Abstract
Accurate mediastinal staging is the hallmark of a sound thoracic oncology program. Mediastinal staging remains the most important validated tool for making treatment decisions for patients with non-small-cell lung cancer. The last few years have seen the emergence of several new techniques to improve mediastinal staging. This article summarizes the current state of the art of this rapidly evolving field.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journals/expertrespiratory; (4) view/print certificate.
Release date: November 14, 2011; Expiration date: November 14, 2012
Learning objectives
• Describe the general approach to mediastinal staging of NSCLC
• Describe criteria determining the need for invasive mediastinal staging in patients with NSCLC
• Describe selection of the best tests to be used for mediastinal staging in patients with NSCLC
Financial & competing interests disclosure
EDITOR
Elisa Manzotti,Editorial Director, Future Science Group, London, UK
Disclosure:Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay, MD,Freelance writer and reviewer, Medscape, LLC
Disclosure:Laurie Barclay, MD, has disclosed no relevant financial relationships.
AUTHORS
Samjot Singh Dhillon,Department of Medicine, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14268, USA
Disclosure:Samjot Singh Dhillon has disclosed no relevant financial relationships.
Jaspreet Kaur Dhillon,Department of Medicine, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14268, USA
Disclosure:Jaspreet Kaur Dhillon has disclosed no relevant financial relationships.
Sai Yendamuri,Department of Thoracic Surgery, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14268, USA
Disclosure:Sai Yendamuri has disclosed no relevant financial relationships.
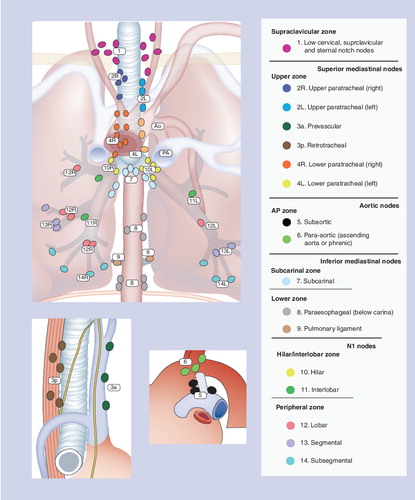
Adapted with permission courtesy of the International Association for the Study of Lung Cancer. Copyright © 2009 Memorial Sloan-Kettering Cancer Center, NY, USA.
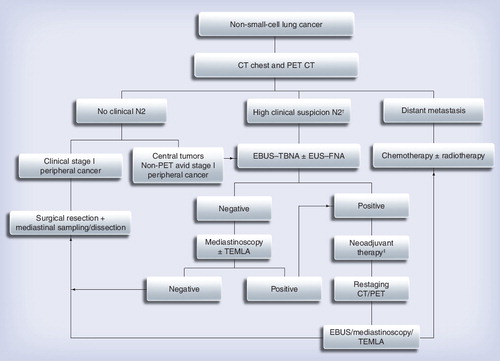
This should be tailored to individual patient and institution-specific factors as discussed in the text.
†Enlarged or PET positive N2 nodes.
‡This choice depends on the practicing physician.
CT: Computed tomography; EBUS: Endobronchial ultrasound; EUS: Endoscopic ultrasound; FNA: Fine-needle aspiration; TEMLA: Transcervical extended mediastinal lymphadenectomy; TBNA: Transbronchial needle aspiration.
Lung cancer is the leading cause of mortality in both men and women in the USA. Non-small-cell lung cancer (NSCLC) accounts for the majority (84%) of lung cancers Citation[1]. While small-cell cancer is broadly staged as ‘limited stage’ and ‘extensive stage’ based on field of radiation, a meticulous Tumor Nodal Metastasis (TNM) system is imperative for classifying NSCLC into distinct stages I–IV as the prognosis and treatment of each stage is different Citation[1,2]. This staging paradigm is crucial for making clinical decisions – the potential for curative surgical resection, the decision to administer adjuvant chemotherapy, to appropriately enroll patients in clinical trials and to compare outcomes. Incorrect staging of NSCLC results in errors in management including inaccurate prognostic information for the patients, futile and potentially toxic treatment and inaccuracies in research. After excluding distant metastasis, the ‘N’ staging becomes paramount in making treatment decisions, as patients with N0 and N1 involvement are generally candidates for surgery and patients with N2 disease are usually offered definitive or neoadjuvant chemoradiation. Additionally, a wide variety of investigative procedures and tests are available for mediastinal staging and an in-depth understanding of the potential benefits and pitfalls of each diagnostic test is important to make optimal decisions for work-up of NSCLC patients. This review aims to provide an update on the latest diagnostic tests, procedures and surgical techniques so that they can be used judiciously for ensuring accurate mediastinal staging of NSCLC and to provide optimal work-up prior to treatment.
‘Mapping’ the mediastinal lymph nodes
Understanding the lymphatic drainage of the lung is obviously relevant to staging the mediastinum. Intrapulmonary lymph nodes generally drain through the hilar lymph nodes to the mediastinal lymph nodes, but there is substantial variability in this drainage pattern. Segmental and subpleural lymphatics may drain directly to paratracheal or supraclavicular nodes and crossover lymphatic drainage across the midline is not infrequent Citation[3]. Tumors of the right upper lobe commonly metastasize to the right paratracheal station (4R), those of the left upper lobe to the subaortic and paraaortic (5 and 6) lymph node stations and those of the right middle and both lower lobes spread to the subcarinal (7) station and then to right paratracheal nodes (4R) Citation[4]. Lymph node maps based on tumor drainage patterns have been developed for facilitating staging and patient management. Several different lymph node maps have been used in the past, which hampers the comparison of various research studies. The latest (seventh) TNM system by the International Association for the Study of Lung Cancer has attempted to provide a uniform lymph node map by combining the Naruke map used in Japan and Europe with the Mountain–Dressler map used in the USA and readers are encouraged to thoroughly review the new map. The basic N0–N3 station system has been retained and seven distinct nodal zones have been described for future use in survival analysis, although the clinical and survival significance of these nodal zones is not clear currently and the zones are not for current standard nomenclature Citation[1,4]. Precise radiological, vascular, airway and surgical landmarks for accurate staging that can be unvaryingly recognized by all members of the multidisciplinary team taking care of NSCLC patients were introduced Citation[4]. For example, the N1 and N2 zone in the old map was distinguished by the pleural reflection, which could only be recognized during surgery. The new map uses major thoracic vessels as landmarks to separate these two important zones that can now be easily recognized on imaging studies. Distinct vascular landmarks have been designated to separate upper and lower paratracheal nodes. The subjective landmark of midline of trachea to separate right and left paratracheal nodes has been shifted to the left lateral margins of trachea based on actual lymphatic drainage pattern in the lung and owing to the fact that most of lymph nodes in front of trachea are usually removed en bloc with right paratracheal nodes during surgery.
The alteration in landmarks used to identify various lymph node stations has the unintended consequence of making it difficult to compare the new studies with the old ones. The upper boundary of right hilar N1 station has shifted up by utilizing the caudal margin of azygous as the perimeter between right paratracheal and right hilar stations Citation[5]. The N2 subcarinal zone has become bigger. The right paratracheal station has become larger as the boundary between right and left paratracheal stations has been changed from midline of trachea to the left lateral tracheal wall. Thus, a previous metastatic N2 node involvement with left upper lobe NSCLC may now qualify as N3 Citation[5]. Physicians taking care of patients need to recognize these changes while managing patients during this transition. Overall, the new staging system satisfies the long-awaited goal of having a uniform system with clear distinguishing landmarks and will better serve the patients and caregivers in the long term.
What constitutes a positive lymph node?
It is important for the practicing clinician to be aware of what a positive lymph node means and how the lymph node specimens are processed. There is considerable variability in surgical sampling of lymph nodes that includes selected lymph node biopsy, selective lymph node sampling, systemic nodal dissection, lobe-specific systemic nodal dissection or extended lymph node dissection Citation[3]. The systemic lymph node dissection that involves systematic dissection and removal of all mediastinal tissue containing the lymph nodes within anatomical landmarks is ideally recommended Citation[3]. Unfortunately there is no unanimity about the processing and histopathological examination of these nodes. The European Society of Thoracic Surgeons (ESTS) guidelines recommend that in the presence of gross tumor, one section from the most suspicious site should have hematoxylin and eosin (H and E) staining to look for metastasis and extracapsular extension Citation[3]. If this is negative, then several 2-mm sections of the node in longitudinal plane are recommended, which can improve the rate of detection of metastasis Citation[3]. Each block should be examined separately. Further yield can be improved with serial sectioning and immunohistocytochemistry (IHC) Citation[3]. However, all these techniques to detect less obvious foci of metastasis are arduous, and are not uniformly adhered to. Many times, only limited sections of lymph nodes are reviewed by H and E stain examination. IHC is still not the current standard of care.
Metastasis to the lymph nodes does not always occur macroscopically in an easily recognizable fashion. Micrometastasis is the presence of microscopic disease that may escape detection by standard H and E stains. By definition, it is often defined as tumor cells in lymph node measuring 0.2–2 mm in greatest diameter Citation[6]. The prevalence of micrometastasis is approximately 19%, but this may in fact may be underestimation due to sampling issues and inability of current techniques to adeptly detect it Citation[6]. Even enhanced techniques, such as IHC, may not be fully successful in detecting micrometastasis. The exact significance of the presence of micrometastasis on the patient prognosis is not yet fully clear, but it may explain the high frequency of recurrence of resected NSCLC. Isolated tumor cells are a term used to describe presence of only a single tumor cell or small cluster of tumor cells <0.2 mm in diameter, which show no stromal reaction or proliferative potential Citation[3,6]. The exact clinical impact of separating such minor foci of metastasis in lymph nodes into micrometastasis and isolated tumor cells is still not clearly understood. However, in future, this may hold promise in predicting patients at high risk of recurrence and selecting candidates for adjuvant chemotherapy.
Skip or discontinuous metastasis is the direct metastasis to the mediastinal or N2 nodes, bypassing the intrapulmonary/hilar or N1 nodes. This phenomenon can be seen in approximately 25% (18–38%) of cases, and more so in upper lobe tumors and in adenocarcinomas Citation[4,6–8]. It is uncertain whether skip metastasis occurs owing to unclear pathophysiological mechanisms, direct drainage from intrapulmonary lymphatics or simply represents undetected cases of micrometastases Citation[6].
Methods of mediastinal staging
A variety of invasive and noninvasive tests are available to stage the mediastinum. The practising clinician should be well versed in the performance characteristics of each staging modality and be aware of specific limitations and the contexts that can maximize the value of each technique. The practical application of each of these methods also depends on available expertise and the presence of specific resources that can optimize results. Continuous quality assessments should be part of the practice to achieve valid results.
Noninvasive methods
Noninvasive staging methods, though not confirmatory, provide detailed anatomical information, aid in determining the need for additional invasive staging procedures and assist in deciding the best mode of invasive staging.
Chest radiograph
Chest radiographs may be helpful in the initial recognition of a tumor and the recognition of bulky mediastinal disease, but is neither sensitive nor specific enough to warrant detailed discussion.
CT scan of the chest
CT scan of the chest is often the first step in mediastinal staging of NSCLC owing to the benefit of obtaining detailed anatomical information. Intravenous contrast enhancement is desirable, although not mandatory, as it helps to distinguish lymph nodes from vascular structures. A normal-sized mediastinal node is defined as a lymph node with a short axis diameter of ≤1 cm on a transverse CT scan image. Various CT criteria have been suggested to define features suggestive of malignant involvement, but none including the size criteria are very sensitive or specific. Almost 20% of the lymph nodes smaller than 1 cm are malignant and nearly 40% of lymph nodes larger than 1 cm are benign Citation[9–11]. In one meta-analysis, the pooled sensitivity of chest CTs for detecting mediastinal lymph node metastatic involvement using size criteria is only 51% (95% CI: 47–54%) and pooled specificity is 86% (95% CI: 84–88%) in a population with a median prevalence of 28% Citation[9]. Of note, the specificity of CT scans can be influenced by confounding factors that lead to anatomic changes, such as postobstructive pneumonia Citation[11]. In spite of the low accuracy of CT scan in diagnosing mediastinal nodal metastasis, the anatomical information obtained from a CT scan cannot be underemphasized as it helps with further decision making and appropriate biopsy site direction. Most current guidelines recommend a CT scan in the initial evaluation of NSCLC Citation[9,12].
PET
Whole body PET scan provides functional imaging by evaluating the preferential uptake of radiolabeled 18fluorodeoxyglucose (18F-FDG) by tumor cells and combined PET–CT improves this technology further by adding the anatomical details to the functional image Citation[13,14]. A standard uptake value (SUV) cut off of >2.5 is considered abnormal and suggestive of neoplastic involvement Citation[9,15]. However, this criteria has not been uniformly used in all studies. Additionally, there is a concerning variation in the quality of PET reporting among institutions, with some centers not even reporting SUV values – this impairs a fair comparison of research studies Citation[13].
A meta-analysis of 44 studies of PET scan (not integrated PET–CT) calculated a pooled sensitivity of 74% (95% CI: 69–79%) and specificity of 85% (95% CI: 82–88%) when the median prevalence of mediastinal metastasis is 29% Citation[9]. Thus, based on this, PET has better accuracy than contrast-enhanced CT in staging NSCLC, but is still not good enough to do away with histologic confirmation. Additional criteria to improve accuracy of PET scan in detecting malignant involvement of the mediastinal, such as using a higher cutoff for SUV or using the ratio of maximum SUV of mediastinal node to that of main tumor have been suggested, but these criteria need further validation Citation[16]. The performance of PET scans in specific circumstances appears to be better. PET scan is accurate in the setting of enlarged nodes – a meta-analysis by Gould reported a median sensitivity of 100% and specificity of 78% Citation[17]. However, almost 25% of the enlarged nodes can be PET-positive for nonmalignant reasons, such as infection or inflammation, and tissue diagnosis is needed to confirm malignancy. PET is less sensitive but more specific in patients with normal-sized nodes as the same meta-analysis by Gould showed that in the presence of normal-sized nodes, the sensitivity of PET is 82% and the specificity is 93% Citation[17]. False-negative (FN) PET scan can occur in micrometastasis, bronchioalveolar carcinoma, mucoepidermoid carcinomas and in typical carcinoid tumors.
Integrated PET–CT can assist in detecting lesions not well observed on PET or CT and can provide better anatomical localization and demarcation of adjacent structures Citation[14]. Based on pooled results of several recent studies, PET–CT has slightly higher accuracy than PET Citation[14]. It is better in detecting metastatic lesions, provides better anatomical correlation for invasive staging and is more useful in mediastinal restaging than remediastinoscopy (accuracy for restaging: 83 vs 60%, respectively; p < 0.05) and PET or CT alone Citation[14,18]. As the multidetector CT imaging apect of integrated PET–CT has improved, it has replaced conventional PET-only scanners. Additional advantages of PET scan include the ability to detect extrathoracic disease that may save futile mediastinoscopy and thoracotomies. It can detect unanticipated stage IV disease in distant sites, such as neck nodes, adrenals, liver and bone (5–20% of cases), and can also obviate the need for a bone scan Citation[9,13,14]. PET also aids in deciding the most appropriate invasive test by helping to identiy the most appropriate sites for biopsy and plan radiation therapy fields.
The PET scan continues to be an important recommended test for initial evaluation of lung cancer Citation[9,12]. Recent studies suggest that PET–CT prevents unnecessary surgical procedures. Fisher and colleagues randomly assigned 189 NSCLC patients undergoing surgical evaluation for thoracotomy to PET–CT and conventional staging (blood work, contrast-enhanced CT chest and upper abdomen bronchoscopy) Citation[19]. These patients subsequently underwent clinically required diagnostic procedures, such as endobronchial ultrasound (EBUS) or endoscopic ultrasound (EUS), and more than 90% patients in each group underwent mediastinoscopy. In the PET–CT group, 21 out of 60 thoracotomy procedures (35%) were considered futile as compared with 38 out of 73 (52%) in the conventional staging group (p = 0.05). Another recent randomized Canadian trial of 337 patients by Maziak and colleagues comparing PET–CT with conventional staging (CT abdomen and bone scan) in suspected early stage NSCLC on routine CT (all patients also had brain CT or MRI) demonstrated that PET–CT correctly upstaged disease in 13.8% of patients as compared with only 6.8% in conventional staging group (p = 0.046) and prevented unnecessary thoracotomies Citation[20]. In this context, it is important to note that studies of PET–CT have not clearly shown any improvement in mortality in NSCLC Citation[19,20] and the issue of cost–effectiveness is still not fully clear. In spite of its pros and cons, PET–CT has been overwhelmingly adopted by the medical community and is recommended by current guidelines in the initial evaluation of all NSCLC patients Citation[9,12].
MRI
MRI is not routinely used for mediastinal staging of NSCLC. MRI can detect the difference in intensity between tumor and normal tissue, such as bone, vessels, fat and soft tissue, and is helpful in certain specific situations where the possibility of invasion of mediastinum, major vessels, chest wall, diaphragm or vertebral bodies arises Citation[9]. It is also useful in evaluating superior sulcus tumors for brachial plexus involvement. MRI may have some role in evaluation of subcarinal and A–P window nodes due to absence of partial volume average artifact, which can be seen in axial CT imaging Citation[21]. However, the poor spatial resolution sometimes makes it difficult to recognize small discrete individual lymph nodes in a group and may make them look like a single enlarged abnormal node. MRI is also unable to recognize benign patterns of calcifications in nodes Citation[21]. The addition of MRI findings to routine CT imaging has not been shown to improve accuracy of mediastinal lymph node staging Citation[22]. Diffusion-weighted imaging is a MRI technique that provides tissue contrast based on the difference of diffusion of water molecules amongst various tissues. Tumors have less diffusion of water due to increased cellularity, more macromolecular proteins and less extracellular space and hence lower apparent diffusion coefficient Citation[22]. Nomori et al. reported lower rate of false positives (FPs) and a higher accuracy of diffusion-weighted imaging MRI as compared with PET–CT in mediastinal staging of NSCLC Citation[23], but these results have not been replicated by others Citation[24]. Further work is needed to determine whether this technology will be useful in routine mediastinal staging of NSCLC.
Invasive methods
Noninvasive investigations may aid in mediastinal staging but are not accurate enough to make critical management decisions. Even with the best currently available noninvasive staging tests, almost one in every three thoracotomies may be futile Citation[19]. Thus, invasive methods have a vital role in mediastinal staging of NSCLC. The choice of invasive procedure depends on the location of suspected mediastinal nodes, as some nodal stations are more easily accessed by one procedure as compared with another. Therefore, patient cohorts in research studies of various invasive procedures are different owing to selection bias. Two invasive staging tests may have the same sensitivity and specificity, but may have different predictive values based on prevalence of mediastinal lymph node metastasis Citation[25]. Additionally, the proficiency of the physician performing the procedure may also play a part in the varying sensitivity and specificity observed. Thus, comparing the sensitivity and specificity of various invasive procedures of mediastinal staging are fraught with difficulty – procedures should be considered complementary rather than competitive Citation[1]. With this background, we present three kinds of summary data in a tabular fashion. compares the lymph node station accessible by various staging procedures, is an overview of diagnostic yields of common procedures and provides a broad comparison of various staging procedures.
Cervical mediastinoscopy
Cervical mediastinoscopy is the historical ‘gold standard’ for mediastinal lymph node staging. It is performed in the operating room under general anesthesia and patients are usually discharged on the same day. A small incision is made over the suprasternal notch and dissection is performed out into the pretracheal space up to the carina. Nodes are sampled under direct visualization. Mediastinoscopy typically samples right and left upper and lower paratracheal (2R, 2L, 4R, 4L), and subcarinal (7) stations. The subaortic or aortopulmonary window (5), para-aortic (6), inferior mediastinal (8 and 9), hilar (10) and lobar/intralobar (11–14) stations are inaccessible to routine mediastinoscopy. This method of sampling has the advantage of improved detection of micrometastasis and extracapsular tumor spread. Contraindications are few and include presence of tracheostomy, unstable cervical spine or limited extension of neck due to arthritis.
The average sensitivity of mediastinoscopy is 80% and the average FN rate is 10% Citation[1]. Half of the FN cases can be attributed to nodes not accessible by mediastinoscopy and some can be due to operator-related issues. Video mediastinoscopes allow better visualization, more extensive sampling, including better access to posterior subcarinal node, and even complete lymph node dissection (video-assisted mediastinal lymphadenectomy [VAMLA]) and can also help to achieve higher accuracy of staging and better standardization of technique. Sensitivity of 90% and FN rate of 7% has been reported Citation[1]. It is recommend that all five nodal stations (stations 2R/L, 4R/L and 7) be examined in a cervical mediastinoscopy and, if nodes are present, at least one node should be sampled from each station Citation[1,12]. Systematic nodal sampling results in more accurate staging than selective sampling while complete lymphadenectomy can detect more multistation disease even though it does not result in a stage shift Citation[26]. Current evidence suggests that the utilization and sampling of mediastinoscopy is suboptimal. A survey of 729 hospitals (11,668 patients of NSCLC) conducted by the American College of Surgeons in 2001 showed that only 27.1% of patients had preoperative mediastinoscopy and that tissue biopsy material was obtained from <50% of these procedures Citation[27]. A Dutch study from one nonteaching university and three community hospitals reported mediastinoscopy sampling per expected standard in only 40% of cases Citation[28]. Another recent retrospective study from the USA suggested that mediastinal lymph node sampling was suboptimal in patients undergoing resection for NSCLC and suboptimal resection was associated with poorer prognosis; likely as a consequence of imprecise staging Citation[29].
The procedure is safe and carries a morbidity of 0.6–3.7% and mortality of 0–0.2% Citation[30]. The major concern is vascular injury (0.4%) and major bleeding, but that is rare and mostly associated with dissection around station 4R. Other complications include pneumothorax, nerve injury (recurrent laryngeal or phrenic), esophageal or tracheal injury and wound infection Citation[30,31]. Some patients may have concern about the residual neck scar.
Extended mediastinoscopy
Left upper lobe tumors can involve the subaortic (station 5) and para-aortic (station 6) lymph node stations that are not accessible by standard cervical mediastinoscopy. Extended mediastinoscopy enables sampling of these stations. Essentially, after standard mediastinoscopy, the mediastinoscope is directed lateral to the aortic arch and these stations are sampled. It is a challenging procedure performed only in a few centers.
Anterior or parasternal mediastinotomy (Chamberlain procedure)
Left anterior mediastinotomy involves an incision over the left second or third intercostal space and insertion of mediastinoscope after retraction of left internal mammary artery. It allows biopsy of subaortic (station 5) and para-aortic (station 6) lymph node stations. This procedure is also used to evaluate left upper lobe tumors.
VAMLA & transcervical extended mediastinal lymphadenectomy
These techniques allow complete bilateral lymph node excision through the cervical mediastinotomy approach. Whereas VAMLA relies on the use of video mediastinoscopy to perform the dissection, transcervical extended mediastinal lymphadenectomy (TEMLA) involves the use of a sternal retractor to increase the mediastinal space to perform an open transcervical dissection Citation[101]. VAMLA can access stations 2, 4, 7 and 8 while TEMLA can access 1, 2, 3a, 3p, 4, 5, 6, 7 and 8 Citation[32,33]. The advantage of complete lymphadenectomy is the potential reduction in the FN rate. In a study by Witte et al., VAMLA performed in 144 patients with resectable lung cancer with a technically adequate dissection was achieved in 86.8%. Additional disease on thoracotomy was identified in only two patients Citation[34]. However, the complication rate was a significant 3.98%, with five recurrent nerve paralyses. In an initial experience, Kuzdzal and colleagues performed TEMLA on 83 patients and reported a sensitivity, specificity and accuracy of the detecting mediastinal node metastases as 90, 100 and 96%, respectively, whereas the positive predictive value was 100% and negative predictive value (NPV) was 95% Citation[33]. The same authors performed a randomized prospective study in NSCLC patients, 21 in the TEMLA arm and 20 in the cervical mediastinoscopy arm Citation[35]. Owing to significantly higher number of FN in cervical mediastinoscopy group (5 vs 0; p = 0.019), the study was terminated prior to the goal of recruiting 100 patients. The sensitivity of mediastinoscopy was 37.5% and NPV was 66.7%, compared with sensitivity of 100% and NPV of 100% in the TEMLA group. An updated series published by Zielinski reported a sensitivity of 94.1%, specificity of 100% and NPV of 97.2% among 256 patients that had TEMLA. The recurrent laryngeal nerve palsy rate was lower at 2.3%, with only 0.8% having permanent recurrent laryngeal nerve palsy Citation[36]. Recent data suggest that TEMLA is better than remediastinoscopy, EBUS and PET–CT in restaging NSCLC patients after induction chemotherapy or chemoradiotherapy Citation[37]. While the technique of TEMLA is not very popular yet, the theoretical advantage of complete mediastinal lymphadenectomy and the excellent results in recent studies may make TEMLA the new gold standard of invasive mediastinal staging procedures. However, all the results of TEMLA are from a single institution, similar to VAMLA, and a wider adoption of these techniques with validation of results is essential.
Video-assisted thoracic surgery
Video-assisted thoracic surgery (VATS) or thoracoscopy is a less invasive alternative to thoracotomy for mediastinal staging. It can assist in T and N staging simultaneously. It is performed under general anesthesia and a scope is passed through a small incision in the chest wall as well as additional working ports allowing dissection of lung and lymph nodes. Nodes on only one side of the mediastinum can be accessed. Lymph node stations 4R/L, 5, 6, 7, 8, 9 and 10–14 can all be dissected. Many times the primary purpose of VATS may be resection of lung mass, but lymph nodes are sampled initially to determine the exact stage and to decide whether to continue with the resection. Complication rate is low (range: 0–9%) Citation[38]. In a meta-analysis, the pooled sensitivity of VATS was 75%, but the range of sensitivity was wide (37–100%), highlighting differences in expertise in VATS lymphadenectomy Citation[38].
Transthoracic needle aspiration
Transthoracic needle aspiration (TTNA) can be performed using fluoroscopy or CT guidance. Almost all mediastinal nodal stations are accessible by TTNA as stations 2, 4, 5 and 6 can be approached using an anterior parasternal approach and stations 4, 7, 8 and 9 can be accessible by a posterior approach Citation[12]. The pooled sensitivity of TTNA for mediastinal lymph node aspiration is 89%, specificity is 100% and FP is 0% when prevalence is 81% Citation[38]. However, in addition to high prevalence, most of these patient cohorts had extensive bulky mediastinal lymphadenopathy and these results are probably only relevant for such patients. The risk of pneumothorax is high and has been reported to be in the range of 10–60% Citation[39]. Presence of extensive bullous disease with the risk of persistent air leak can be a contraindication for the procedure.
Conventional transbronchial needle aspiration
Transbronchial aspiration of mediastinal nodes involves passing a transbronchial needle aspiration (TBNA) needle through the working channel of a bronchoscope and penetrating the bronchial wall at the expected location of lymph node based on CT correlation. Wang popularized the technique and provided detailed descriptions of several lymph node stations including specific sites to access them from the airway Citation[40]. While a high rate of success with this technique was reported from a few tertiary centers, a meta-analysis on TBNA showed a pooled sensitivity of 39%, specificity of 100% and a FN rate of 28% when the prevalence of mediastinal metastasis is 34% Citation[41]. This analysis suggested that sensitivity was critically dependent on prevalence of mediastinal metastasis. Despite being a useful technique, it has not become popular among physicians. A 1991 American College of Chest Physicians (ACCP) survey of 871 physicians, 98% of whom were pulmonologists, found that only 11.8% of physicians routinely used TBNA for staging malignancies. TBNA received the most negative comments amongst all bronchoscopy procedures. Reasons for not doing TBNA were issues of scope damage, inadequacy of specimens and lack of training. CT guidance, education and experience can improve diagnostic yield Citation[42]. Four to five passes of lymph nodes critical for staging are generally recommended Citation[43]. Rapid onsite cytology may reduce the number of total needle passes and lowers the rate of complications, but does not improve diagnostic yield Citation[44]. Conventional TBNA is safe, easy, inexpensive and useful when performed correctly. Core biopsy with a 19 G needle can be obtained with TBNA and this is a distinct advantage when compared with the more refined EBUS fine-needle aspiration technique.
Electromagnetic navigational bronchoscopy-guided TBNA
Electromagnetic navigational (EMN) bronchoscopy is a new tool available for airway interventionalists. A virtual bronchoscopy image is generated from CT images and superimposed upon the real tracheobronchial tree during the procedure. The patient is placed in an electromagnetic field and an eight-way steerable flexible catheter with a position sensor (locatable guide) inside a sheath is passed through the regular bronchoscope. The exact position of the sensor when placed within the electromagnetic field is seen on the system monitor, which helps in navigating it to the predetermined target. Once the target is reached, the sheath is secured and the probe is removed. Instruments can be passed through this sheath for specimen collection. Most studies of EMN involve accessing peripheral lung lesions. However the EMN system can also be used to target lymph nodes similar to peripheral lesions. Gildea and colleagues reported a 100% sampling success in all the 31 lymph nodes during one of the initial clinical trials of EMN Citation[45]. However, validation studies of the utility of EMN in this setting are lacking. EMN can be used to sample peripheral lymph nodes (stations 12–14) that may not be accessible by linear EBUS due to the larger size of the scope. Radial EBUS passed through the EMN sheath can be used to confirm the exact location to further improve yield.
EBUS-guided TBNA
EBUS is a comparatively new minimally invasive technique for mediastinal staging and allows visualization of lymph nodes adjacent to the large airways usually in anterior and superior mediastinum. Two types of EBUS are available: radial probe and linear probe. A 20-MHz radial probe ultrasound with an inflatable balloon can be passed through the working channel of a bronchoscope that can provide a 360° view and has a resolution of less than 1 mm. This is helpful in assessing the bronchial wall to determine the extent of invasion and the surrounding structures including the lymph nodes. However, the radial probe is removed after localization of the lymph node and the TBNA needle is passed through the same working channel and so the ultrasound guidance is not real time. A peripheral probe without a balloon is also available, which helps in accessing peripheral lymph nodes and masses. The convex probe linear EBUS scope has a 7.5-MHz convex transducer at the tip of a flexible bronchoscope. The outer diameter of the insertion tube of the scope is 6.2 mm and that of the tip is 6.9 mm. Therefore, owing to its bigger size, this scope has to be inserted through the mouth or through an endotracheal tube greater than size 8.0. A balloon at the tip can be inflated with normal saline and can be helpful in obtaining a superior view due to better contact with the airway wall. Simultaneous white light and ultrasound images can be viewed, although the white light image is not as good as that of standard bronchoscope. A needle system with a 22 or 21 G needle can be passed through the EBUS scope and introduced into the lymph node under real-time ultrasound guidance, which has several safety knobs/locks to prevent bronchoscope damage. The length of the needle protrusion is 2 cm but can be increased up to 4 cm (conventional TBNA needle length is generally 1.3–1.5 cm). A stylet prevents contamination of the needle when passing through the bronchial wall and helps push the tissue out of the needle hub at the time of specimen collection. The color Doppler feature allows recognition of blood vessels to prevent inadvertent puncture and also helps in recognition of blood vessels as landmarks for the new lymph-node staging system. The procedure can be performed under conscious sedation, monitored anesthesia care, general anesthesia or a laryngeal mask airway. After identifying a lymph node, the needle is advanced and seen entering the node in ultrasound view. Negative pressure is applied with a syringe and the needle is moved back and forth inside the node several times. Variations of this technique include not using negative pressure with a syringe when the initial specimen is bloody or leaving the stylet partially in during sample acquisition Citation[46]. The sample obtained is smeared on glass slides, air dried, fixed with 95% alcohol and stained for rapid on-site cytological analysis or added to cell block medium. The optimal number of passes for getting good diagnostic yield is at least three per lymph node station Citation[47,48]. An expert bronchoscopist and cytologist form the backbone of a successful EBUS program.
The EBUS scope can access all mediastinal nodes reachable by cervical mediastinoscopy and has the additional ability to reach the hilar lymph node stations. It can access stations 2, 3p, 4, 7, 10, 11 and at times, 12. Station 3a is anterior to the airway with intervening vasculature and usually not accessible. Owing to its size, the linear EBUS scope generally cannot go beyond the large airways but the peripheral radial probe ultrasound can allow assessment and aspiration of nodes adjacent to distal airways. Several studies have shown high sensitivity and specificity of EBUS for mediastinal lymph node staging, including nodes that are enlarged or are PET positive Citation[47,49–51]. Yasufuku and colleagues studied 102 patients with proven or suspected lung cancer prior to surgery to compare the accuracy of CT, PET and EBUS Citation[49]. They were able to perform EBUS on 147 mediastinal and 53 hilar lymph nodes without any complications. The sensitivity of CT, PET and EBUS-TBNA for correct detection of malignant involvement was 76.9, 80.0 and 92.3%, respectively, and specificity was 55.3, 70.1 and 100%, with diagnostic accuracy of 60.8, 72.5 and 98.0%, respectively. Thus, in this study, EBUS was superior to both CT and PET in mediastinal lymph node staging. The performance of EBUS based on meta-analysis of 12 studies involving 1292 patients (103 of them had radial ultrasound) showed a sensitivity of 93%, specificity of 100%, FN of 9% (range: 1–37%) and FP of 0%, with a mean prevalence of metastatic disease of 63% Citation[52]. Thus, the prevalence of metastatic mediastinal disease in EBUS studies was higher than the typical range of 20–40% reported in studies of other tests/procedures and this fact should be kept in mind when deciding the most appropriate diagnostic procedure for a patient.
Few studies have compared EBUS directly with mediastinoscopy. In a commonly quoted prospective crossover trial of 66 patients with suspected NSCLC and enlarged lymph nodes (>1 cm) in the paratracheal and subcarinal area (nodal stations accessible by both procedures), EBUS had better diagnostic yield than mediastinoscopy in per lymph node analysis (91 vs 78%; p = 0.007), but this difference was predominantly due to performance in the subcarinal station Citation[53]. These results may be owing to the fact that the mediastinoscope may not be able to access the posterior subcarinal station well and this may well be operator dependent. Furthermore, the prevalence of metastatic disease in this study cohort was very high at 89%, which could have favored EBUS. Overall, no significant difference was seen in terms of determination of true pathological N staging per patient between in two procedures. PET–CT, which is commonly used by clinicians nowadays to direct site of biopsy, was also not utilized for this study. A recent retrospective review showed that eight out of 29 (28%) patients with high risk of N2 disease and negative EBUS had metastatic disease on mediastinoscopy Citation[54]. Prospective trials of EBUS compared with mediastinoscopy are in progress and may shed more light on these issues. Generally, mediastinoscopy is better at determining small foci of metastasis and may allow complete lymphadenectomy Citation[55], while EBUS has the advantage of being less invasive and accessing more nodes, including hilar nodes. It is also important to appreciate the fact that both techniques are operator dependent and studies may be confounded by local expertise.
EBUS may be useful in a few other special situations. EBUS has been shown to be useful in staging patients with clinical stage 1 NSCLC and normal mediastinal lymph nodes on CT along with a negative PET scan. Herth and colleagues performed EBUS-TBNA on 156 lymph nodes ranging from 5 to 10 mm on 97 such patients with NSCLC followed by surgical staging Citation[56]. Out of ten patients with confirmed mediastinal metastatic disease, nine were correctly diagnosed by EBUS. The sensitivity, specificity and NPV of EBUS for detecting malignancy in this group with low prevalence were 89, 100 and 98.9%, respectively. Another situation where EBUS has been evaluated is restaging after neoadjuvant therapy. While EBUS has been shown to be safe in restaging the mediastinum after neoadjuvant chemotherapy, the results are poorer when compared with the staging the mediastinum prior to initiation of therapy. In two studies looking at this question Citation[55,57], the NPV of EBUS was 78 and 20%, respectively. Therefore, whenever possible, surgical confirmation is still needed in negative cases. The distinct advantage of EBUS in this situation is the safety of performing the procedure in patients that have had mediastinoscopy prior to the initiation of neoadjuvant therapy, as is often the case. While small case series have demonstrated the safety of remediastinoscopy, most surgeons are reluctant to perform this procedure.
Overall, EBUS is a very safe procedure and complications, such as pneumothorax or bleeding have been described only rarely Citation[52]. The risk of bleeding is low; even in cases where major thoracic blood vessels have been intentionally punctured by ultrasound-guided needles to access lymph nodes, no significant bleeding has been reported Citation[58–60]. The major limitation of EBUS is that the amount of tissue obtained is so small that it is prone to sampling and interpretation error. There is a concern of over-staging as some International Association for the Study of Lung Cancer nodal station boundaries are sometimes hard to detect on EBUS/EUS and consequently over-staging can occur when the needle passes through dysplastic or neoplastic airway mucosa or when the main tumor is inadvertently sampled instead of the node Citation[61].
Hopefully, future studies will help further define and refine the role of EBUS in mediastinal staging of NSCLC. With the recent emergence of targeted therapy based on mutation testing, more and more tissue is being requested by pathology. Preliminary studies suggest that it may be possible to perform such analysis on EBUS specimens but further work is needed to clarify this issue Citation[62].
Transesophageal EUS-needle aspiration
EUS is similar to EBUS but uses a larger echoendoscope (13 mm) that is inserted into the esophagus to access posterior and inferior mediastinal nodes. Both radial and curvilinear forms are available with an ability to recognize lymph nodes as small as 4–6 mm Citation[63]. A variety of needles ranging from 19–25 G are available. The procedure is generally performed under conscious sedation. Lymph node stations that can be accessed are level 2L, 4L, 3p, 7, 8 and 9. The inferior border of station 7 is sometimes difficult to observe. Station 5 can occasionally be accessible and some case reports have described accessing station 6 with or without transaortic puncture Citation[59,60,64]. An additional advantage of EUS includes the capability to diagnose M1 disease by accessing retroperitoneal and celiac nodes, lesions in the left lobe of the liver and the left and sometimes even the right adrenal gland.
Several studies have shown high sensitivity and specificity of EUS for mediastinal lymph node staging Citation[63,65–67]. A meta-analysis of 16 studies of EUS needle aspiration in diagnosis of mediastinal nodal involvement with NSCLC showed a pooled sensitivity of 84% and pooled specificity of 99.5%, FP of 0.7% and FN of 19% (range: 0–61%) when the mean prevalence is 61% Citation[38]. Size of lymph node, sonographic features and drainage patterns are not helpful in deciding which lymph node to biopsy. When multiple lymph nodes are present, the nodal site that will upstage the patient the most is chosen first and three to five passes per site are suggested for adequate sampling Citation[63]. Central versus peripheral site of aspirate of lymph node and application of suction or no suction do not seem to alter diagnostic yield Citation[68].
EUS has been shown to have better accuracy and higher predictive value than CT and PET for posterior mediastinal nodes (stations 5, 7, 8 and 9) Citation[69]. Even in patients with normal mediastinum by CT criteria, performing EUS precluded surgery in 12% and altered management in 25% of patients Citation[70]. Similar to EBUS, a study of EUS showed that it was superior to mediastinoscopy for subcarinal and paratracheal nodes Citation[67]. However, mediastinoscopy appears to have been performed suboptimally in this study as only 24 out of 60 patients had subcarinal lymph nodes sampled and the sensitivity of mediastinoscopy in the subcarinal region was unacceptably low at 7%. Well-designed, randomized, controlled trials are needed to compare the performance of EUS to mediastinoscopy. Another study implied that the routine addition of EUS to mediastinoscopy improves the performance of either procedure alone by detecting more N2 and T4 disease and can avoid up to 16% of thoracotomies Citation[66]. However, caution should be exercised as some studies have shown that EUS may not be sufficient to diagnose T4 disease and surgical confirmation is also needed Citation[71]. Interestingly, in one study, EUS was able to detect malignant N2/N3 disease in 37% (13 out of 35) of patients with anterior and posterior mediastinal lymphadenopathy even after a prior negative mediastinoscopy Citation[72]. EUS could have precluded mediastinoscopy in one third of these patients in this study if it was chosen as the initial procedure. EUS seems to be a very promising procedure and is slowly making its place in the paradigms of mediastinal staging of NSCLC.
The limitations of EUS needle aspiration include the inability to perform direct airway inspection and inability to access the primary lung mass. Some stations commonly involved with metastatic involvement, such as 2R/L and 4R are not visualized owing to intervening tracheal air. The FN rate is high and so surgical confirmation is needed for all negative cases. Sometimes, recognition of exact boundaries of lymph node stations and separation of the lymph node from the main mass is difficult Citation[63]; 2% of FP cases have been reported when left lower lobe tumors were misrecognized as subcarinal node. The contraindications of this procedure are few but include esophageal obstruction or inability to achieve satisfactory sedation owing to cardiorespiratory issues. However, theoretically, it may be better than EBUS in patients with significant coughing and with marginal respiratory status. Lymph node puncture is also easier owing to absence of tracheal rings in this transesophageal approach.
Combining EBUS & EUS (medical mediastinoscopy)
While EBUS can reach lymph nodes proximate to large airways in the upper and anterior mediastinum, EUS can access nodes in para-esophageal location along the posterior and inferior mediastinum with additional ability to approach few M1 sites. While the reach of these two procedures overlaps for some lymph node stations, they are also complementary in other stations. Combining EBUS and EUS can improve the reach of either procedure and an initial observational study reported a sensitivity of 93% and an NPV of 97% of the combined procedure Citation[73]. Subsequently, Annema and colleagues performed a randomized controlled trial comparing combined EBUS/EUS (medical staging) with surgical staging (mediastinoscopy with or without left parasternal mediastinotomy or VATS) in patients with abnormal CT or PET and showed that medical staging had a higher sensitivity (79 vs 85%; sensitivity of EBUS/EUS followed by surgical staging was 94%) and resulted in less futile thoracotomies in the medical mediastinoscopy group (9 vs 18%; p = 0.02) Citation[74]. Thus, performing EBUS/EUS prior to surgical staging is a reasonable option. Even in a study of patients with radiologically normal mediastinum where final surgical confirmation was obtained by TEMLA and the prevalence of N1/N2 disease was only 22%, the combined approach has a sensitivity of 68% and NPV of 91%, which was better than either EBUS or EUS performed alone Citation[75].
As EUS may not be available in all centers, some physicians have introduced the EBUS scope into the esophagus and used it as EUS to access stations not reached by EBUS. This approach has shown reasonable success and is being termed EUS with bronchoscope-guided NA (EUS-B-NA) Citation[76,77]. Herth and colleagues evaluated 150 patients with EUS-B-NA, 139 of whom had NSCLC and the sensitivities of EBUS, EUS and EUS-B-NA were 92, 89 and 96%, respectively, and the NPV of EUS-B-NA was 96% Citation[77]. This approach seems promising and hopefully more studies on this will be seen in the near future.
Molecular staging of NSCLC
The recurrence rate of NSCLC remains high, probably owing to inaccurate staging and inability of current methods to detect micrometastasis. IHC methods using antibodies directed towards the epithelial markers, such as cytokeratin AE1/AE3 and Ber-EP4 or towards genetic abnormalities appear to be promising Citation[6]. Vollmer and colleagues utilized IHC with the AE1/AE3 antibodies on 193 patients with clinical T1–2, N0, M0, NSCLC and were able to detect twice as many positive lymph nodes as compared with routine H and E Citation[78]. The average size of metastatic deposits detected by IHC was significantly smaller. However, cytokeratin is present in noncancerous epithelial and nonepithelial cells and the FP rate can be high. Reverse transcriptase (RT)-PCR, flow cytometry and imprint cytology may also aid in detecting micrometastasis Citation[6]. Success has been reported in using RT-PCR targeted to p53 or K-Ras mutations Citation[79]. However, these mutations are not present in all tumors. Further work is needed to be able to bring these techniques to the staging paradigm of NSCLC.
Clinical approach
In total, two main questions arise when performing mediastinal staging of NSCLC. When is invasive mediastinal staging needed and what is the best invasive test?
All patients suspected of having NSCLC should undergo at least a CT scan of the chest and upper abdomen (including liver and adrenals), if possible with the use of intravenous contrast and a PET scan. As per the current ACCP guidelines, if there is mediastinal infiltration encircling airways and vessels and no distinct node is seen, then mediastinum involvement by NSCLC can be assumed based on imaging and an investigative procedure is needed simply to verify the diagnosis Citation[9]. Under most circumstances, it is better to err on the side of minimally invasive or invasive staging if the patient can tolerate such a procedure rather than inaccurately staging a patient.
Invasive mediastinal staging is recommended if:
• Mediastinal nodes are enlarged (>1 cm in axial diameter);
• Mediastinal nodes are normal in size but the tumor is central or N1 involvement is suspected (as N1 involvement increases the probability of N2, N3 involvement to approximately 20–25% Citation[9]). In the case of a central tumor, it can be hard to determine the N1 nodal status and additionally the FN rate of both CT and PET is high. In this case invasive staging is suggested by ACCP guidelines irrespective of CT or PET results;
• Mediastinal nodes are normal in size but are positive on PET scan.
For peripheral stage I tumor, the prevalence of metastatic disease is low and NPV of PET scan is high enough Citation[80] that invasive staging of normal-sized mediastinal nodes can be omitted unless the primary tumor itself is not fludeoxyglucose avid as mediastinal metastasis of such tumors will also not show fludeoxyglucose uptake Citation[12]. However, during surgical resection of such cases, we still routinely perform mediastinal sampling of lymph nodes to further improve our accuracy of staging Citation[81].
Currently there is no single best test for mediastinal staging of NSCLC and all tests are complimentary. CT scanning provides anatomical information and PET can help additionally in deciding the best site and test for tissue diagnosis and to avoid futile procedures by discovering distant metastatic disease. The minimally invasive tests may be cost effective and help avoid surgical procedures, but once the patient is deemed to be a surgical candidate these negative results ultimately need invasive confirmation and mediastinal nodes should always be sampled to ensure that no micrometastasis or metastatic disease is present. Finally, the decision to perform a particular procedure depends on the location of the enlarged nodes along with the location of primary lesion, patients’ comorbidities and fitness for procedure along with institution-specific factors such as the availability of equipment, along with proficiency of clinicians, cytopathologist and support staff. shows a flowchart of mediastinal staging of NSCLC, but every institution needs to tailor it according to specific patient factors and availability of resources, as discussed earlier. A multidisciplinary approach is vital for the management of these complicated patients so that the best available resources can be utilized for accurate mediastinal staging, which in turn serves as the basis for all further management decision. The ACCP and ESTS guidelines can serve as valuable resources of each institution to develop their own strategy Citation[9,12,38].
Expert commentary
Accurate mediastinal staging is critical in the management of patients of NSCLC. While considerable progress has been made in recent years in refining the imaging and surgical techniques and development of minimally invasive techniques, current data suggest that a significant proportion of patients are still not accurately staged. Based on the information presented in this article, noninvasive tests are not sufficient and surgical staging may not be performed optimally. Detection of metastasis and micrometastasis in resected tissue is suboptimal and advancement of molecular staging may have an important role to play in near future. Handheld gamma probes after intravenous injection of 18F-FDG that may be able to detect metastasis intraoperatively are also being studied Citation[82].
In addition to continuing research into new techniques and molecular markers to accurately stage NSCLC, it is imperative to have standardization in staging procedures. Considerable variability exists in all modalities of staging – the size criteria for abnormal lymph node has been variable, the criteria for PET positivity and SUV cut off has not been uniform, endoscopic and surgical staging patterns are diverse, mediastinoscopy is performed suboptimally and tissue is not even retrieved in many cases. Additionally, a clear consensus about the processing and pathological evaluation of retrieved lymph nodes is needed. Variable availability of technique and expertise makes the development of guidelines a daunting task. The recent ACCP and ESTS guidelines, TNM 7 staging system and the uniform lymph node map are steps in the right direction. As the staging step is so critical in NSCLC management, clear guidelines about each and every step are needed and all thoracic oncology programs need to be evaluated based on their adherence with standard of care guidelines.
Five-year view
The next few years promise to be exciting in the field of mediastinal lymph node staging. The TNM staging is expected to continue to evolve based on new data and the next staging system can hopefully incorporate molecular markers for staging and prognostication. The role of minimal EUS techniques will be further refined. Several ongoing clinical trials are directly comparing EBUS and EUS to mediastinoscopy and these procedures are expected to be a vital part of staging algorithms. Virtual bronchoscopy navigation for TBNA is being investigated. Exciting surgical techniques, such as TEMLA, are appearing on the horizon and may become the new gold standard of mediastinal staging. Finally with the current advances in NSCLC, personalized treatment may soon become the standard of care. More and more tissue will be needed to fulfill the need of IHC, molecular and mutational studies and that may be the biggest challenge to the minimally invasive techniques as compared with surgical staging.
Table 1. Lymph node station accessibility of various invasive procedures.
Table 2. Overview of common invasive mediastinal staging techniques†.
Table 3. Strengths and weaknesses of common mediastinal staging procedures.
Key issues
• Accurate mediastinal staging is critical in the management of non-small-cell lung cancer (NSCLC).
• A variety of invasive and noninvasive techniques are available. These techniques are complimentary and physicians taking care of patients with NSCLC need to be thoroughly familiar with these techniques and current guidelines.
• Current noninvasive methods are not always sufficient to accurately stage NSCLC and invasive staging may be needed to guide definitive therapy.
• The minimally invasive procedures are becoming popular in the staging paradigm.
• Surgery remains the current gold standard for mediastinal staging. All negative endobronchial/endoscopic ultrasound cases need surgical confirmation owing to high false-negative rate.
• Thoroughness of staging is an area where there is a significant scope of improvement.
• Additional work is needed in mediastinal staging at the molecular level and to detect micrometastasis and skip metastasis and to comprehend the exact clinical significance of these findings.
References
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest136(1), 260–271 (2009).
- Lababede O, Meziane M, Rice T. Seventh edition of the cancer staging manual and stage grouping of lung cancer: quick reference chart and diagrams. Chest139(1), 183–189 (2011).
- Lardinois D, De Leyn P, Van Schil P et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg.30(5), 787–792 (2006).
- Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J. Thorac. Oncol.4(5), 568–577 (2009).
- Van Schil PE. From individual lymph nodes to stations and zones: east and west reconciled? J. Thorac. Oncol.4(5), 561–562 (2009).
- Kim AW. Lymph node drainage patterns and micrometastasis in lung cancer. Semin. Thorac. Cardiovasc. Surg.21(4), 298–308 (2009).
- Riquet M, Manac’h D, Saab M, Le Pimpec-Barthes F, Dujon A, Debesse B. Factors determining survival in resected N2 lung cancer. Eur. J. Cardiothorac. Surg.9(6), 300–304 (1995).
- Libshitz HI, McKenna RJ Jr, Mountain CF. Patterns of mediastinal metastases in bronchogenic carcinoma. Chest90(2), 229–232 (1986).
- Silvestri GA, Gould MK, Margolis ML et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest132(3 Suppl.), S178–S201 (2007).
- Kerr KM, Lamb D, Wathen CG, Walker WS, Douglas NJ. Pathological assessment of mediastinal lymph nodes in lung cancer: implications for non-invasive mediastinal staging. Thorax47(5), 337–341 (1992).
- McLoud TC, Bourgouin PM, Greenberg RW et al. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology182(2), 319–323 (1992).
- De Leyn P, Lardinois D, Van Schil PE et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur. J. Cardiothorac. Surg.32(1), 1–8 (2007).
- Ung YC, Maziak DE, Vanderveen JA et al.18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J. Natl Cancer Inst.99(23), 1753–1767 (2007).
- De Wever W, Stroobants S, Coolen J, Verschakelen JA. Integrated PET/CT in the staging of nonsmall cell lung cancer: technical aspects and clinical integration. Eur. Respir. J.33(1), 201–212 (2009).
- Hellwig D, Graeter TP, Ukena D et al. 18F-FDG PET for mediastinal staging of lung cancer: which SUV threshold makes sense? J. Nucl. Med.48(11), 1761–1766 (2007).
- Cerfolio RJ, Bryant AS. Ratio of the maximum standardized uptake value on FDG-PET of the mediastinal (N2) lymph nodes to the primary tumor may be a universal predictor of nodal malignancy in patients with nonsmall-cell lung cancer. Ann. Thorac. Surg.83(5), 1826–1829 (2007).
- Gould MK, Kuschner WG, Rydzak CE et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann. Intern. Med.139(11), 879–892 (2003).
- De Leyn P, Stroobants S, De Wever W et al. Prospective comparative study of integrated positron emission tomography–computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J. Clin. Oncol.24(21), 3333–3339 (2006).
- Fischer B, Lassen U, Mortensen J et al. Preoperative staging of lung cancer with combined PET-CT. N. Engl. J. Med.361(1), 32–39 (2009).
- Maziak DE, Darling GE, Inculet RI et al. Positron emission tomography in staging early lung cancer: a randomized trial. Ann. Intern. Med.151(4), 221–228, W-48 (2009).
- Quint LE, Francis IR, Wahl RL, Gross BH, Glazer GM. Preoperative staging of non-small-cell carcinoma of the lung: imaging methods. AJR Am. J. Roentgenol.164(6), 1349–1359 (1995).
- Webb WR, Gatsonis C, Zerhouni EA et al. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology178(3), 705–713 (1991).
- Nomori H, Mori T, Ikeda K et al. Diffusion-weighted magnetic resonance imaging can be used in place of positron emission tomography for N staging of non-small cell lung cancer with fewer false-positive results. J. Thorac. Cardiovasc. Surg.135(4), 816–822 (2008).
- Pauls S, Schmidt SA, Juchems MS et al. Diffusion-weighted MR imaging in comparison to integrated [(18)F]-FDG PET/CT for N-staging in patients with lung cancer. Eur. J. Radiol. doi: 10.1016/j.ejrad.2010.09.001 (2010) (Epub ahead of print).
- Ceron L, Michieletto L, Zamperlin A. Mediastinal staging in lung cancer: a rational approach. Monaldi Arch. Chest Dis.71(4), 170–175 (2009).
- Detterbeck F, Puchalski J, Rubinowitz A, Cheng D. Classification of the thoroughness of mediastinal staging of lung cancer. Chest137(2), 436–442 (2010).
- Little AG, Rusch VW, Bonner JA et al. Patterns of surgical care of lung cancer patients. Ann. Thorac. Surg.80(6), 2051–2056 (2005).
- Smulders SA, Smeenk FW, Janssen-Heijnen ML, Wielders PL, de Munck DR, Postmus PE. Surgical mediastinal staging in daily practice. Lung Cancer47(2), 243–251 (2005).
- Osarogiagbon RU, Allen JW, Farooq A, Berry A, O’Brien T. Pathologic lymph node staging practice and stage-predicted survival after resection of lung cancer. Ann. Thorac. Surg.91(5), 1486–1492 (2011).
- Park BJ, Flores R, Downey RJ, Bains MS, Rusch VW. Management of major hemorrhage during mediastinoscopy. J. Thorac. Cardiovasc. Surg.126(3), 726–731 (2003).
- Lemaire A, Nikolic I, Petersen T et al. Nine-year single center experience with cervical mediastinoscopy: complications and false negative rate. Ann. Thorac. Surg.82(4), 1185–1189 (2006).
- Hurtgen M, Friedel G, Toomes H, Fritz P. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA) – technique and first results. Eur. J. Cardiothorac. Surg.21(2), 348–351 (2002).
- Kuzdzal J, Zielinski M, Papla B et al. Transcervical extended mediastinal lymphadenectomy – the new operative technique and early results in lung cancer staging. Eur. J. Cardiothorac. Surg.27(3), 384–390 (2005).
- Witte B, Wolf M, Huertgen M, Toomes H. Video-assisted mediastinoscopic surgery: clinical feasibility and accuracy of mediastinal lymph node staging. Ann. Thorac. Surg.82(5), 1821–1827 (2006).
- Kuzdzal J, Zielinski M, Papla B et al. The transcervical extended mediastinal lymphadenectomy versus cervical mediastinoscopy in non-small cell lung cancer staging. Eur. J. Cardiothorac. Surg.31(1), 88–94 (2007).
- Zielinski M. Transcervical extended mediastinal lymphadenectomy: results of staging in two hundred fifty-six patients with non-small cell lung cancer. J. Thorac. Oncol.2(4), 370–372 (2007).
- Zielinski M, Hauer L, Hauer J, Nabialek T, Szlubowski A, Pankowski J. Non-small-cell lung cancer restaging with transcervical extended mediastinal lymphadenectomy. Eur. J. Cardiothorac. Surg.37(4), 776–780 (2010).
- Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest132(3 Suppl.), S202–S220 (2007).
- Zwischenberger JB, Savage C, Alpard SK, Anderson CM, Marroquin S, Goodacre BW. Mediastinal transthoracic needle and core lymph node biopsy: should it replace mediastinoscopy? Chest121(4), 1165–1170 (2002).
- Wang KP. Staging of bronchogenic carcinoma by bronchoscopy. Chest106(2), 588–593 (1994).
- Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax60(11), 949–955 (2005).
- Navani N, Spiro SG, Janes SM. Mediastinal staging of NSCLC with endoscopic and endobronchial ultrasound. Nat. Rev. Clin. Oncol.6(5), 278–286 (2009).
- Diacon AH, Schuurmans MM, Theron J et al. Transbronchial needle aspirates: how many passes per target site? Eur. Respir. J.29(1), 112–116 (2007).
- Trisolini R, Cancellieri A, Tinelli C et al. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest139(2), 395–401 (2011).
- Gildea TR, Mazzone PJ, Karnak D, Meziane M, Mehta AC. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am. J. Respir. Crit. Care Med.174(9), 982–989 (2006).
- Wang KP, Turner JF, Symanowski J. A retrospective review of different methods of endobronchial ultrasound-guided transbronchial needle aspiration: a preliminary study. J. Bronchol. Interven. Pulmonol.18(1), 94–96 (2011).
- Lee HS, Lee GK, Kim MS et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest134(2), 368–374 (2008).
- Block MI. Endobronchial ultrasound for lung cancer staging: how many stations should be sampled? Ann. Thorac. Surg.89(5), 1582–1587 (2010).
- Yasufuku K, Nakajima T, Motoori K et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest130(3), 710–718 (2006).
- Rintoul RC, Tournoy KG, El Daly H et al. EBUS-TBNA for the clarification of PET positive intra-thoracic lymph nodes – an international multi-centre experience. J. Thorac. Oncol.4(1), 44–48 (2009).
- Hwangbo B, Kim SK, Lee HS et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest135(5), 1280–1287 (2009).
- Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc. Am. Thorac. Soc.6(2), 180–186 (2009).
- Ernst A, Anantham D, Eberhardt R, Krasnik M, Herth FJ. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J. Thorac. Oncol.3(6), 577–582 (2008).
- Defranchi SA, Edell ES, Daniels CE et al. Mediastinoscopy in patients with lung cancer and negative endobronchial ultrasound guided needle aspiration. Ann. Thorac. Surg.90(6), 1753–1757 (2010).
- Szlubowski A, Herth FJ, Soja J et al. Endobronchial ultrasound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy – a prospective study. Eur. J. Cardiothorac. Surg.37(5), 1180–1184 (2010).
- Herth FJ, Eberhardt R, Krasnik M, Ernst A. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest133(4), 887–891 (2008).
- Herth FJ, Annema JT, Eberhardt R et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J. Clin. Oncol.26(20), 3346–3350 (2008).
- Vincent B, Huggins JT, Doelken P, Silvestri G. Successful real-time endobronchial ultrasound-guided transbronchial needle aspiration of a hilar lung mass obtained by traversing the pulmonary artery. J. Thorac. Oncol.1(4), 362–364 (2006).
- Wallace MB, Woodward TA, Raimondo M, Al-Haddad M, Odell JA. Transaortic fine-needle aspiration of centrally located lung cancer under endoscopic ultrasound guidance: the final frontier. Ann. Thorac. Surg.84(3), 1019–1021 (2007).
- von Bartheld MB, Rabe KF, Annema JT. Transaortic EUS-guided FNA in the diagnosis of lung tumors and lymph nodes. Gastrointest. Endosc.69(2), 345–349 (2009).
- Tournoy KG, Annema JT, Krasnik M, Herth FJ, van Meerbeeck JP. Endoscopic and endobronchial ultrasonography according to the proposed lymph node map definition in the seventh edition of the tumor, node, metastasis classification for lung cancer. J. Thorac. Oncol.4(12), 1576–1584 (2009).
- Nakajima T, Yasufuku K, Nakagawara A, Kimura H, Yoshino I. Multi-gene mutation analysis of metastatic lymph nodes in non-small cell lung cancer diagnosed by EBUS-TBNA. Chest doi:10.1378/chest.10-3186 (2011) (Epub ahead of print).
- McComb BL, Wallace MB, Pascual JM, Othman MO. Mediastinal staging of nonsmall cell lung carcinoma by endoscopic and endobronchial ultrasound-guided fine needle aspiration. J. Thorac. Imaging26(2), 147–161 (2011).
- Liberman M, Duranceau A, Grunenwald E et al. New technique performed by using EUS access for biopsy of para-aortic (station 6) mediastinal lymph nodes without traversing the aorta (with video). Gastrointest. Endosc.73(5), 1048–1051 (2011).
- Fritscher-Ravens A, Soehendra N, Schirrow L et al. Role of transesophageal endosonography-guided fine-needle aspiration in the diagnosis of lung cancer. Chest117(2), 339–345 (2000).
- Annema JT, Versteegh MI, Veselic M et al. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA294(8), 931–936 (2005).
- Larsen SS, Vilmann P, Krasnik M et al. Endoscopic ultrasound guided biopsy versus mediastinoscopy for analysis of paratracheal and subcarinal lymph nodes in lung cancer staging. Lung Cancer48(1), 85–92 (2005).
- Wallace MB, Kennedy T, Durkalski V et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest. Endosc.54(4), 441–447 (2001).
- Eloubeidi MA, Cerfolio RJ, Chen VK, Desmond R, Syed S, Ojha B. Endoscopic ultrasound-guided fine needle aspiration of mediastinal lymph node in patients with suspected lung cancer after positron emission tomography and computed tomography scans. Ann. Thorac. Surg.79(1), 263–268 (2005).
- LeBlanc JK, Devereaux BM, Imperiale TF et al. Endoscopic ultrasound in non-small cell lung cancer and negative mediastinum on computed tomography. Am. J. Respir. Crit. Care Med.171(2), 177–182 (2005).
- Varadarajulu S, Schmulewitz N, Wildi SM et al. Accuracy of EUS in staging of T4 lung cancer. Gastrointest. Endosc.59(3), 345–348 (2004).
- Eloubeidi MA, Tamhane A, Chen VK, Cerfolio RJ. Endoscopic ultrasound-guided fine-needle aspiration in patients with non-small cell lung cancer and prior negative mediastinoscopy. Ann. Thorac. Surg.80(4), 1231–1239 (2005).
- Wallace MB, Pascual JM, Raimondo M et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA299(5), 540–546 (2008).
- Annema JT, van Meerbeeck JP, Rintoul RC et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA304(20), 2245–2252 (2010).
- Szlubowski A, Zielinski M, Soja J et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging – a prospective trial. Eur. J. Cardiothorac. Surg.37(5), 1175–1179 (2010).
- Hwangbo B, Lee GK, Lee HS et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest138(4), 795–802 (2010).
- Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest138(4), 790–794 (2010).
- Vollmer RT, Herndon JE II, D’Cunha J et al. Immunohistochemical detection of occult lymph node metastases in non-small cell lung cancer: anatomical pathology results from Cancer and Leukemia Group B Trial 9761. Clin. Cancer Res.9(15), 5630–5635 (2003).
- Hashimoto T, Kobayashi Y, Ishikawa Y et al. Prognostic value of genetically diagnosed lymph node micrometastasis in non-small cell lung carcinoma cases. Cancer Res.60(22), 6472–6478 (2000).
- Farrell MA, McAdams HP, Herndon JE, Patz EF Jr. Non-small cell lung cancer: FDG PET for nodal staging in patients with stage I disease. Radiology215(3), 886–890 (2000).
- Gomez-Caro A, Garcia S, Reguart N et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur. J. Cardiothorac. Surg.37(5), 1168–1174 (2010).
- Nwogu C. Sentinel node and positron emission tomography mapping in lung cancer. Semin. Thorac. Cardiovasc. Surg.21(4), 323–326 (2009).
Website
- Transcervical extended mediastinal lymphadenectomy. www.ctsnet.org/sections/clinicalresources/thoracic/expert_tech-44.html
Mediastinal staging of non-small-cell lung cancer
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertrespiratory. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Which of the following statements about the overall approach to mediastinal staging of non-small cell lung cancer (NSCLC) is most likely correct?
□ A Optimal treatment decisions for patients with NSCLC can be made without mediastinal staging
□ B Upper abdominal CT is not needed in most patients
□ C According to the American College of Chest Physicians (ACCP) guidelines, a distinct node must be visible on chest CT to diagnose mediastinal involvement
□ D It is better to perform mini-invasive or invasive staging if a patient can tolerate it rather than risk inaccurate staging
2. A 66-year-old man is suspected of having NSCLC. Which of the following findings would make you most likely to recommend invasive staging?
□ A Peripheral location of tumor
□ B Largest mediastinal node is 0.8 cm
□ C Suspected N1 involvement
□ D Negative PET scan
3. The patient has suspected N1 involvement and central tumor. Which of the following statements about tests used for invasive mediastinal staging is most likely correct?
□ A Endoscopic ultrasound is the gold standard for mediastinal staging
□ B Mini-invasive tests do not require invasive confirmation
□ C At present there is no single best test for mediastinal staging of NSCLC, and all tests are complementary
□ D Patient factors do not affect choice of staging tests