Abstract
The high rate of brain metastasis in patients with advanced melanoma has been a clinical challenge for oncologists. Despite considerable progress made in the management of advanced melanoma over the past two decades, improvement in overall survival has been elusive. This is due to the high incidence of CNS metastases, which progress relentlessly and which are only anecdotally responsive to systemic therapies. Surgery, stereotactic radiosurgery and whole-brain radiotherapy with or without cytotoxic chemotherapy remain the mainstay of treatment. However, new drugs have been developed based on our improved understanding of the molecular signaling mechanisms responsible for host immune tolerance and for melanoma growth. In 2011, the US FDA approved two agents, one antagonizing each of these processes, for the treatment of advanced melanoma. The first is ipilimumab, an anti-CTLA-4 monoclonal antibody that enhances cellular immunity and reduces tolerance to tumor-associated antigens. The second is vemurafenib, an inhibitor that blocks the abnormal signaling for melanoma cellular growth in tumors that carry the BRAFV600E mutation. Both drugs have anecdotal clinical activity for brain metastasis and are being evaluated in clinical trial settings. Additional clinical trials of newer agents involving these pathways are also showing promise. Therefore, targeted therapies must be incorporated into the multimodality management of melanoma brain metastasis.
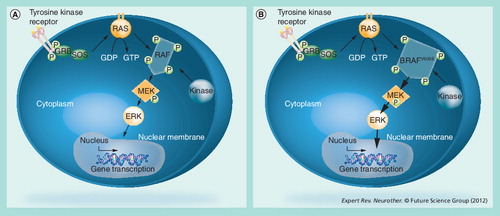
(A) Protein signaling cascade commences with the onset of Raf activation by activated growth factor receptors, leading to the induction of specific gene transcription. Homeostatic control is regulated by feedback mechanisms along each chain on the pathway. (B) Mutations in the BRAF gene results in the presence of the constitutively activated form of BRAF and the persistent activation of ERK signaling that stimulate the proliferation and survival of melanoma cells.
The large molecular weight at 489.9 and the polar chlorophenyl and sulfonic acid groups may prevent this molecule from readily crossing the blood–brain barrier.
Propane-1-sulfonic acid {3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2.4-difluorophenyl}-amide: C23H18ClF2N3O3S: Mr = 489.9.
Adapted from Citation[69].
![Figure 2. Vemurafenib.The large molecular weight at 489.9 and the polar chlorophenyl and sulfonic acid groups may prevent this molecule from readily crossing the blood–brain barrier.Propane-1-sulfonic acid {3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2.4-difluorophenyl}-amide: C23H18ClF2N3O3S: Mr = 489.9.Adapted from Citation[69].](/cms/asset/78f66e90-c8b0-4f98-aa1e-de5010fec0a5/iern_a_11212837_f0002_b.jpg)
Patients with advanced melanoma have a high propensity of developing melanoma brain metastases Citation[1,2]. However, the treatment of these metastases has not changed in the past two decades. Neuro-oncologists and neurosurgeons have achieved limited success using conventional treatments that include radiotherapy, either local treatment with stereotactic radiosurgery or global therapy with whole-brain radiotherapy (WBRT), with or without antecedent surgical resection. These treatment approaches have limitations because melanoma is typically radioresistant and multiple brain metastases are often found at the time of neurological presentation Citation[3]. However, two treatments have recently become available for advanced melanoma. Ipilimumab (Yervoy™), which received US FDA approval on 25 March 2011 Citation[101], is a humanized monoclonal antibody against the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) receptor, thereby allowing the persistent and increased activation of immune response against metastatic melanomas. Later, the FDA also approved vemurafenib (Zelboraf®) on 17 August 2011 Citation[102] for the treatment of BRAFV600E-mutated malignant melanoma. Vemurafenib is a small-molecule inhibitor of the serine threonine kinase BRAF capable of correcting the abnormal activation of the MAPK pathway. It has significant activity against tumor cells harboring the mutated BRAFV600E protein. Although definitive data are lacking at present, these two therapeutic strategies may offer an alternative to the management of metastatic melanoma in the brain and the rest of the CNS.
Genetic mutations in melanoma
DNA damage of melanocytes from ultraviolet radiation is the basis for the oncogenesis of melanoma. Similar to other epithelial malignancies, there are cumulative somatic mutations detected as the tumor evolves from benign nevus to invasive melanoma Citation[4,5]. Notably, loss of the germ-line mutation CDKN2A/p16INK4a has been linked to the development of inherited melanoma Citation[6]. As this mutation is also found in 10–25% of sporadic melanoma, mutated CDKN2A/p16INK4a appears to be a key initiator of melanoma oncogenesis Citation[6]. In addition, a number of somatic mutations are found in melanomas and the accumulation of such mutations is likely to enable the subsequent tumorigenic progression of benign melanotic nevus to dysplastic nevus and then superficial spreading melanoma. Furthermore, there is a high frequency of BRAF mutations, in the order of 26–70% Citation[5], found in invasive melanoma. In particular, the activating mutation BRAFV600E is linked to the efficacy of vemurafenib for metastatic melanoma Citation[7,8]. Last, the final steps of melanoma tumorigenesis probably also involve the co-option of the microenvironment near the tumor, including evading the immune system, in order to allow growth, proliferation and eventual metastasis of melanoma cells Citation[9]. It is unclear at this time how differences among patients’ genetic and immunological background influence the efficacy of new immunotherapy and targeted drugs.
The growing problem of melanoma brain metastasis
The cumulative incidence of melanoma brain metastasis is <10% according to a large series Citation[10,11]. However, brain involvement increases substantially to 45–50% in those having advanced melanoma and 75% at autopsy Citation[12,13]. This is because melanoma micrometastases Citation[14,15] are common in the brain and patients can harbor numerous metastases in the brain without neurological deficits. Furthermore, while melanoma can present in the brain as the first site of metastasis, it is more common for brain metastasis to present later in the course of disease, most often acting as a harbinger of terminal disease with a median survival of approximately 4 months after detection. While surgery and radiotherapy interventions can prolong the disease-free interval when solitary, large metastases in the brain are found early in the course of melanoma metastasis, these treatments provide only short-term, but nevertheless important palliation. Additionally, symptomatic leptomeningeal spread of metastatic melanoma, which occurs in 1–5% of melanoma patients Citation[16], is not as common as parenchymal brain metastases, but carries a poor prognosis and continues to be an area of unmet need. Therefore, the application of efficacious treatments for advanced melanoma that concomitantly cross the blood–brain barrier remains the best strategy to control brain metastasis.
The role of surgery & radiotherapy for melanoma brain metastasis
Surgical resection and radiotherapy have been the conventional treatment for brain metastasis from melanoma Citation[17–19]. Although it has been shown in randomized trials that resection of a solitary brain metastasis followed by whole-brain cranial irradiation would improve patient survival Citation[20,21], this strategy may not be relevant to melanoma because of the high frequency of multiple and miliary brain metastases and their radioresistance. Furthermore, most patients enrolled in prior randomized studies had lung cancer and those with melanoma comprised only 2–8% of the cohort Citation[20,21]. However, a retrospective analysis by Raizer et al. demonstrated that there is a lower hazard rate of death in patients who had neurosurgical resection incorporated as part of their overall disease management Citation[22]. Therefore, patient selection for neurosurgical intervention, which is primarily directed at symptom management, is essential to achieving a good outcome. For example, removal of a large brain metastasis of ≥4 cm in diameter may relieve elevated intracranial pressure and reverse neurological impairment by alleviating mass effect across the midline due to brain shift or subfalcine herniation, herniation of the uncus of the temporal lobe causing compression of the brainstem and posterior cerebral artery or hydrocephalus secondary to blockage of cerebrospinal fluid outflow. For patients with smaller and few metastases (less than six), neurosurgical evacuation of a hemorrhagic tumor may be indicated because this would alleviate neurological deficits as the mass effect from blood does not typically respond to dexamethasone. Last, tumors that are near sensitive areas of brain, such as the language areas, sensorimotor homunculus and visual cortex, or those that cause intractable seizures, should also be strongly considered for resection. The overarching goal of neurosurgical intervention should be directed at preservation of neurological function so that patients can later receive systemic treatment for their advanced melanoma.
Melanoma cells have low responsiveness to radiation in vitro and this correspond to the low efficacy of WBRT Citation[23,24]. After WBRT, the median survival of patients with melanoma brain metastasis is only 14 weeks Citation[25,26]. However, Smalley et al. demonstrated that adjuvant WBRT given after resection of a solitary melanoma brain metastasis resulted in a lower relapse rate than those who were just observed, 12 versus 85%, respectively Citation[27]. However, past clinical trials have shown that escalating the radiation dose per fraction, adding temozolomide as radiosensitizer, or combining temozolomide and thalidomide to WBRT did not result in prolonged survival Citation[28–30]. The Graded Prognostic Assessment, in which a score is calculated based on the Karnofsky performance status and the number of brain metastases, may offer a better estimate of survival with median survivals vary between 15 and 57 months in the worst and best prognostic groups, respectively Citation[31]. In sum, the available data indicate that WBRT has limited efficacy against patients with multiple melanoma brain metastases but WBRT can benefit those who have minimal residual disease after resection of a solitary brain metastasis.
Stereotactic radiosurgery is another modality of treatment that delivers highly conformal radiation to a metastasis target in a high dose per fraction fashion Citation[32]. In general, the local control rate is in the order of 90% for brain metastasis from systemic malignancy Citation[32]. However, retrospective studies have shown that the 1-year local control rate is only 63–75% for melanoma Citation[33]. Tumor size seems to be an important determinant of local control, with 1-year local control of 75 versus 42% in tumors ≤2 versus >2 cm3, respectively Citation[34]. Furthermore, the prospective RTOG 9508 trial Citation[35] and retrospective data from Sperduto et al. using the Grade Prognostic Assessment Citation[36] both showed that radiosurgery plus WBRT is better than WBRT alone. In addition, Sperduto et al. showed that radiosurgery alone is better than WBRT and this benefit is predominantly found in patients with good performance status and limited number of brain metastases Citation[36]. Although randomized prospective data demonstrating benefit for melanoma brain metastases are still lacking, radiosurgery alone is definitely less invasive and carries fewer comorbidities than neurosurgical resection.
Conventional chemotherapies
Chemotherapy has limited efficacy against metastatic melanoma to the brain. Temozolomide, at a dose of 150 mg/m2/day × 5 days, only had an objective response rate of 3–5% Citation[37,38]. When temozolomide is combined with WBRT, the objective response rates improved to 9–44% Citation[39,40], while the objective response rates for temozolomide plus thalidomide was 0–12% Citation[41], temozolomide plus thalidomide and WBRT was 8% Citation[28], and temozolomide plus sorafenib was 21.7% Citation[42]. The nitrosourea fotemustine offers a higher response rate, in the order of 25–28% Citation[43], but it is not available in the USA. Although patients treated with paclitaxel have a 12–14% response rate Citation[44], its penetrance across the blood–brain barrier is rather limited. New immunotherapy and small-molecule serine threonine kinase inhibitors, as well as those that are currently in clinical trial testing, are beginning to show promise for the prevention and treatment of brain metastases while exhibiting tolerable side effects.
Ipilimumab (BMS-734016) immunotherapy
Ipilimumab immunotherapy was granted FDA approval on 25 March 2011 Citation[101] for use against metastatic melanoma. It works by blocking the CTLA-4 antigen on the T-cell surface and resulting in the persistent and heightened activation of anti-tumor adaptive immunity Citation[45]. In Phase I and II dose-finding studies, ipilimumab appears to have a linear dose–response relationship (overall response rate: 11.1% at 10 mg/kg, 4.2% at 3 mg/kg and 0% at 0.3 mg/kg) that is also accompanied by a linear dose–toxicity effect (adverse event at any grade: 70.4% at 10 mg/kg, 64.8% at 3 mg/kg and 26.4% at 0.3 mg/kg) Citation[46]. Like other biological agents, ipilimumab did not have a maximum tolerated dose or a significant number of adverse events at grade 3 or 4. The most common immune-mediated toxicity was associated with the highest dose used (10 mg/kg), resulting in diarrhea that occurred at a rate of 16% Citation[47]. In Phase III trials ipilimumab has been shown to have efficacy in the prolongation of overall survival of patients with metastatic melanoma. In the trial comparing ipilimumab at a dose of 3 mg/kg plus gp100 vaccine versus gp100 vaccine alone and ipilimumab alone, patients treated with ipilimumab plus gp100 had a median survival of 10.0 versus 6.4 months for those treated with gp100 alone, while those had ipilimumab alone had 10.1 months, with no statistical difference between the ipilimumab groups Citation[48]. In another trial of patients with untreated metastatic melanoma, ipilimumab plus dacarbazine combination was better than dacarbazine alone and the median survival was 11.2 versus 9.1 months, respectively Citation[49]. The respective proportions of patients who were alive were 47.3 versus 36.3% at 1 year, 28.5 versus 17.9% at 2 years, and 20.8 versus 12.2% at 3 years Citation[49]. Therefore, the data indicate that ipilimumab has an acceptable toxicity profile and significant efficacy in the prolongation of patient survival.
While ipilimumab cannot cross the blood–brain barrier, activated T cells can migrate into the brain and exert an anti-tumor effect. However, data on its efficacy against melanoma brain metastases are limited. Only one prior trial enrolled patients with known brain metastases and the inclusion criteria required them to be treated and to have achieved radiologic stability prior to enrollment Citation[49]. Nevertheless, anecdotal reports have indicated that ipilimumab may have efficacy against metastatic melanoma in the brain and the spinal cord. Hodi et al. reported a case of a woman with melanoma that had metastasized to the brain and spinal cord from an unknown primary Citation[50]. After initial treatment with neurosurgical resection of a symptomatic metastasis, adjuvant radiosurgery to other brain metastases, external beam irradiation to spinal metastases and adjuvant temozolomide, she received ipilimumab at the time of disease progression in the brain. Although a new brain metastasis was found during the maintenance phase of ipilimumab treatment, surgical removal of the metastasis demonstrated predominantly CD8+ cytotoxic T cells but few FoxP3+ Tregs, suggesting a fulminant immune response against this brain metastasis rather than tumor progression. Her melanomas remained stable for 7 months and she lived for 2 years from the time of initial diagnosis. In a separate analysis of clinical response in the Phase III trial of ipilimumab plus gp100 versus gp100 or ipilimumab alone, Downey et al. reported one complete response and two partial responses that were associated with ipilimumab treatment out of ten patients with brain metastases who were previously treated with adjuvant radiotherapy Citation[51]. Last, Schartz et al. reported a patient with multiple melanoma brain metastases who had a complete response after ipilimumab treatment alone Citation[52]. Therefore, these anecdotal cases suggest that CD8+ cytotoxic T cells can travel into the immunologically privileged CNS and mount an antimelanoma immune response. Similar to the variable kinetics of response to ipilimumab in systemic melanoma Citation[53], the response heterogeneity seen in the population with brain metastasis probably has to do with the background immunogenicity and immunoediting capability of the individual melanoma brain metastases, as well as the host’s ability to recognize melanoma antigens. However, the efficacy of ipilimumab against leptomeningeal metastasis remains unknown.
In order to adequately evaluate the efficacy of ipilimumab against active melanoma brain metastases, a separate Phase II multi-institutional trial is being conducted in melanoma patients with active brain metastases. This trial is using a high-dose regimen that was not FDA approved at the time of this review; specifically, 10 mg/kg every 3 weeks for four doses followed by the same dose every 12 weeks. The primary end point is ‘disease control rate’, specifically the total complete responses, partial responses, and stable disease at 12 weeks. In the preliminary report Citation[54], arm A (51 patients) consisted of asymptomatic patients not requiring steroids and arm B (21 patients) consisted of patients requiring steroids for symptomatic brain metastases. About three-quarters of the patients had received prior systemic therapy (78.4% in arm A and 71.4% in arm B) and approximately 40% had received prior radiotherapy (39.2% in arm A and 42.9% in arm B). Disease control with ipilimumab at 12 weeks was achieved in 23.5% of brain metastases, 27.5% of nonbrain metastases and 17.6% of total tumor burden in arm A. Not surprisingly, the values were lower in arm B: 9.5, 4.8 and 4.8%, respectively. Grade 3 immune-mediated adverse reactions were reported in 21.6% of patients in arm A (including diarrhea, rash, adrenal insufficiency and hypophysitis) and 9.5% of patients in arm B (including diarrhea, rash, increased alanine aminotransferase or aspartate aminotransferase). One death in arm A from septic shock was considered drug-related. With a third of the patients not yet evaluable, and by using either modified WHO criteria or immune-related response criteria, there were eight partial responses in brain metastases in arm A and one in arm B, some of which were durable responses. These preliminary data point to the encouraging efficacy of ipilimumab against active melanoma brain metastases.
Other immunotherapies in development
While there is as yet no published controlled data on iplimumab activity against melanoma metastases in the CNS, the following new agents (not inclusive) are being tested alone or in combination with ipilimumab and other agents in order to further augment cellular immune responses against melanoma. Anti-PD-1 (also known as MDX 1106/BMS-936558/ONO-4538) is a fully human monoclonal IgG4 antibody directed against another negative immunoregulator programmed death-1 (PD-1; or programmed cell death-1 [PCD-1]), a transmembrane protein receptor and member of the immunoglobulin superfamily. Activated PD-1 negatively regulates T-cell activation and effector function through the suppression of PI3K/Akt pathway activation, which provides the necessary signaling for cellular survival Citation[55–57]. In early clinical trials, anti-PD-1 antibody has induced partial responses in melanoma patients and they experienced less severe immune-related toxicity than ipilimumab Citation[58]. In a recent report, 104 patients with heavily pretreated advanced melanoma, but with brain metastasis stabilized by other means, received anti-PD-1 at 1, 3 or 10 mg/kg every 2 weeks followed by cohort expansion, resulted in 25% (26 out of 104) objective responses of systemic melanomas and many were durable Citation[59]. Two other anti-PD-1 antibodies, CT011 and MK-3475, are also in development Citation[60,61]. Given the favorable response and toxicity profiles, anti-PD-1 antibody could have efficacy against melanoma brain metastases but more clinical data are needed.
Other immunomodulatory monoclonal antibodies currently being tested including BMS-663513Citation[62]. This is an agonist human monoclonal antibody that targets CD137 (a member of the TNF receptor family), which acts as a costimulatory molecule for CD8+ and CD4+ T cells. In a Phase I trial, there were three partial responses among 54 enrolled melanoma patients without brain metastasis. A second agonist humanized monoclonal antibody against CD134 (OX40, a member of the TNF receptor superfamily of receptors) that results in secondary costimulatory signaling, is being tested in Phase I trials Citation[63,64]. Last, lymphocyte activation gene-3 (LAG-3) is a cell-surface molecule that maintains tolerance to self and tumor antigens. Naive CD8+ T cells express low levels of LAG-3, and expression increases upon antigen stimulation. In vivo antibody blockade of LAG-3 or genetic ablation of the Lag-3 gene results in increased accumulation and effector function of antigen-specific CD8+ T cells within organs and tumors Citation[65,66]. In one study, combining LAG-3 blockade with specific anti-tumor vaccination resulted in a significant increase in activated CD8+ T cells in the tumor and disruption of the tumor parenchyma, supporting that LAG-3 blockade may have potential as an anti-melanoma treatment Citation[65]. These novel strategies of immunomodulatory treatments for systemic malignant melanoma may hold promise in the prevention and/or treatment of melanoma brain metastasis.
Vemurafenib for BRAFV600E
Vemurafenib received FDA approval on 17 August 2011 Citation[102] for the treatment of BRAFV600E-positive malignant melanoma. Vemurafenib is a small-molecule serine threonine kinase inhibitor of BRAF and its action blocks the mutated BRAFV600E and its abnormal activation of the MAP kinase pathway Citation[67,68]. BRAF is estimated to be mutated in over 40% of advanced melanomas and 90% of the mutations involve a valine-to-glutamic acid substitution at residue 600 (V600E) Citation[67,69]. In the initial Phase I testing of the drug, the overall response rate was 76% (37 out of 49 patients with mutated BRAFV600E responded) and 34 (69%) had a partial response while three (6%) had a complete response Citation[8]. This impressive result led to a pivotal Phase III trial comparing vemurafenib plus dacarbazine versus dacarbazine alone showing that overall survival at 6 months was 84 versus 64%, and the median progression-free survival was 5.3 versus 1.6 months, respectively Citation[7]. The clinical data indicate that vemurafenib is efficacious against malignant melanoma.
The efficacy of vemurafenib against brain metastasis from melanoma is unknown. This is because past clinical trials excluded patients with active brain metastases Citation[7,8]. However, the physiochemical property of vemurafenib suggests that it may have difficulty crossing the blood–brain barrier. This is because drug penetrance into the CNS depends on its size, preferably <300 in molecular weight, and its lipid solubility, preferably >1 in the octanol:water partition coefficient. Vemurafenib has a molecular weight of 489.9 Citation[67] and its large size makes it difficult to passively diffuse across the blood–brain barrier. Furthermore, the molecular structure of the drug, which has the polar sulfonic acid and chlorophenyl groups at either end making it hydrophilic and hydroscopic Citation[67,69], also limits its ability to cross the blood–brain barrier. However, additional pharmacokinetic data, by measuring drug levels in the cerebrospinal fluid or extracellular fluid from the brain parenchyma by microdialysis, would be needed in order to determine the extent of CNS penetration.
Despite these unfavorable chemical properties, there have been anecdotal reports of vemurafenib efficacy against melanoma brain metastases. An open-label single-arm trial evaluated the safety and efficacy of 960 mg of vemurafenib taken twice daily in patients with BRAFV600E-positive metastatic melanoma and unresectable CNS metastases that required corticosteroids for symptom control Citation[70]. Preliminary results were presented for seven out of a planned 20 total patients enrolled in the trial. Median number of brain metastases per patient was nine (range: 3–18), which were previously treated with whole-brain radiation in six and another type of radiotherapy in one Citation[70]. Four patients had received one prior systemic therapy while three others had three or more treatments for their metastases in the body Citation[70]. Of the five patients with staging data available after 1–4 months of vemurafenib therapy, one had a confirmed partial response in the brain and body, one had a minor response in the brain and body, and three achieved stable disease. Adverse events possibly related to vemurafenib were only of grade 1 or 2 vomiting, liver enzyme elevation, arthralgia, cough, facial paresis, fatigue, cutaneous herpes zoster eruption, muscle spasm, nausea, peripheral edema, oral herpes, maculopapular rash and sinusitis Citation[70].
A case report described the use of vemurafenib in a 16-year-old woman with rapidly progressive and hemorrhagic CNS metastases from BRAFV600E mutation-positive melanoma despite prior high-dose IL-2, ipilimumab and stereotactic radiosurgery Citation[71]. At baseline she was receiving dexamethasone as the largest of several lesions was 5 cm with associated vasogenic edema. She had an excellent response while being dosed at 960 mg twice daily, and after 6 months an MRI showed reduction in all brain metastases Citation[71].
Other BRAFV600E inhibitors
Dabrafenib (GSK2118436) is another orally bioavailable and specific inhibitor of mutated BRAFV600E protein currently in development. It has the lowest IC50 against BRAFV600E among its competitors and may have a more favorable chemical profile for penetrating the CNS compared with vemurafenib. In a Phase I/II trial presented at the ESMO 2010 meeting in Milan (Italy), dabrafenib shrank the previously untreated brain metastases in seven out of ten patients Citation[72]. The overall reductions ranged from 20 to 100% of brain metastases that were 3 mm or larger in diameter before treatment Citation[72]. Furthermore, preliminary results of another Phase I–II study with this agent were also reported in which 57 melanoma patients with activating mutation of BRAF received dabrafenib, including ten out of 16 at the planned Phase II dose, where 27 objective responses were observed including activity against previously untreated brain metastases Citation[73]. These data suggest that dabrafenib may be more effective than vemurafenib or ipilimumab for patients with advanced melanoma with brain metastases.
Other agents targeting BRAF and the MAP kinase pathway include RAF-265, which is a potent inhibitor of RAF with a highly selective profile and inhibits all three isoforms of RAF, as well as mutant BRAF, with high potency. RAF265 is now being investigated in Phase I and II clinical trials in melanoma Citation[74]. Moreover, XL281 is a specific inhibitor of RAF kinases, including the mutant form of BRAF, and has completed Phase I testing so far Citation[75].
Quelling resistance to BRAF inhibitors
The median time to progression for vemurafenib is only 6 months. Hence, a lot of work is being done to understand and thwart the induction of vemurafenib resistance. Efforts are centered around developing stronger inhibitors, that is, dabrafenib (GSK2118436), that bind tighter to the kinase domains or that bind to nearby conserved domains that can obstruct the ATP-binding pockets, while others are aimed at developing pan-Raf inhibitors such as RAF-265 that might also inhibit CRAF implicated in the pathway to developing skin cancers and keratoacanthomas. Investigators are also testing combinations of other agents with Raf inhibitors in order to block signaling pathways that are downstream from Raf (i.e., MEK), parallel to Raf (i.e., PI3K) or upstream from Raf (i.e., EGF receptor) Citation[74]. Concomitant blockade of such redundant or bypass pathways may prevent the development of resistant cells and enhance the efficacy of targeted therapies.
Expert commentary
The era of personalized cancer therapy against melanoma has begun with the introduction of vemurafenib. This is based on the principle of applying a drug that specifically targets an activating mutation on which the tumor cells depend for their growth and proliferation. The initial response rate to vemurafenib was impressive and 70–80% of treated patients having the BRAFV600E mutation had an objective response Citation[7]. However, resistant disease eventually develops due to the selection pressure imposed on the tumor cells by the medication and the eventual escape by these cells via other signal transduction survival pathways. It is noteworthy that resistance to vemurafenib occurred rather quickly in melanoma patients and the median progression-free survival was only 5.3 months Citation[7]. Therefore, treatment of metastatic melanoma may require the inhibition of several targets by multiple drugs that are applied sequentially or in a combinatorial fashion. The combined approach may be less desirable due to a potential increase in off-target effects resulting in greater clinical toxicities. Furthermore, the development cost of each drug is already high and the combined cost of developing a set of drugs that only benefit a small subset of melanoma patients may be prohibitive. Therefore, cost–benefit analysis may become a necessary endeavor in the future drug development process.
Cancer immunotherapy can result in durable responses because it activates the patient’s intrinsic immune system to destroy melanoma metastases. Because of the heterogeneous immunological profiles among individual patients, ipilimumab efficacy as measured by response, as well as the kinetics of such response, was found to be different among patients Citation[49,50,54]. However, those who experienced a benefit had durable systemic responses and the complete response seen in some often lasted for years. It is also noteworthy that ipilimumab can effect anti-melanoma responses in the CNS Citation[51–53]. It remains to be determined whether or not there is synergistic efficacy when targeted therapy is combined with immunotherapy for metastatic melanoma to the CNS and other systemic organs.
Five-year view
Five years from now, there will most likely be additional targeted therapies and immunotherapies for metastatic melanoma. Because of the high rate of metastasis to the CNS, the treatment of brain, spinal cord and leptomeningeal metastases will become an even more pressing issue in the management of patients with advanced melanoma. The critical issues will include the design of small molecule inhibitors that have a high penetrance across the blood–brain barrier as well as immunotherapies that can drive more anti-melanoma cytotoxic T cells into the CNS.
Table 1. Known genetic mutations in melanomas.
Key issues
– The efficacy of ipilimumab and vemurafenib against melanoma brain metastasis is unknown.
– Ipilimumab can most likely induce an anti-melanoma immune response in the CNS, based on the anecdotal reports of response in patients with melanoma brain metastases and the cytotoxic CD8+ T cells that were found in resected specimens.
– Separate clinical trials would be needed in order to evaluate the extent of benefit from ipilimumab and vemurafenib against metastatic melanoma in the CNS.
– For the small-molecule inhibitor vemurafenib, serum and cerebrospinal fluid pharmacokinetic studies would be needed in order to assess the extent of CNS penetration.
– The cost associated with specific targeted therapy in serial and combinatorial personalized cancer therapies should be studied further.
Acknowledgements
The authors thank Nkengafeh Asong and Edwin Lok for their assistance with this review.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br. J. Dermatol. 150(2), 179–185 (2004).
- Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J. Neurosurg. 88(1), 11–20 (1998).
- Madajewicz S, Karakousis C, West CR, Caracandas J, Avellanosa AM. Malignant melanoma brain metastases. Review of Roswell Park Memorial Institute experience. Cancer 53(11), 2550–2552 (1984).
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 61(5), 759–767 (1990).
- Gray-Schopfer VC, da Rocha Dias S, Marais R. The role of B-RAF in melanoma. Cancer Metastasis Rev. 24(1), 165–183 (2005).
- Monzon J, Liu L, Brill H et al. CDKN2A mutations in multiple primary melanomas. N. Engl. J. Med. 338(13), 879–887 (1998).
- Chapman PB, Hauschild A, Robert C et al. ; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364(26), 2507–2516 (2011).
- Flaherty KT, Puzanov I, Kim KB et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363(9), 809–819 (2010).
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024), 1565–1570 (2011).
- Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 22(14), 2865–2872 (2004).
- Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94(10), 2698–2705 (2002).
- Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer 42(2), 660–668 (1978).
- Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am. J. Surg. 135(6), 807–810 (1978).
- Bedikian AY, Wei C, Detry M et al. Predictive factors for the development of brain metastasis in advanced unresectable metastatic melanoma. Am. J. Clin. Oncol. 34(6), 603–610 (2011).
- Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1(2), 149–153 (1995).
- Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 5(5), 443–452 (2006).
- Kyritsis AP, Markoula S, Levin VA. A systematic approach to the management of patients with brain metastases of known or unknown primary site. Cancer Chemother. Pharmacol. 69(1), 1–13 (2012).
- McWilliams RR, Brown PD, Buckner JC, Link MJ, Markovic SN. Treatment of brain metastases from melanoma. Mayo Clin. Proc. 78(12), 1529–1536 (2003).
- Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control 16(3), 248–255 (2009).
- Patchell RA, Tibbs PA, Regine WF et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280(17), 1485–1489 (1998).
- Patchell RA, Tibbs PA, Walsh JW et al. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 322(8), 494–500 (1990).
- Raizer JJ, Hwu WJ, Panageas KS et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro-oncology 10(2), 199–207 (2008).
- Fertil B, Malaise EP. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 7(5), 621–629 (1981).
- Fertil B, Malaise EP. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int. J. Radiat. Oncol. Biol. Phys. 11(9), 1699–1707 (1985).
- Nieder C, Marienhagen K, Geinitz H, Grosu AL. Can current prognostic scores reliably guide treatment decisions in patients with brain metastases from malignant melanoma? J. Cancer Res. Ther. 7(1), 47–51 (2011).
- Rate WR, Solin LJ, Turrisi AT. Palliative radiotherapy for metastatic malignant melanoma: brain metastases, bone metastases, and spinal cord compression. Int. J. Radiat. Oncol. Biol. Phys. 15(4), 859–864 (1988).
- Smalley SR, Schray MF, Laws ER Jr, O’Fallon JR. Adjuvant radiation therapy after surgical resection of solitary brain metastasis: association with pattern of failure and survival. Int. J. Radiat. Oncol. Biol. Phys. 13(11), 1611–1616 (1987).
- Atkins MB, Sosman JA, Agarwala S et al. Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a Phase II Cytokine Working Group study. Cancer 113(8), 2139–2145 (2008).
- Margolin K, Atkins B, Thompson A et al. Temozolomide and whole brain irradiation in melanoma metastatic to the brain: a Phase II trial of the Cytokine Working Group. J. Cancer Res. Clin. Oncol. 128(4), 214–218 (2002).
- Sause WT, Cooper JS, Rush S et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int. J. Radiat. Oncol. Biol. Phys. 20(3), 429–432 (1991).
- Sperduto PW, Kased N, Roberge D et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 30(4), 419–425 (2012).
- Phillips MH, Stelzer KJ, Griffin TW, Mayberg MR, Winn HR. Stereotactic radiosurgery: a review and comparison of methods. J. Clin. Oncol. 12(5), 1085–1099 (1994).
- Selek U, Chang EL, Hassenbusch SJ 3rd et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int. J. Radiat. Oncol. Biol. Phys. 59(4), 1097–1106 (2004).
- Powell JW, Chung CT, Shah HR et al. Gamma knife surgery in the management of radioresistant brain metastases in high-risk patients with melanoma, renal cell carcinoma, and sarcoma. J. Neurosurg. 109(Suppl.), 122–128 (2008).
- Andrews DW, Scott CB, Sperduto PW et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 363(9422), 1665–1672 (2004).
- Sperduto PW, Chao ST, Sneed PK et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int. J. Radiat. Oncol. Biol. Phys. 77(3), 655–661 (2010).
- Agarwala SS, Kirkwood JM, Gore M et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a Phase II study. J. Clin. Oncol. 22(11), 2101–2107 (2004).
- Boogerd W, de Gast GC, Dalesio O. Temozolomide in advanced malignant melanoma with small brain metastases: can we withhold cranial irradiation? Cancer 109(2), 306–312 (2007).
- Antonadou D, Paraskevaidis M, Sarris G et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J. Clin. Oncol. 20(17), 3644–3650 (2002).
- Hofmann M, Kiecker F, Wurm R et al. Temozolomide with or without radiotherapy in melanoma with unresectable brain metastases. J. Neurooncol. 76(1), 59–64 (2006).
- Hwu WJ, Lis E, Menell JH et al. Temozolomide plus thalidomide in patients with brain metastases from melanoma: a Phase II study. Cancer 103(12), 2590–2597 (2005).
- Amaravadi RK, Schuchter LM, McDermott DF et al. Phase II trial of temozolomide and sorafenib in advanced melanoma patients with or without brain metastases. Clin. Cancer Res. 15(24), 7711–7718 (2009).
- Jacquillat C, Khayat D, Banzet P et al. Chemotherapy by fotemustine in cerebral metastases of disseminated malignant melanoma. Cancer Chemother. Pharmacol. 25(4), 263–266 (1990).
- Einzig AI, Hochster H, Wiernik PH et al. A Phase II study of taxol in patients with malignant melanoma. Invest. New Drugs 9(1), 59–64 (1991).
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 3(7), 611–618 (2002).
- Weber JS, O’Day S, Urba W et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 26(36), 5950–5956 (2008).
- Wolchok JD, Weber JS, Hamid O et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun. 10, 9 (2010).
- Hodi FS, O’Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363(8), 711–723 (2010).
- Robert C, Thomas L, Bondarenko I et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364(26), 2517–2526 (2011).
- Hodi FS, Oble DA, Drappatz J et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat. Clin. Pract. Oncol. 5(9), 557–561 (2008).
- Downey SG, Klapper JA, Smith FO et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. Cancer Res. 13(22 Pt 1), 6681–6688 (2007).
- Schartz NE, Farges C, Madelaine I et al. Complete regression of a previously untreated melanoma brain metastasis with ipilimumab. Melanoma Res. 20(3), 247–250 (2010).
- Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 8, 1 (2008).
- Lawrence DP, Hamid HO, Mcdermott DF et al. Phase II trial of ipilimumab monotherapy in melanoma patients with brain metastases. J. Clin. Oncol. 28(15 Suppl.), Abstract 8523 (2010).
- Cantrell D. Protein kinase B (Akt) regulation and function in T lymphocytes. Semin. Immunol. 14(1), 19–26 (2002).
- Freeman GJ, Long AJ, Iwai Y et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192(7), 1027–1034 (2000).
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 99(19), 12293–12297 (2002).
- Brahmer JR, Drake CG, Wollner I et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28(19), 3167–3175 (2010).
- Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366(26), 2443–2454 (2012).
- Berger R, Rotem-Yehudar R, Slama G et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 14(10), 3044–3051 (2008).
- Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J. Clin. Oncol. 29(36), 4828–4836 (2011).
- Sznol M, Hodi FS, Margolin K. Phase I study of BMS-663513, a fully human anti-CD137 against monoclonal antibody, in patients (pts) with advanced cancer. J. Clin. Oncol. 2008(26), Abstract 3007 (2008).
- Gough MJ, Weinberg AD. OX40 (CD134) and OX40L. Adv. Exp. Med. Biol. 647, 94–107 (2009).
- Sznol M. Betting on immunotherapy for melanoma. Curr. Oncol. Rep. 11(5), 397–404 (2009).
- Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 24(12), 619–622 (2003).
- Grosso JF, Kelleher CC, Harris TJ et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 117(11), 3383–3392 (2007).
- Buckmelter AJ, Ren L, Laird ER et al. The discovery of furo[2,3-c]pyridine-based indanone oximes as potent and selective B-Raf inhibitors. Bioorg. Med. Chem. Lett. 21(4), 1248–1252 (2011).
- Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351(Pt 2), 289–305 (2000).
- Flaherty KT, Yasothan U, Kirkpatrick P. Vemurafenib. Nat. Rev. Drug Discov. 10(11), 811–812 (2011).
- Dummer R, Rinderknecht J, Goldinger SM et al. An open-label pilot study of vermurafenib in previously treated metastatic melanoma patients with brain metastases. J. Clin. Oncol. 29(15 Suppl.), Abstract 8548 (2011).
- Rochet NM, Kottschade LA, Markovic SN. Vemurafenib for melanoma metastases to the brain. N. Engl. J. Med. 365(25), 2439–2441 (2011).
- Falchook GS, Long GV, Kurzrock R et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a Phase 1 dose–escalation trial. Lancet 379(9829), 1893–1901 (2012).
- Kefford RF, Arkenau H, Brown MP. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other soild tumors. J. Clin. Oncol. 28(15 Suppl.), Abstract 8503 (2010).
- Alcalá AM, Flaherty KT. BRAF inhibitors for the treatment of metastatic melanoma: clinical trials and mechanisms of resistance. Clin. Cancer Res. 18(1), 33–39 (2012).
- Schwartz GK, Robertson S, Shen A. A Phase I study of XL281, a selective oral RAF kinase inhibitor, in patients (pts) with advanced solid tumors. J. Clin. Oncol. 27(15 Suppl.), Abstract 3513 (2009).
Websites
- US FDA news release. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2011/ucm1193237.htm
- US FDA news release. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm268241.htm