ABSTRACT
We studied the course of primary succession following deglaciation and the convergence/divergence of plant community development with respect to topographic factors at Koryto Glacier Valley on Kamchatka’s Pacific coast. Vegetation changes over an ∼270-yr-old chronosequence were related to concurrent changes in substrate and soil properties. Ordination analyses showed that substrate texture and topography are the most important environmental factors influencing the course of succession. About 25 yrs after surface deglaciation, belowground stagnant ice melts, and moraine consolidation causes the successional communities to diverge. Species-poor communities, dominated by alder and grasses (Alnus fruticosa, Calamagrostis purpurea), occurred on the fine-grained substrate of moraine crests, while species-rich communities, dominated by legumes and forbs (Oxytropis kamtschatica, Saxifraga species), developed on the coarse-grained substrate of moraine flanks, and in depressions communities dominated by willows and sedges (Salix arctica, Juncus beringensis) developed. In depressions and plains adjacent to the ridges, succession toward Alnus stands is hindered by late-melting snow and flooding. Plant-species richness peaked at the 80-yr-old moraine, but thereafter decreased as the rapid growth of Alnus led to dense stands that dominated resources and inhibited colonization and growth of earlier, as well as later, successional species. Mat-forming capacity, high litter production, an extensive root system, and snow-pressure tolerance enable A. fruticosa to maintain dominance without replacement by Betula ermanii. This potential climax species remains scattered on rock terraces and elevated locations above the valley basin where it escapes snow avalanches and accumulation, a factor responsible for the inversion of vegetation zones in this maritime region.
Introduction
The climate of the northern hemisphere changed over recent centuries. Many records suggest the mid-19th century experienced particularly cold conditions and served as the culmination of the Little Ice Age (CitationMatthews, 1992), whereas temperatures in the 20th century were significantly higher (CitationBriffa et al., 1995), as indicated by the recession of many alpine and arctic glaciers (CitationLawrence, 1958; CitationSolomina et al., 1995). The ecological impacts of these changes are being documented in several terrestrial ecosystems, incorporating detailed botanical field observation (CitationJones and del Moral, 2005; CitationRaffl et al., 2006). We report here on results of a study of long-term vegetation and soil development on moraines deposited by the retreating Koryto Glacier in the Kronotsky Mountains, Kamchatka, which is thought to be the first extensive illustration of ecosystem response to changing climate in this part of the Russian Far East.
Our knowledge about long-term successional trends spanning more than a century is primarily based on studies comparing differently aged stages under similar site conditions (CitationWalker and del Moral, 2003). This comparative (chronosequence) approach is often the only method available when studying an intrinsically slow process like primary succession in alpine or arctic environments (CitationSvoboda and Henry, 1987; CitationJones and Henry, 2003). However, a basic assumption in any chronosequence study is that (1) all progressively older sites are well dated, and (2) the communities at the oldest sites have developed through stages similar to the younger communities (CitationFastie, 1995). There is ample evidence that progressively older sites behind retreating glaciers are associated with a different successional course and rarely converge to only one final community if their environmental conditions vary markedly from one to another (CitationVetaas, 1997). Divergence of seral stages is expected to arise over a broad range of elevations if later species in succession are more sensitive to an elevational gradient than early species (CitationLepš and Rejmánek, 1991). Newly exposed glacial deposits are extreme habitats, characterized by low nitrogen content (CitationKohls et al., 1994), and initial stages of succession host relatively uniform vegetation (CitationMatthews, 1979). Pioneer species usually have high colonization potential and a broader ecological niche. For instance, high-elevation succession on the foreland of Storbreen Glacier in southern Norway slowly progressed from pioneer Poa–Cerastium stages to a Salix–Polygonum snowbed assemblage, while at lower elevation the same initial colonists were succeeded by a Phyllodoce–Salix assemblage over a century, which were replaced successively by Betula–Vaccinium heaths (CitationMatthews, 1979). The present study shows a successional pattern on a glacier forefront with almost no elevational differences across terminal moraine ridges, but with pronounced substrate and microtopographic heterogeneity within particular sites, causing a different pattern of succession to develop over small spatial scales.
Recessions of glaciers are a worldwide phenomenon, but they are particularly frequent and fast in maritime areas. The faster retreat of maritime glaciers relative to land-locked alpine and arctic glaciers has long been recognized (CitationMatthews, 1992). In Kamchatka, there are 448 glaciers with a total area of about 905 km2. More than 80% of glacial area is concentrated in the Central (Serediny) Mountains and the Klyuchevskaya Volcanic Group, where average altitude exceeds 3000 m. About 10% of the glacial area is found in the Kronotsky Peninsula, which is an extinct volcanic massif of Tertiary origin near the Pacific Ocean. It is strongly glaciated, although its summits seldom exceed 1000 m elevation. CitationMuravyev (1999) refers to 32 glaciers that occur as low as 250 m a.s.l. and extend over an area of 92 km2. Such glaciation is the result of a maritime climate with high snow precipitation and plateau-shape landforms that allow glaciers to have extensive accumulation zones (CitationShiraiwa et al., 1997). The Koryto Glacier is the third largest glacier, occupying ca. 7.5 km2. It extends northwest from 1100 m to 300 m elevation with the terminus lying below the treeline. Its fast retreat, about 1000 m over the past 150 years, exposed bare surfaces of varying ages for plant colonization. Their relatively precise dating, combining historical accounts on glacier dynamics during the last 50 years with lichonometric (CitationSolomina and Calkin, 2003) and tephra-layer dating of older moraines (CitationBraisteva and Ponomareva, 1997; CitationYamagata et al., 1999), provided plant ecologists with a rare opportunity to infer the successional changes in species composition and diversity, and patterns and rates of vegetation and soil development.
This study is a result of the joint Japanese-Russian expedition to Kamchatka in 1999, which aimed to describe and explain the pattern of postglacial plant succession at Koryto Glacier Valley. Plant succession was studied indirectly through spatial vegetation pattern, and an attempt was made to relate floristic changes over the 270-yr-old chronosequence to concurrent changes in substrate and soil properties. In this paper, we first reconstruct the course of revegetation, analyze plant species' responses to edaphic heterogeneity, and finally discuss the role of autogenic and allogenic factors in determining successional changes.
Study Area
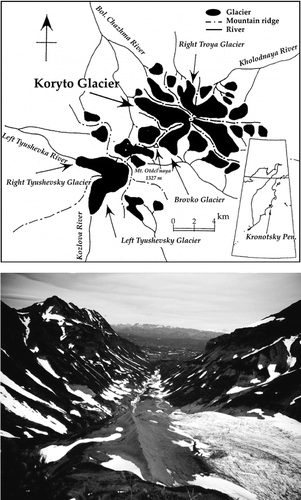
The study site is situated in the eastern part of the Kronotsky Biosphere State Reserve (). The general vegetation description of this area is provided by CitationKuvaev (1993) and CitationYakubov (1997). Koryto Glacier (54°45′N, 161°40′E) is located in the upper part of the Bolshaya Chazma River. It occupies a U-shaped valley bounded by lateral moraine slopes and cliffs that rise up to ca. 1200 m a.s.l. The elevation of the present terminus is about 320 m. Snowfields that persist into late summer are a characteristic feature of the study area. A mosaic of deciduous Betula ermanii forests, and Alnus fruticosa and Pinus pumila thickets are typical for eastern Kamchatka (CitationKhomentovsky, 1998; CitationKrestov, 2003). The valley walls above the deglaciated foreland are mostly covered with Alnus fruticosa. The parent materials are composed of pillow lava and hyaloclastite of submarine volcanic origin overlain by heterogeneous glacio-fluvial sediments, and long distance transported volcanic ash. The maritime climate consists of short, cool summers and mild snowy winters. Climatic data taken from the nearest meteorological stations at Stopozh (1939–1997) and Koryto Glacier (1996–1997) show a mean annual temperature of about 1°C. The monthly minimum (February) and maximum (July) temperatures are −22.6 and 17.4°C, and mean winter and summer temperatures are −7.5 and 8.2°C, respectively. Most precipitation in the Kronotsky Peninsula falls as snow and comes from the Pacific. On average, the study area receives 2.5–3 m winter snowfall and 0.4 m summer rainfall. Snowpack at accumulation zones is 5–10 m deep. Deep snow accumulates mainly in leeward valleys where it persists into late summer and reduces time for plant growth.
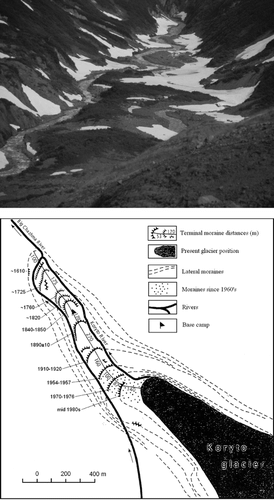
FIGURE 1 (Top) Location of the Koryto Glacier in the Kronotsky Peninsula, eastern Kamchatka. (Bottom) Northwestward view of the Koryto Glacier Valley in mid-August 1999.
Since Kamchatka lies between the Siberian High and Aleutian Low, the annual amount of precipitation and consequently fluctuation of glaciers depends on variation of these pressure systems. From the late 1970s, for instance, the Siberian High invaded Kamchatka, to shift the Aleutian Low into Alaska, with the resulting positive balance of its coastal glaciers, while a negative trend prevailed over Kamchatka (CitationMcCabe and Fountain, 1995). During the warm second half of the 20th century, the surface of the Koryto Glacier decreased by about 35 m, its front retreated about 310 m, and the glaciated area decreased from 8.9 to 7.55 km2 (Muravyev, 1999). This rapid recession was stopped by a positive snow balance in 1954–1957, 1970–1976, and 1984–1985, when the glacier slightly readvanced, depositing three terminal moraine ridges that were about 330, 230, and 140 m distant from the glacier terminus in 1999 (). The moraine ridge located 490 m from the present terminus is 70–80 years old, as inferred from ring counts (60–70 rings) of the largest Alnus, and taking into account the time (10–15 yrs) it needs to establish on new substrate. This corresponded with an age estimate of 83 yrs obtained from Rhizocarpon growth curves (CitationSolomina and Calkin, 2003). The moraine situated 930 m from the glacier terminus must have been deposited shortly before 1854, as it is overlain directly by the ash deposited on this date by the Shiveluch Volcano. The Shiveluch 1854 tephra layer was absent on the next moraine ridge, ∼120 years old, with a till deposit overlain by about 3 cm of loamy soil. Overall, the Koryto Glacier retreated 1300 m since the beginning of the 17th century, and 930 m since the middle of the 19th century.
Methods
Access to the Koryto Glacier is logistically difficult, and the use of a helicopter is necessary. Field work was conducted in August 1999 by placing sampling plots on the 5-, 15-, 25-, 40-, 80-, 120-, 180-, and 270-yr-old moraines comprising the major stages of vegetation development. These were chosen on the basis of known age and adequate area. Plant composition and abiotic factors were surveyed in 2.5-m × 2.5-m plots, placed at 15-m intervals along line transects crossing the moraines. Transects were oriented along moraine ridges to cross all major plant associations. There were 106 relevés recorded in total. Vascular plants were identified to species, their percent cover estimated, and the tallest individuals measured for height. Percentage area covered by sand (<0.4 cm), gravel (0.5–5 cm), and boulders (stones > 5 cm in diameter) was estimated and the slope measured in each plot. Soil and litter depths were measured at two random points in plots with developed organic horizons. Total soil carbon and nitrogen concentrations were analyzed in the topsoil and mineral soil, but only from one profile within each moraine due to field-time restrictions ().
TABLE 1 Total carbon and nitrogen concentrations (mg g−1) from soil horizons within moraines of the Koryto Glacier foreland. C—soil parent material just above bedrock, B—illuvial horizon, A—eluvial dark colored horizon, L-H—litter/humus horizon.
In the search for mechanisms responsible for declining species richness later in succession, an attempt was made to relate the turnover of species to life-history traits of the dominant shrub Alnus fruticosa. We first compared species richness and diversity values, and edaphic parameters between Alnus-dominated stands (coverage > 40%) and stands where Alnus coverage and, hence, competitive pressure was much lower. Subsequently, stem height and maximum diameter were measured for 3 shrubs in each Alnus-dominated plot (42 in total). From these shrubs, the largest individual was selected at each moraine for tree-ring analyses. Disks were taken at the stem base, and then at 70 and 140 cm to determine radial and height growth patterns. To quantify if a decline in radial growth occurs in shrubs, individual cumulative growth curves, representing a general size-age relationship, were fitted with simple linear and non-linear logistic models. To assess the specific leaf area (leaf area per unit dry weight) and total foliar carbon and nitrogen concentration in these shrubs, 10–15 leaves were collected from the upper, well-lit crown regions (504 leaves in total). After returning to the Sapporo laboratory, leaf blades were measured using a LI-COR-3100 area meter, oven-dried at 70°C, weighed individually, combined by plot, ground into powder, and analyzed using a C-H-N-S analyzer (EA 1108, Carlo Erba Instruments, Italy). Stem disks were dried, smooth-cut, and sanded, and tree-ring widths measured to the nearest 0.01 mm with the aid of a microscope interfaced to a computer. Since it was difficult to synchronize accurately disks from our shrubs due to irregular growth and the occurrence of some missing rings, we refrained from cross-dating tree-ring sequences, but were still able to estimate shrub age and calculate mean ring width for each disk. For between-site comparisons, we used ring widths from disks taken at 70 cm (except for <70-cm-tall Alnus on the 25-yr-old moraine), to avoid the occurrence of too wide rings at the base of old leaning Alnus stems, which are associated with the creation of tension wood. Temporal trends (increase, decrease, or peak) in Alnus traits were tested using GLM in S-plus (CitationCrawley, 2002). Second order polynomials were used to assess the peaks along the chronosequence. Finally, we dug out 27 small- to medium-sized (from 3 to 81 cm tall) Alnus fruticosa individuals at the 80-yr-old moraine and measured their maximum stem diameter, crown height, and diameter in two perpendicular directions, and root length, width, and depth.
Three major factors are thought to limit species diversity in plant communities: harshness of the environment, competitive exclusion, and species pool limitation (Citationdel Moral and Ellis, 2004). To assess the pool of available species and its relationship to primary successional communities, a floristic survey was conducted on both sides of Koryto Glacier Valley. This included recording the species encountered and making standard relevés in various plant communities (Dolezal, unpublished data). Since travel by foot was slow due to rough terrain and not all areas were possible to visit, it was necessary to supplement our records by unpublished data collected by Yakubov in 1981. The complete floristic list is available upon request.
Data Analysis
To analyze the variability in plant species composition, both classification and ordination methods were used. TWINSPAN cluster analysis (CitationHill and Šmilauer, 2005) with standard default values was used to generate a floristic classification. The vegetation was divided after three levels of division into eight ecologically interpretable groups. For each community type, we calculated a mean value of plant cover, environmental characteristics (percent proportion of sand, gravel, etc.), species richness as the total number of vascular plant species present in a plot, Shannon diversity and evenness, Simpson dominance, and percent share of seven growth forms: (1) shrubs, (2) subshrubs, (3) perennial nonlegume forbs, (4) legumes, (5) annuals, (6) grasses, and (7) rushes and sedges (graminoids). Growth form proportions were weighted by species cover-abundance in each plot, and then averaged for a plant community. The heterogeneity of the vegetation might be an important factor increasing the species richness of vegetation at larger spatial scales. We characterized heterogeneity by averaging the standardized Euclidean distance (chord distance) between all pairs of plots within each community type derived from TWINSPAN.
Detrended Correspondence Analysis (DCA) and Canonical Correspondence Analysis (CCA) were used to analyze vegetation responses to environmental gradients; both methods were conducted using CANOCO (Citationter Braak and Šmilauer, 1998). The use of these ordination methods is based on the assumption of unimodal species response (the lengths of the 1st and 2nd DCA axes were 3.97 and 3.82, respectively, which permits the use of unimodal methods). Due to the presence of many rare species with very small cover, the option “downweighting of rare species” was used (see Citationter Braak and Šmilauer, 1998, for technical details). The constrained and unconstrained methods are complementary: DCA axes correspond to the dominant gradients in species composition, whereas CCA axes to gradients in species composition best correlated with measured environmental factors. We used DCA to visualize a pattern of similarity between samples and their relationships to environmental factors. In CCA, the significant environmental factors obtained by forward selection were used as explanatory variables. Age since deglaciation, slope inclination, maximum vegetation height, and soil and litter depth were log-transformed to improve normality and homogeneity of variance before analysis. Percentage values were arcsin-square-root transformed. We used constrained ordinations (1) to determine zones of plant species optimum performance with respect to surface age and site characteristics (substrate texture, slope, etc.); (2) to ascribe the explained variability to particular variables using a variance partitioning procedure in which case the factors not used as predictors were defined as covariables, to remove their effects and obtain a net effect of individual factor (using this approach we constructed tests analogous to the testing of particular terms in ANOVA models but for multivariate data); and (3) to determine what site characteristics best determine species composition on particular moraines. Monte Carlo permutation tests (999 permutations) were used to assess the significance of the relationships found in multivariate analyses.
Result
Soil Development
The deglaciated forefront had distinctive terminal moraine ridges located entirely below the treeline, with only slight elevational differences (). All studied terminal moraines on the valley bottom were at about 300 m a.s.l. The soil layer thickness on the till deposits increased with moraine age. The older moraines (≥80 yrs) had volcanic ash deposits (tephra layers) with developed soil horizon, while younger moraines (≤40 yrs), to a distance 330 m from the glacier front, were pure mineral substrate. Organic carbon and total nitrogen levels showed marked changes during succession (), from low contents in the bare soil followed by rapid increases in the older soils, probably due to the presence of Alnus and other plants capable of fixing atmospheric nitrogen.
Plant Communities
Within the 106 2.5 × 2.5 m surveyed plots, 85 vascular plant taxa were recorded, belonging to 60 genera and 31 families, which is 29.4% of species present in the Koryto Glacier Valley. The local species pool consisted of 289 vascular plants, 158 genera and 53 families (total list of vascular plants for the Kronotsky Biosphere Reserve includes 728 species, 308, genera and 86 families, while the flora of vascular plants of Kamchatka includes 1166 authentically registered species and subspecies, 410 genera, and 89 families. Of species participating in the succession, 95.2% were perennial plants (5 shrubs, 12 subshrubs, 46 nonlegume forbs, 3 legumes, 8 grasses, 4 graminoids, and 3 ferns) and only four species were annuals: Boschniakia rossica (Cham. & Schlecht.) Fedtsch. (Orobanchaceae), an achlorophyllous parasitic plant, entirely dependent on its Alnus host; Erigeron caespitans Komar.; Erigeron kamtschaticus DC.; and Stellaria calycantha (Ledeb.) Bong., but these are also referred to as annual to perennial.
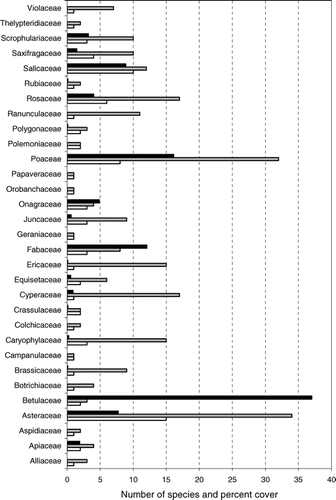
The most frequently occurring species present in more then 40% of the surveyed plots were Poa malacantha (78.3%), Alnus fruticosa (60.3%), Chamaenerion latifolium (64.2%), Veronica grandiflora (54.7%), Aruncus dioicus (50%), Artemisia opulentha (48.1%), and Salix arctica (43.4%). The most species-rich plant families were Asteraceae (14), Salicaceae (10), and Poaceae (8 species) (). The most abundant plant families were Betulaceae (37% of total vegetation cover, most of which was due to Alnus fruticosa), Poaceae (16.1%), Fabaceae (12.1%) and Salicaceae (8.9%).
FIGURE 3 List of plant families participating during the succession with the number of species (white bar) and share of total vegetation cover (black bar). Also shown is the number of species for each family in the local species pool (gray bar).
Eight vegetation units were distinguished on the basis of the TWINSPAN analysis. shows the list of 32 major species and their frequencies in the respective clusters. summarizes their main ecological and floristic characteristics. These communities are as follows:
TABLE 2 Species frequency of occurrence (% of plots in each community for 32 major species from a total of 85) for the eight vegetation communities on the Koryto Glacier foreland.
TABLE 3 The eight vegetation communities derived from TWINSPAN and their characteristics: age of moraines on which they were located, mean (±SD) and maximum number of species per 2.5-m × 2.5-m plots, Shannon diversity and evenness, Simpson dominance, vegetation disimilarity (Chord distance), % proportion of seven growth forms, mean plant cover, and substrate and edaphic characteristics.
Epilobium hornemanni–Poa malacantha unit represented the pioneer stage that dominated initial succession up to 10–15 yrs, characterized by scattered individuals of wind-dispersed species, whose colonization was concentrated where some ameliorating factor occurred. The pioneer vegetation had the lowest mean plant cover and thus the greatest amount of bare area, the lowest richness but the second highest equitability, apparently due to similar percent cover (0.02–0.1%) among species. These plots had the highest proportion of gravel. Cryptograms, usually the primary colonists on freshly exposed nutrient-depleted moraines, were missing in our pioneer community, except for some scattered individuals of the lichen Stereocaulon pascale.
Chamerion latifolium–Sagina saginoides unit occurred on slightly older but still unconsolidated deposits (15–25 yrs old). This unit remains affected by disturbances associated with moraine consolidation, melting of belowground stagnant ice, freezing/thawing cycles, etc., so that plant cover was still low, being 9% on average. Although they still contained stagnant ice, these moraines were colonized by the first individuals of Alnus fruticosa, a native actinorhizal nitrogen fixer, roughly 10–15 yrs after surface deglaciation, as indicated by tree-ring analysis (). Of the eight TWINSPAN communities, this type had the lowest diversity and highest Simpson dominance due to the prevalence of only a few species. Small patches of Chamerion latifolium, Poa malacantha, Sagina saginoides, and Alnus fruticosa, typical of this vegetation unit, occurred also on older substrates (25–40 yrs old), confined to depressions between moraine ridges disturbed by erosion associated with late-melting snow and regular floods.
Salix arctica–Alnus fruticosa unit was a type of vegetation representing a transition from the initial pioneer stages to Alnus dominated stands. It first appeared on the 25-yr-old moraine in a few spots, in particular on the fine-grained substrate of the moraine crest. This unit was the major vegetation on the 40-yr-old moraine, where the substrate stabilized after belowground stagnant ice had melted, and a typical toposequence appeared, composed of a flat moraine crest, dry stony flanks, and floodplains. The ground surface of the moraine crest was covered with clumps of shrubs, forbs, and grasses, clustered around young Alnus fruticosa individuals. Facilitation may have occurred through both physical protection and increased nitrogen availability. The common associates included Salix chamissonis, Juncus beringensis, Salix udensis, Salix pulchra, Astragalus alpinus, Veronica grandiflora, Cardaminopsis lyrata, and Saxifraga porsildiana. Of the eight TWINSPAN communities, this type had the highest species richness. This community remained also on older moraines (80–120 yrs old), in depressions with moist soil where the water table is temporarily high or reaches the surface, and on slopes adjacent to flat moraine crests, with marked surface erosion and a high proportion of sand and a lower proportion of gravel and large boulders.
Oxytropis kamtschatica–Veronica grandiflora unit was represented by spatially distinct species-rich vegetation dominated by legumes and grasses that developed on the coarse-grained substrate of moraine flanks and well-drained boulder fields that accumulated at the bottom of the flanks. Common associates were Saxifraga funstonii, Artemisia opulentha, Trisetum spicatum, Solidago spireifolia, Saussurea pseudo-tilesii and Saxifraga cherlerioides. This unit, which first appeared on the 40-yr-old moraine, and then was repeatedly sampled on all older moraines, had the second highest species richness and Shannon diversity, low Simpson dominance, intermediate values of plant cover, and shallow litter layer.
Artemisia opulentha–Alnus fruticosa unit represented the young Alnus thickets (52% mean cover and 1.5 m mean stand height) on the 80-yr-old moraine crest with species rich understory vegetation (13–21 species/plot, 40 species in total). Common associated species in this community were Poa platyantha (7.3% mean cover), Veronica grandiflora (8.9%), and Aruncus dioicus (5.3%).
Salix arctica–Chamerion angustifolium–Alnus fruticosa unit occurred repeatedly on older moraines (120–270 yrs) and represented a transitional assemblage which evolved in the contact zones of moraine flanks below the main ridges; these are composed of deep litter and soil layers and a low percentage of bare ground. The three major species had similar cover (15–25%, 1.1 m mean stand height) and their frequently associated species were Saussurea pseudo-tilesii (8.5%) and Calamagrostis purpurea (5.9%).
Aruncus dioicus–Alnus fruticosa unit represented a dense and tall Alnus thicket (65% mean Alnus cover and 3.3 m mean stand height) on the 120-yr-old moraine crest with low light levels and species-poor vegetation in the understory (6–13 species/plot, 16 species in total). Common associates included Heracleum lanatum (3.8%), Salix pulchra (8.8%), Saussurea pseudo-tilesii (4.5%), and Calamagrostis purpurea (2.5%).
Calamagrostis purpurea–Alnus fruticosa unit represented the “final stage” of succession. These old and relatively opened thickets (51% mean Alnus cover and 2.1 m mean stand height), composed of several generations of shrubs, occurred along narrow ridges on the 180- and 270-yr-old moraines and were characterized by species poor understories (4–12 species/plot, 19 species in total) dominated by 60 cm tall C. purpurea grass.
TABLE 4 Between-site comparison of Alnus fruticosa leaf traits (mean ± SE), ring counts (stem base, 70 and 140 cm), and mean ring width (min–max). Shown are also vegetation and soil characteristics for two types of stands A: Alnus coverage > 40%, and B: Alnus coverage < 40%. ns—non-significant differences among moraines (P > 0.05), ▴significant increase, ▾decrease, or • peak in the values of tested characteristics.
Species-Environment Relationships
The DCA on species percent cover provided the basic overview of the compositional gradients in the data. The TWINSPAN communities in the ordination diagram maintained their coherency (). The first two ordination axes accounted for 12.9 and 9.7% of variability, respectively. A high value of species-environment correlations on the first two DCA axes (r = 0.94 and 0.88) revealed that the selected environmental factors were strong determinants of species variation in the data set. The relatively high proportion of variance explained by axis 2 suggests that there is no single dominant gradient. The first gradient in species composition was the successional change from pioneer assemblages on fresh deposits with Chamerion latifolium and Sagina saginoides through the progression of revegetation on flat morainal crests dominated by Alnus fruticosa, where the undergrowth dominants change over time from Artemisia opulenta and Aruncus dioicus to Heracleum lanatum and Calamagrostis purpurea (). The second floristic gradient was associated with the pronounced substrate and microtopographic heterogeneity within particular sites, i.e. with compositional change from Oxytropis-dominated boulder fields at the moraine bottoms, over transitional assemblages evolved in contact zones of substrate types, to Alnus-dominated stands on the fine-grained substrate of ridge crests. Most investigated explanatory variables were correlated with both ordination axes (), indicating that the gradient at a small spatial scale (within-moraine toposequences) resembles the broad-scale gradient (chronosequence across moraine ridges).
FIGURE 4 DCA–ordination diagrams: (a) pattern of compositional similarity between samples of successional vegetation (different symbols denote different Twinspan communities [1-○, 2-□, 3-◊, 4-l, 5-•, 6-▪, 7-♦, 8-□]; the first two ordination axes accounted for 12.9 and 9.7% of variability, respectively); (b) successional changes in species diversity; (c) plant species optimum performance; and (d) relationships to environmental factors and proportions of seven growth forms, with isoclines of species richness of samples.
![FIGURE 4 DCA–ordination diagrams: (a) pattern of compositional similarity between samples of successional vegetation (different symbols denote different Twinspan communities [1-○, 2-□, 3-◊, 4-l, 5-•, 6-▪, 7-♦, 8-□]; the first two ordination axes accounted for 12.9 and 9.7% of variability, respectively); (b) successional changes in species diversity; (c) plant species optimum performance; and (d) relationships to environmental factors and proportions of seven growth forms, with isoclines of species richness of samples.](/cms/asset/4d809111-1ad9-4969-a37b-7c3a12fc1771/uaar_a_11957319_f0008.gif)
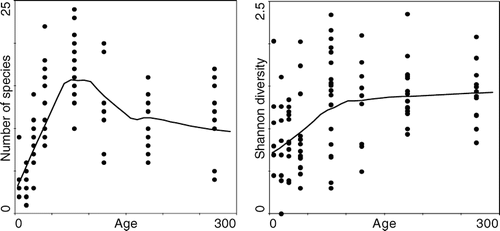
The CCA on species percent cover with the habitat variables is summarized in . In the analysis of all plots, CCA showed that 28.5% of the compositional variability was explained by 8 significant factors selected by forward selection out of 9 explanatory variables tested (P = 0.001). The variance partitioning procedure (, analyses 1–9) revealed that stand height is the most important factor for species composition and was positively correlated with the first axis, followed by percent proportion of boulders, sand, substrate age, litter depth, total plant cover, percent proportion of gravel, and slope inclination. Several investigated habitat variables are closely correlated, as expected from their causal relationships (e.g. soil depth is a function of substrate age and litter accumulation); consequently, after litter depth and age were selected, the conditional effects of soil depth decreased dramatically. In fact, the conditional effects of all investigated variables were significant by Monte Carlo permutation test (p < 0.05), except for soil depth (p = 0.097). The separate CCA analyses for individual moraines (analyses 10–16) showed that, in the early years after deglaciation, the relationship between species composition and substrate texture was non-significant and the only significant variable was surface age of moraines, which explained 10.9% of the variation in the species data. However, environmental determination of plant community composition increased with increasing moraine age, causing differentiation of successional communities (). Percent boulder and gravel proportions are two significant factors on the 40-yr-old moraine, clearly separating two plant assemblages (Twinspan community types 3 and 4). Interestingly, at this stage, the sparse vegetation with Alnus fruticosa has higher species richness (and even the highest richness recorded along the chronosequence) than the community with Oxytropis kamtschatica, while the reverse pattern is typical of older moraines (), where Alnus is taller with greater cover abundance, thereby decreasing understory species richness. Overall, species richness of vascular plants showed a unimodal relationship with the surface age of moraines (). Mean number of plant species increased within the first 80 yrs, i.e. accumulation rather than species turnover prevails within this period. Subsequently, a pronounced decrease in species richness was found with stand age and Alnus canopy consolidation. In the plots where Alnus dominance is lacking (vegetation of moraine flanks and boulder fields), species richness with moraine age decreased much less (). Compositional heterogeneity evaluated as the mean Chord distance varied widely, showing no simple relationship with successional age (). It increased beneath the closed Alnus canopy on the 120-yr-old moraine, as the undergrowth species do not form compact patches. A decline of heterogeneity in the oldest and more open Alnus stands, being composed of several generations, reflects the consolidation of established clonal dominants, such as Calamagrostis purpurea.
TABLE 5 Canonical correspondence analyses of compositional variability in primary successional vegetation. Analyses were conducted for all plots (1–9) and for each moraine separately (10–16). Explained variability and significance levels (*** P < 0.001, ** P < 0.01, * P < 0.05) are shown for the marginal effects—variability explained by a given factor without considering other factors, and the partial (net) effects—variability explained by a given factor after accounting for the effects of other factors (covariables). In analyses 10–16, significant factors selected by forward selection are shown.
Life-history analysis for Alnus revealed a peak in SLA and total foliar nitrogen concentration at the 80-yr-old moraine, and a decrease towards both younger and older substrates. Foliar carbon concentrations showed a peak at the 120-yr-old moraine (). Shrub age, leaf size and stem diameter increased steadily with surface age of the moraines. Maximum height of Alnus stands peaked at the 120-yr-old moraine and then declined as old and senescent shrubs became more leaned. Tree-ring analysis showed that all shrubs from differently aged moraines still grow actively in stem diameter, and thus have no apparent asymptote (figure not shown). Their individual cumulative growth curves were thus better fitted with the linear than logistic model. Alnus also exhibited steady height growth, reaching 140 cm at about 10 years (). Alnus also creates an extensive root system: the 27 excavated individuals had an average height (min–max) of 22.6 cm (3–81), mean diameter of 7.8 mm (1.6–15.5), mean crown length of 30.9 cm (6–90), and crown width of 27.2 (4.8–78), while the roots had a mean depth of 24.5 cm (9–63), root length of 37.5 cm (2.1–160), and root width of 23.9 cm (3–115).
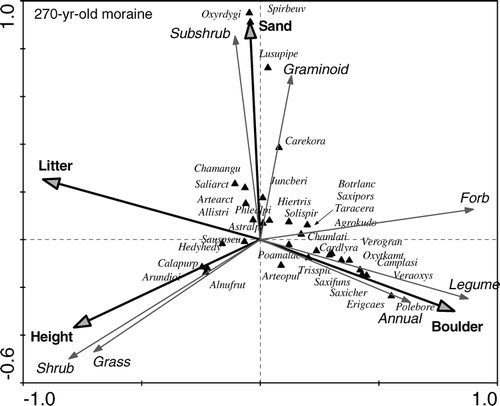
FIGURE 5 CCA ordination diagram displays the relationship between species cover and significant environmental factors on the 270-yr-old moraine. Growth form proportions were made passive so as not to influence the ordination axes, but their relation to dependent and explanatory variables can be judged from the ordination diagram. For abbreviation of species names see Table 2. The remaining species: Phlealpi—Phleum alpinum L., Allistric—Allium strictum Schard., Oxyrdygi—Oxyria dygina L., Spirbeau—Spirea beauverdiana Scheid., Taracera—Taraxacum ceratophorum (Ledeb.) DC., Botrlanc—Botrychium lanceolatum (Gmel.) Angstr., Luzupipe— Luzula piperi (Cov.) Jones., Agrokudo—Agrostis kudoi Honda, Camplasi—Campanula lasiocarpa Cham., Veraoxys—Veratrum oxysepalum Turcz., Polebore—Polemonium boreale Adams.
Discussion
The floristic composition of same age terrain on the foreland of the Koryto Glacier markedly changed from wet depressions, over stony flanks, to flat ridge crests. Thus, several spatially distinct assemblages of vegetation evolved simultaneously (cf. CitationVetaas, 1997). Their spatial distribution was primarily controlled by topography and substrate characteristics. These factors largely influence moisture availability, soil development, organic matter accumulation, and length of growing season due to timing of snowmelt. The progression of revegetation in depressions and plains adjacent to the ridges was retarded by long-persisting snowpack and river floods. Elevated ridges represented the least limiting sites for both soil and plant development. Compared to other habitats, these areas are less likely to have differed with respect to their successional pattern.
Given the limited time available for fieldwork, not all combinations of plant species were surveyed; for instance, sparsely vegetated floodplains and depressions between old moraine ridges, because they were still under snow cover during our fieldwork (). In fact, the previous winter was extremely snowy, resulting in snowfields persisting into very late summer. Despite this, several important successional stages were described on this foreland. During the first 5–15 yrs after deglaciation, recently exposed surfaces of unconsolidated deposits are colonized by pioneer forbs and grasses (Sagina saginoides, Epilobium hornemanii, Festuca rubra, Poa malacantha). From this initial pioneer stage, there is a gradual transition to a willow-alder stage after 25 yrs, where the ground surface is covered with scattered clumps of shrubs, forbs and grasses. About 25–40 yrs after surface deglaciation, belowground stagnant ice melts out, and moraine consolidation causes the successional communities to diverge. On the fine-grained substrate of moraine crests, Alnus increases in abundance to form dense thickets 60–80 yrs after deglaciation. Oxytropis kamtschatica–dominated communities with higher species richness evolved on the coarse-grained substrates of moraine flanks, while willow-dominated communities developed in depressions between moraines with late-melting snow. Successional communities after 40 yrs are dependent on soil characteristics (litter depth) and topography and, consequently, environmental determination of plant community composition increases over the course of succession and causes the communities to diverge. Different successional seres form a complex pattern in close proximity, with transitional assemblages evolved in contact zones of substrate types.
FIGURE 6 Changes in recorded number of species and calculated Shannon diversity indices with surface age of moraines. The curves were fitted by local polynomial regression fitting (loess).
A number of studies have shown that primary succession proceeds from communities, where species composition is determined by diaspor availability (colonization potential; CitationFastie, 1995), presence of microsites suitable for germination (CitationJumpponen et al., 1999), or stochastic factors such as favorable climatic events (Citationdel Moral et al., 1995), towards communities where species composition depends on macroclimate and relief (CitationSvoboda and Henry, 1987), soil properties as well as the species ability to avoid competitive exclusion (Citationdel Moral et al., 2005). Moreover, several empirical studies (e.g. CitationChapin et al., 1994) have demonstrated that the pattern of succession is mainly a consequence of the prevailing strategies of constituent species. Symbiotic nitrogen fixers are often keystone species in early primary succession (CitationBlundon and Dale, 1990) with the potential to facilitate colonization of later successional species by increasing soil fertility; however, their dense stands often inhibit or even block further progress. Alnus fruticosa is a prominent species in early primary succession in Kamchatka (CitationHulten, 1924). This indigenous woody shrub is common throughout NE Eurasia and forms dense thickets over large areas of alpine and maritime sites (CitationKrestov, 2003). Good seed dispersal and symbiosis with nitrogen-fixing bacteria allow A. fruticosa to colonize infertile glacial or volcanic substratum early after deposition (CitationGrishin et al., 1996; CitationSolomina and Calkin, 2003). Our tree-ring analysis suggests that Alnus fruticosa enters the successional trajectory on the Koryto Glacier moraines approximately 10–20 yrs after surface deglaciation. Scattered patches originating from these early colonizers tend to coalesce during the following 50–100 yrs, particularly on the consolidated substratum of elevated moraine ridges. A mat-forming capacity, high litter production, an extensive root system, and snow-pressure tolerance are life-history traits that likely enable A. fruticosa to maintain dominance without an eventual replacement by Betula ermanii Cham. This climax tree species in subalpine forests and particularly snowfall areas throughout Kamchatka (CitationShamshin, 1999) remains rare on the youthful surfaces and steep slopes surrounding the Koryto Glacier. It occurs on scattered rock terraces and elevated locations above the valley basin where it escapes snow avalanches.
Vascular plant species richness on this foreland increased within the first 80 yrs, i.e. accumulation rather than turnover of species prevailed until Alnus fruticosa formed a dense canopy. Increase in species richness in the early stages of succession, starting on nutrient-poor substrate, is usually related to increasing soil organic matter (CitationReiners et al., 1971), presence of pioneer plants with N-fixing symbionts (CitationKohls et al., 1994), as well as decreasing physical stress as early colonizing organisms ameliorate site conditions (CitationHunter and Aarssen, 1998). Decreasing species richness in older stages is usually ascribed to increasing asymmetry of competition for resources that causes local extinction of some species (CitationRejmánek and Rejmánková, 2002). In the early stages of this succession, Alnus seemed to increase diversity by facilitating colonization of other species, but later rapid growth of Alnus led to dense stands that dominate resources and inhibit colonization and growth of earlier as well as later successional species. Moreover, the decline of species richness in the oldest Alnus stands composed of several generations of shrubs was accompanied by decreasing compositional heterogeneity. The decline of heterogeneity reflects consolidation of established understory clonal dominants, such as Calamagrostis purpurea, that suppress simultaneously species richness and heterogeneity by forming compact patches.
Most pioneer species possess some mode of vegetative reproduction. The importance of clonal reproduction and spread in succession has been suggested to increase both in early stages due to the rapid capture of available space, and then in late-successional stages when establishment from seed becomes limited by dense cover and a compact litter layer (CitationPrach and Pyšek, 1994). Available space was not likely the main reason for the presence of clonal plants on fresh deposits. Probably these perennials with sufficient belowground resources can more easily tolerate frequent and intense disturbances associated with freezing and thawing processes, stone avalanches, flooding, and late-melting snow than annual species. For example, Sagina saginoides has a tap root that serves as a storage organ and vascular link between shoots, which emerge from the senescent and disintegrating tap root causing plant fragmentation (CitationKlimeš et al., 1997). Other pioneer species on fresh deposits were Chamerion latifolium, a long-lived clonal herb that grows as small clumps of connected shoots, and Epilobium hornemanii, with short-lived epigeotropic rhizomes serving as storage organs and as a bud bank. These species have a rather limited ability for horizontal vegetative spread compared to late-successional species such as Chamerion angustifolium, which is a root-sprouting perennial that forms clones over large areas, or Aruncus dioicus and Calamagrostis purpurea, understory dominants of Alnus thickets, that can spread over long distances by hypogeotropic rhizomes. This supports the concept that species capable of “guerilla” type of growth are more successful later in succession than “phalanx” species, because they produce widely spaced modules with a greater chance of penetrating into closed vegetation and deep litter (CitationRydin and Borgegard, 1991).
The lack of Betula ermanii adult trees on this foreland is unlikely due to dispersal and recruitment limitations or insufficient time since deglaciation. We regularly found Betula seedlings on sparsely vegetated younger moraines, while older individuals were found on the oldest moraines, usually on spots where Alnus was missing or its cover was low; no one was taller than 2 m, indicating that small Betula trees were under snow cover during winter, thus protected against avalanches. Consequently, on the narrow deglaciated foreland of the Koryto Glacier, local site conditions prevent succession towards Betula dominance, especially frequent avalanches that probably kill all trees that protrude above snowpack during winter. The shorter growing seasons due to the longer snowpack duration in the valley basin may also play a role. Hence, if the climate becomes drier with less snow precipitation, Betula can eventually overgrow Alnus and become dominant.
Conclusion
In accordance with previous studies (e.g. CitationLepš et al., 2000), succession on fine-grained substrate resulted in species-poor vegetation dominated by Alnus fruticosa, while succession on coarse-grained substrate led to a species-rich community without a strong dominant. Our observation suggests that Alnus was first involved in nucleation, as pioneer forbs and grasses on young moraine ridges occurred near shrubs. Thus, the first vegetation with Alnus was more diverse than the Oxytropis boulder field vegetation. Facilitation by Alnus may have occurred through both increased nitrogen availability and physical protection. Shrubs probably serve as a trap and shield against the strong katabatic wind which blows from the glacier and erodes the fine surface material, delaying the start of soil formation (CitationYamagata et al., 1999). The shrubs catch eolian particles, prevent soil erosion, and enable plant colonization. Later in succession, Alnus became a strong competitor and only a few species able to tolerate low light levels and deep litter remained in the shrub thickets. The higher species richness on coarse-grained soils can be explained by the following factors simultaneously: (1) the low Alnus cover due to the inability to create an extensive root system on boulder fields, (2) the shorter growing season due to longer snowpack duration limiting shrub growth, or (3) soil and plant erosion caused by snow and water actions outcropping base-rich substratum, which can be beneficial for rare plant species (CitationGrishin et al., 1996). An interesting property of this moraine ecosystem is that high snow accumulations and avalanches in certain environments (boulder fields, moraine flanks, and depressions) prevent Alnus from becoming dominant, but in other environments (moraine crest) help maintain its dominance by blocking succession toward Betula forest. The resulting landscape mosaic with inversion communities of Betula ermanii on elevated locations and the warmest slope positions, and Alnus fruticosa (or communities of tall herbs) on snow-accumulation sites are similar to the general vegetation pattern for maritime eastern Kamchatka (CitationKrestov, 2003).
Acknowledgments
We thank A. Ovsanikov, K. Yamagata, and T. Sone for their field assistance, and the members of the Kamchatka Institute of Ecology and Nature Management in Petropavlovsk-Kamchatsky for their hospitality and logistic support. This study was supported by a Monbusho Grant-in-Aid for International Scientific Research (11691166) from the Ministry of Education, Science, Sports and Culture of Japan. Dolezal was supported during the elaboration of this paper by GAČR 206/05/0119 and GA AV IAA600050802.
References Cited
- Blundon, D. J. and M. R. T. Dale . 1990. Dinitrogen fixation (acetylene reduction) in primary succession near Mount Robson, British Columbia, Canada. Arctic and Alpine Research 22:255–263.
- Braisteva, O. A. and V. V. Ponomareva . 1997. Holocene key-marker tephra layers in Kamchatka, Russia. Quaternary Research 47:125–139.
- Briffa, K. R. , P. D. Jones , F. H. Schweingruber , S. G. Shiyatow , and E. R. Cook . 1995. Unusual twentieth-century summer warmth in a 1,000-year temperature record from Siberia. Nature 376:156–159.
- Chapin III, F. S. , L. R. Walker , C. L. Fastie , and L. C. Sharman . 1994. Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecological Monographs 64:149–175.
- Crawley, M. J. 2002. Statistical Computing: an Introduction to Data Analysis Using S-Plus Chichester J. Wiley.
- del Moral, R. and E. E. Ellis . 2004. Gradients in heterogeneity and structure on lahars, Mount St. Helens, Washington, USA. Plant Ecology 175:273–286.
- del Moral, R. , J. H. Titus , and A. M. Cook . 1995. Early primary succession on Mount St. Helens, Washington, USA. Journal of Vegetation Science 6:107–120.
- del Moral, R. , D. M. Wood , and J. H. Titus . 2005. Proximity, microsites, and biotic interactions during early succession. In Dale, V. H. , F. Swanson , and C. Crissafuli , editors. Ecological responses to the 1980 eruption of Mount St. Helens New York: Springer-Verlag. 93–110.
- Fastie, C. L. 1995. Causes and ecosystem consequences of multiple pathways of primary succession at Glacier Bay, Alaska. Ecology 76:1899–1916.
- Grishin, S. Y. , R. del Moral , P. V. Krestov , and V. P. Verkholat . 1996. Succession following the catastrophic eruption of Ksudach volcano (Kamchatka, 1907). Vegetatio 127:129–153.
- Hill, M. O. and P. Šmilauer . 2005. TWINSPAN for Windows version 2.3. Huntingdon and České Budejovice Centre for Ecology and Hydrology and University of South Bohemia.
- Hulten, E. 1924. Eruption of a Kamchatka volcano and its atmospheric consequences. Geologiska Foreningens Forhandlinger 46:407–417.
- Hunter, A. F. and L. W. Aarssen . 1998. Plants helping plants: new evidence indicates that beneficence is important in vegetation. BioScience 38:34–40.
- Jones, C. C. and R. del Moral . 2005. Patterns of primary succession on the foreland of Coleman Glacier, Washington, USA. Plant Ecology 180:105–116.
- Jones, G. P. and G. H. R. Henry . 2003. Primary plant succession on recently deglaciated terrain in the Canadian High Arctic. Journal of Biogeography 30:277–296.
- Jumpponen, A. , H. Vare , K. G. Mattson , R. Ohtonen , and J. M. Trappe . 1999. Characterization of ‘safe sites’ for pioneers in primary succession on recently deglaciated terrain. Journal of Ecology 87:98–105.
- Khomentovsky, P. A. 1998. Pinus pumila (Siberian dwarf pine) on the Kamchatka Peninsula, Northeast Asia. In Laderman, A. D. , editor. Coastally restricted forest Oxford Oxford University Press. 199–220.
- Klimeš, L. , J. Klimešová , R. Hendriks , and J. van Groenendael . 1997. Clonal plant architectures: a comparative analysis of form and function. In de Kroon, H. and J. van Groenendael , editors. The Ecology and Evolution of Clonal Plants Leiden Backhuys Publishers. 1–29.
- Kohls, S. J. , C. Venkessel , D. D. Baker , D. F. Grigal , and D. B. Lawrence . 1994. Assessment of N2 fixation and N cycling by Dryas along a chronosequence within the forelands of the Athabasca Glacier, Canada. Soil Biology and Biochemistry 26:623–632.
- Krestov, P. V. 2003. Forest vegetation of the easternmost Russia (Russian Far East). In Kolbek, J. , M. Šrůtek , and E. Box , editors. Forest Vegetation of Northeast Asia Dordrecht Kluwer. 93–180.
- Kuvaev, V. B. 1993. The altitudinal distribution of vascular plants in the mountains of the Kronotsky Reservation (eastern Kamchatka). Botanicheskii Zhurnal (St. Petersburg) 78:5 25–48.
- Lawrence, D. B. 1958. Glaciers and vegetation in southeast Alaska. American Scientist 46:89–122.
- Lepš, J. , J. Michálek , O. Rauch , and P. Uhlík . 2000. Early succession on plots with the upper soil horizon removed. Journal of Vegetation Science 11:259–264.
- Lepš, J. and M. Rejmánek . 1991. Convergence or divergence: what should we expect from vegetation succession. Oikos 62:261–264.
- Matthews, J. A. 1979. A study of the variability of some successional and climax plant assemblage-type using multiple discriminant analysis. Journal of Ecology 67:255–271.
- Matthews, J. A. 1992. The Ecology of Recently Deglaciated Terrain. a Geoecological Approach to Glacier Forelands and Primary Succession Cambridge Cambridge University Press. 386 pp.
- McCabe, G. J. and A. G. Fountain . 1995. Relationships between atmospheric circulation and mass-balance at South Cascade Glacier, Washington, USA. Arctic and Alpine Research 27:226–233.
- Muravyev, Y. D. 1999. Present-day glaciation in Kamchatka: distribution of glaciers and snow. In Naruse, R. , editor. Cryospheric Studies in Kamchatka II The Institute of Low Temperature Science, Hokkaido University. 1–7.
- Prach, K. and P. Pyšek . 1994. Clonal plants—What is their role in succession. Folia Geobotanica and Phytotaxonomica 29:307–320.
- Raffl, C. , M. Mallaun , R. Mayer , and B. Erschbamer . 2006. Vegetation succession pattern and diversity changes in a glacier valley, central Alps, Austria. Arctic, Antarctic, and Alpine Research 38:421–428.
- Reiners, W. A. , I. A. Worley , and D. B. Lawrence . 1971. Plant diversity in a chronosequence at Glacier Bay, Alaska. Ecology 52:55–69.
- Rejmánek, M. and E. Rejmánková . 2002. Biogeography of artificial islands: the effects of age, area, elevation, and isolation on plant species richness. Preslia 74:307–314.
- Rydin, H. and S. O. Borgegard . 1991. Plant characteristics over a century of primary succession on island: Lake Hjälmaren. Ecology 72:1089–1101.
- Shamshin, V. A. 1999. The Stone Birch Forests of Kamchatka: Biology, Ecology and Stand Structure Moscow GEOS. 170. (in Russian).
- Shiraiwa, T. , Y. D. Muravyev , S. Yamaguchi , G. E. Glazirin , Y. Kodama , and T. Matsumoto . 1997. Glaciological features of Koryto Glacier in the Kronotsky Peninsula, Kamchatka, Russia. Bulletin of Glaciological Research 15:27–36.
- Solomina, O. and P. E. Calkin . 2003. Lichenometry as applied to moraines in Alaska, U.S.A., and Kamchatka, Russia. Arctic, Antarctic, and Alpine Research 35:129–143.
- Solomina, O. N. , Y. D. Muravyev , and L. I. Bazanova . 1995. Little Ice Age glaciers in Kamchatka. Annals of Glaciology 216:240–244.
- Svoboda, J. and G. H. R. Henry . 1987. Succession in marginal arctic environments. Arctic and Alpine Research 19:373–384.
- ter Braak, C. J. F. and P. Šmilauer . 1998. CANOCO Reference Manual and User's Guide to Canoco for Windows. Software for Canonical Community Ordination (version 4) Wageningen Centre for Biometry. 351 pp.
- Vetaas, O. R. 1997. Relationships between floristic gradients in a primary succession. Journal of Vegetation Science 8:665–676.
- Walker, L. R. and R. del Moral . 2003. Primary Succession and Ecosystem Rehabilitation Cambridge Cambridge University Press. 442 pp.
- Yakubov, V. V. 1997. Vascular Plants of the Kronotsky Biosphere Reserve (Kamchatka) Vladivostok Russian Academy of Sciences. 100 pp.
- Yamagata, K. , S. Sawaguchi , Y. D. Muravyev , and O. N. Solomina . 1999. Soil development in relation to vegetation and topography at the Koryto Glacier Basin, Kamchatka. In Naruse, R. , editor. Cryospheric Studies in Kamchatka II The Institute of Low Temperature Science, Hokkaido University. 76–78.