Abstract
When contemplating a pregnancy, women treated for multiple sclerosis (MS) with a disease-modifying drug must decide to discontinue their medication before conception or risk exposing their unborn child to potential drug toxicity. Few studies exist as reference for patients and physicians, and of those available, the majority are less than ideal due to real-world constraints, ethical issues and methodological shortcomings. The authors provide a brief summary of existing animal and human data with current recommendations regarding the safety of IFN-β, glatiramer acetate, natalizumab, mitoxantrone, fingolimod and teriflunomide during pregnancy and lactation in women with MS. We also assess the quality, strengths and limitations of the existing studies including challenges with study design. The investigation of outcomes such as spontaneous abortion and congenital anomalies are highlighted with potential methodological improvements for future studies on drug safety in pregnancy suggested. The authors explore the pharmacokinetics and pharmacodynamics of the MS disease-modifying drugs for their possible mechanistic role in fetal harm and discuss the potential role of clinical trials. Future pharmacovigilance studies should continue to pursue multicenter collaboration with an emphasis on appropriate study design.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertneurothera; (4) view/print certificate.
Release date: 1 March 2013; Expiration date: 1 March 2014
Learning objectives
Upon completion of this activity, participants will be able to:
• Describe general principles of DMD management during pregnancy for women with MS, based on a review
• Describe DMD management during breastfeeding for women with MS, based on a review
• Describe findings regarding safety of specific DMDs in pregnancy, based on a review
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Publisher, Future Science Group, London, UK
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay, MD, Freelance writer and reviewer, Medscape, LLC.
Disclosure: Laurie Barclay, MD, has disclosed no relevant financial relatinoships.
AUTHORS AND CREDENTIALS
Ellen Lu, BSc
Department of Medicine (Division of Neurology), Faculty of Medicine, University of British Columbia, Vancouver, Canada.
Disclosure: Ellen Lu, BSc, has received funding from the Canadian Institutes of Health Research (Canada Graduate Scholarships: Master's and Doctoral Research Awards), the Multiple Sclerosis Society of Canada (MSc and PhD Research Studentships) and the University of British Columbia (Graduate Entrance Scholarship, Faculty of Medicine Graduate Awards and Four Year Doctoral Fellowship)..
Bing Wei Wang
Department of Medicine (Division of Neurology), Faculty of Medicine, University of British Columbia, Vancouver, Canada.
Disclosure: Bing Wei Wang has received funding from the Canadian Institutes of Health Research (Professional Student Research Award) and the Albert and Mary Steiner Summer Research Award.
Colleen Guimond, MSc
Department of Medical Genetics, Faculty of Medicine, University of British Columbia, Vancouver, Canada
Disclosure: Colleen Guimond, MSc, has disclosed no relevant financial relationships.
Anne Synnes, MDCM, MHSc
Department of Pediatrics, Faculty of Medicine, University of British Columbia, Vancouver, Canada
Disclosure: Anne Synnes, MDCM, MHSc, has disclosed no relevant financial relationships.
A Dessa Sadovnick, PhD
Department of Medicine (Division of Neurology); Department of Medical Genetics, Faculty of Medicine, University of British Columbia, Vancouver, Canada
Disclosure: Dessa Sadovnick, PhD, has disclosed receiving research support from the MS Society of Canada Scientific Research Foundation, and CIHR; speaker honoraria and/or travel expenses to attend conferences from: Biogen-Idec, Merck-Serono, Teva Neurosciences, Bayer and unrestricted educational funding to hold a workshop from CIHR, Biogen-Idec and Teva Neurosciences.
Leanne Dahlgren, MD, MHSc
Department of Obstetrics and Gynecology, Faculty of Medicine, University of British Columbia, Vancouver, Canada
Disclosure: Leanne Dahlgren, MD, MHSc, has disclosed no relevant financial relationships.
Anthony Traboulsee, MD
Department of Medicine (Division of Neurology), Faculty of Medicine, University of British Columbia, Vancouver, Canada
Disclosure: Anthony Traboulsee, MD, has received honoraria from EMD Serono, Teva Neurosciences, Bayer, Biogen Idec, Chugai Pharmaceuticals and Roche.
Helen Tremlett, PhD
Department of Medicine (Division of Neurology), Faculty of Medicine, University of British Columbia, Vancouver, Canada
Disclosure: Helen Tremlett, PhD, is funded by the Multiple Sclerosis Society of Canada (Don Paty Career Development Award), Michael Smith Foundation for Health Research and is the Canada Research Chair for Neuroepidemiology and Multiple Sclerosis. She has received: research support from the US National Multiple Sclerosis Society, CIHR, and UK MS Trust; speaker honoraria and/or travel expenses to attend conferences from the Consortium of MS Centres, US National MS Society, the University of British Columbia Multiple Sclerosis Research Program, Bayer Pharmaceutical (speaker, 2010, honoraria declined), Teva Pharmaceuticals (speaker 2011), ECTRIMS (2011), UK MS Trust (2011) and the Chesapeake Health Education Program, US Veterans Affairs (2012, honorarium declined). Unless otherwise stated, all speaker honoraria are either donated to an MS charity or to an unrestricted grant for use by her research group.
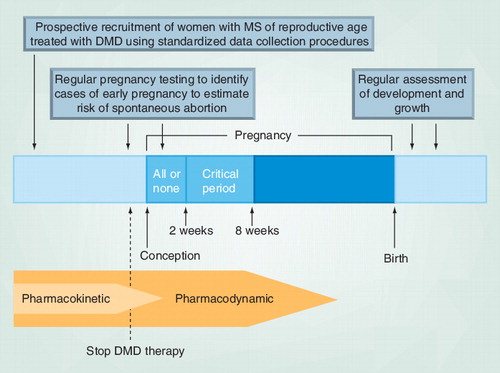
‘All or none’ refers to the time from conception until implantation when insults to the embryo are likely to result in either death or intact survival of the embryo. The ‘critical period’ refers to early organogenesis, during which all the major organ systems of the body are being formed; drug exposure during this period can result in significant congenital anomalies (although exposure at any time during pregnancy has the potential for adverse effects).
DMD: Disease-modifying drug; MS: Multiple sclerosis.
Sophia, aged 29 years, was diagnosed with multiple sclerosis (MS) 4 years ago. Her symptoms began with unilateral optic neuritis and transverse myelitis. Since the beginning of IFN-β therapy 2 years ago, she has experienced a decrease in relapse frequency (from two per year before treatment to <1 per year on treatment). Sophia wishes to start a family and has been advised by her neurologist to discontinue her disease-modifying drug (DMD) at least 3 months before conceiving. She is concerned about the risk of relapse following discontinuation of disease-modifying therapy as well as the conflicting findings regarding the potential harm to her unborn child of using a DMD during pregnancy.
MS is an autoimmune disease of the CNS that commonly first presents in men and women of childbearing age Citation[1]. Although considered a progressive disease, the severity and rate of progression is highly variable; some patients may only experience occasional relapses of MS disease interspersed with periods of relatively stable disease Citation[1]. Nonetheless, patients with relapsing–remitting disease are encouraged by some clinicians to start DMDs early to reduce the frequency of relapse with the hopes of slowing disease progression Citation[2], although actual evidence is mixed Citation[3,4]. IFN-β and glatiramer acetate (GA) are currently considered as first-line therapies, whereas natalizumab, fingolimod, mitoxantrone and teriflunomide are typically reserved as second-line options Citation[5]. Patients with MS are generally recommended to discontinue DMDs before conception Citation[6]; however, for those with severe or highly active disease, some recommend GA or IFN-β be continued throughout pregnancy (clinical opinion) Citation[6]. Natalizumab, mitoxantrone, fingolimod and teriflunomide are not recommended and do not play a major role in the management of a planned pregnancy because the known (or potential) risks currently outweigh the benefits Citation[7,8]. Although the risk of a relapse is decreased during pregnancy, particularly in the third trimester Citation[9], some risk remains and relapse rates can increase immediately postpartum, such that some patients and physicians may be reluctant to stop DMD therapy. In other cases, women with MS may have an unplanned pregnancy while taking a DMD. In either situation, there is a real need for clear information regarding the safety of DMD exposure in pregnancy. Women who intend to breastfeed are typically advised to not take a DMD Citation[8,10]; nonetheless, those with highly active disease may opt to reinitiate DMD therapy and forego breastfeeding Citation[6,11]. Here, the authors discuss the evidence from studies exploring DMD safety during pregnancy and highlight major challenges and key considerations for future studies of drug safety in pregnancy.
Summary of findings from the existing literature
Prospective cohort studies appear to be the best way to assess the risk of adverse perinatal outcomes in patients with MS, as clinical trials of pregnant women pose significant ethical issues (discussed later). The authors recently systematically reviewed the literature (2005–2012) surrounding DMD safety during pregnancy in MS Citation[7]. With data from 15 studies and 893 pregnancies in MS women with in utero DMD exposure, we were able to examine perinatal outcomes such as spontaneous abortion (i.e., miscarriage), cesarean delivery, birthweight, birth length, gestational age and congenital anomaly (i.e., birth defects). The best evidence from one prospective cohort study suggested that IFN-β exposure was associated with preterm birth, shorter mean birth length and lower mean birthweight Citation[12]. However, the growth of newborns exposed to IFN-β still fell within the normal expected general population range Citation[12]. IFN-β exposure was not associated with low birthweight (<2500 g), cesarean delivery, congenital anomaly or spontaneous abortion Citation[12]. Fewer studies (with limited sample size) were available regarding the safety of GA Citation[13–16] or natalizumab Citation[17]. Therefore, while GA exposure was not associated with lower mean birthweight, congenital anomaly, preterm birth or spontaneous abortion Citation[13], and natalizumab did not appear to increase the risk of shorter birth length, lower birthweight or lower gestational age Citation[17], conclusive statements surrounding safety cannot be made. There were also no reported cohort studies of mitoxantrone, fingolimod or teriflunomide exposure during pregnancy in MS. Since women with MS are at an increased risk of relapse during the postpartum period Citation[18,19] and breastfeeding has been found in some studies (although not all Citation[20,21]) to reduce postpartum relapses Citation[19,22], a discussion of the potential (and wider-known) benefits of breastfeeding versus reinitiation of a DMD would be appropriate. shows a summary of pregnancy and breastfeeding outcomes following DMD exposure in animals and humans with current recommendations.
Strength & limitations of existing studies
At the time of our systematic review, few studies were considered of high quality when evaluated using internationally accepted criteria Citation[23]. However, these criteria must be balanced against the real-world limitations inherent in research involving pregnant women Citation[24]. Nonetheless, most studies were susceptible to recall, voluntary participation, surveillance or reporting bias. Furthermore, studies with small sample size are often unable to adjust for potential confounders including family history, maternal age, previous obstetric history, comorbid illnesses, or exposure to other medications or recreational drugs. There was also considerable heterogeneity in methodology. For example, when classifying DMD exposure, some defined in utero DMD exposure as within 1 month prior to conception, whereas others used the estimated time of conception. All these issues outlined here could contribute to the differences in reported findings between studies – with some studies reporting harm Citation[12,13,25–27] and others not Citation[14–17,27–33].
Some of the best available studies are prospective, which, by their nature, minimize reporting and recall bias (a common challenge in pregnancy-related pharmacovigilance studies). Three such cohort studies have been published to date and all examined IFN-β exposure Citation[12,13,25]. Of these, two had relatively large sample sizes (n = 69 Citation[13] and 88 Citation[12]), which also allowed for adjustment of important confounders (including gestational age, parity, socioeconomic status and smoking or alcohol use) to better estimate the true effect of IFN-β exposure. In addition, some studies investigated DMD exposure late in pregnancy; three small studies (n = 9 Citation[14], 11 Citation[16] and 12 Citation[15] pregnancies) reported on GA exposure up to the third trimester of pregnancy, whereas others followed long-term developmental outcomes beyond the immediate perinatal period in offspring exposed to GA Citation[16] or IFN-β Citation[12,26] in utero. Individual studies have also investigated maternal natalizumab exposure during pregnancy Citation[17] and paternal DMD use around conception Citation[28] – both represent the first cohort studies published on these topics. These studies did not find evidence of harm; however, given their relatively small sample sizes, further studies should seek to confirm these findings.
Challenges with ascertainment of specific outcomes
Perhaps one of the biggest challenges to date when examining pregnancy outcomes in MS relates to study power, because small sample sizes limit the ability to examine many important outcomes. Most studies of DMD exposure in pregnancy have involved relatively few women. From our own experience in British Columbia (Canada), this related largely to good clinical practice, with women following advice to discontinue DMDs before conception Citation[30]. In addition, given the relatively short period of time that some of the recently approved DMDs have been licensed for MS, especially fingolimod and teriflunomide, there are limited long-term postmarketing data available to assess safety. Consequently, many studies have been unable to adequately assess rarer outcomes such as specific birth defects or syndromes. Other outcomes, such as spontaneous abortion, are difficult to ascertain. These are discussed in more detail below.
Spontaneous abortion
IFN-β has been found to cause spontaneous abortion in animals Citation[101,102]; however, there was mixed evidence from human observational studies Citation[12,25,29]. Spontaneous abortions are difficult to detect in practice, especially those that occur early enough in gestation to avoid routine detection by patient or clinician. The symptoms of early pregnancy can be vague and easily confused with transitory illnesses (e.g., viral illnesses) or normal menstrual cycle variability Citation[34]. If pregnancy is not recognized, a spontaneous abortion may be mistaken for heavier-than-normal menses or a passed clot – underestimating the true risk of spontaneous abortion. Prospective enrollment through pregnancy registries as well as regular use of objective pregnancy detection methods, such as home pregnancy kits and/or serum β-human chorionic gonadrotropin levels, may improve ascertainment of spontaneous abortions.
Congenital anomalies & related rare outcomes
Roughly 260 DMD-exposed pregnancies are needed for a study to achieve 80% power, with a type I error of 5%, to identify a 5% absolute increased risk of congenital anomaly from a baseline risk of 3% in the general population Citation[35]. Consequently, most studies to date have lacked a sufficient sample size to investigate these rarer outcomes. In addition, since the majority of identified cases of in utero DMD exposure occur within the first trimester, the risk of congenital anomalies (or other adverse outcomes) associated with exposure beyond the first trimester remain largely unknown Citation[30].
One potential solution to improve ascertainment of rare outcomes is the creation of universal standardized research templates to investigate drug safety in pregnancy. Multicenter pregnancy registries with prospective recruitment of women initiated on DMD therapy may be the ideal platform to investigate newly licensed drugs using these standardized forms; presently, there are some drug-specific worldwide registries, including those for natalizumab Citation[103] and fingolimod Citation[104], that are actively recruiting patients. Such an approach would permit future meta-analyses because these templates would include key demographic, obstetrical and medical data using common definitions. These standardized forms should also capture key developmental outcomes such as gross and fine motor milestones, intellectual development and behavioral measures among children exposed to DMD during pregnancy or breastfeeding – all of which are important and largely understudied. Another approach is through data linkage where population-based registries and/or data sources (often collated for purposes other than research, e.g., health administrative data) are linked together to create powerful, comprehensive datasets Citation[36–39]. Data on confounders are also crucial because drugs are estimated to be responsible for only 1% of birth defects Citation[105] – albeit an important preventable cause.
Potential confounding factors
Data from population-based studies suggest that some diseases including epilepsy, migraine, irritable bowel syndrome, systemic lupus erythematosus, depression, anemia and rheumatoid arthritis are more prevalent among individuals with MS compared to the general population Citation[40,41]. These comorbid medical conditions, or the medications used to treat them, may have effects on the unborn child. Lifestyle factors such as smoking, alcohol intake, exercise and obesity may also play a role; for example, there is evidence that individuals with MS may be more likely to engage in behaviors that are known to be harmful to developing fetuses including smoking and alcohol consumption Citation[42]. These potential confounders should be considered in any analysis of birth outcomes of individuals with MS. In addition to the well-established contribution of maternal factors on birth outcomes, it is recognized that paternal ethnic origin, height and birthweight and the degree of paternal involvement during pregnancy may influence birth outcomes Citation[43,44]. Furthermore, as most women with MS and their clinicians would take precautions to avoid DMD exposure in planned pregnancies Citation[30], it is very likely that a higher proportion of unintended pregnancies make up the DMD-exposed cohort relative to the DMD-unexposed cohort. Planned pregnancies are associated with significantly less fetal and maternal morbidity and mortality Citation[6], potentially due to increased parental precaution regarding recreational substance use, better nutrition, as well as better management of comorbid medical conditions. Hence, births in a DMD-exposed cohort may be biased toward a greater risk of adverse birth outcomes.
Potential biological mechanism(s)
Both IFN-β and GA are large macromolecules Citation[45] that are unlikely to cross the placenta to directly affect the developing fetus Citation[46]. However, indirect effects are possible. For instance, IFN-β is a cytokine that has immune, antiproliferative and antiviral effects Citation[47]. It is known to increase transcription of over 100 different genes Citation[48]; hence, IFN-β and its metabolites could trigger downstream production of maternal cytokines that affect the developing placenta or cross the placental barrier to affect the fetus. This could disrupt the complex, sequential pattern of chemical signals required for normal fetal growth and development Citation[49].
Natalizumab is an antibody that crosses the placenta to cause in utero exposure, leading to reduced platelet counts and decreased survival of offspring in animals Citation[106]. Mitoxantrone is toxic to DNA, causing inhibition of topoisomerase II and DNA strand breakage, with a cytocidal effect on cells Citation[50]. It is known to cause growth retardation and preterm birth in animals Citation[107]. Spontaneous abortion and decreased fetal growth were observed in newborns of women with in utero mitoxantrone exposure for cancer treatment Citation[51]; women using mitoxantrone may also have secondary amenorrhea Citation[107]. In men undergoing cancer chemotherapy, mitoxantrone may cause azoospermia with a return to normospermic levels 3–4 months after chemotherapy for most patients Citation[52]. Fingolimod binds to the sphingosine 1-phosphate receptor that is involved in vascular formation during embryogenesis in animals Citation[108]. Teriflunomide is known to cause fetal death and malformations in animals Citation[109]; the putative mechanism of harm is suspected to involve the inhibition of dihydroorotate dehydrogenase, an enzyme involved in pyrimidine synthesis Citation[109].
The relationship between DMD exposure and adverse birth outcomes is obscured by our limited understanding of the pharmacokinetic and pharmacodynamic properties of these agents. On the basis of studies of healthy human subjects, IFN-β has a half-life of hours to days (depending on the specific formulation) Citation[101,102], whereas the half-life of GA is unknown, although most of the drug appears to be hydrolyzed locally at the injection site Citation[110]. Natalizumab has a half-life of 11 ± 4 days Citation[106], mitoxantrone 3 days Citation[107], fingolimod 6–9 days Citation[108] and teriflunomide 18–19 days Citation[109]. One commonly studied biomarker of IFN-β bioactivity, the protein MxA, remains in circulation for days to weeks after administration Citation[53]. Even when the drug is no longer detectable in the body, the biochemical and physiological effects of drugs on the body may persist such that harm to the fetus is still possible. in utero exposure at different stages of pregnancy with the same drug can result in different outcomes Citation[54]. Pregnancy loss occurs most commonly during the first 2 weeks after conception, whereas congenital anomaly and impaired brain or growth development often occur later in pregnancy Citation[54].
Proposed methodological improvements as well as pharmacokinetic and pharmacodynamic considerations for future observational studies of drug exposure in pregnancy have been summarized in . In addition, substantial physiological changes occur during pregnancy Citation[55], which can affect the pharmacokinetic/dynamic properties of drugs. However, these physiological changes may not be as relevant in women with DMD exposure since most cases of DMD exposure (based on studies to date) have occurred early in gestation when these physiological effects may be not as prominent.
Potential role of clinical trials
The active recruitment of pregnant women (or those actively planning pregnancy) into a randomized controlled trial of a drug for MS is typically considered unethical. Nonetheless, women enrolled in clinical trials Citation[29,31] do occasionally become pregnant accidentally. Collectively, these data can be invaluable as they often represent the first human exposures to drugs during pregnancy. However, these women represent a specific MS subpopulation that may not be generalizable to the wider MS population. Nonetheless, increasingly, there has been debate about the merits of including a limited number of pregnant women in clinical trials Citation[56]. There are some situations where the use of drug therapy during pregnancy may be justifiable. For example, a woman with very active MS may remain on DMDs during pregnancy to minimize the risk of a relapse. Likewise, a woman with epilepsy may be safer continuing anticonvulsants throughout pregnancy rather than risk experiencing significant hypoxic events due to seizures that could be life-threatening to her and her child Citation[57]. When clinically justified, a smaller scale clinical trial with regular, close follow-up of mothers has been suggested – especially if drug therapy during pregnancy is unavoidable due to the mother’s medical condition Citation[56].
Expert commentary & five-year view
Future research on drug safety in pregnancy should strive to minimize methodological limitations and fully consider pharmacokinetic and pharmacodynamic factors. One potential solution to improve ascertainment of rare outcomes is the creation of universal standardized research templates to investigate drug safety in pregnancy. In addition, pharmacovigilance studies in pregnancy highlight the necessity for international, multicenter collaboration. It is encouraging to find several active pregnancy registries with prospective recruitment of women initiated on DMD therapy (including the newer agents natalizumab, fingolimod and teriflunomide); these international multicenter pregnancy registries may be the ideal platform to investigate newly licensed drugs using standardized forms. Such an approach would permit future meta-analyses because these templates would include key demographic, obstetrical and medical data using common definitions. It is also promising to find recent studies investigating longer-term developmental outcomes in offspring associated with in utero drug exposure Citation[12,26] as well as the potential effects of paternal drug use on pregnancy Citation[28]; future studies should continue to expand on these areas of research. The potential benefits of clinical trials involving a limited number of pregnant women warrant further consideration as a viable approach to investigating drug safety in pregnancy.
Table 1. Disease-modifying drugs for multiple sclerosis during pregnancy and breastfeeding: summary of current evidence and recommendations.
Key issues
• Women with multiple sclerosis should discontinue disease-modifying drugs (DMDs) before conception; those with severe or highly active disease may consider continuing with glatiramer acetate or IFN-β during pregnancy (clinical opinion).
• Women intending to breastfeed are advised to remain off DMD therapy; those with severe or highly active disease may choose to reinitiate therapy and forgo breastfeeding (clinical opinion).
• Future studies should strive to account for pharmacodynamic and pharmacokinetic considerations as well as investigate the risk of spontaneous abortion and the safety of breastfeeding associated with DMD therapy, ideally with large, multicenter collaborations.
• Confounding factors including maternal obstetrical history, lifestyle factors, comorbidities and medication use should be assessed and accounted for in future studies.
References
- Compston A, Coles A. Multiple sclerosis. Lancet 372(9648), 1502–1517 (2008).
- Frohman EM, Havrdova E, Lublin F et al. Most patients with multiple sclerosis or a clinically isolated demyelinating syndrome should be treated at the time of diagnosis. Arch. Neurol. 63(4), 614–619 (2006).
- Pittock SJ, Weinshenker BG, Noseworthy JH et al. Not every patient with multiple sclerosis should be treated at time of diagnosis. Arch. Neurol. 63(4), 611–614 (2006).
- Shirani A, Zhao Y, Karim ME et al. Association between use of interferon β and progression of disability in patients with relapsing–remitting multiple sclerosis. JAMA 308(3), 247–256 (2012).
- Buck D, Hemmer B. Treatment of multiple sclerosis: current concepts and future perspectives. J. Neurol. 258(10), 1747–1762 (2011).
- Tsui A, Lee MA. Multiple sclerosis and pregnancy. Curr. Opin. Obstet. Gynecol. 23(6), 435–439 (2011).
- Lu E, Wang BW, Guimond C, Synnes A, Sadovnick AD, Tremlett H. Disease-modifying drugs for multiple sclerosis in pregnancy: a systematic review. Neurology 79, 1130–1135 (2012).
- Borisow N, Döring A, Pfueller CF, Paul F, Dörr J, Hellwig K. Expert recommendations to personalization of medical approaches in treatment of multiple sclerosis: an overview of family planning and pregnancy. EPMA J. 3(1), 9 (2012).
- Finkelsztejn A, Brooks JB, Paschoal FM Jr, Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG 118(7), 790–797 (2011).
- Ferrero S, Esposito F, Pretta S, Ragni N. Fetal risks related to the treatment of multiple sclerosis during pregnancy and breastfeeding. Expert Rev. Neurother. 6(12), 1823–1831 (2006).
- Houtchens MK, Kolb CM. Multiple sclerosis and pregnancy: therapeutic considerations. J. Neurol. doi:10.1007/s00415-012-6653-9 (2012) (Epub ahead of print).
- Amato MP, Portaccio E, Ghezzi A et al.; MS Study Group of the Italian Neurological Society. Pregnancy and fetal outcomes after interferon-b exposure in multiple sclerosis. Neurology 75(20), 1794–1802 (2010).
- Weber-Schoendorfer C, Schaefer C. Multiple sclerosis, immunomodulators, and pregnancy outcome: a prospective observational study. Mult. Scler. 15(9), 1037–1042 (2009).
- Salminen HJ, Leggett H, Boggild M. Glatiramer acetate exposure in pregnancy: preliminary safety and birth outcomes. J. Neurol. 257(12), 2020–2023 (2010).
- Fragoso YD, Finkelsztejn A, Comini-Frota ER et al. Pregnancy and multiple sclerosis: the initial results from a Brazilian database. Arq. Neuropsiquiatr. 67(3A), 657–660 (2009).
- Fragoso YD, Finkelsztejn A, Kaimen-Maciel DR et al. Long-term use of glatiramer acetate by 11 pregnant women with multiple sclerosis: a retrospective, multicentre case series. CNS Drugs 24(11), 969–976 (2010).
- Hellwig K, Haghikia A, Gold R. Pregnancy and natalizumab: results of an observational study in 35 accidental pregnancies during natalizumab treatment. Mult. Scler. 17(8), 958–963 (2011).
- Vukusic S, Hutchinson M, Hours M et al.; Pregnancy In Multiple Sclerosis Group. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain 127(Pt 6), 1353–1360 (2004).
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N. Engl. J. Med. 339(5), 285–291 (1998).
- Portaccio E, Ghezzi A, Hakiki B et al.; MS Study Group of the Italian Neurological Society. Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology 77(2), 145–150 (2011).
- Neuteboom RF, Hintzen RQ. Breast-feeding, postpartum and prepregnancy disease activity in multiple sclerosis. Neurology 76(17), 1532; author reply 1532–1532; author reply 1533 (2011).
- Langer-Gould A, Huang SM, Gupta R et al. Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch. Neurol. 66(8), 958–963 (2009).
- Sayre MR, O’Connor RE, Atkins DL et al. Part 2: evidence evaluation and management of potential or perceived conflicts of interest: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122(18 Suppl. 3), S657–S664 (2010).
- Strong C. How should risks and benefits be balanced in research involving pregnant women and fetuses? IRB 33(6), 1–5 (2011).
- Boskovic R, Wide R, Wolpin J, Bauer DJ, Koren G. The reproductive effects of β interferon therapy in pregnancy: a longitudinal cohort. Neurology 65(6), 807–811 (2005).
- Patti F, Cavallaro T, Lo Fermo S et al. Is in utero early-exposure to interferon β a risk factor for pregnancy outcomes in multiple sclerosis? J. Neurol. 255(8), 1250–1253 (2008).
- Fernández Liguori N, Klajn D, Acion L et al. Epidemiological characteristics of pregnancy, delivery, and birth outcome in women with multiple sclerosis in Argentina (EMEMAR study). Mult. Scler. 15(5), 555–562 (2009).
- Hellwig K, Haghikia A, Gold R. Parenthood and immunomodulation in patients with multiple sclerosis. J. Neurol. 257(4), 580–583 (2010).
- Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon β-1a therapy. Mult. Scler. 17(4), 423–430 (2011).
- Lu E, Dahlgren L, Sadovnick A, Sayao A, Synnes A, Tremlett H. Perinatal outcomes in women with multiple sclerosis exposed to disease-modifying drugs. Mult. Scler. 18(4), 460–467 (2012).
- Sandberg-Wollheim M, Frank D, Goodwin TM et al. Pregnancy outcomes during treatment with interferon β-1a in patients with multiple sclerosis. Neurology 65(6), 802–806 (2005).
- De Las Heras V, De Andrés C, Téllez N, Tintoré M; EMPATIE Study Group. Pregnancy in multiple sclerosis patients treated with immunomodulators prior to or during part of the pregnancy: a descriptive study in the Spanish population. Mult. Scler. 13(8), 981–984 (2007).
- Finkelsztejn A, Fragoso YD, Ferreira ML et al. The Brazilian database on pregnancy in multiple sclerosis. Clin. Neurol. Neurosurg. 113(4), 277–280 (2011).
- Bastian LA, Piscitelli JT. Is this patient pregnant? Can you reliably rule in or rule out early pregnancy by clinical examination? JAMA 278(7), 586–591 (1997).
- Corsello G, Giuffrè M. Congenital malformations. J. Matern. Fetal Neonatal Med. 25(Suppl. 1), 25–29 (2012).
- Daw JR, Mintzes B, Law MR, Hanley GE, Morgan SG. Prescription drug use in pregnancy: a retrospective, population-based study in British Columbia, Canada (2001–2006). Clin. Ther. 34(1), 239–249 (2012).
- Ban L, Tata LJ, West J, Fiaschi L, Gibson JE. Live and non-live pregnancy outcomes among women with depression and anxiety: a population-based study. PLoS ONE 7(8), e43462 (2012).
- Andersen AB, Erichsen R, Farkas DK, Mehnert F, Ehrenstein V, Sørensen HT. Prenatal exposure to acid-suppressive drugs and the risk of childhood asthma: a population-based Danish cohort study. Aliment. Pharmacol. Ther. 35(10), 1190–1198 (2012).
- Kieler H, Artama M, Engeland A et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ 344, d8012 (2012).
- Marrie RA, Yu BN, Leung S et al. The utility of administrative data for surveillance of comorbidity in multiple sclerosis: a validation study. Neuroepidemiology 40(2), 85–92 (2012).
- Kang JH, Chen YH, Lin HC. Comorbidities amongst patients with multiple sclerosis: a population-based controlled study. Eur. J. Neurol. 17(9), 1215–1219 (2010).
- Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. High frequency of adverse health behaviors in multiple sclerosis. Mult. Scler. 15(1), 105–113 (2009).
- Misra DP, Caldwell C, Young AA Jr, Abelson S. Do fathers matter? Paternal contributions to birth outcomes and racial disparities. Am. J. Obstet. Gynecol. 202(2), 99–100 (2010).
- Shah PS; Knowledge Synthesis Group on determinants of preterm/low birthweight births. Paternal factors and low birthweight, preterm, and small for gestational age births: a systematic review. Am. J. Obstet. Gynecol. 202(2), 103–123 (2010).
- Neuhaus O, Kieseler BC, Hartung HP. Pharmacokinetics and pharmacodynamics of the interferon-βs, glatiramer acetate, and mitoxantrone in multiple sclerosis. J. Neurol. Sci. 259, 27–37 (2007).
- Bernick SJ, Kane S. Drug transfer to the fetus and to the breastfeeding infant: what do we know? Curr. Drug Deliv. 9(4), 350–355 (2012).
- Dhib-Jalbut S, Marks S. Interferon-β mechanisms of action in multiple sclerosis. Neurology 74(Suppl. 1), S17–S24 (2010).
- Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl Acad. Sci. USA 95(26), 15623–15628 (1998).
- Moore KL, Persaud TVN. Before We Are Born: Essentials of Embryology and Birth Defects (7th Edition). Saunders/Elsevier, PA, USA (2008).
- Vollmer T, Stewart T, Baxter N. Mitoxantrone and cytotoxic drugs’ mechanisms of action. Neurology 74(Suppl. 1), S41–S46 (2010).
- Briggs GG, Freeman RK, Yaffe SJ. Mitoxantrone. In: Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins, PA, USA (2008).
- Meistrich ML, Wilson G, Mathur K et al. Rapid recovery of spermatogenesis after mitoxantrone, vincristine, vinblastine, and prednisone chemotherapy for Hodgkin’s disease. J. Clin. Oncol. 15(12), 3488–3495 (1997).
- Ronni T, Melén K, Malygin A, Julkunen I. Control of IFN-inducible MxA gene expression in human cells. J. Immunol. 150(5), 1715–1726 (1993).
- Buhimschi CS, Weiner CP. Medications in pregnancy and lactation: part 1. Teratology. Obstet. Gynecol. 113(1), 166–188 (2009).
- Hodge LS, Tracy TS. Alterations in drug disposition during pregnancy: implications for drug therapy. Expert Opin. Drug Metab. Toxicol. 3(4), 557–571 (2007).
- Goldkind SF, Sahin L, Gallauresi B. Enrolling pregnant women in research – lessons from the H1N1 influenza pandemic. N. Engl. J. Med. 362(24), 2241–2243 (2010).
- Battino D, Tomson T. Management of epilepsy during pregnancy. Drugs 67(18), 2727–2746 (2007).
- Hale TW, Siddiqui AA, Baker TE. Transfer of interferon b-1a into human breastmilk. Breastfeed. Med. 7(2), 123–125 (2012).
- Hellwig K, Haghikia A, Rockhoff M, Gold R. Multiple sclerosis and pregnancy: experience from a nationwide database in Germany. Ther. Adv. Neurol. Disord. 5(5), 247–253 (2012).
- Stuart M, Bergstrom L. Pregnancy and multiple sclerosis. J. Midwifery Womens Health 56(1), 41–47 (2011).
- Miller AE, Rustgi S, Farrell C. Use of glatiramer acetate during pregnancy: offering women a choice. Mult. Scler. 18(Suppl. 4), P733 (2012).
- Giannini M, Portaccio E, Ghezzi A et al. Pregnancy and fetal outcomes after glatiramer acetate exposure in patients with multiple sclerosis: a prospective observational multicentric study. BMC Neurol. 12, 124 (2012).
- Cristiano LM, Bozic C, Bloomgren G. Preliminary evaluation of pregnancy outcomes from the Tysabri (natalizumab) pregnancy exposure registry. Mult. Scler. 17(Suppl. 10), S457 (2011).
- De Santis M, Straface G, Cavaliere AF, Rosati P, Batocchi AP, Caruso A. The first case of mitoxantrone exposure in early pregnancy. Neurotoxicology 28(3), 696–697 (2007).
- Hellwig K, Schimrigk S, Chan A, Epplen J, Gold R. A newborn with Pierre Robin sequence after preconceptional mitoxantrone exposure of a female with multiple sclerosis. J. Neurol. Sci. 307(1–2), 164–165 (2011).
- Geissbuhler Y, Butzkueven H, Hernandez-Diaz S et al. Pregnancy outcomes from fingolimod clinical trials and post-marketing experience and the need for a multinational Gilenya™ (fingolimod) Pregnancy Exposure Registry in multiple sclerosis. Mult. Scler. 18(Suppl. 4), P141 (2012).
- Kieseier B, Benamor M, Benzerdjeb H, Stuve O. Pregnancy outcomes from the teriflunomide clinical development programme: retrospective analysis of the teriflunomide clinical trial database. Mult. Scler. 18(Suppl. 4), P737 (2012).
Websites
- Biogen Idec. Avonex® (interferon β-1a). www.accessdata.fda.gov/drugsatfda_docs/label/2007/103628s5115lbl.pdf (Accessed 10 June 2012)
- Bayer HealthCare. Betaseron® (interferon β-1b). http://berlex.bayerhealthcare.com/html/products/pi/Betaseron_PI.pdf (Accessed 10 June 2012)
- US NIH. TYSABRI Pregnancy Exposure Registry. www.clinicaltrials.gov/ct2/show/NCT00472992(Accessed 30 December 2012)
- US NIH. The Multi-National Pregnancy Fingolimod Exposure Registry in Multiple Sclerosis. www.clinicaltrials.gov/ct2/show/NCT01285479 (Accessed 30 December 2012)
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Reviewer guidance: evaluating the risks of drug exposure in human pregnancies. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071645.pdf (Accessed 3 June 2012)
- Biogen Idec/Elan Pharmaceuticals I. Tysabri® (natalizumab). www.accessdata.fda.gov/drugsatfda_docs/label/2006/125104s015lbl.pdf (Accessed 10 June 2012)
- EMD Serono I/C. Novantrone® (mitoxantrone). www.accessdata.fda.gov/drugsatfda_docs/label/2009/019297s030s031lbl.pdf (Accessed 14 September 2012)
- Novartis Pharmaceuticals Corp. Gilenya™ (fingolimod). www.accessdata.fda.gov/drugsatfda_docs/label/2010/022527s000lbl.pdf (Accessed 10 June 2012)
- Sanofi Aventis US. Aubagio® (teriflunomide).www.accessdata.fda.gov/drugsatfda_docs/label/2012/202992s000lbl.pdf (Accessed 30 December 2012).
- Teva Pharmaceuticals. Copaxone® (glatiramer acetate). www.copaxone.com/pdf/prescribinginformation.pdf (Accessed 10 June 2012)
Safety of disease-modifying drugs for multiple sclerosis in pregnancy: current challenges and future considerations for effective pharmacovigilance
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertneurothera. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Your patient is a 28-year-old woman with highly active multiple sclerosis (MS) who plans to become pregnant. Based on the review by Dr. Lu and colleagues, which of the following statements about disease-modifying drug (DMD) management is most likely correct?
□ A Many studies support the safety of continuing her current treatment with DMDs
□ B Risk of MS relapse is increased during pregnancy
□ C Clinical opinion is that women with severe or highly active MS may consider continuing glatiramer acetate or interferon beta during pregnancy
□ D There is high-quality evidence available regarding the risks of spontaneous abortion and congenital anomalies associated with DMD use in pregnancy
2. The patient described in question 1 continued interferon beta therapy and had an uneventful pregnancy and delivery of a healthy boy. Based on the review by Dr. Lu and colleagues, which of the following statements about use of DMDs during breastfeeding is most likely correct?
□ A She should continue interferon beta therapy while breastfeeding
□ B Clinical opinion is that women with severe or highly active disease may choose to reinitiate DMD therapy and forgo breastfeeding
□ C Natalizumab is not excreted in human milk and is therefore safe to use during breastfeeding
□ D Use of glatiramer acetate while breastfeeding has been proven to harm the nursing infant
Based on the review by Dr. Lu and colleagues, which of the following statements about use of specific DMDs during pregnancy would most likely be correct?
□ A In 1 prospective cohort study, use of interferon beta in pregnancy was associated with preterm birth, shorter mean birth length, and lower mean birth weight
□ B Use of interferon beta in pregnancy was associated with cesarean delivery, congenital anomaly, and spontaneous abortion
□ C Many large studies have proven that use of glatiramer acetate in pregnancy is not associated with lower mean birth weight, congenital anomaly, preterm birth, or spontaneous abortion
□ D Findings of cohort studies of exposure to mitoxantrone during pregnancy have been inconclusive