term for a group of syndromes. Many physicians still have limited knowledge about this clinco–radiological syndrome, even though this syndrome can actually be encountered in multiple specialties. RCVS is still in the process of being defined, but large series from different countries supported that this syndrome is globally prevalent Citation[12–18]. The clinical presentation of RCVS has been better characterized, but it is still under-recognized. The etiological heterogeneity and controversies of spectral or overlapping disorders make it difficult to establish gold-standard criteria. Using the contemporary term RCVS is appropriate to improve the recognition of this syndrome and to identify more patients. However, we propose that an etiology-specific subgrouping from the seemingly unrelated and diverse range of causes might be more reasonable to understand the essence of disease nature and explore disease-targeting therapy. Dividing the patients into primary and secondary RCVS is the very initial thing that we can do at the present time.
Prospective randomized placebo-controlled trials to evaluate the efficacy of nimodipine is important, since it is currently the most commonly employed treatment. Because the complication rate of RCVS is considerably high, putting patients into clinical trials, obtaining consents, choice of study end points and criteria for drop-outs and unblinding all involve tough ethical issues. Collaboration studies from multiple centers are preferred to increase study power and generalizability. This can serve as a platform for exploring the safety and efficacy of future disease-targeting therapy.
Five-year view
To date, research on the complications of RCVS has shown large interpopulation variability, and those regarding the pathophysiology and treatment are still in paucity. We propose several avenues for study in the next 5 years.
Biomarkers in establishing the diagnosis & monitoring the course
Reversible cerebral vasoconstriction syndromes are conditions where diagnosis cannot definitively be made in the acute stage (awaiting for reversibility of vasoconstrictions), but treatment should be implemented as soon as possible. The diagnostic criteria should also be refined to clarify the significance and necessity of ‘reversibility’. An expert committee is required to reach a consensus. On the other hand, identifying disease-specific biomarkers that can help diagnose the syndrome as early as possible, such as in the early stage, is imperative. The difficulty in identifying a disease-specific biomarker and exploring the complex pathophysiology in such an enigmatic syndrome is that it is like searching for a needle in the haystack. Future research using system biology techniques, such as genomics, proteomics or metabolomics, are probably more efficient and holistic than hypothesis-driven approach. Analyzing the pattern differences will also be helpful for mechanism-based subgrouping of this syndrome. Based on the study results, it might be possible to develop animal models of this syndrome.
Distribution & evolution of the vasoconstriction
A goal of future investigations could also be the analysis of the distribution and evolution of segmental vasoconstrictions using high-field MR equipment. Whether there is really a centripetal evolution is of interest. Investigating the more distal arteries and even arterioles in the early stage of disease is crucial to answer this question and important in elucidating the pathogenesis of the disease. In addition, whether patients with RCVS also have an increased risk of extracranial or systemic vasoconstrictions remains unexplored. A systematic investigation is required.
Hemorrhagic complications
Prospective studies on hemorrhagic complications are required to explore whether the discrepancy of complication rate is intrinsically owing to the difference of study population or ethnic predisposition. Future studies might examine the prevalence of vasoconstrictions among intracranial hemorrhages in the general population and analyze the pattern of hemorrhage between those with and without vasoconstrictions.
Risk factors
The association of drugs with RCVS requires further elaboration. To date, most of the associations are derived from observational studies. Case–control or prospective trials are needed to evaluate whether these drug associations hold up in comparison to female predominant populations with other forms of headaches. Some pathological mechanisms, such as genetic polymorphism or external triggers, still need to be identified in patients with RCVS with unknown etiologies. BDNF Val66Met polymorphism may affect the degree of vasoconstrictions, but is not associated with the risks of developing RCVS Citation[18], suggesting that there could be some other genetic or epigenetic factors contributing to disease susceptibility. Although the phenotype is distinct, RCVS is more likely to be a complex disease than a monogenic disorder following Mendenlian inheritance. A genome-wide approach is a more appropriate method, but the required case number to reach adequate statistical power is a great concern.
Potential therapeutic agents
A last issue for research concerns potential trials of therapeutic agents. Therapies that are tailored to the underlying causes, mechanisms or genetic predisposition will be a trend. Whether these approaches can effectively reduce the ischemic or hemorrhagic complications should be proved by well-designed clinical trials. Based on currently available information, we speculate that therapies targeting on the restoration of endothelial function might be promising and deserves further explorations.
Table 1. Possible etiologies and associated conditions of reversible cerebral vasoconstriction syndrome.
Box 1. Current diagnostic criteria of primary thunderclap headache and benign (or reversible) angiopathy of the CNS in the International Classification of Headache Disorders, 2nd Edition.
4.6 Primary thunderclap headache:
A. Severe head pain fulfilling criteria B and C
B. Both of the following characteristics:
1. Sudden onset, reaching maximum intensity in <1 min
2. Lasting from 1 h to 10 days
C. Does not recur regularly over subsequent weeks or months
D. Not attributed to another disorder
6.7.3 Headache attributed to benign (or reversible) angiopathy of the CNS:
A. Diffuse, severe headache of abrupt or progressive onset, with or without focal neurological deficits and/or seizures and fulfilling criteria C and D
B. ‘Strings and beads’ appearance on angiography and subarachnoid hemorrhage ruled out by appropriate investigations
C. One or both of the following:
1. Headache develops simultaneously with neurological deficits and/or seizures
2. Headache leads to angiography and discovery of ‘strings and beads’ appearance
D. Headache (and neurological deficits, if present) resolves spontaneously within 2 months
Box 2. Summary of critical elements for the diagnosis of reversible cerebral vasoconstriction syndromes proposed by Calabrese et al.
A. Transfemoral angiography or indirect computed tomography angiography or magnetic resonance angiography documenting multifocal segmental cerebral artery vasoconstriction
B. No evidence for aneurysmal subarachnoid hemorrhage
C. Normal or near-normal cerebrospinal fluid analysis (protein level <80 mg/dl, leukocytes <10 mm3 and normal glucose level)
D. Severe, acute headaches, with or without additional neurologic signs or symptoms
E. Reversibility of angiographic abnormalities within 12 weeks after onset. If death occurs before the follow-up studies are completed, autopsy rules out such conditions as vasculitis, intracranial atherosclerosis and aneurysmal subarachnoid hemorrhage, which can also manifest with headache and stroke
Key issues
• Reversible cerebral vasoconstriction syndromes (RCVS) are comprised of diverse conditions characterized by repetitive thunderclap headaches and reversible cerebral vasoconstrictions.
• Primary thunderclap headache and RCVS belong to the same disease spectrum.
• RCVS can be either primary or evoked by various factors, such as puerperium or exposure to vasoactive substances.
• Up to 80% of sufferers have identifiable triggers, such as Valsalva-like maneuvers (exertion, defecation and sex), bathing and emotional disturbance.
• Thunderclap headaches recur for a period of approximately 2 weeks, while the vasoconstrictions are pervasive and may last for months.
• Complications include posterior reversible encephalopathy syndrome, ischemic stroke and intracranial hemorrhage (intracerebral hemorrhage, cortical subarachnoid hemorrhage and subdural hemorrhage).
• The severity of vasoconstrictions determines the risk of ischemic complications. Migraine and female gender are risk factors for hemorrhagic complications.
• Dysregulation of cerebral vascular tone is central to the pathophysiology. Overlapping syndromes, such as posterior reversible encephalopathy syndrome and eclampsia, suggest possible pathogenic mechanisms.
• Treatment is empirical and includes calcium-channel blockers. Glucocorticoids may be harmful.
• Most patients recover well. Cerebral infarction and hemorrhagic complications are predictive of permanent neurological deficits.
References
- Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann. Intern. Med.146(1), 34–44 (2007).
- Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM. Reversible cerebral segmental vasoconstriction. Stroke19(9), 1159–1170 (1988).
- Dodick DW, Brown RD Jr, Britton JW, Huston J 3rd. Nonaneurysmal thunderclap headache with diffuse, multifocal, segmental, and reversible vasospasm. Cephalalgia19(2), 118–123 (1999).
- Chen SP, Fuh JL, Lirng JF, Wang SJ. Is vasospasm requisite for posterior leukoencephalopathy in patients with primary thunderclap headaches? Cephalalgia26(5), 530–536 (2006).
- Calabrese LH, Gragg LA, Furlan AJ. Benign angiopathy: a distinct subset of angiographically defined primary angiitis of the central nervous system. J. Rheumatol.20(12), 2046–2050 (1993).
- Bogousslavsky J, Despland PA, Regli F, Dubuis PY. Postpartum cerebral angiopathy: reversible vasoconstriction assessed by transcranial Doppler ultrasounds. Eur. Neurol.29(2), 102–105 (1989).
- Dodick DW, Eross EJ. A not so uncommon cause of thunderclap headache. Headache42(6), 555 (2002).
- Jackson M, Lennox G, Jaspan T, Jefferson D. Migraine angiitis precipitated by sex headache and leading to watershed infarction. Cephalalgia13(6), 427–430 (1993).
- Singhal AB, Caviness VS, Begleiter AF, Mark EJ, Rordorf G, Koroshetz WJ. Cerebral vasoconstriction and stroke after use of serotonergic drugs. Neurology58(1), 130–133 (2002).
- Kaye BR, Fainstat M. Cerebral vasculitis associated with cocaine abuse. JAMA258(15), 2104–2106 (1987).
- Chen SP, Fuh JL, Wang SJ. Reversible cerebral vasoconstriction syndrome: an under-recognized clinical emergency. Ther. Adv. Neurol. Disord.3(3), 161–171 (2010).
- Chen SP, Fuh JL, Lirng JF, Chang FC, Wang SJ. Recurrent primary thunderclap headache and benign CNS angiopathy: spectra of the same disorder? Neurology67(12), 2164–2169 (2006).
- Chen SP, Fuh JL, Chang FC, Lirng JF, Shia BC, Wang SJ. Transcranial color doppler study for reversible cerebral vasoconstriction syndromes. Ann. Neurol.63(6), 751–757 (2008).
- Chen SP, Fuh JL, Wang SJ et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann. Neurol.67(5), 648–656 (2010).
- Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain130(Pt 12), 3091–3101 (2007).
- Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser M. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke41(11), 2050–2511 (2010).
- Singhal AB, Hajj-Ali RA, Topcuoglu MA et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch. Neurol.68(8), 1005–1012 (2011) .
- Chen SP, Fuh JL, Wang SJ, Tsai SJ, Hong CJ, Yang AC. Brain-derived neurotrophic factor gene Val66Met polymorphism modulates reversible cerebral vasoconstriction syndromes. PLoS One6(3), e18024 (2011).
- Schwedt TJ, Matharu MS, Dodick DW. Thunderclap headache. Lancet Neurol.5(7), 621–631 (2006).
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia24(Suppl. 1), 9–160 (2004).
- Liao YC, Fuh JL, Lirng JF, Lu SR, Wu ZA, Wang SJ. Bathing headache: a variant of idiopathic thunderclap headache. Cephalalgia23(9), 854–859 (2003).
- Wang SJ, Fuh JL, Wu ZA, Chen SP, Lirng JF. Bath-related thunderclap headache: a study of 21 consecutive patients. Cephalalgia28(5), 524–530 (2008).
- Yeh YC, Fuh JL, Chen SP, Wang SJ. Clinical features, imaging findings and outcomes of headache associated with sexual activity. Cephalalgia30(11), 1329–1335 (2010).
- Polmear A. Sentinel headaches in aneurysmal subarachnoid haemorrhage: what is the true incidence? A systematic review. Cephalalgia23(10), 935–941 (2003).
- Day JW, Raskin NH. Thunderclap headache: symptom of unruptured cerebral aneurysm. Lancet2(8518), 1247–1248 (1986).
- Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J. Neurol. Neurosurg. Psychiatry77(9), 1021–1024 (2006).
- Kumar S, Goddeau RP Jr, Selim MH et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology74(11), 893–899 (2010).
- Field DK, Kleinig TJ, Thompson PD, Kimber TE. Reversible cerebral vasoconstriction, internal carotid artery dissection and renal artery stenosis. Cephalalgia30(8), 983–986 (2010).
- Singhal AB, Bernstein RA. Postpartum angiopathy and other cerebral vasoconstriction syndromes. Neurocrit. Care3(1), 91–97 (2005).
- Kirton A, Diggle J, Hu W, Wirrell E. A pediatric case of reversible segmental cerebral vasoconstriction. Can. J. Neurol. Sci.33(2), 250–253 (2006).
- Liu HY, Fuh JL, Lirng JF, Chen SP, Wang SJ. Three paediatric patients with reversible cerebral vasoconstriction syndromes. Cephalalgia30(3), 354–359 (2010).
- Buttner A. Review: the neuropathology of drug abuse. Neuropathol. Appl. Neurobiol.37(2), 118–134 (2011).
- Levine RJ, Maynard SE, Qian C et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med.350(7), 672–683 (2004).
- Rana S, Karumanchi SA, Levine RJ et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension50(1), 137–142 (2007).
- Hajj-Ali RA, Furlan A, Abou-Chebel A, Calabrese LH. Benign angiopathy of the central nervous system: cohort of 16 patients with clinical course and long-term followup. Arthritis Rheum.47(6), 662–669 (2002).
- Petryshen TL, Sabeti PC, Aldinger KA et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol. Psychiatry15(8), 810–815 (2010).
- Dodick DW. Thunderclap headache. J. Neurol. Neurosurg. Psychiatry72(1), 6–11 (2002).
- Salvarani C, Brown RD Jr, Calamia KT et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann. Neurol.62(5), 442–451 (2007).
- Ducros A, Bousser MG. Reversible cerebral vasoconstriction syndrome. Pract. Neurol.9(5), 256–267 (2009).
- Armstrong FS, Hayes GJ. Segmental cerebral arterial constriction associated with pheochromocytoma: report of a case with arteriograms. J. Neurosurg.18, 843–846 (1961).
- Im SH, Kim NH. Thunderclap headache after micturition in bladder pheochromocytoma. Headache48(6), 965–967 (2008).
- Heo YE, Kwon HM, Nam HW. Thunderclap headache as an initial manifestation of phaeochromocytoma. Cephalalgia29(3), 388–390 (2009).
- Tang-Wai DF, Phan TG, Wijdicks EF. Hypertensive encephalopathy presenting with thunderclap headache. Headache41(2), 198–200 (2001).
- Dietrich HH, Dacey RG Jr. Molecular keys to the problems of cerebral vasospasm. Neurosurgery46(3), 517–530 (2000).
- Pluta RM. Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacol. Ther.105(1), 23–56 (2005).
- Nishizawa S, Laher I. Signaling mechanisms in cerebral vasospasm. Trends Cardiovasc. Med.15(1), 24–34 (2005).
- Kasselman LJ, Sideris A, Bruno C et al. BDNF: a missing link between sympathetic dysfunction and inflammatory disease? J. Neuroimmunol.175(1–2), 118–127 (2006).
- Zukowska Z, Pons J, Lee EW, Li L. Neuropeptide Y: a new mediator linking sympathetic nerves, blood vessels and immune system? Can. J. Physiol. Pharmacol.81(2), 89–94 (2003).
- Chen SP, Fuh JL, Chung YT, Liu TY, Wang SJ. Oxidative stress in reversible cerebral vasoconstriction syndrome: a urine 8-iso-PGF2α study. Cephalalgia31(Suppl. 1), 35 (2011).
- Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am. J. Neuroradiol.29(3), 447–455 (2008).
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am. J. Neuroradiol.29(6), 1043–1049 (2008).
- Fletcher JJ, Kramer AH, Bleck TP, Solenski NJ. Overlapping features of eclampsia and postpartum angiopathy. Neurocrit. Care11(2), 199–209 (2009).
- Donaldson JO. Eclampsia and postpartum cerebral angiopathy. J. Neurol. Sci.178(1), 1 (2000).
- Levine RJ, Lam C, Qian C et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med.355(10), 992–1005 (2006).
- Singhal AB, Kimberly WT, Schaefer PW, Hedley-Whyte ET. Case records of the Massachusetts General Hospital. Case 8–2009. A 36-year-old woman with headache, hypertension, and seizure 2 weeks post partum. N. Engl. J. Med.360(11), 1126–1137 (2009).
- Lambru G, Manzoni GC, Torelli P, Grisendi I, Zanferrari C. Reversible cerebral vasoconstriction phenomena following indomethacin administration. Headache51(5), 813–818 (2011).
- Lu SR, Liao YC, Fuh JL, Lirng JF, Wang SJ. Nimodipine for treatment of primary thunderclap headache. Neurology62(8), 1414–1416 (2004).
- Bouchard M, Verreault S, Gariepy JL, Dupre N. Intra-arterial milrinone for reversible cerebral vasoconstriction syndrome. Headache49(1), 142–145 (2009).
- Chik Y, Hoesch RE, Lazaridis C, Weisman CJ, Llinas RH. A case of postpartum cerebral angiopathy with subarachnoid hemorrhage. Nat. Rev. Neurol.5(9), 512–516 (2009).
- Grande PO, Lundgren A, Bjartmarz H, Cronqvist M. Segmental cerebral vasoconstriction: successful treatment of secondary cerebral ischaemia with intravenous prostacyclin. Cephalalgia30(7), 890–895 (2010).
- Elstner M, Linn J, Muller-Schunk S, Straube A. Reversible cerebral vasoconstriction syndrome: a complicated clinical course treated with intra-arterial application of nimodipine. Cephalalgia29(6), 677–682 (2009).
- Klein M, Fesl G, Pfister HW et al. Intra-arterial nimodipine in progressive postpartum cerebral angiopathy. Cephalalgia29(2), 279–282 (2009).
- Linn J, Fesl G, Ottomeyer C et al. Intra-arterial application of nimodipine in reversible cerebral vasoconstriction syndrome: a diagnostic tool in select cases? Cephalalgia31(10), 1074–1081 (2011).
- Farid H, Tatum JK, Wong C, Halbach VV, Hetts SW. Reversible cerebral vasoconstriction syndrome: treatment with combined intra-arterial verapamil infusion and intracranial angioplasty. AJNR Am. J. Neuroradiol. DOI: 10.3174/ajnr.A2341 (2011) (Epub ahead of print).
Reversible cerebral vasoconstriction syndrome: current and future perspectives
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/expertneurothera. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Your patient is a 53-year-old man seen in the emergency department for what he describes as “the worst headache of his life,” which came on suddenly and without apparent trigger. On the basis of the above review by Dr. Chen and colleagues, which of the following statements about the presentation and diagnosis is most likely correct?
□ A The headache reaching maximal intensity within 1 hour would be characteristic of thunderclap headache (TCH)
□ B If his presentation is consistent with TCH, a neuroimaging study is not necessary
□ C Primary TCH and reversible cerebral vasoconstriction syndrome (RCVS) are considered spectra of the same disorder
□ D For primary TCH without initial vasoconstriction, follow-up angiography is unnecessary
2. On the basis of the review by Dr. Chen and colleagues, which of the following statements about the pathophysiology and complications of RCVS is most likely correct?
□ A Vasoconstriction typically resolves spontaneously in a few hours
□ B Most patients with RCVS have no identifiable triggers
□ C Complications of posterior reversible encephalopathy syndrome (PRES), ischemic stroke, and intracranial hemorrhage are rare
□ D Possible pathophysiology includes sympathetic overactivity, oxidative stress, endothelial dysfunction, and imbalance of proangiogenic and antiangiogenic factors
3. The patient described in question 1 is diagnosed with RCVS. Per the review by Dr. Chen and colleagues, which of the following statements is most likely to apply to his treatment?
□ A Treatment is empiric and includes calcium-channel blockers
□ B Optimal dosing regimen and duration of treatment with nimodipine are well defined according to randomized, placebo-controlled trials
□ C Glucocorticoids have been proven to be effective in RCVS
□ D Blood pressure monitoring is not necessary with intravenous nimodipine
Notes
Taken from Citation[1].