Abstract
Plants are an attractive platform for the production of N-glycosylated subunit vaccines. Wild type glycosylation of plants can be exploited to produce vaccines that antigen-presenting cells effectively take up, degrade and present to cells of the adaptive immune system. Alternatively, glycoengineered plants can be used to produce humanized antigens. Glycoengineering also allows the construction of plants that are able to produce vaccines with custom-made N-glycan structures aiding the construction of vaccines that can be delivered to antigen-presenting cells in a target-oriented approach. The knowledge of innate immune receptors and their role in antigen uptake and presentation is rapidly increasing. In this article, aspects of plant glycosylation and immunology are reviewed and we discuss the possibilities to use this knowledge for the rational design of plant-expressed vaccines.
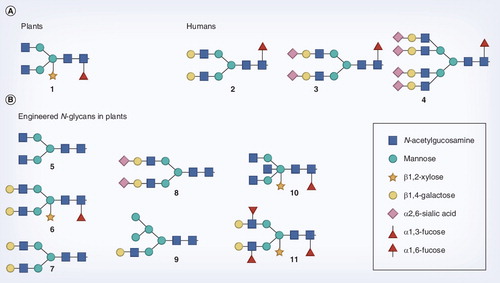
(A) Some typical plant (1) and human (2–4) type N-glycans as can be found in these organisms. The plant N-glycan is of the bi-antennary type, and decorated with the characteristic β1,2-xylose and α1,3-fucose residues. The N-glycans found in humans lack these epitopes, but can be extended with β1,4-galactose and sialic acid and further branching can occur. (B) Some of the N-glycan structures that have been generated in plants by glycoengineering.
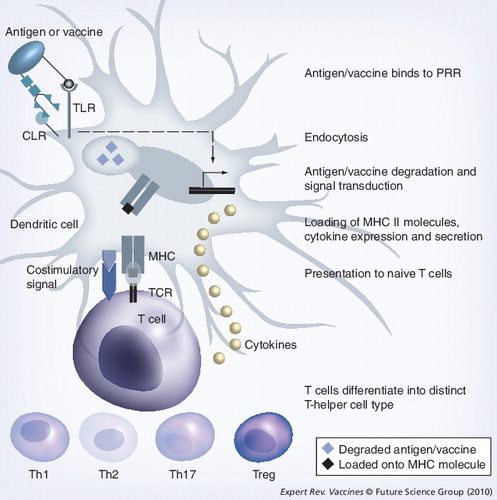
Different classes of PRRs bind different ligands. An important PRR class is formed by the TLRs binding microbial lipids, lipoproteins, lipopolysaccharides, nucleic acids and, in the case of tissue damage, heat-shock proteins. These compounds are considered as danger signals. Another class of PRRs is formed by the CLRs binding carbohydrates, for example, N-glycans. CLR binding typically leads to endocytosis of the recognized ligand. However, to mount an effective immune response, concomitant signaling through both a TLR and a CLR receptor is required. Binding to TLRs leads to signal transduction, resulting in the activation of transcription factors that lead to the expression of cytokines. CLR binding leads to endocytosis of the antigen or vaccine molecule as well as signal transduction that also results in the activation of transcription factors and cytokine expression. TLR and CLR signaling may influence each other, leading to the expression of specific cytokines that determine the differentiation of naive T cells into distinct T-helper cells (see text for more details). Parallel to signaling, the endocytosed antigen or vaccine is degraded in the endosome/lysosome, loaded onto MHC class II molecules and presented to the TCR of naive T-helper cells. During the process of antigen/vaccine degradation and MHC loading, the dendritic cell matures and expresses costimulatory molecules (CD80/86) that provide an extra signal required by the naive T cell to proliferate and differentiate into effector T-helper cells.
CLR: C-type lectin receptor; PRR: Pattern-recognition receptor; TCR: T-cell receptor; TLR: Toll-like receptor.
Since mass-vaccination programs started in the 1950s, the incidence of many infectious diseases has rapidly decreased. The success of mass vaccination was shown by the eradication of smallpox in 1979 Citation[1]. Because vaccination is the most effective and cost-efficient method to control and even eradicate disease, research efforts to develop new and improve existing vaccines continue to be important. Most new vaccines are recombinant subunit vaccines that comprise one antigen of a pathogen that leads to a protective immune response. Subunit vaccines are usually produced in heterologous expression systems such as the bacterium Escherichia coli, the yeast Saccharomyces cerevisiae or Spodoptera insect cells.
Plants are a promising production platform for vaccines. Many proteins of pharmacological interest have been produced in plants, including vaccines, over the last two decades. The first vaccine was a hepatitis B surface antigen produced in tobacco Citation[2]. The expression hosts, tissues and expression systems for plant-made vaccines have recently been reviewed extensively Citation[3,4].
In the secretory pathway of all eukaryotic cells, specific oligosaccharide structures (so-called N-glycans) may be coupled to secreted proteins, irrespective of whether they are endogenous or recombinant proteins such as subunit vaccines. However, the structure of N-glycans from plants deviate to some extent from mammalian N-glycans , and on plant-produced vaccines this can have both detrimental and beneficial effects. It is known that these plant-specific N-glycans can be immunogenic, at least for some animals Citation[5]. In humans, pre-existing anti-plant-glycan antibodies in serum may lead to adverse effects Citation[6]; if such antibodies are of the IgE isotype allergic reactions may occur. Fortunately, anticarbohydrate IgE antibodies seem to be of little clinical relevance Citation[7]. On the other hand, plant-specific N-glycan epitopes can be exploited as a target of the host’s immune response and may be beneficial for immune protection. The presence of these or other carbohydrates as ‘cis-adjuvants’ may help in antigen uptake via lectin receptors, subsequent degradation and antigen presentation of dendritic cells (DCs) to T cells. In this article, we provide an overview of plant glycosylation and glycoengineering, and discuss the immunological opportunities this may offer for the efficacy of plant-produced subunit vaccines.
N-glycosylation: differences between plants & mammals
In eukaryotes, secreted proteins may be modified on specific asparagines by oligosaccharides upon entry into the endoplasmic reticulum (ER) in a process called N-glycosylation. The initial addition of a N-glycan structure is to aid the folding process of the protein, and subsequent modifications of the N-glycans in the ER have a signaling function in the protein-folding quality-control mechanism. In mammals, N-glycans of glycoproteins that arise after further processing in the secretory pathway play crucial roles in many biological processes Citation[8–10]. Biosynthesis of N-glycans can approximately be separated in two phases, which take place sequentially in the ER and in the downstream Golgi apparatus. Plants also possess N-glycosylation and the differences and similarities with the mammalian machinery will now be discussed.
In the first ER-associated phase, N-glycan biosynthesis between mammals and plants is highly similar and does not result in differences in N-glycans found on mature glycoproteins. N-glycan biosynthesis starts with the assembly of a precursor Man5GlcNAc2, linked to dolichol-phosphate lipid at the cytoplasmic side of the ER membrane. Then, this precursor flips to the luminal side of the ER, where the residual four mannose and three glucose residues are added by distinctive glycosyl transferases in a stepwise manner Citation[11,12]. The lipid-linked Glc3Man9GlcNAc2 moiety is then transferred en bloc by the multisubunit oligosaccharyltransferase complex (OST) to selected asparagines of nascent polypeptides during their translocation into the ER Citation[9]. In Arabidopsis thalianaalg3 mutants, the resulting truncated N-glycans are efficiently transferred from lipids to proteins. This is in contrast to what is observed in other organisms and suggests that the Arabidopsis OST is remarkably substrate tolerant Citation[13]. By subsequent trimming reactions catalyzed by exoglycosidases of the ER and the Golgi apparatus, the so-called high-mannose type (Man9GlcNAc2 to Man5GlcNAc2) glycans are generated. Removal of the glucose residues is part of a quality-control process in the folding of newly synthesized glycoproteins Citation[14,15].
When released from the quality-control cycle, N-glycosylation enters the second, Golgi-associated phase. Here, differences occur between plants and mammals that are reflected in the final complex-type N-glycan profile . The first obligatory step in complex-type glycan formation is catalyzed by the enzyme N-acetylglucosaminyltransferase (GnT)I. Only when this enzyme has acted, the subsequent reactions can take place. In plants, these include those catalyzed by β1,2-xylosyltransferase and α1,3-fucosyltransferase enzymes that are not found in mammals. As a consequence, complex-type glycans in plants are characterized by a β1,2-xylose residue and/or an α1,3-fucose residue linked to the core glycan Citation[16]. A second N-acetylglucosamine (GlcNAc) is enzymatically added to the mannose core by GnTII. The terminal GlcNAc residues on N-glycans of plant glycoproteins, which are stored in the vacuoles, are often removed by exoglycosidases, resulting in Man3XylFucGlcNAc2 complex-type glycans Citation[16,17]. Alternatively, although this occurs at a very low frequency, the terminal GlcNAc residues of secreted proteins may be extended by β1,3-galactose and α1,4-fucose residues by the respective glycosyltransferases. These structures are called Lewis A epitopes and can also be found on glycoconjugates in mammals Citation[18,19]. Since any of the above-described processing reactions may not go to completion, N-glycan structures, even on a single type of glycoprotein, can be heterogeneous and may include complex glycans as well as various intermediate high-mannose structures Citation[20,21]. Although plants make complex-type glycans, plant glycoproteins lack the characteristic β1,4-galactose- and sialic acid-containing complex-type glycans found in mammals (e.g., ). They also lack homologs of the mammalian N-acetylglucosaminyltransferases involved in further branching of the bi-antennary N-glycans .
In summary, plants are able to produce GlcNAc-terminated complex-type bi-antennary glycans, but these glycans are substituted by plant-specific xylose and fucose residues and are not extended by some typical mammalian residues . These characteristics appear to be conserved over the entire plant kingdom and have to be taken into account when plants are used as a production platform for medicinal proteins.
Relevance of serum antibodies directed against plant glycans
There has been quite some debate on the possible consequences of the ‘non-mammalian’ xylose and fucose epitopes on N-glycans of plant-produced biopharmaceuticals or vaccines. Carbohydrate-specific IgE antibodies have been found in patients allergic to pollen or venom allergens (reviewed in Citation[7]). These IgE antibodies predominantly bind α1,3-fucose and β1,2-xylose on N-glycans of plants and invertebrates and are most likely raised in response to pollen or insect venom exposure. The presence of these carbohydrate-specific antibodies is important in view of possible adverse immune reactions to plant-produced vaccines. Mari investigated the role of IgE to cross-reacting carbohydrate determinants Citation[22]. Skin prick tests revealed a poor biological activity of these carbohydrate-specific antibodies. These results are seemingly in contrast with those obtained with in vitro basophil histamine-release assays using purified glycoprotein allergens from tomato Citation[23] or celery Citation[24]. These assays clearly show that N-glycans are important in mediator release. Nevertheless, the overall impression is that pollen-induced carbohydrate-specific IgE antibodies are of limited clinical relevance as biological activity mediated by N-glycans is only observed in a selected group of food-allergic patients and often requires relatively high concentrations of allergen Citation[25].
Not only have IgE antibodies specific for carbohydrates been found in human sera, but IgG specific for α1,3-fucose and β1,2 xylose has also been detected, albeit at low levels Citation[26]. Dietary antigens of plant origin do not seem to be the cause of serum antibodies as exposure in the gut normally leads to tolerance. Similar to the carbohydrate-specific IgE antibodies, IgG antibodies also probably develop in humans as a consequence of pollen (and venom) allergen exposure. Rabbits were shown to elicit specific antibodies after parenteral exposure of both α1,3-fucose and β1,2-xylose present on antibodies produced by plants Citation[5,27]. These antibodies developed upon immunization of rabbits with complete Freund’s adjuvant, which is unlikely to be used in humans. Immunogenicity of these plant-specific N-glycans may also depend on the organism, since they do not seem to be immunogenic to mice Citation[28]. As could be expected, topical application of glycoproteins from plants does not have adverse effects on humans, not even on human allergenic patients with IgE antibodies against plant N-glycans Citation[29,30].
Glycoengineering
In order to prevent any adverse consequences of xylose and fucose epitopes, as well as to broaden the scope of applications of plant-made pharmaceuticals, efforts have been undertaken to control N-glycan biosynthesis in plants. Humanization of glycosylation has focused on two areas: preventing the addition of the plant-specific xylose and fucose residues and diversification of N-glycans by the introduction of typical mammalian biosynthesis components .
Genetic knock-out of xylosyltranferases and fucosyltransferases has been established in the moss Physcomitrella patens via homologous recombination Citation[31] and in the model plant A. thaliana by screening mutant libraries Citation[32]. However, given the presence of gene families in plants, combined with the fact that homologous recombination in plants is very rare, the implementation of these approaches to other plant species is very difficult. As an alternative, gene silencing by RNAi of xylosyl- and fucosyltranferases has been established in several plant species, including Nicotiana benthamianaCitation[33], Medicago sativaCitation[34] and Lemna minorCitation[35]. Through yet another approach, the addition of xylose and fucose was strongly inhibited in Nicotiana tabacumCitation[36] via the expression of mutant galactosyltransferases, which appear to result in intermediate galactosylated N-glycan structures that are not the substrates for the xylosyl- and fucosyltransferases . All of these plants have been used to produce recombinant glycoproteins that are indeed essentially devoid of the plant-specific xylose and fucose residues.
Nicotiana tabacum was the first plant wherein glycans were extended by typical mammalian residues through the introduction of human β1,4-galactosyltranferases Citation[37,38]. N-glycans of antibodies produced by these plants were to a significant amount extended by terminal β1,4-galactose residues, but these glycans still carried xylose and fucose Citation[38]. Subsequently, RNAi of xylosyl- and fucosyltranferases was combined with expression of β1,4-galactosyltransferase, and from these engineered plants, efficiently galactosylated antibodies that were also devoid of xylose and fucose could be isolated . Most interestingly, glycosylation of these antibodies was very homogeneous and the antibodies appeared to perform superior in a HIV-neutralization assay Citation[39]. Very recently, in addition to the xylose and fucose knockdown and β1,4-galactose introduction, terminal sialylation was also introduced in N. benthamianaCitation[40]. This was established by transient expression of the entire sialylation pathway in the galactosylating host plant via the coordinate expression of mammalian genes for substrate biosynthesis, nucleotide sugar activation, transport and the sialyltransferase for transfer to the N-glycan. Furthermore, a wide variety of typical mammalian N-glycan epitopes were generated in plants by expression of the corresponding glycosyltranferases. For example, simultaneous expression of β1,4-galactosyltransferase and α1,3-fucosyltransferase has generated Lewis X structures in tobacco Citation[41]. Expression of GnTIII has resulted in bisected glycans Citation[42] and the generation of tri-antennary N-glycans has also been reported, therefore, further branching of N-glycans in plants seems feasible [Nagels, Unpublished Data].
Interestingly, no significant phenotypes have been reported for the glycoengineered knock-in or knock-out plants. Although it cannot be excluded that phenotypes may appear as a consequence of future, novel glycoengineering approaches in plant species used thus far, or in not yet engineered plant species, the high added value of such plants allows the application of controlled conditions that would minimize undesired phenotypes. This flexibility further increases the potential of plants as a host for therapeutic glycoproteins as the variety in glycoforms that can be produced via plants seems unlimited.
Role of carbohydrates in antigen uptake & presentation
N-linked glycans on proteins used in subunit vaccines play an important role in the recognition and endocytosis by cells of the innate immune system, notably DC as the most important antigen-presenting cell (APC) type (see for a detailed explanation of this process including the steps described later). Sets of different classes of pattern-recognition receptors (PRRs) expressed on the surface of these cells bind distinct molecular patterns present on antigens. Two important classes of these PRRs are Toll-like receptors (TLRs) Citation[43] and C-type lectin receptors (CLRs) Citation[44]. TLRs bind characteristic pathogen-associated molecular patterns (PAMPs) present in microbial lipids, lipoprotein, lipopolysaccharide (LPS), nucleic acids and, in the case of tissue damage, heat-shock proteins. All these PAMPs function as danger signals and upon binding, TLRs induce signal transduction events leading to the maturation of DCs, the secretion of inflammatory cytokines and subsequent effector T-cell induction. CLRs recognize carbohydrate structures regardless of whether these are of self or nonself origin. The most important function of CLRs is to recognize and internalize glycosylated antigens to allow antigen presentation by MHC class II molecules. Uptake of antigen by CLRs without concomitant binding of a PAMP to a TLR often leads to tolerance and immune suppression Citation[45]. Usually, the joint action of TLRs and CLRs shape the immune response.
Upon binding of an antigen to a CLR and/or a TLR, the antigen is endocytosed Citation[44,46,47]. The antigen is subsequently degraded in the endosome/lysosome that is formed upon endocytosis and it is loaded onto a MHC class II molecule Citation[48,49]. During this process, the DC matures and the cell migrates to a lymph node where a fragment of the antigen is presented to a naive T cell, resulting in differentiation and proliferation. This only takes place upon costimulation via costimulatory molecules (CD80 or CD86) also present on the cell surface of the DCs that bind to CD28 receptor molecules expressed on the CD4+ T-helper cell. Simultaneously with antigen presentation, cytokines are secreted by the DCs. Which cytokines are secreted depends on the PRRs that are activated by the specific antigen. The profile of secreted cytokines determines the outcome of different T-cell responses. Thus, IL-12 is secreted as a consequence of intracellular pathogens (i.e., viruses and intracellular bacteria), leading to the Th1-type response. This type of T-cell response should develop upon vaccination against a virus. Helminths lead to secretion of IL-4 inducing a Th2-type response leading, among others, to the production of IgE by B cells. A Th2-type response is also induced by antigens, leading to an allergy. Extracellular pathogens such as fungi and extracellular bacteria lead to the release of IL-6 and TGF-β, with the subsequent development of a Th17-type response. Vaccines against extracellular bacteria should mount a Th17-type response. If there is no or little binding to TLR receptors, the APC may secrete IL-10 or TGF-β, which leads to the development of regulatory T cells that suppress immune responses. Thus, plant (glyco)proteins binding to different (sets of) PRRs leads to differential and specific immune signaling. This can potentially be exploited to improve the efficacy of plant-produced subunit vaccines.
Plant sugar structures induce tailored immune responses through CLRs
Plant polysaccharides, not necessarily N-glycans, have been shown to be effective immunological response modifiers having few adverse effects. They are increasingly considered as adjuvant in combination with subunit vaccines. The combination of a plant polysaccharide and synthetic subunit vaccine is assumed to be a safer and more tolerable combination when compared with live-attenuated or killed vaccines with aluminium compounds as adjuvant. Thus, inulin Citation[50], a storage polysaccharide of Compositae, has been shown to be a promising adjuvant inducing both Th1 and Th2 immune responses. New plant polysaccharides with adjuvant activity have recently been discovered such as a polysaccharide from the seeds of Plantago asiaticaCitation[51] and from the roots of Actinidia erinathaCitation[52]. The latter also showed a dual Th1 and Th2-potentiating activity. Not only are such plant polysaccharides considered as adjuvant, but also plant glycosides, that is, oligosaccharide moieties that are attached to plant compounds, are considered promising adjuvants. QuilA, isolated from the bark of Quillaja saponariaCitation[53], is the best-studied glycosidic adjuvant. Plant polysaccharides and glycosides probably exert their adjuvant activity through the carbohydrate-binding CLRs on the antigen-presenting DCs. The seeds of Plantago asiatica not only contain a polysaccharide but also phenylethanoid glycosides showing adjuvant activity. Coculturing immature DCs with either the polysaccharide or one of the phenylethanoid glycosides leads to DC maturation as demonstrated by increased expression of MHC class II molecules and the costimulatory molecule CD86 Citation[51]. Immature DCs capture antigens that are endocytosed, degraded and presented to T cells via MHC class II molecules. The CLRs play a key role in endocytosis. In the same study, Huang et al. show that Plantago polysaccharides and glycosides both stimulate endocytosis through the mannose receptor, a CLR, leading to antigen presentation and T-cell proliferation Citation[51].
Although plant carbohydrates are promising candidates to stimulate protective immune responses in combination ‘in trans’ with subunit vaccines, the combination of both properties in one molecule would be even more promising. The antigen and adjuvant are then presented in a ‘cis configuration’, making optimal use of the synergistic relationships between CLRs, TLRs and intracellular PRRs such as NOD-like receptors. The possibility of this approach was, among others, demonstrated by Singh et al. using the carbohydrates Lewis X and Lewis B chemically cross-linked to ovalbumin (OVA) Citation[54]. In a transgenic mouse model, it was demonstrated that this leads to increased MHC class I and II presentation of OVA, which was mediated through the CLR DC-specific ICAM-grabbing nonintegrin (DC-SIGN). In a similar fashion, Xie et al. showed that the algal β1,3-glucan laminarin coupled to OVA can be used to target the CLR dectin-1 and enhance antigen-specific immune responses Citation[55]. Thus, combining carbohydrates with subunit vaccines in cis, allows the targeting of DCs leading to protective immune responses in situations where the natural response is insufficient as exemplified by HIV and various cancers Citation[56]. Plants are promising production hosts for such vaccines by engineering appropriate glycosylation of subunit vaccines.
Important lessons for the engineering of plant-produced ‘cis vaccines’ can be learned from glycoallergens wherein the carbohydrate moiety binds a CLR. The peanut glycoallergen Ara h 1 acts as a Th2-stimulating adjuvant by binding to DC-SIGN on DCs Citation[57]. Consequently, the DC-SIGN/Ara h 1 complex is endocytosed, degraded and presented to T cells, skewing the immune response towards a Th2 ‘allergic’ phenotype. Deglycosylation of Ara h 1 abolished activation of DCs proving the necessity of the carbohydrate moiety. Ara h 1 glycans mainly consist of xylosylated N-glycans with the composition Man3(-4)XylGlcNAc2Citation[58], which like mannose-terminating glycan, functions as a ligand for DC-SIGN Citation[59]. Various pathogens target DC-SIGN to modulate TLR signaling and regulate adaptive immune responses Citation[60]. However, the DC first has to sense the pathogen through TLR activation before DC function is altered through DC-SIGN signaling. TLR activation by Ara h 1 has not yet been reported, however, in view of the findings of Gringhuis et al. seems required Citation[60]. Coordinate binding of glycosylated antigens to both a CLR and a TLR determine the outcome of the immune response through the cytokine pattern elicited by the DC as outlined in the previous section. Binding to CLRs, TLRs and other PRRs leads to the differential activation of transcription factors such as those belonging to the NF-κB family that regulate the expression of cytokines and chemokines that shape the adaptive immune response. Increased insight into these signaling routes and their cross-talk allows the design of ‘intelligent’ vaccines that mount desired immune responses. Combining these insights with our knowledge of plant glycosylation and glycoengineering and how plant glycans bind PRRs allows a rational design of plant-produced vaccines.
Expert commentary
The production of vaccines in plants is promising because plants can be considered as flexible production hosts that allow the development of a large variety of custom-glycosylated vaccines in a cheap fashion. A large variety of vaccines have been expressed in plants Citation[3] leading to immune protection in animals, with Dow’s Newcastle disease virus vaccine for chickens as the first registered product. Human vaccines have also been successfully produced in plants with some being tested in humans Citation[61]. Since the first description of a plant-produced vaccine Citation[2], the focus has mainly been on the possibilities that plants offer for vaccine production. Little attention has been paid to glycosylation and often only focused on possible adverse reactions of plant glycans in humans.
A key development that allows improved vaccine efficacy is the targeting of DCs to yield strong protective adaptive immune responses Citation[62] through various PRRs. An important role in targeting DCs is foreseen for CLRs binding carbohydrate groups, but other PRRs may also be used. Currently, different classes of PRRs are known that are distinguished on the basis of their structure and function. The TLRs are the best-studied class of PRRs, however, the description of the first TLR only dates back to 1997 Citation[63]. CLRs play a key role in endocytosis of glycan-conjugated antigens by APCs and the first CLR to be discovered was dectin-1 in 2001 Citation[64]. As such, it is not surprising that the biology of PRRs is a field that is still developing, particularly with the involvement of CLRs. Although the knowledge with regard to the role of PRRs in activating adaptive immune responses is rapidly increasing, much has still to be discovered.
It is worth noting that plants appear to be very tolerant for glycoengineering and many different glycotraits have been introduced without obviously affecting plant growth, as discussed earlier. Different CLRs are specific for distinct carbohydrates. Since plants can be engineered in such a way that many different glycans can be added to proteins, plants are an ideal platform for the production of rationally designed vaccines. Increased understanding of the biology of PRRs with regard to antigen uptake and signaling functions will further aid the rational design of vaccines.
An important aspect in the production of vaccines is the homogeneity of the product. Glycosylation is prone to result in a heterogeneous population of N-glycans in many heterologous production systems. In cultured cells, the glycoforms formed differ as a consequence of different environmental conditions such as pH, nutrient availability and cell status Citation[65]. Plants have been demonstrated to yield highly homogenous glycan profiles on recombinant proteins after glycoengineering. For example, antibodies produced by glycoengineered Lemna minor contained a single major N-glycan species with two terminal GlcNAc residues and no plant-specific N-glycans were detected on the antibodies Citation[35]. From glycoengineered N. benthamiana, highly homogeneously glycosylated antibodies could be isolated of which the galactosylated structures represent approximately 80% of all glycoforms. In this work, they outperformed the CHO cell line producing the same antibody Citation[39]. Based on these observations, it can be concluded that plants are excellent production hosts for glycosylated subunit vaccines yielding a homogeneous product.
Five-year view
The glycoengineering results in the plant field demonstrate that plants may have an added advantage as they are very flexible and subunit vaccines containing any glycan structure can be produced, with potentially very high product homogeneity. Not all relevant glycotraits are already present in all relevant plant species and in the near future various glycotraits will be transferred to the specific plant species of preference. In addition, full control over specific glycotraits, such as glycan homogeneity and full (genetic) knock-out of xylose and fucose, will be implemented. This would allow in these species the commercial production of many native (humanized) glycovaccines. Whether the full potential of glycoengineering plants will be used to produce vaccines carrying dedicated N-glycans that function as ‘cis-adjuvants’ will largely depend on developments in the field of immunology. Consolidation of plant species will occur based on other factors such as cost of goods (including downstream processing), speed to market, intellectual property issues and compatibility with existing regulations. Furthermore, these factors and developments in these areas codetermine the competitive position of plants as a platform for the production of glycovaccines. Taken together, our view is that the current status quo in the fields of glycobiology, immunology and plant expression will lead to glycosylated plant-produced vaccines that will contribute to improved public healthcare.
Key issues
• Plants hold the promise to be an excellent production platform for N-glycosylated subunit vaccines with high product homogeneity.
• Plants are highly flexible production hosts that can be engineered to produce virtually any desired glycoform without affecting the plant’s phenotype.
• Plants can be engineered to produce N-glycosylated vaccines that can potentially be targeted to dendritic cells yielding highly efficacious and protective immune responses.
• Although antibodies against plant N-glycans have been detected in allergic patients their clinical relevance seems limited, hence plants form a safe production platform.
• Glycosylated plant-produced vaccines will contribute to improved public healthcare.
Acknowledgements
The authors thank Irma van Die for helpful discussions and Maurice Henquet, Ruud Wilbers, Lotte Westerhof and Jules Beekwilder for critically reading the manuscript. The authors also thank Cost Action FA0804: ‘Molecular farming: plants as a production platform for high value proteins’ for facilitating stimulating discussions.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Fenner F, Henderson DA, Arita I, JeZek Z, Ladnyi ID. Smallpox and its eradication. World Health Organization, Geneva, Switzerland (1988).
- Mason HS, Lam DM, Arntzen CJ. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl Acad. Sci. USA89(24), 11745–11749 (1992).
- Yusibov V, Rabindran S. Recent progress in the development of plant derived vaccines. Expert Rev. Vaccines7(8), 1173–1183 (2008).
- Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnol. J.8, 1–18 (2010).
- Jin C, Altmann F, Strasser R et al. A plant-derived human monoclonal antibody induces an anti-carbohydrate immune response in rabbits. Glycobiology18(3), 235–241 (2008).
- Faye L, Boulaflous A, Benchabane M, Gomord V, Michaud D. Protein modifications in the plant secretory pathway: current status and practical implications in molecular pharming. Vaccine23(15), 1770–1778 (2005).
- van Ree R. Carbohydrate epitopes and their relevance for the diagnosis and treatment of allergic diseases. Int. Arch. Allergy Immunol.129(3), 189–197 (2002).
- Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell81(3), 309–312 (1995).
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem.54, 631–664 (1985).
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell126(5), 855–867 (2006).
- Helenius J, Ng DT, Marolda CL, Walter P, Valvano MA, Aebi M. Translocation of lipid-linked oligosaccharides across the ER membrane requires Rft1 protein. Nature415(6870), 447–450 (2002).
- Snider MD, Sultzman LA, Robbins PW. Transmembrane location of oligosaccharide-lipid synthesis in microsomal vesicles. Cell21(2), 385–392 (1980).
- Henquet M, Lehle L, Schreuder M et al. Identification of the gene encoding the α1,3-mannosyltransferase (ALG3) in Arabidopsis and characterization of downstream N-glycan processing. Plant Cell20(6), 1652–1664 (2008).
- Jin H, Yan Z, Nam KH, Li J. Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of UGGT in ER quality control. Mol. Cell26(6), 821–830 (2007).
- Lederkremer GZ. Glycoprotein folding, quality control and ER-associated degradation. Curr. Opin. Struct. Biol.19(5), 515–523 (2009).
- Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Laine AC, Gomord V, Faye L. N-glycoprotein biosynthesis in plants: recent developments and future trends. Plant Mol. Biol.38(1–2), 31–48 (1998).
- Vitale A, Chrispeels MJ. Transient N-acetylglucosamine in the biosynthesis of phytohemagglutinin: attachment in the Golgi apparatus and removal in protein bodies. J. Cell Biol.99(1 Pt 1), 133–140 (1984).
- Fitchette-Lainè A-C, Gomord V, Cabanes M et al.N-glycans harboring the Lewis A epitope are expressed at the surface of plant cells. Plant J.12(6), 1411–1417 (1997).
- Bakker H, Schijlen E, de Vries T et al. Plant members of the α1,3/4-fucosyltransferase gene family encode an α 1,4-fucosyltransferase, potentially involved in Lewis(A) biosynthesis, and two core α 1,3-fucosyltransferases. FEBS Lett.507(3), 307–312 (2001).
- Elbers IJ, Stoopen GM, Bakker H et al. Influence of growth conditions and developmental stage on N-glycan heterogeneity of transgenic immunoglobulin G and endogenous proteins in tobacco leaves. Plant Physiol.126(3), 1314–1322 (2001).
- Sturm A, Van Kuik JA, Vliegenthart JF, Chrispeels MJ. Structure, position, and biosynthesis of the high mannose and the complex oligosaccharide side chains of the bean storage protein phaseolin. J. Biol. Chem.262(28), 13392–13403 (1987).
- Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int. Arch. Allergy Immunol.129(4), 286–295 (2002).
- Foetisch K, Westphal S, Lauer I et al. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J. Allergy Clin. Immunol.111(4), 889–896 (2003).
- Bublin M, Radauer C, Wilson IB et al. Cross-reactive N-glycans of Api g 5, a high molecular weight glycoprotein allergen from celery, are required for immunoglobulin E binding and activation of effector cells from allergic patients. FASEB J.17(12), 1697–1699 (2003).
- van Ree R. Clinical importance of cross-reactivity in food allergy. Curr. Opin. Allergy Clin. Immunol.4(3), 235–240 (2004).
- Bardor M, Faveeuw C, Fitchette A-C et al. Immunoreactivity in mammals of two typical plant glyco-epitopes, core α1,3-fucose and core xylose. Glycobiology13(6), 427–434 (2003).
- Faye L, Chrispeels MJ. Common antigenic determinants in the glycoproteins of plants, molluscs and insects. Glycoconjugate J.5(3), 245–256 (1988).
- Chargelegue D, Vine ND, van Dolleweerd CJ, Drake PM, Ma JK. A murine monoclonal antibody produced in transgenic plants with plant-specific glycans is not immunogenic in mice. Transgenic Res.9(3), 187–194 (2000).
- Ma JK, Hikmat BY, Wycoff K et al. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med.4(5), 601–606 (1998).
- Mari A, Ooievaar-de Heer P, Scala E et al. Evaluation by double-blind placebo-controlled oral challenge of the clinical relevance of IgE antibodies against plant glycans. Allergy63(7), 891–896 (2008).
- Huether CM, Lienhart O, Baur A et al. Glyco-engineering of moss lacking plant-specific sugar residues. Plant Biol. (Stuttg.)7(3), 292–299 (2005).
- Schähs M, Strasser R, Stadlmann J, Kunert R, Rademacher T, Steinkellner H. Production of a monoclonal antibody in plants with a humanized N-glycosylation pattern. Plant Biotechnol. J.5(5), 657–663 (2007).
- Strasser R, Stadlmann J, Schahs M et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J.6(4), 392–402 (2008).
- Sourrouille C, Marquet-Blouin E, D’Aoust MA et al. Down-regulated expression of plant-specific glycoepitopes in alfalfa. Plant Biotechnol. J.6(7), 702–721 (2008).
- Cox KM, Sterling JD, Regan JT et al. Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat. Biotech.24(12), 1591–1597 (2006).
- Bakker H, Rouwendal GJ, Karnoup AS et al. An antibody produced in tobacco expressing a hybrid β1,4-galactosyltransferase is essentially devoid of plant carbohydrate epitopes. Proc. Natl Acad. Sci. USA103(20), 7577–7582 (2006).
- Palacpac NQ, Yoshida S, Sakai H et al. Stable expression of human β1,4-galactosyltransferase in plant cells modifies N-linked glycosylation patterns. Proc. Natl Acad. Sci. USA96(8), 4692–4697 (1999).
- Bakker H, Bardor M, Molthoff JW et al. Galactose-extended glycans of antibodies produced by transgenic plants. Proc. Natl Acad. Sci. USA98(5), 2899–2904 (2001).
- Strasser R, Castilho A, Stadlmann J et al. Improved virus neutralization by plant-produced anti-HIV antibodies with a homogeneous β1,4-galactosylated N-glycan profile. J. Biol. Chem.284(31), 20479–20485 (2009).
- Castilho A, Strasser R, Stadlmann J et al.In planta protein sialylation through over-expression of the respective mammalian pathway. J. Biol. Chem.285(21), 15923–15930 (2010).
- Rouwendal GJ, Florack DE, Hesselink T, Cordewener JH, Helsper JP, Bosch D. Synthesis of Lewis X epitopes on plant N-glycans. Carbohydr. Res.344(12), 1487–1493 (2009).
- Rouwendal GJ, Wuhrer M, Florack DE et al. Efficient introduction of a bisecting GlcNAc residue in tobacco N-glycans by expression of the gene encoding human N-acetylglucosaminyltransferase III. Glycobiology17(3), 334–344 (2007).
- Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol.1(2), 135–145 (2001).
- Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and langerhans cells. Nat. Rev. Immunol.2(2), 77–84 (2002).
- Geijtenbeek TBH, van Vliet SJ, Engering A, ‘t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Ann. Rev. Immunol.22(1), 33–54 (2004).
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat. Immunol.9(4), 361–368 (2008).
- Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol.20(1), 89–95 (2008).
- Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Ann. Rev. Immunol.20(1), 621–667 (2002).
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHC II pathway. Cell117(5), 677–687 (2004).
- Petrovsky N. Novel human polysaccharide adjuvants with dual Th1 and Th2 potentiating activity. Vaccine24(Suppl. 2), S26–S29 (2006).
- Huang D-F, Tang Y-F, Nie S-P, Wan Y, Xie M-Y, Xie X-M. Effect of phenylethanoid glycosides and polysaccharides from the seed of Plantago asiatica L. on the maturation of murine bone marrow-derived dendritic cells. Eur. J. Pharmacol.620(1–3), 105–111 (2009).
- Sun H-X, Wang H, Xu H-s, Ni Y. Novel polysaccharide adjuvant from the roots of Actinidia eriantha with dual Th1 and Th2 potentiating activity. Vaccine27(30), 3984–3991 (2009).
- Kensil C, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol.146(2), 431–437 (1991).
- Singh SK, Stephani J, Schaefer M et al. Targeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentation. Mol. Immunol.47(2–3), 164–174 (2009).
- Xie J, Guo L, Ruan Y et al. Laminarin-mediated targeting to dectin-1 enhances antigen-specific immune responses. Biochem. Biophys. Res. Comm.391(1), 958–962 (2010).
- Ahlers JD, Belyakov IM. Strategies for recruiting and targeting dendritic cells for optimizing HIV vaccines. Trends Mol. Med.15(6), 263–274 (2009).
- Shreffler WG, Castro RR, Kucuk ZY et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J. Immunol.177(6), 3677–3685 (2006).
- Kolarich D, Altmann F. N-glycan analysis by matrix-assisted laser desorption/ionization mass spectrometry of electrophoretically separated nonmammalian proteins: application to peanut allergen Ara h 1 and olive pollen allergen Ole e 1. Anal. Biochem.285(1), 64–75 (2000).
- Koppel EA, Ludwig IS, Appelmelk BJ, van Kooyk Y, Geijtenbeek TB. Carbohydrate specificities of the murine DC-SIGN homologue mSIGNR1. Immunobiology210(2–4), 195–201 (2005).
- Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity26(5), 605–616 (2007).
- Tacket CO. Plant-based oral vaccines: results of human trials. Curr. Top. Microbiol. Immunol.332, 103–117 (2009).
- Tacken PJ, de Vries IJM, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol.7(10), 790–802 (2007).
- Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature388(6640), 394–397 (1997).
- Brown GD, Gordon S. A new receptor for β-glucans. Nature413(6851), 36–37 (2001).
- Brooks SA. Strategies for analysis of the glycosylation of proteins: current status and future perspectives. Mol. Biotechnol.43(1), 76–88 (2009).