Abstract
Refractory dyspnea is breathing difficulty that persists at rest or with minimal activity despite optimal therapy of the underlying condition. Both endogenous (β-endorphin) and exogenous (morphine) opioids modulate the perception of dyspnea by binding to opioid receptors. Proposed mechanisms whereby opioids relieve refractory dyspnea include: decreasing respiratory drive with an associated decrease in corollary discharge; altering central perception; altering activity of peripheral opioid receptors located in the lung and decreasing anxiety. As patients respond variably to opioid therapy, a low dose of an opioid should be prescribed initially to manage refractory dyspnea. The dose should be titrated to achieve the lowest effective dose based on patient ratings of breathing difficulty. Research is needed to address clinical uncertainties and to identify genetic factors to improve the use of opioids to relieve refractory dyspnea.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertrespiratory; (4) view/print certificate.
Release date: 2 April 2013; Expiration date: 2 April 2014
Learning objectives
Upon completion of this activity, participants will be able to:
• Describe proposed mechanisms for the effect of systemic opioids in relieving refractory dyspnea, based on a review
• Describe management of refractory dyspnea with systemic opioids, based on a review
• Describe barriers to opioid use for management of refractory dyspnea and directions for future research
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Publisher, Future Science Group, London, UK.
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay, MD
Freelance writer and reviewer, Medscape, LLC.
Disclosure: Laurie Barclay, MD, has disclosed no relevant financial relationships.
AUTHORS AND CREDENTIALS
Donald A Mahler, MD
Professor of Medicine, Geisel School of Medicine at Dartmouth, Hanover, NH, USA; Section of Pulmonary and Critical Care Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA.
Disclosure: Donald A Mahler, MD, has disclosed no relevant financial relationships.
†Treatment of psychological factors includes relaxation techniques, behavior modification, breathing strategies, anxiolytic medications and support for caregivers.
‡Multidisciplinary and comprehensive pulmonary rehabilitation Citation[93].
NIVS: Noninvasive ventilatory support.
![Figure 1. General approach to treating patients with dyspnea/refractory dyspnea.†Treatment of psychological factors includes relaxation techniques, behavior modification, breathing strategies, anxiolytic medications and support for caregivers.‡Multidisciplinary and comprehensive pulmonary rehabilitation Citation[93].NIVS: Noninvasive ventilatory support.](/cms/asset/50b82190-44ce-466d-8c42-5eb5f886c817/ierx_a_11219346_f0001_b.jpg)
This review focuses on the use of opioids in the management of refractory dyspnea, which has been defined as breathing difficulty that persists at rest or with minimal activity despite optimal therapy of the underlying condition Citation[1]. Refractory dyspnea is prominent and disabling among patients with advanced lung and heart disease. The mechanisms that contribute to dyspnea are presented in this review to illustrate the complexity of this symptom and to provide a rationale for treating the underlying disease with standard therapies prior to prescribing an opioid medication. For example, many patients with advanced pulmonary or cardiac disease develop hypoxemia at rest and/or with physical activities; oxygen should be prescribed for these patients to improve cellular oxygenation and to improve breathing difficulty. The experience of dyspnea can be quite distressing and typically evokes strong emotional responses including anxiety, panic and/or fear. In these patients, relaxation techniques, behavior modification, breathing strategies and anxiolytic medications should be considered.
The pharmacology of opioids is reviewed along with description of the types and location of opioid receptors. This information provides a framework for understanding the possible mechanisms whereby opioids reduce breathlessness. The results of randomized controlled trials (RCTs) that compared opioid and placebo treatments on dyspnea are summarized. An approach for prescribing opioids in the management of patients with refractory dyspnea is presented including consideration of possible barriers among physicians and patients/families.
Mechanisms of dyspnea
Breathing is normally an unconscious activity as neurons in the brainstem transmit efferent impulses to control the cyclical contraction and relaxation of the respiratory muscles. When this process is perturbed, the act of breathing reaches consciousness and the individual may experience unpleasant and distressful breathing. In a 2012 update, the American Thoracic Society reaffirmed the definition of dyspnea as, “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” Citation[2]. Dyspnea is a warning signal that the usual unconscious awareness of breathing has been altered.
Neurobiological model
Although the precise mechanisms of dyspnea are not completely understood, a neurobiological model provides a framework for understanding this symptom Citation[3,4]. Various respiratory stimuli may activate one or more sensory receptors which transmit afferent information to the brain, where dyspnea is perceived Citation[5,6]. Common stimuli that activate these sensory receptors are summarized in .
Davenport and Vovk proposed that different patterns of stimulation of sensory receptors and corresponding afferent transmission result in the various qualities of dyspnea (e.g., ‘work/effort’ of breathing, unsatisfied inspiration/‘air hunger’ and ‘chest tightness’) Citation[6].
Different pathways have been proposed to integrate and process respiratory sensations to the CNS. One pathway reflects discriminative processing – awareness of intensity. Afferent information is directed from respiratory muscle receptors to the brainstem medulla, then to the ventroposterior thalamus area and, subsequently, to the primary and secondary somatosensory cortex Citation[5,6]. These structures are considered to process the intensity domain and sensory qualities of dyspnea Citation[5]. A second pathway involves affective processing – awareness of unpleasantness Citation[6]. Afferent information is relayed from airway and lung receptors via the vagal nerve to the brainstem medulla, then to the amygdala and medial dorsal areas of the thalamus and, subsequently, to the insular and cingulate cortex Citation[5,6]. These limbic system structures are considered to process the affective domain of dyspnea Citation[7,8]. Davenport and Vovk proposed that activation of neural processes in the cortex requires a gating mechanism, whereby sensory information is distributed to specific areas of the brain for cognitive processing Citation[6]. The thalamus and hippocampus are considered critical neural areas for the gating of respiratory sensory input Citation[6].
The perception of dyspnea has been attributed to a mismatch, or imbalance, of afferent impulses from one or more sensory receptors and the outgoing central respiratory motor activity (demand to breathe) to the muscles of respiration (ability to breathe) Citation[2,9,10]. Dyspnea is recognized as a multidimensional sensory experience that includes not only intensity and unpleasantness, but also distinct sensory qualities Citation[2,11–15]. The major descriptors of breathlessness are classified as the work/effort of breathing, chest tightness and air hunger that reflect different receptor activation Citation[2,9,11,12]. Patients across a variety of cardiorespiratory diseases consistently report work/effort, whereas chest tightness appears specific for bronchoconstriction and is reported by some, but not all, patients with asthma Citation[12,16]. Air hunger correlates with the automatic drive to breathe as can be provoked by breathing a carbon dioxide gas mixture Citation[10]. Lansing et al. Citation[11] have proposed that air hunger is synonymous with unsatisfied inspiration, as has been described by patients with chronic obstructive pulmonary disease (COPD) immediately after exercise Citation[13]. Consideration of the domains and qualities of dyspnea may be relevant to understanding how specific therapies, such as opioids, relieve refractory dyspnea.
Psychological factors
An individual’s personality and emotional state can affect the perception of breathing difficulty Citation[17]. Anxiety, panic and depression can have a major impact on all patients with chronic cardiorespiratory disease, but especially in those who experience refractory dyspnea. Lansing et al. proposed that emotional and behavioral responses result from the unpleasantness domain of dyspnea, and that thoughts, attention, hypervigilance, and memories can modulate breathlessness Citation[11].
The prevalence of anxiety is higher in patients with COPD (15% of 1202 patients) compared with matched controls (6% of 302 subjects) Citation[18]. In fact, greater levels of anxiety have been reported in patients with COPD compared with those who have heart disease or cancer Citation[19]. Patients may experience frustration and depression when their breathing difficulty limits their ability to be physically active, and may be fearful of their next breathing exacerbation. Anxious patients may limit or even avoid social situations because of their concern that such activities may precipitate or aggravate breathlessness Citation[20]. Once anxiety develops among patients with COPD, it has been shown to relate to poorer health outcomes Citation[18]. Dyspnea can be a potent stimulus for the development of anxiety, which may then contribute to more intense breathlessness and perpetuate a dyspnea → anxiety → dyspnea cycle Citation[21,22].
Depression is also more prevalent in patients with chronic cardiorespiratory disease. In the ECLIPSE study, 26% of 2118 patients with COPD suffered from depression Citation[23]. A higher prevalence of depression has been observed in females, current smokers and those with severe airflow obstruction Citation[23]. Anxiety, panic and depression may enhance or magnify the individual’s experience of dyspnea, and the patient’s report of his/her breathing discomfort may seem out of proportion when considered with the severity of physiological impairment.
Neuroimaging
Neuroimaging studies have suggested that dyspnea is processed in cortical and limbic structures of the brain Citation[2]. In particular, the anterior insula cortex, anterior cingulate cortex, amygdala and thalamus are activated on PET and functional MRI in response to different respiratory stimuli as used in experimental studies involving healthy individuals Citation[6,8,24–26]. von Leupoldt et al. investigated the affective dimension (i.e., unpleasantness) of dyspnea in 14 healthy subjects in whom dyspnea was induced by inspiratory resistance loading and concomitant positive and negative emotional stimuli were provided Citation[8]. Their results suggested that unpleasantness of perceived dyspnea was processed in the right anterior insula and amygdala structures Citation[8].
Peiffer et al. used PET imaging to study brain activity with the onset of acute dyspnea induced by resistive load breathing in ten healthy male subjects and again after its relief Citation[26]. Dyspnea relief involved characteristic activity in areas of the brain that were distinct from those subserving the development of dyspnea. These investigators suggested that activation of different areas of brain may reflect a neural modulation network for dyspnea perception Citation[7].
Neuromodulation
Neuromodulation is the process in which neurotransmitters are secreted by discrete groups of neurons and diffuse through large areas of the nervous system to produce cellular (e.g., control of breathing) and perceptual (e.g., pain and dyspnea) effects. Using naloxone to block opioid receptor signaling, investigators have shown that endogenous opioids (e.g., β-endorphin) affect the perception of both intensity and unpleasantness of dyspnea Citation[27–29]. Bellofiore et al. reported that dyspnea intensity ratings were significantly lower with placebo compared with naloxone in six patients with asthma who inhaled methacholine to constrict airways (3.4 ± 0.8 vs 5.1 ± 0.9; p < 0.05) Citation[27]. Mahler et al. showed that the regression slope of breathlessness (intensity) and oxygen consumption was significantly lower with normal saline than naloxone in 17 patients with COPD during treadmill exercise (2.51 ± 2.10 vs 3.34 ± 1.93; p = 0.02) Citation[28]. Gifford et al. found that both ratings of the intensity (77 ± 21 vs 83 ± 18 mm; p = 0.0004) and unpleasantness (77 ± 19 vs 81 ± 20 mm; p = 0.024) of breathlessness were lower with normal saline than naloxone during inspiratory load breathing in 14 patients with COPD Citation[29].
At the present time, endogenous opioids are the only class of neurotransmitters that have been shown to modulate the perception of breathlessness. However, based on our current knowledge of other sensory experiences such as pain, it is likely that other neuropeptides contribute to and/or modulate dyspnea.
Refractory dyspnea
A general strategy for the treatment of dyspnea is displayed in . When dyspnea persists despite standard therapies for the underlying disease, it is considered to be refractory. The word ‘refractory’ has been defined as unmanageable or resistant to treatment or cure. In one review, the overall prevalence of breathlessness was greater than 90% in those with advanced COPD and greater than 60% in those with advanced heart disease Citation[30]. About 94% of patients with chronic lung disease reported the presence of dyspnea in the last year of life Citation[31]. The development of refractory dyspnea usually occurs along a continuum of progressive lung (e.g., COPD; idiopathic pulmonary fibrosis) and heart (left ventricular failure) disease until the end of a person’s life. As noted earlier, anxiety, panic and depression are common comorbidities in patients who experience refractory dyspnea, and these conditions can clearly affect the patient’s breathing difficulty.
Pharmacology of opioids
Opioids are classified as natural opiates contained in the resin of the opium poppy (morphine and codeine), esters of morphine (morphine diacetate and heroin), semisynthetic opioids (hydromorphone and oxycodone) and fully synthetic (fentanyl, methadone, tramadol and dextropropoxyphene). Opioids bind to one or more of the three principal opioid receptor types named µ, δ and κ Citation[32]. The anatomic locations of opioid receptors are provided in Box 1. The pharmacodynamic response to an opioid depends upon the receptor to which it binds and its affinity for that receptor. Morphine and related opioids bind preferentially to the µ receptor, as do endogenous opioids such as β-endorphin Citation[33]. There are variants of the µ receptor due to individual genetic factors, and different opioids have varying potencies based on the µ opioid receptor subtypes.
Both exogenous as well as endogenous opioids relieve pain by binding to µ receptors in the peripheral and central nervous systems. For example, opioids reduce transmission of pain signals to the CNS by directly inhibiting the release of nociceptive substances (e.g., substance P) at peripheral sensory neurons and decrease the central processing of pain Citation[34,35]. Secondary pharmacological effects may include alterations in mood, drowsiness, euphoria, confusion, peripheral vasodilation, constipation, nausea/vomiting and cough suppression.
Opioids are effective in relieving dyspnea by one or more different mechanisms Citation[1,36]: decreasing respiratory drive (associated decreased corollary discharge); altering central perception; altering activity of peripheral opioid receptors located in the lung and decreasing anxiety.
Opioids depress respiratory drive by a direct effect on the responsiveness of brainstem respiratory centers to hypoxia and hypercapnia Citation[37]. This effect is directly proportional to the dose of opioid drug and its analgesic potency Citation[38]. With the decrease in respiratory output, there is a presumed corresponding decrease in corollary discharge from the brainstem to perceptual areas in the cerebral cortex.
Results of neuroimaging studies demonstrate that µ opioid receptor agonists can modulate the central processing of dyspnea similar to pain relief. As examples: intramuscular hydromorphone increased regional cerebral blood flow in the anterior cingulate cortex, amygdala and thalamus Citation[39]; intravenous remifentanyl was associated with increased activity in the anterior cingulate cortex, thalamus and brain stem Citation[40–42] and intravenous fentanyl was associated with increases in cerebral blood flow consistent with regional neuronal activation in the anterior cingulate, orbitofrontal and prefrontal cortices of the brain compared with saline infusion Citation[43]. These areas of the brain are activated when dyspnea is provoked in healthy individuals in laboratory investigations Citation[2].
A third possible mechanism involves peripheral opioid receptors located predominantly in bronchioles and alveolar walls of the respiratory tract Citation[44]. Approximately 50–80% of the analgesic effect of systemically administered opioids can be mediated by peripheral opioid receptors Citation[34]. It is possible, although unproven, that opioids may modulate breathing difficulty by a putative effect of binding to peripheral opioid receptors in the respiratory tract. However, Mahler et al. found that an increase in blood levels of β-endorphin, an endogenous opioid, by pharmacological manipulation did not alter ratings of the intensity or unpleasantness of dyspnea during resistive load breathing in patients with COPD Citation[45]. These results suggest that the modulatory effect of endogenous opioids, and presumably exogenous opioids, occurs within the CNS Citation[45].
Another consideration is that opioids may improve dyspnea by reducing the associated anxiety that develops in response to the distress of breathing difficulty Citation[46,47]. Using the multidimensional dyspnea profile, Banzett et al. showed that intravenous morphine reduced both air hunger and anxiety ratings associated with breathlessness induced by breathing carbon dioxide during restricted ventilation in six healthy individuals Citation[46]. These laboratory findings are consistent with the proposal of Horton and Rocker that exogenous opioids may be more effective in relieving the affective dimension, especially anxiety and fear that contribute to breathlessness, rather than intensity Citation[48]. However, Gifford et al. did not find any difference in intensity and unpleasant ratings by patients with COPD when the effect of endogenous opioids was blocked with naloxone Citation[29]. Oxymorphone, which is only available for parental administration, is approved by the US FDA for treatment of ‘relief of anxiety in patients with dyspnea associated with pulmonary edema secondary to acute left ventricular dysfunction’ according to Drug Facts and Comparisons 2012 Citation[49].
Results of RCTs
Opioids have been used since the late 19th century to relieve breathlessness in patients with respiratory disease Citation[50–52]. The first RCT was published in 1981 when Woodcock et al. demonstrated a 20% reduction in dyspnea during treadmill exercise with dihydrocodeine (1 mg/kg) in 12 patients with COPD and normal arterial blood gases Citation[53].
In 2002, Jennings et al. published a systematic review of 18 RCTs that compared opioids with placebo for the treatment of dyspnea secondary to any cause Citation[36]. In the nine trials in which patients received oral opioids (n = 8) or subcutaneous morphine (n = 1), there was a significant beneficial effect with parenteral opioids on reducing dyspnea compared with placebo (mean D: -0.40; CI: -0.32 to -0.17) Citation[36]. However, in eight of these nine studies, exercise was used as a stimulus to provoke dyspnea, whereas only one study examined patients who were short of breath at rest and might be considered to have refractory dyspnea Citation[36]. In a study by Johnson et al., the mean daily score of breathlessness recorded at home by patients with emphysema was 7–10 mm lower on a 100-mm Visual Analog Scale (VAS) with 15 mg of dihydrocodeine compared with placebo Citation[54]. Jennings et al. summarized various limitations in the study designs of the 18 trials that were reviewed: opioid doses were relatively small; doses were not titrated in any studies and dosing intervals were too long in certain trials Citation[36].
In 2003, Abernethy et al. published a randomized, double-blind, placebo-controlled crossover trial that compared 4 days of 20-mg oral sustained-release morphine with 4 days of oral placebo Citation[55]. Thirty eight participants who were opioid naive and had dyspnea at rest in spite of optimal therapy for their underlying condition (mainly patients with COPD) completed the trial. Patients experienced significant improvements (i.e., less dyspnea) by 6.6 mm on a VAS (CI: 1.6–11.6 mm; p = 0.011) in the morning and by 9.5 mm on a VAS (CI: 3.0–16.1 mm; p = 0.006) in the evening Citation[55]. More patients reported constipation while taking morphine despite using laxatives. The investigators concluded that “sustained release, oral morphine at low dosage provides significant symptomatic improvement in refractory dyspnea in the community setting” Citation[55].
In a pilot study, Johnson et al. found a significant decrease in median breathlessness reported by ten patients with advanced heart failure after receiving 5-mg oral morphine four-times daily for 4 days, whereas there was no change with placebo Citation[56]. However, the investigators did not report statistical comparison between the two treatments Citation[56]. Subsequently, Oxberry et al. compared the effects of 5-mg morphine four-times daily, 2.5-mg oxycodone four-times daily and placebo, all administered as liquid medications for 4 days each with 3-day washout between treatments in 35 patients with advanced congestive heart failure Citation[57]. The investigators found no significant differences between opioid treatments and placebo for the change in breathlessness severity from baseline to day 4 with the treatments Citation[57]. The authors commented that their study “may have been underpowered” and also proposed that the study may have missed a “delayed benefit” Citation[57]. Another possibility was that the doses of morphine and oxycodone were suboptimal for the patient population. In a meta-analysis of patients with cancer-related dyspnea, Ben-Aharon et al. showed that opioids had a positive effect in reducing dyspnea (weighted mean difference: -1.31 [95% CI: -2.49 to -0.13]) Citation[58].
Nebulized opioids have been considered based on the rationale that inhaled medications would bind to opioid receptors in the respiratory tract to relieve dyspnea with minimal or no side effects Citation[1]. In their review, Jennings et al. found that appropriate outcomes were available in only three out of nine studies that examined nebulized opioids Citation[36]. The meta-analysis of these three RCTs showed no significant improvement in breathlessness ratings with nebulized opioids compared with normal saline (mean Δ: -0.11; CI: -0.32 to + 0.10) Citation[36]. Two subsequent RCTs reported conflicting results. Jensen et al. found no differences in the intensity or unpleasantness of perceived dyspnea during cycle ergometry with a single dose of nebulized fentanyl citrate (50 µg) compared with saline in patients with COPD Citation[59]. In contrast, Shohrati et al. reported that 1 mg of nebulized morphine given daily for 5 days provided a significant reduction in patient-reported dyspnea on a VAS compared with nebulized normal saline in 40 patients who were thought to develop COPD in part due to inhaling sulfur mustard during military conflict Citation[60].
Recommendations for opioid use
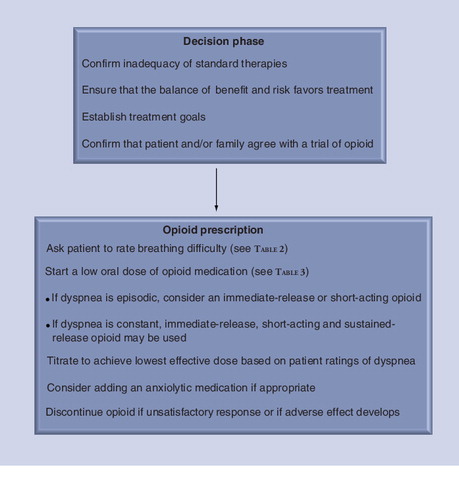
The American Thoracic Society has described a continuum model for management of symptoms, including dyspnea, during any stage of illness, whether chronic or end of life Citation[61]. The recommended approach is individualized and integrated while incorporating concurrent curative–restorative and palliative care Citation[61]. The possible use of opioid therapy should be discussed with the patient and family members as the chronic disease progresses and breathing difficulty becomes distressful and disabling. As such, the healthcare provider can inform the patient that there is available treatment for her/his refractory dyspnea and offer initial treatment prior to end-of-life care. Oxberry et al. found that patients with congestive heart failure were willing to consider morphine for refractory dyspnea if recommended by a trusted physician Citation[62]. presents a suggested approach for prescribing an opioid medication for relief of refractory dyspnea.
Recent statements and guidelines emphasize the obligation of physicians and nurses to use opioids to relieve refractory dyspnea Citation[1,61,63,64]. One barrier to their use is the concern that opioids may cause respiratory depression that might hasten death. However, results of clinical studies do not support this consideration. In different prospective, but nonrandomized studies, Clemens et al. found significant decreases in the intensity of dyspnea and respiratory rate with initial opioid treatment in patients receiving palliative care, but no evidence of respiratory depression Citation[65,66]. Chan et al. found that higher doses of opioids and anxiolytic medications used in withdrawal of life support were not associated with a decreased time from withdrawal of life support to death Citation[67]. Other studies found that survival time after withdrawal of mechanical ventilation in the last week of life was unrelated to the dose of prescribed morphine Citation[68–70]. Of numerous studies that provided information on oxygen saturation or arterial blood gases, only one investigation reported any changes in oxygenation with use of opioids Citation[1]. Although the arterial carbon dioxide partial pressure may increase with the use of opioids, the arterial carbon dioxide partial pressurevalue did not exceed 40 mmHg Citation[71].
These results are reassuring for physicians and other healthcare providers, particularly if the dose of opioid is titrated based on balancing the benefit – dyspnea relief – with possible side effects in individual patients. In addition to the concern of respiratory depression, other possible adverse effects with opioids include confusion, drowsiness, nausea/vomiting, constipation and hallucinations. The principle of double effect addresses the potential for causing harm with the use of opioids in palliative care. This principle provides justification for using opioids, as well as anxiolytics, that might hasten death as long as the purpose of administering opioids is to relieve distressful breathing difficulty Citation[61]. In a survey of 28 physicians, Rocker et al. reported that “most physicians were reluctant to prescribe opioids for refractory dyspnea because of lack of related knowledge and experience, and fears related to potential adverse effects and legal censure” Citation[72].
Another barrier for the use of opioids is that patients and family members may have different perspectives about who should be involved in the decision of treating refractory dyspnea, the importance of maintaining consciousness for as long as possible, and any perceived value of suffering. Conflict may arise on treating refractory dyspnea with opioids among physicians, patients and family members who have different cultural perspectives. Open communication and frequent discussions about these issues are important and essential. In a survey of patients and their caregivers, patients reported that opioids provided a sense of calm and relief from severe breathing difficulty, whereas family members observed improvements in patients’ symptoms of anxiety and depression while also experiencing reductions in their own stress Citation[72].
A reasonable approach is to offer/recommend a low dose of an opioid to a patient who experiences refractory dyspnea and to then titrate the dose based on his/her response. The goal would be to use the lowest effective dose to relieve breathing difficulty. If the patient agrees to a trial at home, in the clinic or in the hospital, treatment should focus on both psychological and physical components of dyspnea Citation[61]. The patient may be asked to rate the severity of ‘total dyspnea’ and/or one or more domains of dyspnea using available scales (Box 2) Citation[1,2,73]. The intensity domain refers to the level or magnitude of breathlessness (‘how intense or strong is your breathlessness?’), which is typically rated on single-item scales such as the VAS, Numerical Rating Scale or the 0–10 Category-Ratio (Borg) Scale Citation[2]. The affective domain refers to the distress and unpleasantness of breathlessness (‘how unpleasant or uncomfortable is your breathlessness?’), which may be rated on single- or multiple-item scales Citation[2]. The third domain of dyspnea measurement is symptom impact or burden which assesses how breathing difficulty affects behavior and functional performance Citation[2]. To evaluate the efficacy of therapy on the impact of dyspnea in ambulatory patients, multidimensional instruments (e.g., the self-administered computerized transition dyspnea index) are more responsive than unidimensional rating scales (e.g., Medical Research Council scale) Citation[74]. Patients should be asked to routinely and regularly rate their breathlessness as part of a comprehensive care plan, and this information should be documented in the medical record Citation[1,75].
Oral administration of an opioid is appropriate initially for most patients. Morphine sulfate is the principal alkaloid of opium and has been used frequently for the treatment of refractory dyspnea. Hydromorphone and oxycodone have also been recommended for the treatment of refractory dyspnea Citation[48,61]. Clearly, the use of opioids for treatment of refractory dyspnea should be individualized. Rocker et al. proposed that patients will most likely respond favorably to opioids if the onset of dyspnea is acute, if it is strongly associated with negative emotions such as fear and anxiety, and if the patient has a sense of loss of control even when reducing physical activities Citation[76]. In a retrospective analysis of pooled data from four studies, Johnson et al. found that younger age and worse baseline breathlessness intensity were predictors of a beneficial response for refractory dyspnea with morphine or oxycodone therapy, whereas disease group or functional status did not predict improvement Citation[77].
Proposed doses and frequency of administration for treatment of opioid-naive patients with refractory dyspnea are listed in . The variability in an individual patient’s response underscores the need to start with a low dose and titrate subsequent doses based on whether dyspnea relief has been achieved and whether any side effects have developed. Both the pharmacology of the opioid (immediate release [IR], short-acting and sustained release) and starting dose will depend on the frequency (episodic, daily and/or constant) and the severity of dyspnea. Rocker et al. reported that patients in Canada and in the UK are usually started on oral IR morphine with doses described in Citation[76]. IR morphine has an onset of action of approximately 30 min and duration of approximately 4 h. The American Thoracic Society recommended that the same approach can be used to treat “moderate-to-severe pain or dyspnea”, starting with an initial dose of 5–10 mg of short-acting morphine Citation[61]. Once a stable dose of IR or short-acting opioid has been achieved, it is appropriate to switch to a sustained-release preparation at a comparable daily dose. Long-acting or sustained-release opioids have an onset of approximately 3–4 h and duration of approximately 8–12 h. A third approach is to start with a sustained-release opioid as used by Abernethy et al. in their RCT, in which patients took either 20-mg oral sustained-release morphine or placebo for 4 days Citation[55]. Currow et al. reported that 52 out of 83 patients with chronic refractory dyspnea achieved at least 10% improvement in ratings of dyspnea on a VAS with 10-mg daily dose of sustained-release morphine (62% response rate) Citation[78]. However, this benefit was maintained at 3 months in only 28 of the 83 patients (33%) Citation[78]. The different approaches shown in reflect unique experiences in different countries and provide options for prescribing opioids to treat refractory dyspnea.
Other methods of opioid delivery are also available. As previously noted, analyses and reviews of published RCTs have concluded that nebulized opioids are not effective for relief of dyspnea Citation[1,36,63]. For patients who cannot swallow or in whom oral opioids are ineffective, transmucosal, transdermal, subcutaneous or intravenous routes of administration of opioids may be used to treat persistent dyspnea. Continuous intravenous infusion of an opioid can be used for end of life care Citation[79]. Anxiolytic medications are often prescribed along with opioids as combined modalities to palliate patients who experience refractory dyspnea Citation[80].
Expert commentary
The opioid neurotransmitter system is complex and involves various physiological activities in the body. Over the past decade, there has been increased acceptance to prescribe opioids to relieve refractory dyspnea in patients with advanced lung and heart disease. Published guidelines and statements of major medical organizations support the approach that “opioids be dosed and titrated for relief of dyspnea in the individual patient” Citation[1,61,63,64]. Physicians and other healthcare providers can suggest or recommend a trial of an oral opioid to a patient who reports distressing and disabling breathlessness that interferes with her/his daily activities and quality of life. Open communication with patients and family members about expected benefits and possible side effects is crucial. Certainly, not all patients will accept an opioid trial for different reasons. Nonetheless, it is important to reassure patients that such therapy is available if and when the patient is ready, and that it is not necessary to wait for end of life care.
Five-year view
Clinical and basic research studies are needed to expand our knowledge and understanding of the role of opioids in the treatment of refractory dyspnea. Over the next 5 years, it will be important not only to address clinical uncertainties, but also to identify genetic factors that influence the effectiveness of opioids for relief of dyspnea in symptomatic individuals.
Affective dimension of dyspnea
Previous studies reported the benefits of opioids on the intensity dimension of dyspnea in patients with respiratory disease Citation[36,55]. In future clinical trials involving patients, it will be important to investigate whether opioids modulate unpleasantness/anxiety/fear components of dyspnea. In addition, by combining data obtained from functional MRI with perceptual ratings of dyspnea in symptomatic patients, it may be possible to demonstrate the neuropathways involved with opioid therapy. Although such studies are challenging to perform, the information obtained will enhance our understanding and clinical application of using opioids to relieve refractory dyspnea in patients.
Psychological factors
Psychological factors clearly affect a patient’s perception of dyspnea. Studies on the impact of specific psychological traits, particularly anxiety, panic and depression, on neural processing and behavioral responses are important, particularly as they relate to opioid efficacy. Measuring psychological outcomes along with breathlessness ratings can provide a comprehensive assessment of the overall effect of opioids in patients with refractory dyspnea.
Other clinical uncertainties
There is considerable variability in the efficacy of opioids to relieve pain and dyspnea among individuals. Important gaps remain in our knowledge about clinical factors that affect the current use of opioids to treat patients with refractory dyspnea Citation[81]. Clinical uncertainties include:
• Are there subgroups of patients in whom opioids are more beneficial?
• What are the relative effects of IR versus long-acting opioid medications to manage refractory dyspnea?
• Do opioids relieve breathlessness solely, or predominantly, by activity within the CNS, or do peripheral opioid receptors play a role?
• What are the current barriers for prescribing opioids to treat patients with refractory dyspnea?
Endogenous opioids
Endogenous opioids are natural substances produced in the body that act as neuromodulators to alter the perception of both breathlessness and pain as well as produce a feeling of euphoria. In addition, these endogenous neurotransmitters do not generate adverse effects commonly associated with opioid medications. Studies have shown that listening to soothing music, laughing and crying, eating chocolate, taking capsaicin (found in chili peppers) and receiving acupuncture (acu) can stimulate the release of endogenous opioids Citation[101].
It is interesting to consider whether these and/or other interventions that promote release of endogenous opioids would be useful clinically to relieve refractory dyspnea. For example, Ngai et al. compared transcutaneous electrical nerve stimulation (TENS) over acu sites (n = 22) versus placebo-TENS (n = 22) for 45 min in patients with COPD Citation[82] . With acu-TENS, patients reported significant decreases in ratings of dyspnea (mean Δ = -21%) and exhibited significant increases in both forced expiratory volume in 1 s (mean Δ = +24%) and β-endorphin levels (mean Δ = +18%) compared with placebo-TENS Citation[82].
Opioid pharmacology
Genetic factors regulate pharmacokinetics (metabolizing enzymes and transporters), and pharmacodynamics (receptors and signal transduction) contribute to individual variability for pain relief. However, similar data are not currently available for dyspnea Citation[83]. The µ-opioid receptor gene generates multiple receptor subtypes that may explain variable clinical effects of opioids among patients Citation[84]. These receptor variants are widely distributed throughout the nervous system including areas of the brain and spinal cord involved in pain processing Citation[84]. The most common single-nucleotide polymorphism is A118G. Current evidence suggests that the A118G variant results in reduced opioid effect (e.g., miosis and response to experimental pain) and consequently leads to increased opioid dosage requirements for analgesia Citation[83]. Whether the A118G variant and/or other polymorphisms affect dyspnea relief is unknown.
Polymorphisms impact the expression and function of a number of drug metabolizing enzymes, and thereby influence the overall clinical opioid response. Opioid drugs are metabolized by the CYP system which can affect receptor site concentrations. The CYP2D6 gene is highly polymorphic (~100 alleles) and of clinical interest because some of the variants are ‘poor metabolizers’ and unable to metabolize weaker opioids into their more potent metabolites (e.g., codeine → morphine) Citation[83]. Differences in codeine metabolism have been shown to impact patient-controlled analgesia, such that patients who are poor metabolizers receive more frequent codeine dosing because of inadequate pain relief Citation[85].
Drug transporters are structural proteins that influence the absorption, distribution and elimination of opioids. For example, transporters can influence the bioavailability of orally administered opioids via intestinal absorption, and expression at the blood–brain barrier has the potential to facilitate transfer into the CNS Citation[83]. Opioid substrates are transported by P-glycoprotein which has substantial variability in expression and function among individuals. However, the role of genetic polymorphisms in transporters on opioid efficacy is unclear Citation[83].
Table 1. Various stimuli that activate receptors to cause dyspnea.
Table 2. Proposed doses of morphine for treatment of refractory dyspnea in opioid-naive patients.
Box 1. Anatomic location of opioid receptors.
• Inflammatory cells
• Peripheral nervous system
– Dorsal-root ganglia
– Central terminals of primary afferent neurons
– Peripheral sensory-nerve fibers and their terminals
– Respiratory tract: highest density in bronchioles and alveolar walls
– Myocardial cells
• CNS
– Midbrain, brain stem, thalamus and hypothalamus
– Limbic system
– Cerebral cortex
Box 2. Scales for patients to rate or quantify their breathing difficulty to assess the efficacy of opioid therapy†.
• Visual Analog Scale: a 100 mm horizontal or vertical line with descriptors such as no breathlessness anchored at 0 mm and greatest breathlessness anchored at 100 mm. The patient places a mark on the line that matches his/her breathlessness at the present time or over a specific time period (e.g., past 12 h) Citation[87,88].
• Numerical Rating Scale: a scale with 11 numbers ranging from 0 (no shortness of breath) to 10 (shortness of breath as bad as can be). These descriptors were proposed by Gift et al. Citation[89]. The patient selects (circles or marks) a number that matches his/her breathlessness at the present time or over a specific time period (e.g., past 12 h). The descriptor for number 10 should be modified if the Numerical Rating Scale is used for patients to rate unpleasantness.
• 0–10 category-ratio (Borg) scale: a scale that ranges from 0 (nothing at all) to 10 (very, very severe [almost maximal]) and incorporates nonlinear spacing of verbal descriptions of severity which correspond to specific numbers. The patient selects a number that matches his/her breathlessness at the present time or over a specific time period (e.g., past 12 h) Citation[90].
Key issues
• Refractory dyspnea is prominent in patients with advanced lung and heart disease.
• A continuum care model recommends an individualized and integrated approach for the management of refractory dyspnea.
• Randomized controlled trials demonstrate that systemic opioids are effective in relieving dyspnea. Proposed mechanisms for this effect include decreasing respiratory drive and the associated decrease in corollary discharge, altering central perception, altering peripheral opioid receptor activity in the lung and/or decreasing anxiety.
• Physicians and other healthcare providers should offer a trial of an oral opioid to a patient who experiences refractory breathlessness that affects his/her daily activities and quality of life.
• Barriers for the use of opioids include concern about side effects, particularly respiratory depression, and possibly different perspectives among patients and their family members.
• As individual patients exhibit variable responses to opioid therapy, a low dose should be prescribed initially.
• Subsequent doses of opioids should be titrated to achieve the lowest effective dose, based on assessment of whether dyspnea relief has been achieved by patient ratings and the possible development of side effects.
• There are important gaps in our knowledge about clinical and genetic factors that impact our understanding and use of opioids to treat patients who experience refractory dyspnea.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Mahler DA, Selecky PA, Harrod CG et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 137(3), 674–691 (2010).
- Parshall MB, Schwartzstein RM, Adams L et al.; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 185(4), 435–452 (2012).
- O’Donnell DE, Webb KA. Mechanisms of dyspnea in COPD. In: Dyspnea Mechanisms, Measurement, and Management (Volume 208). Mahler DA, O’Donnell DE (Eds). Taylor & Francis, FL, USA, 29–58 (2005).
- Mahler DA. Mechanisms and measurement of dyspnea in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 3(3), 234–238 (2006).
- von Leupoldt A, Dahme B. Cortical substrates for the perception of dyspnea. Chest 128(1), 345–354 (2005).
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neurobiol. 167(1), 72–86 (2009).
- Peiffer C, Costes N, Hervé P, Garcia-Larrea L. Relief of dyspnea involves a characteristic brain activation and a specific quality of sensation. Am. J. Respir. Crit. Care Med. 177(4), 440–449 (2008).
- von Leupoldt A, Sommer T, Kegat S et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am. J. Respir. Crit. Care Med. 177(9), 1026–1032 (2008).
- O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am. Rev. Respir. Dis. 148(5), 1351–1357 (1993).
- Banzett RB, Lansing RW, Reid MB, Adams L, Brown R. ‘Air hunger’ arising from increased PCO2 in mechanically ventilated quadriplegics. Respir. Physiol. 76(1), 53–67 (1989).
- Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir. Physiol. Neurobiol. 167(1), 53–60 (2009).
- Mahler DA, Harver A, Lentine T, Scott JA, Beck K, Schwartzstein RM. Descriptors of breathlessness in cardiorespiratory diseases. Am. J. Respir. Crit. Care Med. 154(5), 1357–1363 (1996).
- O’Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am. J. Respir. Crit. Care Med. 155(1), 109–115 (1997).
- Harver A, Mahler DA, Schwartzstein RM, Baird JC. Descriptors of breathlessness in healthy individuals: distinct and separable constructs. Chest 118(3), 679–690 (2000).
- Williams M, Cafarella P, Olds T, Petkov J, Frith P. The language of breathlessness differentiates between patients with COPD and age-matched adults. Chest 134(3), 489–496 (2008).
- Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am. Rev. Respir. Dis. 142(5), 1009–1014 (1990).
- Harver A, Mahler DA. Dyspnea: sensation, symptom, and illness. In: Dyspnea. Mahler DA (Ed.). Marcel Dekker, Inc., NY, USA, 1–34 (1998).
- Eisner MD, Blanc PD, Yelin EH et al. Influence of anxiety on health outcomes in COPD. Thorax 65(3), 229–234 (2010).
- Kvaal K, Macijauskiene J, Engedal K, Laake K. High prevalence of anxiety symptoms in hospitalized geriatric patients. Int. J. Geriatr. Psychiatry 16(7), 690–693 (2001).
- Smoller JW, Pollack MH, Otto MW, Rosenbaum JF, Kradin RL. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am. J. Respir. Crit. Care Med. 154(1), 6–17 (1996).
- von Leupoldt A, Dahme B. Psychological aspects in the perception of dyspnea in obstructive pulmonary diseases. Respir. Med. 101(3), 411–422 (2007).
- Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur. Respir. J. 31(3), 667–677 (2008).
- Hanania NA, Müllerova H, Locantore NW et al.; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am. J. Respir. Crit. Care Med. 183(5), 604–611 (2011).
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport 11(10), 2117–2120 (2000).
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J. Neurophysiol. 88(3), 1500–1511 (2002).
- Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. Am. J. Respir. Crit. Care Med. 163(4), 951–957 (2001).
- Bellofiore S, Di Maria GU, Privitera S, Sapienza S, Milic-Emili J, Mistretta A. Endogenous opioids modulate the increase in ventilatory output and dyspnea during severe acute bronchoconstriction. Am. Rev. Respir. Dis. 142(4), 812–816 (1990).
- Mahler DA, Murray JA, Waterman LA et al. Endogenous opioids modify dyspnoea during treadmill exercise in patients with COPD. Eur. Respir. J. 33(4), 771–777 (2009).
- Gifford AH, Mahler DA, Waterman LA et al. Neuromodulatory effect of endogenous opioids on the intensity and unpleasantness of breathlessness during resistive load breathing in COPD. COPD 8(3), 160–166 (2011).
- Bausewein C, Farquhar M, Booth S, Gysels M, Higginson IJ. Measurement of breathlessness in advanced disease: a systematic review. Respir. Med. 101(3), 399–410 (2007).
- Edmonds P, Karlsen S, Khan S, Addington-Hall J. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat. Med. 15(4), 287–295 (2001).
- Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr. Drug Targets 13(2), 230–246 (2012).
- Hughes J, Kosterlitz HW. Opioid Peptides: introduction. Br. Med. Bull. 39(1), 1–3 (1983).
- Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr. Opin. Pharmacol. 9(1), 3–8 (2009).
- Millan MJ. Multiple opioid systems and pain. Pain 27(3), 303–347 (1986).
- Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax 57(11), 939–944 (2002).
- Weil JV, McCullough RE, Kline JS, Sodal IE. Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N. Engl. J. Med. 292(21), 1103–1106 (1975).
- Eckenhoff JE, Oech SR. The effects of narcotics and antagonists upon respiration and circulation in man. A review. Clin. Pharmacol. Ther. 1, 483–524 (1960).
- Schlaepfer TE, Strain EC, Greenberg BD et al. Site of opioid action in the human brain: µ and κ agonists’ subjective and cerebral blood flow effects. Am. J. Psychiatry 155(4), 470–473 (1998).
- Leppä M, Korvenoja A, Carlson S et al. Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage 31(2), 661–669 (2006).
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia – imaging a shared neuronal network. Science 295(5560), 1737–1740 (2002).
- Pattinson KT, Governo RJ, MacIntosh BJ et al. Opioids depress cortical centers responsible for the volitional control of respiration. J. Neurosci. 29(25), 8177–8186 (2009).
- Firestone LL, Gyulai F, Mintun M, Adler LJ, Urso K, Winter PM. Human brain activity response to fentanyl imaged by positron emission tomography. Anesth. Analg. 82(6), 1247–1251 (1996).
- Zebraski SE, Kochenash SM, Raffa RB. Lung opioid receptors: pharmacology and possible target for nebulized morphine in dyspnea. Life Sci. 66(23), 2221–2231 (2000).
- Mahler DA, Gifford AH, Waterman LA et al. Effect of increased blood levels of beta-endorphin on perception of breathlessness. Chest doi:10.1378/chest.12-1541 (2012) (Epub ahead of print).
- Banzett RB, Adams L, O’Donnell CR, Gilman SA, Lansing RW, Schwartzstein RM. Using laboratory models to test treatment: morphine reduces dyspnea and hypercapnic ventilatory response. Am. J. Respir. Crit. Care Med. 184(8), 920–927 (2011).
- Banzett RB, Moosavi SH. Dyspnea and pain: similarities and contrasts between two very unpleasant sensations. APS Bulletin 11, 1–8 (2001).
- Horton R, Rocker G. Contemporary issues in refractory dyspnoea in advanced chronic obstructive pulmonary disease. Curr. Opin. Support. Palliat. Care 4(2), 56–62 (2010).
- Williams AL. Drug Facts and Comparisons. Wolters Kluwer Health, MI, USA, 1315–1317 (2012).
- Powell DR, Hartley PH. Diseases of the Lungs and Pleurae. HK Lewis, London, UK (1911).
- Reigl F. Diseases of the respiratory organs. In: Cyclopedia of Medical Practice (Volume 4). Von Ziemsen H (Ed.). Samson Law, London, UK, 523–586 (1876).
- West S. Diseases of the Organs of Respiration (Volume 1). Charles Griffin, London, UK (1909).
- Woodcock AA, Gross ER, Gellert A, Shah S, Johnson M, Geddes DM. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. N. Engl. J. Med. 305(27), 1611–1616 (1981).
- Johnson MA, Woodcock AA, Geddes DM. Dihydrocodeine for breathlessness in ‘pink puffers’. Br. Med. J. (Clin. Res. Ed). 286(6366), 675–677 (1983).
- Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 327(7414), 523–528 (2003).
- Johnson MJ, McDonagh TA, Harkness A, McKay SE, Dargie HJ. Morphine for the relief of breathlessness in patients with chronic heart failure – a pilot study. Eur. J. Heart Fail. 4(6), 753–756 (2002).
- Oxberry SG, Torgerson DJ, Bland JM, Clark AL, Cleland JG, Johnson MJ. Short-term opioids for breathlessness in stable chronic heart failure: a randomized controlled trial. Eur. J. Heart Fail. 13(9), 1006–1012 (2011).
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol. 51(8), 996–1008 (2012).
- Jensen D, Alsuhail A, Viola R, Dudgeon DJ, Webb KA, O’Donnell DE. Inhaled fentanyl citrate improves exercise endurance during high-intensity constant work rate cycle exercise in chronic obstructive pulmonary disease. J. Pain Symptom Manage. 43(4), 706–719 (2012).
- Shohrati M, Ghanei M, Harandi AA, Foroghi S, Harandi AA. Effect of nebulized morphine on dyspnea of mustard gas-exposed patients: a double-blind randomized clinical trial study. Pulm. Med. 2012, 610921 (2012).
- Lanken PN, Terry PB, Delisser HM et al.; ATS End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am. J. Respir. Crit. Care Med. 177(8), 912–927 (2008).
- Oxberry SG, Jones L, Clark AL, Johnson MJ. Attitudes to morphine in chronic heart failure patients. Postgrad. Med. J. 88(1043), 515–521 (2012).
- Marciniuk DD, Goodridge D, Hernandez P et al.; Canadian Thoracic Society COPD Committee Dyspnea Expert Working Group. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can. Respir. J. 18(2), 69–78 (2011).
- Qaseem A, Wilt TJ, Weinberger SE et al.; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann. Intern. Med. 155(3), 179–191 (2011).
- Clemens KE, Klaschik E. Symptomatic therapy of dyspnea with strong opioids and its effect on ventilation in palliative care patients. J. Pain Symptom Manage. 33(4), 473–481 (2007).
- Clemens KE, Quednau I, Klaschik E. Is there a higher risk of respiratory depression in opioid-naive palliative care patients during symptomatic therapy of dyspnea with strong opioids? J. Palliat. Med. 11(2), 204–216 (2008).
- Chan JD, Treece PD, Engelberg RA et al. Narcotic and benzodiazepine use after withdrawal of life support: association with time to death? Chest 126(1), 286–293 (2004).
- Daly BJ, Thomas D, Dyer MA. Procedures used in withdrawal of mechanical ventilation. Am. J. Crit. Care 5(5), 331–338 (1996).
- Stone P, Phillips C, Spruyt O, Waight C. A comparison of the use of sedatives in a hospital support team and in a hospice. Palliat. Med. 11(2), 140–144 (1997).
- Thorns A, Sykes N. Opioid use in last week of life and implications for end-of-life decision-making. Lancet 356(9227), 398–399 (2000).
- Bar-Or D, Marx JA, Good J. Breathlessness, alcohol, and opiates. N. Engl. J. Med. 306(22), 1363–1364 (1982).
- Rocker G, Young J, Donahue M, Farquhar M, Simpson C. Perspectives of patients, family caregivers and physicians about the use of opioids for refractory dyspnea in advanced chronic obstructive pulmonary disease. CMAJ 184(9), E497–E504 (2012).
- Horton R, Rocker G, Currow D. The dyspnea target: can we zero in on opioid responsiveness in advanced chronic obstructive pulmonary disease? Curr. Opin. Support. Palliat. Care 4(2), 92–96 (2010).
- Mahler DA, Waterman LA, Ward J, McCusker C, ZuWallack R, Baird JC. Validity and responsiveness of the self-administered computerized versions of the baseline and transition dyspnea indexes. Chest 132(4), 1283–1290 (2007).
- Mularski RA, Campbell ML, Asch SM et al. A review of quality of care evaluation for the palliation of dyspnea. Am. J. Respir. Crit. Care Med. 181(6), 534–538 (2010).
- Rocker G, Horton R, Currow D, Goodridge D, Young J, Booth S. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax 64(10), 910–915 (2009).
- Johnson MJ, Bland JM, Oxberry SG, Abernethy AP, Currow DC. Opioids for chronic refractory breathlessness: patient predictors of beneficial response. Eur. Respir. J. doi:10.1183/09031936.00139812 (2012) (Epub ahead of print).
- Currow DC, McDonald C, Oaten S et al. Once-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J. Pain Symptom Manage. 42(3), 388–399 (2011).
- Cohen MH, Anderson AJ, Krasnow SH et al. Continuous intravenous infusion of morphine for severe dyspnea. South. Med. J. 84(2), 229–234 (1991).
- Gomutbutra P, O’Riordan DL, Pantilat SZ. Management of moderate-to-severe dyspnea in hospitalized patients receiving palliative care. J. Pain Symptom Manage. doi:10.1016/j.jpainsymman.2012.05.004 (2012) (Epub ahead of print).
- Johnson MJ, Abernethy AP, Currow DC. Gaps in the evidence base of opioids for refractory breathlessness. A future work plan? J. Pain Symptom Manage. 43(3), 614–624 (2012).
- Ngai SP, Jones AY, Hui-Chan CW, Yu HP. Acute effects of Acu-TENS on FEV1 and blood β-endorphin level in chronic obstructive pulmonary disease. Altern. Ther. Health Med. 17(5), 8–13 (2011).
- Somogyi AA, Barratt DT, Coller JK. Pharmacogenetics of opioids. Clin. Pharmacol. Ther. 81(3), 429–444 (2007).
- Pasternak GW. Molecular insights into µ opioid pharmacology: from the clinic to the bench. Clin. J. Pain 26(Suppl. 10), S3–S9 (2010).
- Persson K, Sjöström S, Sigurdardottir I, Molnár V, Hammarlund-Udenaes M, Rane A. Patient-controlled analgesia (PCA) with codeine for postoperative pain relief in ten extensive metabolisers and one poor metaboliser of dextromethorphan. Br. J. Clin. Pharmacol. 39(2), 182–186 (1995).
- Ventura C, Bastagli L, Bernardi P, Caldarera CM, Guarnieri C. Opioid receptors in rat cardiac sarcolemma: effect of phenylephrine and isoproterenol. Biochim. Biophys. Acta 987(1), 69–74 (1989).
- Gift AG. Visual analogue scales: measurement of subjective phenomena. Nurs. Res. 38(5), 286–288 (1989).
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 93(3), 580–586 (1988).
- Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am. J. Crit. Care 7(3), 200–204 (1998).
- Borg GA. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14(5), 377–381 (1982).
- Booth S, Bausewein C, Higginson I, Moosavi SH. Pharmacological treatment of refractory breathlessness. Expert Rev. Respir. Med. 3(1), 21–36 (2009).
- Dudgeon D. Management of dyspnea at the end of life. In: Dyspnea. Mahler DA, O’Donnell DE (Eds). Taylor & Francis, NY, USA, 429–461 (2005).
- Nici L, Donner C, Wouters E et al.; ATS/ERS Pulmonary Rehabilitation Writing Committee. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 173(12), 1390–1413 (2006).
Website
- Weir SB. How endorphins work. 2012, 1–2 (2012). http://shine.yahoo.com/healthy-living/endorphins-232500595.html
Opioids for refractory dyspnea
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertrespiratory. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
Based on the review by Dr. Mahler, which of the following statements about proposed mechanisms for the effect of systemic opioids in relieving refractory dyspnea is most likely correct?
□ A Opioids decrease respiratory drive with an associated increase in corollary discharge
□ B Opioids depress respiratory drive by a direct effect on the responsiveness of brainstem respiratory centers to hypoxia and hypercapnia
□ C Opioid effects on dyspnea are mediated primarily by peripheral opioid receptors in the lung
□ D Reducing anxiety plays no role in opioid effects on dyspnea
2. Your patient is a 78-year-old man with cardiac disease, congestive heart failure, and refractory dyspnea affecting daily activities and quality of life despite treatment with oxygen and relaxation techniques. Based on the review by Dr. Mahler, which of the following statements about management with systemic opioids is most likely correct?
□ A To date, no randomized controlled trials have shown that systemic opioids are effective in relieving dyspnea
□ B A moderate dose of opioid should be chosen initially to maximize the likelihood of dyspnea relief
□ C The opioid dose should be titrated to optimize pulmonary function test results
□ D Management of refractory dyspnea demands an individualized and integrated approach, according to the continuum care model
3. Based on the review by Dr. Mahler, which of the following statements about barriers to opioid use for management of refractory dyspnea and directions for future research would most likely be correct?
□ A Respiratory depression by opioids hastens death
□ B Possible adverse effects of opioids include confusion, drowsiness, nausea, vomiting, constipation, and/or hallucinations
□ C Differing perspectives among patients and their family members are not likely to be a barrier to opioid use for management of refractory dyspnea
□ D Clinical and genetic factors affecting opioid use to treat patients with refractory dyspnea are well understood
Notes
Data taken from Citation[32,34,35,44,86].
†These single-item scales were recommended by the American Thoracic Society for patients to rate or quantify dyspnea Citation[2].