ABSTRACT
Arctic and subarctic ecosystems have undergone considerable environmental changes in recent years as the result of climate warming. Fossil records of freshwater diatoms in lakes throughout the circumpolar Arctic, including the central Canadian Arctic treeline region, have revealed marked directional shifts in diatom assemblages in recent lake sediments. These algal changes have been linked to longer growing seasons, decreased duration of ice cover, and/or increased thermal stability. The effects of these recent environmental changes on higher trophic levels, such as the Cladocera, are still unclear. Using cladoceran remains preserved in the sediments of 50 lakes, which were previously examined for changes in diatoms, we show that significant changes in cladoceran species composition have occurred from pre-industrial times to the present. However, these changes are considerably muted compared to the more substantial changes observed in the diatom record. We found no consistent patterns of change in planktonic cladocerans (i.e. Daphnia, Bosmina) within our study lakes, and the response of the Cladocera to environmental changes does not appear to be strongly coupled to recent changes in diatom communities, thus further confirming that the previously observed diatom changes were related to bottom-up limnological controls. These results highlight the complex response of Arctic freshwater food webs to climate change, and the need for incorporating multi-trophic studies into climate change investigations.
Introduction
Freshwater lakes and ponds are one of the most ubiquitous features of Arctic and subarctic landscapes, and represent environments that are highly susceptible to the effects of climate change. Global warming trends are expected to be amplified at higher latitudes (CitationSerreze and Francis, 2006), and, because of the sensitivity of hydro-ecological processes (CitationRouse et al., 1997; CitationWrona et al., 2005), freshwater ecosystems in the north are predicted to be particularly vulnerable to climate stressors. Numerous studies have suggested that high-latitude freshwater ecosystems have undergone significant environmental changes since pre-industrial times, including increasing lake temperatures, increased fluxes of nutrients, reduced periods of ice cover, longer growing seasons, and substantial reorganization of aquatic food webs (e.g. CitationSerreze et al., 2000; CitationWrona et al., 2005; CitationHinzman et al., 2005; CitationSmol et al., 2005; CitationQuinlan et al., 2005; CitationDuguay et al., 2006; CitationSchindler and Smol, 2006).
Several striking examples of the impacts of climatic warming on freshwater ecosystems in the Arctic have been provided by paleolimnological studies of diatom communities. CitationDouglas et al. (1994), for example, recorded dramatic shifts in diatom assemblages over the last ∼200 years in a set of small High Arctic ponds on Ellesmere Island, Canada, whereas relatively stable diatom assemblages were found to have persisted throughout the preceding millennia. CitationSmol et al. (2005) documented a similar restructuring of diatom communities in post-industrial lake sediments throughout most regions of the circumpolar Arctic. In subarctic Finnish Lapland, diatom assemblages shifted from being primarily composed of benthic taxa to having high abundances of planktonic taxa (CitationSorvari and Korhola, 1998; CitationRautio et al., 2000; CitationKorhola et al., 2002; CitationSorvari et al., 2002.). Likewise, CitationRühland et al. (2003a) recorded similar shifts in a series of 50 lakes in the central Canadian Arctic treeline region, with a shift from benthic to planktonic taxa. These widespread shifts in Arctic diatom taxa have been attributed to a shorter duration of ice cover, an increase in the length of the growing season, and/or increased thermal stability resulting from climate warming (CitationSmol et al., 2005).
The Cladocera (Crustacea: Branchiopoda) are an important component of many high-latitude lakes and ponds, and are considered to be key organisms in both pelagic environments (i.e. the Daphniidae and Bosminidae families) as well as benthic habitats (i.e. the Chydoridae family) (CitationFrey, 1988). Because of their crucial role in aquatic food webs, and their abilities to respond rapidly to changing environmental conditions, cladocerans have long been recognized as important ecological and paleolimnological indicators (CitationKorhola and Rautio, 2001; CitationJeppesen et al., 2001; CitationBennike et al., 2004). As the chitinous exoskeletons of these animals are well preserved in lake sediments, changes in the sediment record can be used to examine the past response of cladoceran communities to changing environmental conditions (CitationKorhola and Rautio, 2001). The environmental conditions of northern lakes influence the distributions and abundances of cladocerans (e.g. CitationKorhola 1999; CitationBos and Cumming, 2003; CitationJeppesen et al., 2003; CitationSweetman and Smol, 2006) and, as a result, taxa in Arctic and subarctic lakes should also be expected to be strongly affected by climate warming.
Compared to the well documented shifts in diatom communities (e.g. CitationRühland et al., 2003a; CitationSmol et al., 2005), however, the responses of higher trophic levels, including cladocerans, to recent environmental changes are not as well understood. In their review of the effects of climate change on Arctic and subarctic freshwater ecosystems, CitationRouse et al. (1997) predicted that, while increases in primary production will almost certainly be observed, the responses of higher trophic levels are likely much more variable. As food resources for planktonic grazers increase with higher levels of primary productivity in these ecosystems (e.g. CitationMichelutti et al., 2005), the abundance of herbivorous cladocerans, such as Daphnia, might also be expected to increase. In some systems, this appears to be the case. For instance, in Lake Saanajärvi, located in northwestern Finnish Lapland, CitationRautio et al. (2000) and CitationKorhola et al. (2002) found that increases in the planktonic cladoceran Daphnia were synchronous with the increases in planktonic diatom taxa described previously. CitationO'Brien et al. (2005) also found that, following experimental fertilization of half of a partitioned lake in northern Alaska, Daphnia longiremis abundance was significantly higher in the treated half compared to the reference side. However, the response of food webs to climate warming is often difficult to predict (CitationPetchey et al., 1999; CitationCallaghan et al., 2004; CitationBaulch et al., 2005). Climatic warming can potentially destabilize food webs, and responses of higher trophic levels may be much more complex and unpredictable than in algal communities (e.g. CitationBeisner et al., 1997; CitationStrecker et al., 2004). There is increasing evidence that in many temperate lakes, a decoupling of the algae-zooplankton relationship can occur (CitationWinder and Schindler, 2004; CitationAdrian et al., 2006). In Lake Washington, for example, CitationWinder and Schindler (2004) documented a long-term decline in Daphnia, which they attributed to an increasing temporal mismatch with food resources, due to an earlier spring diatom bloom as the result of climate warming. Relatively few studies, however, have examined the impacts of climate change on Arctic freshwater food webs (e.g. CitationQuinlan et al., 2005).
The objectives of our study were to evaluate the response of cladocerans to recent environmental changes in the central Canadian Arctic treeline region by comparing present-day and pre-industrial cladoceran assemblages from a series of 50 lakes, and to compare the responses of the cladoceran communities to previously observed changes in diatom communities from the same lakes (CitationRühland et al., 2003a). Specifically, we were interested in knowing the following: (1) Have any changes occurred to the community composition or diversity of cladocerans between pre-industrial and present-day periods? (2) If so, was there a shift in abundances from benthic to planktonic cladoceran taxa similar to that observed in the diatom taxa? (3) Are changes in cladoceran and diatom assemblages contemporaneous? (4) Finally, do changes in grazing/predation regimes from cladocerans have a role in driving the response of diatom communities to recent climate warming? If the latter is the case, we would expect changes in cladoceran assemblages to closely correspond to the diatom changes across our 50 lake set.
Study Area
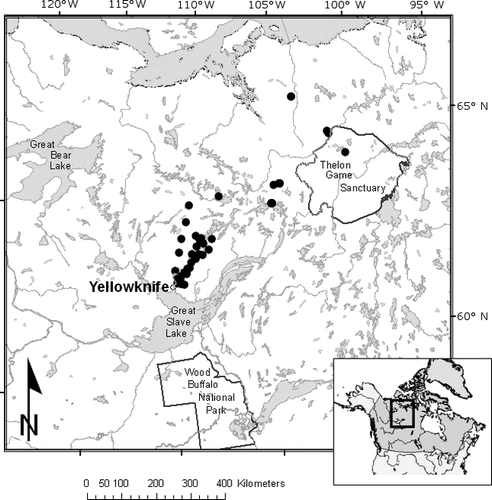
Sediments were analyzed from 50 lakes located in the central Canadian Arctic treeline region, between Yellowknife, Northwest Territories, and the northern boundary of the Thelon Game Sanctuary, Nunavut Territory (). The study lakes span a steep ecoclimatic gradient and are distributed across three major ecozones, from the boreal forest zone in the south, through the forest-tundra transitional zone, and into the Arctic tundra zone in the north (CitationRühland and Smol, 2002; CitationRühland et al., 2003a, Citation2003b).
Methods
Sediment cores were collected at approximately the deepest point of each of the lakes using a Glew gravity corer (CitationGlew et al., 2001). The surface sediments (top 1.0 cm) and bottom sediments (bottom 1.0 cm) of each core were extruded using a vertical extruder (CitationGlew, 1988). Additional details on water chemistry of the lakes, the modern distribution of diatoms and Cladocera, as well as additional details about sediment core retrieval and sampling of these lakes were previously presented in CitationRühland and Smol (2002) and CitationRühland et al. (2003a, Citation2003b).
In order to evaluate the response of cladocerans to recent environmental change, we used a ‘top-bottom’ paleolimnological approach (CitationSmol, 2008), identical to the approach used in the CitationRühland et al. (2003a) diatom study. This ‘snap-shot’ approach involves comparing subfossil assemblages from the surface lake sediments, which represent present-day conditions, to assemblages deposited in downcore lake sediments representing pre-impact conditions (ca. 1850). The goal of the comparisons made between these two discrete points in time (i.e. top vs. bottom) is to assess whether environmental conditions during the pre-industrial era are substantially different than present. These data can then be used to evaluate the magnitude, direction, and nature of cladoceran assemblage changes over the last ca. 200 years on a regional scale.
In addition to the 50-lake regional top-bottom analyses, we examined the detailed changes in cladoceran communities from a sediment core from Slipper Lake (64°35′65″N, 110°50′07″W), a site also examined for changes in diatom communities by CitationRühland et al. (2003a; Citation2005). As there was insufficient sediment from the original Slipper Lake core used by CitationRühland et al. (2003a), we examined cladoceran remains from a second core that was used by CitationRühland and Smol (2005) to verify the trends in the primary core. Both sediment cores were collected at the deepest point in the lake in March 1997 and sectioned into 0.5 cm intervals.
Analysis of cladoceran remains from the sediments of each lake followed standard procedures, outlined in CitationKorhola and Rautio (2001). Approximately 2–4 g wet weight of sediment from each sample was deflocculated in 150 mL of a 10% KOH solution at 80°C for 30 minutes, after which they were rinsed through a 37 µm sieve. For Slipper Lake, the 0.5 cm intervals were combined into 1.0 cm intervals because of the low abundance of cladoceran remains. Slipper Lake samples were separated into >90 µm and 37–90 µm subsamples to facilitate identification of remains. Material retained on the sieves was stored in distilled water and ethanol. Safranin-glycerine solution was added to stain the cladoceran remains. Slides were prepared by pipetting 50 µL aliquots onto slides, and mounting in glycerin jelly. Cladocera were identified at 100–400× magnification using a Leica DMRB compound microscope. For each taxon, the most abundant remains (i.e. head shield, carapace, postabdomen) were used to calculate the number of individuals within a sample. A minimum of 50 individuals were counted per sample. Three lakes (TK-13, TK-21, and TK-40) contained insufficient numbers of Cladocera, and were excluded from subsequent analyses.
Changes in the cladoceran communities between top and bottom samples were quantified using detrended correspondence analysis (DCA). DCA is an unconstrained ordination technique, and has the advantage that the sample scores are scaled in standard deviation (SD), or units of species turnover (β-diversity) (CitationSmol et al., 2005; CitationBirks, 2007). Prior to analysis, all species relative abundance data were square-root transformed to stabilize variances. Bonferroni-adjusted paired t-tests were used to compare both axis 1 and axis 2 sample scores between the two time periods. Changes in the sample scores between top and bottom sediment samples were calculated following CitationQuinlan et al. (2003). Briefly, the axis 1 sample score for the pre-industrial assemblage from each lake was subtracted from the axis 1 sample score of the present-day assemblage to calculate a net change in DCA sample scores along that axis. This calculation was also performed for axis 2 ordination scores. Differences in α-diversity between present-day and pre-industrial cladoceran communities were also compared, using Hill's N2 as a measure of α-diversity. Bonferroni-adjusted paired t-tests were used to compare differences in diversity between time intervals. All ordinations and Hill's N2 calculations were carried out using CANOCO v. 4.5 (Citationter Braak and Šmilauer, 2002); t-tests were calculated using SYSTAT v. 9 (CitationSPSS, 1998).
In order to assess how changes in cladoceran communities matched previously observed changes in diatom communities within the same lakes (CitationRühland et al., 2003a), the degree of dissimilarity between top and bottom samples was calculated using a Bray-Curtis dissimilarity coefficient (CitationClarke et al., 2006). Cladoceran Bray-Curtis dissimilarity coefficients were compared to the matching Bray-Curtis values for the diatom assemblages. In addition, we compared the relative abundance of the dominant planktonic diatom taxa (Cyclotella) to changes in pelagic Cladocera species, using simple Pearson correlations with Bonferroni-adjusted p-values for multiple comparisons. Correlations were calculated using SYSTAT v. 9 (CitationSPSS, 1998).
For the continuous sediment core data from Slipper Lake, a detrended canonical correspondence analysis (DCCA) was used to calculate a quantitative estimate of compositional turnover (β-diversity) following the procedures used by CitationBirks (2007). An estimate of the total amount of compositional change for the last ∼150 years was obtained by constraining the DCCA by using the 210Pb-based sample ages as the sole constraint. Significance was measured using Monte Carlo permutations (999 permutations). For this analysis, species data were square-root transformed, there was no down-weighting of rare taxa in the ordination, detrending was by segments, and scaling was nonlinear. DCCA was performed using CANOCO v. 4.5 (Citationter Braak and Šmilauer, 2002).
Results
Most cladoceran taxa in our study lakes did not demonstrate a uniform pattern of change from the pre-industrial to the present-day assemblages (). This is in contrast to the diatom communities in the same lakes that showed a large and consistent increase in the planktonic species Cyclotella stelligera, and a corresponding decline in benthic Fragilaria taxa (; CitationRühland et al., 2003a). The cladoceran assemblages show no corresponding increase in planktonic species, not withstanding a few lakes that showed changes in the dominant planktonic taxa (i.e. Bosminidae, Daphniidae). Despite no consistent increases or decreases in cladocerans across the study area, relatively subtle shifts were apparent. For example, the benthic cladoceran Pleuroxus trigonellus, which was only found in lake sediments from boreal forest lakes, decreased in abundance in modern sediments compared to pre-industrial periods (negative values in ), whereas a second benthic chydorid taxon (Camptocercus spp.) increased in relative abundance in several lakes across the study region (positive values in ). However, most taxa in our lakes did not show a synchronous shift in their relative abundance from pre-industrial assemblages to modern sediments.
Figure 2 The percentage of change of the main cladoceran species from pre-industrial to modern lake sediments in the 47 central Canadian Arctic treeline lakes that contained sufficient numbers of fossil Cladocera for analysis. Lakes are arranged in order of the distance of each lake from current treeline. Distance to treeline (km) follows the delineation by CitationRühland et al. (2003a) and is based on percentage tree cover, derived from field observations, topographical maps, aerial photographs, and maps derived from satellite imagery. Positive distances along the x-axis indicate lakes north of treeline, and negative distances indicate lakes south of treeline. Positive values on the y-axis indicate an increase in the relative abundance (%) from pre-industrial to present-day lake sediments, while negative values on the y-axis indicates a decrease in the relative abundance (%) from pre-industrial to present-day lake sediments.
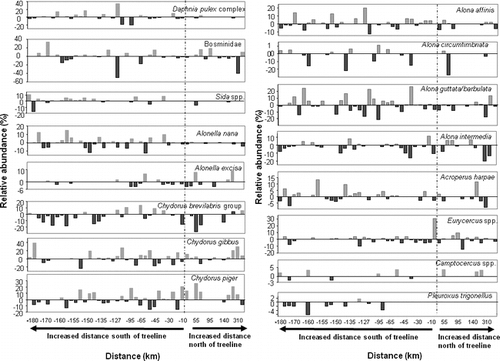
Figure 3 Relative abundances of littoral cladocerans, pelagic cladocerans, and dominant diatom taxa in Slipper Lake, Northwest Territories. The two Chydorus species, C. biovatus and C. brevilabris, were grouped together, as the remains of these taxa were difficult to distinguish from each other, as were the Alona species, A. guttata and A. barbulata. Diatom data are summarized from CitationRühland et al. (2003a) and CitationRühland and Smol (2005).
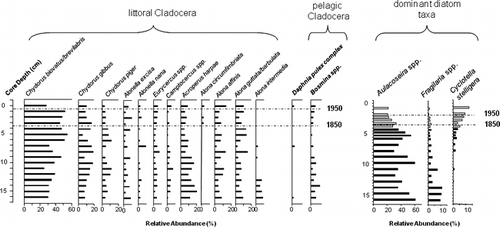
The detailed paleolimnological record from the Slipper Lake sediment core is consistent with the regional top-bottom survey. Throughout the sediment record, the cladoceran assemblage remains relatively stable, and the pelagic cladocerans (Daphnia, Bosmina) present in the sediment record show no apparent change in their abundance (). This is in contrast with the large directional changes occurring in the diatom record (). Beginning at about 5 cm (a.d. ∼1800), there is a shift in the diatom taxa, with pelagic Cyclotella stelligera becoming more abundant and Aulacoseira and Fragilaria spp. decreasing in abundance towards present day.
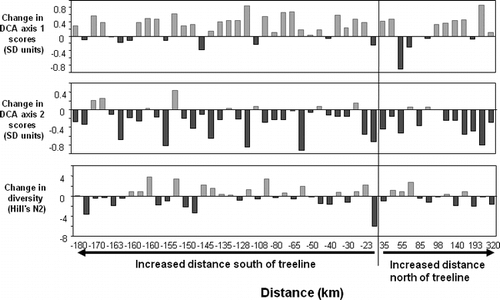
Although there is no clear directional change in individual cladoceran taxa across our regional lake set, there is a significant change in β-diversity (species turnover) (). Both DCA axis 1 and 2 sample scores were significantly different between present-day and pre-industrial times (DCA axis 1, paired t-test, p < 0.0001, t = 4.470; DCA axis 2, paired t-test, p < 0.0001, t = 5.519), indicating a directional shift in the overall composition of species assemblages. There was no significant change in α-diversity (Hill's N2) between pre-industrial and present-day cladoceran assemblages (; paired t-test, p < 0.636, t = 0.477). α-diversity refers to the diversity (i.e. species richness) within a particular area and is usually expressed as a measure of the number of species. Alternatively, ß-diversity is a measure of the change in species diversity between areas, allowing a comparison between ecosystems or sites.
Figure 4 Changes between (top) DCA axis 1 sample scores; (center) DCA axis 2 sample scores, a measure of β-diversity; and (bottom) cladoceran species diversity (Hill's N2), a measure of α-diversity, between pre-industrial lake sediments and modern lake sediments in the 47 central Canadian Arctic treeline lakes. Lakes are arranged in order of the distance of each lake from currenttreeline.
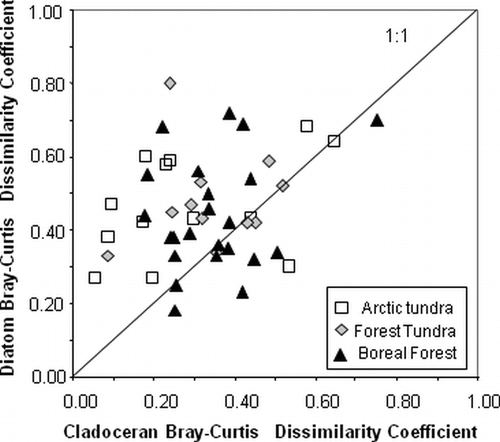
A comparison of the Bray-Curtis dissimilarity coefficients (DC) for diatom taxa (mean DC = 0.46) and cladocerans (mean DC = 0.33) suggests that the species composition of diatom assemblages are more dissimilar between pre-industrial and present-day periods than the cladoceran fauna (). This is not surprising, as the diatom assemblages show striking changes in their relative abundances (CitationRühland et al., 2003a).
Figure 5 Scatter plot comparing Bray-Curtis dissimilarity coefficient values for diatoms and cladocerans for the 47 study lakes. The majority of lakes lie above the 1:1 line, indicating that diatom assemblages are more dissimilar than cladoceran assemblages between pre-industrial and modern lake sediments.
A comparison of the modern distributions of Cyclotella and planktonic cladocerans reveals that the relative abundance of Cyclotella spp. in our 47-lake set is uncorrelated with Daphnia relative abundance (r = 0.19, p = 0.188), Bosminidae relative abundance (r = 0.13, p = 0.377), or total pelagic Cladocera (r = 0.21, p = 0.159). Both Daphnia and Bosmina remains occur at relatively low abundances throughout the Slipper Lake core, and neither pelagic taxa appear as dominant in the paleolimnological record. The cladoceran fauna is comprised primarily of benthic chydorids and is relatively stable throughout the core ().
Discussion
The lowermost cladoceran assemblages from all of our study sites represent environmental conditions pre-dating ca. 1850 (pre-industrial times). Several of our study lakes have undergone detailed paleolimnological analyses that involved establishing a chronology based on 210Pb dating techniques (including Slipper Lake). These sedimentary cores have consistently shown that background conditions (a.d. ∼1850) occurred within the top 6 cm of the core, which is consistent with the typically low rates of sedimentation in Arctic lakes (CitationRühland et al., 2003a, Citation2003b). The mean core depth for all of the bottom sedimentary intervals from our lake set was 19.4 m (), suggesting that most, if not all, of our bottom intervals pre-date the onset of the industrial era.
Table 1 Geographical and limnological data on the 50 study lakes.
CitationRühland et al. (2003a) found that increases in planktonic Cyclotella abundance were most pronounced in the deeper lakes (>6 m) within our study area. They suggested that, among other limnological changes, deeper lakes likely experienced increased water column stability as the warmer temperatures and longer growing season likely allowed longer and stronger periods of thermal stratification. One possible explanation why these deeper lakes did not show comparable increases in planktonic Cladocera is because of strong top-down predation pressure from fish or invertebrate predators. While data on the occurrence of potential predators in our study lakes were not available, it is likely that these deeper lakes sustained fish populations. In Arctic tundra lakes in Alaska, CitationO'Brien et al. (2004) and CitationYurista and O'Brien (2001) found that deeper lakes tend to contain planktivorous fish populations, and in lakes with fish, large planktonic zooplankton species such as Daphnia were generally limited in their abundance. CitationLauridsen et al. (2001) also reported that in a survey of 56 lakes in Arctic Greenland, Daphnia pulex only occurred in lakes without fish.
Invertebrate predators may also have limited the response of Daphnia. CitationO'Brien (2001) found that when the predatory copepod Heterocope septentrionalis was introduced into a pond that lacked both fish and invertebrate predators, D. pulex was eliminated from the pond within a year. Members of the Daphnia pulex complex (i.e. D. middendorffiana and D. pulex) may not be able to increase in abundance in the deeper lakes, despite increased algal food resources, due to suppression by predators.
Cladocerans make up only a component of the zooplankton of lakes. Other zooplankton taxa, such as copepods or rotifers populations, may have increased their abundance and prevented a response in the planktonic Cladocera because of competitive exclusion (CitationMacIsaac and Gilbert, 1989; CitationDzialowski and O'Brien, 2004).
The paleolimnological record indicates that diatoms have shown a large shift in favor of planktonic taxa. While the diatom flora within the phytoplankton may have increased significantly, other algal groups may not have responded to recent environmental changes to the same degree (CitationProwse et al., 2006). In a study of food sources and feeding rates in shallow high-latitude lakes and ponds, CitationRautio and Vincent (2006) found that zooplankton and phytoplankton communities were not strongly coupled, and that 89–98% of the zooplankton resources consisted of detritus. Despite the apparent changes in planktonic algae, cladocerans may not have shown a similar response because of a decoupling of the food web (i.e. CitationBrönmark and Weisner, 1996).
Conclusions
Despite evidence for marked ecological shifts in the diatom communities within the past ∼150 years, the cladoceran assemblages within our treeline lakes do not appear to have experienced comparable ecological changes. CitationRühland et al. (2003a) documented striking shifts in the fossil diatom records within these same lakes, with the relative abundances of planktonic diatom taxa increasing across our study region. Although we found that there was a significant unidirectional shift in the composition of cladoceran species between pre-industrial and modern periods within the same study lakes, we found no consistent pattern of change in the planktonic cladocerans (i.e. Daphnia, Bosmina). The lack of a strong coupling between these two trophic levels suggests that cladoceran and diatom communities may be responding independently to climate change signals within our lakes, and that top-down controls (i.e. grazing) do not appear to have a strong impact in controlling diatom communities within these lakes. If this is true, and given the relatively muted shift in cladoceran assemblage composition, it is probable that the cladoceran fauna in subarctic Canadian lakes may not have reached an equivalent environmental threshold which the diatom communities have apparently surpassed. Cladoceran communities may be relatively more resilient to the impacts of recent climate change. These results highlight the complex response of Arctic freshwater food webs to climate change. Understanding the responses of organisms at multiple trophic levels will be essential for a holistic understanding of the impacts of climate warming on freshwater ecosystems. Given that Arctic ecosystems are predicted to experience an additional 4–6°C warming over the next century, substantial reorganizations of freshwater food webs will undoubtedly continue to occur in the future, particularly if higher trophic levels surpass ecological thresholds.
Acknowledgments
This research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) grant to J. P. Smol, an NSERC special collaborative grant to the Paleoecological Analysis of Circumpolar Treeline (PACT), and the Northern Studies Training Program (NSTP). Logistical support during sample collection was given by the Polar Continental Shelf Project. Tammy Karst, Tamsin Laing, Brent Wolfe, and Dave Porinchu assisted in field work. Assistance in the field was also provided by the Department of Indian Affairs and Northern Development (DIAND).
References Cited
- Adrian, R. , S. Wilhelm , and D. Gerten . 2006. Life-history traits of lake plankton species may govern their phenological response to climate warming. Global Change Biology 12:652–661.
- Baulch, M. D. , D. W. Schindler , M. A. Turner , D. L. Findlay , M. J. Paterson , and R. D. Vinebrooke . 2005. Effects of warming on benthic communities in a boreal lake: implications of climate change. Limnology and Oceanography 50:1377–1392.
- Beisner, B. E. , E. McCauley , and F. J. Wrona . 1997. The influence of temperature and food chain length on plankton predator-prey dynamics. Canadian Journal of Fisheries and Aquatic Sciences 54:586–595.
- Bennike, O. , K. P. Brodersen , E. Jeppesen , and I. R. Walker . 2004. Aquatic invertebrates and high latitude palaeolimnology. In Pienitz, R. , M. S. V. Douglas , and J. P. Smol , editors. Long-term environmental change in Arctic and Antarctic lakes Dordrecht Springer Verlag. 159–186.
- Birks, H. J. B. 2007. Estimating the amount of compositional change in late-Quaternary pollen—Stratigraphical data. Vegetation History and Archaeobotany 16:197–202.
- Bos, D. G. and B. F. Cumming . 2003. Sedimentary cladoceran remains and their relationship to nutrients and other limnological variables in 53 lakes from British Columbia, Canada. Canadian Journal of Fisheries and Aquatic Sciences 60:1177–1189.
- Brönmark, C. and S. E. B. Weisner . 1996. Decoupling of cascading trophic interactions in a freshwater, benthic food chain. Oecologia 108:534–541.
- Callaghan, T. V. , L. O. Bjorn , Y. Chernov , T. Chapin , T. R. Christensen , B. Huntley , R. A. Ims , M. Johansson , D. Jolly , S. Jonasson , N. Matveyeva , N. Panikov , W. Oechel , G. Shaver , S. Schaphoff , and S. Sitch . 2004. Effects of changes in climate on landscape and regional processes, and feedbacks to the climate system. Ambio 33:459–468.
- Clarke, K. R. , P. J. Somerfield , and M. G. Chapman . 2006. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. Journal of Experimental Marine Biology and Ecology 330:55–80.
- de Senerpont, L. N. , W. M. Mooij , S. Hülsmann , E. H. van Nes , and M. Scheffer . 2006. Can overwintering versus diapausing strategy in Daphnia determine match-mismatch events in zooplankton-algae interactions. Oecologia DOI 10.1007/s00443-006-0549-2.
- Douglas, M. S. V. , J. P. Smol , and W. Blake Jr . 1994. Marked post-18th century environmental change in high arctic ecosystems. Science 266:416–419.
- Duguay, C. R. , T. D. Prowse , B. R. Bonsal , R. D. Brown , M. P. Lacroix , and P. Menard . 2006. Recent trends in Canadian lake ice cover. Hydrological Processes 20:781–801.
- Dzialowski, A. R. and W. J. O'Brien . 2004. Is competition important to arctic zooplankton community structure. Freshwater Biology 49:1103–1111.
- Frey, D. G. 1988. Littoral and offshore communities of diatoms, cladocerans and dipterous larvae, and their interpretation in paleolimnology. Journal of Paleolimnology 1:179–191.
- Glew, J. R. 1988. A portable extruding device for close interval sectioning of unconsolidated core samples. Journal of Paleolimnology 1:235–239.
- Glew, J. R. , J. P. Smol , and W. M. Last . 2001. Sediment core collection and extrusion. In Last, W. M. and J. P. Smol , editors. Tracking Environmental Change Using Lake Sediments. Vol. 1: Basin Analysis, Coring, and Chronological Techniques Dordrecht Kluwer Academic Publishers. 73–105.
- Hinzman, L. D. , N. D. Bettez , W. R. Bolton , F. S. Chapin , M. B. Dyurgerov , C. L. Fastie , B. Griffith , R. D. Hollister , A. Hope , H. P. Huntington , A. M. Jensen , G. J. Jia , T. Jorgenson , D. L. Kane , D. R. Klein , G. Kofinas , A. H. Lynch , A. H. Lloyd , A. D. McGuire , F. E. Nelson , W. C. Oechel , T. E. Osterkamp , C. H. Racine , V. E. Romanovsky , R. S. Stone , D. A. Stow , M. Sturm , C. E. Tweedie , G. L. Vourlitis , M. D. Walker , D. A. Walker , P. J. Webber , J. M. Welker , K. Winker , and K. Yoshikawa . 2005. Evidence and implications of recent climate change in northern Alaska and other arctic regions. Climatic Change 72:251–298.
- Jeppesen, E. , P. Leavitt , L. De Meester , and J. P. Jensen . 2001. Functional ecology and palaeolimnology: using cladoceran remains to reconstruct anthropogenic impact. Trends in Ecology and Evolution 16:191–198.
- Jeppesen, E. , J. P. Jensen , T. L. Lauridsen , S. L. Amsinck , K. Christoffersen , M. Sondergaard , and S. F. Mitchell . 2003. Sub-fossils of cladocerans in the surface sediment of 135 lakes as proxies for community structure of zooplankton, fish abundance and lake temperature. Hydrobiologia 491:321–330.
- Korhola, A. 1999. Distribution patterns of Cladocera in subarctic Fennoscandian lakes and their potential in environmental reconstruction. Ecography 22:357–373.
- Korhola, A. and M. Rautio . 2001. Cladocera and other branchiopod crustaceans. In Smol, J. P. , H. J. B. Birks , and W. M. Last , editors. Tracking Environmental Change Using Lake Sediments, Volume 4: Zoological Indicators Dordrecht Kluwer Academic Publishers. 5–41.
- Korhola, A. , S. Sorvari , M. Rautio , P. G. Appleby , J. A. Dearing , N. Rose , A. Lami , and N. G. Cameron . 2002. A multiproxy analysis of climate impacts on recent development of subarctic Lake Saanajärvi in Finnish Lapland. Journal of Paleolimnology 28:59–77.
- Lauridsen, T. , E. Jeppesen , F. Landkildehus , and M. Søndergaard . 2001. Horizontal distribution of cladocerans in arctic Greenland lakes—Impact of macrophytes and fish. Hydrobiologia 442:107–116.
- MacIsaac, H. J. and J. J. Gilbert . 1989. Competition between rotifers and cladocerans of different body sizes. Oecologia 81:295–301.
- Michelutti, N. , A. P. Wolfe , R. D. Vinebrooke , B. Rivard , and J. P. Briner . 2005. Recent primary production increases in arctic lakes. Geophysical Research Letters 32:L19715. , doi:10.1029/2005GL023693.
- O'Brien, W. J. 2001. Long-term impact of an invertebrate predator, Heterocope septentrionalis , on an arctic pond zooplankton community. Freshwater Biology 46:39–45.
- O'Brien, W. J. , M. Barfield , N. D. Bettez , G. M. Gettel , A. E. Hershey , M. E. McDonald , M. C. Miller , H. Mooers , J. Pastor , C. Richards , and J. Schuldt . 2004. Physical, chemical, and biotic effects on arctic zooplankton communities and diversity. Limnology & Oceanography 49:1250–1261.
- O'Brien, W. J. , M. Barfield , N. Bettez , A. E. Hershey , J. E. Hobbie , G. Kipphut , G. Kling , and M. C. Miller . 2005. Long-term response and recovery to nutrient addition of a partitioned arctic lake. Freshwater Biology 50:731–741.
- Petchey, O. L. , P. T. McPhearson , T. M. Casey , and P. J. Morin . 1999. Environmental warming alters food-web structure and ecosystem function. Nature 402:69–72.
- Prowse, T. D. , F. J. Wrona , J. D. Reist , J. E. Hobbie , L. M. J. Levesque , and W. F. Vincent . 2006. General features of the Arctic relevant to climate change in freshwater ecosystems. Ambio 35:330–338.
- Quinlan, R. , A. M. Paterson , R. I. Hall , P. J. Dillon , A. N. Wilkinson , B. F. Cumming , M. S. V. Douglas , and J. P. Smol . 2003. A landscape approach to examining spatial patterns of limnological variables and long-term environmental change in a southern Canadian lake district. Freshwater Biology 48:1676–1697.
- Quinlan, R. , M. S. V. Douglas , and J. P. Smol . 2005. Food web changes in arctic ecosystems related to climate warming. Global Change Biology 11:1381–1386.
- Rautio, M. and W. F. Vincent . 2006. Benthic and pelagic food resources for zooplankton in shallow high-latitude lakes and ponds. Freshwater Biology 51:1038–1052.
- Rautio, M. , S. Sorvari , and A. Korhola . 2000. Crustacean zooplankton and diatom communities, their seasonal variability, and representation in the sediments of subarctic Lake Saanajärvi. Journal of Limnology 59:Suppl. 1 81–96.
- Rouse, W. R. , M. S. V. Douglas , R. E. Hecky , A. E. Hershey , G. E. Kling , L. Lesack , P. Marsh , M. McDonald , B. J. Nicholson , N. T. Roulet , and J. P. Smol . 1997. Effects of climate change on the freshwaters of Arctic and subarctic North America. Hydrological Processes 11:873–902.
- Rühland, K. and J. P. Smol . 2002. Freshwater diatoms from the Canadian Arctic treeline and development of paleolimnological inference models. Journal of Phycology 38:249–264.
- Rühland, K. and J. P. Smol . 2005. Diatom shifts as evidence for recent subarctic warming in a remote tundra lake, NWT, Canada. Palaeogeogaphy, Palaeoclimatology, Palaeoecology 226:1–16.
- Rühland, K. , A. Priesnitz , and J. P. Smol . 2003. Paleolimnological evidence from diatoms for recent environmental changes in 50 lakes across Canadian Arctic treeline. Arctic, Antarctic, and Alpine Research 35:110–123.
- Rühland, K. , J. P. Smol , D. Muir , and X. Wang . 2003. Limnological characteristics of 56 lakes in the central Canadian Arctic treeline region. Journal of Limnology 69:2–27.
- Schindler, D. W. and J. P. Smol . 2006. Cumulative effects of climate warming and other human activities on freshwaters of Arctic and subarctic North America. Ambio 35:160–168.
- Serreze, M. C. and J. A. Francis . 2006. The arctic amplification debate. Climatic Change 76:241–264.
- Serreze, M. C. , J. E. Walsh , F. S. Chapin III , T. Osterkamp , M. Dyurgerov , V. Romanovsky , W. C. Oechel , J. Morison , T. Zhang , and R. G. Barry . 2000. Observational evidence of recent change in the northern high-latitude environment. Climatic Change 46:159–207.
- Smol, J. P. 2008. Pollution of Lakes and Rivers: a Paleoenvironmental Perspective Second edition. Oxford Blackwell. 383 pp.
- Smol, J. P. , A. P. Wolfe , H. J. B. Birks , M. S. V. Douglas , V. J. Jones , A. Korhola , R. Pienitz , K. Rühland , S. Sorvari , A. Antoniades , S. J. Brooks , M. A. Fallu , M. Hughes , B. Keatley , T. Laing , N. Michelutti , L. Nazarova , M. Nyman , A. M. Paterson , B. B. Perren , R. Quinlan , M. Rautio , E. Saulnier-Talbot , S. Siitonen , N. Solovieva , and J. Weckström . 2005. Climate-driven regime shifts in the biological communities of arctic lakes. Proceedings of the National Academy of Sciences 102:4397–4402.
- Sorvari, S. and A. Korhola . 1998. Recent diatom assemblage change in subarctic Lake Saanajärvi, NW Finnish Lapland, and their paleoenvironmental implications. Journal of Paleolimnology 20:205–215.
- Sorvari, S. , A. Korhola , and R. Thompson . 2002. Lake diatom response to recent arctic warming in Finnish Lapland. Global Change Biology 8:171–181.
- SPSS 1998. SYSTAT 8.0 Statistics Chicago SPSS, Inc. 1086 pp.
- Strecker, A. L. , T. P. Cobb , and R. D. Vinebrooke . 2004. Effects of experimental greenhouse warming on phytoplankton and zooplankton communities in fishless alpine ponds. Limnology and Oceanography 49:1182–1190.
- Sweetman, J. N. and J. P. Smol . 2006. Patterns in the distribution of cladocerans (Crustacea: Branchiopoda) in lakes across a north-south transect in Alaska, USA. Hydrobiologia 553:277–291.
- ter Braak, C. J. F. and P. Šmilauer . 2002. CANOCO Reference manual and CanoDraw for Windows user's guide: software for Canonical Community Ordination (version 4.5) Ithaca Microcomputer Power. 500 pp.
- Winder, M. and D. E. Schindler . 2004. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85:2100–2106.
- Wrona, F. J. , T. D. Prowse , J. D. Reist , R. Beamish , J. J. Gibson , J. Hobbie , E. Jeppesen , J. King , G. Koeck , A. Korhola , L. Levêsque , R. Macdonald , M. Power , V. Skvortsov , W. Vincent , R. Clark , B. Dempson , D. Lean , H. Lehtonen , S. Perin , R. Pienitz , M. Rautio , J. Smol , R. Tallman , and A. Zhulidov . 2005. Freshwater ecosystems and fisheries. In . ACIA: Arctic Climate Impact Assessment New York Cambridge University Press. 354–452.
- Yurista, P. M. and W. J. O'Brien . 2001. Growth, survivorship and reproduction of Daphnia middendorffiana in several Arctic lakes and ponds. Journal of Plankton Research 23:733–744.