ABSTRACT
Deposition of atmospheric nitrogen from urban and agricultural sources has caused surface water nitrate concentrations to increase in the Front Range of the Colorado Rocky Mountains. To investigate the effects of sustained increases in nitrate concentrations on phytoplankton dynamics in an alpine lake, we conducted nutrient enrichment experiments in mesocosms amended with nitrate, phosphate, and phosphate plus nitrate on four dates in July and August 2002. During this period, phytoplankton species composition shifted as diatoms decreased in abundance. Phytoplankton chlorophyll a increased in the phosphate and phosphate plus nitrate enrichments, but did not increase in the nitrate only enrichments. Analysis of the phytoplankton community using Principal Component Analysis showed that 34% of the variance was accounted for by the primary axis, which was associated with different time periods, and 21% of the variance was explained by the secondary axis, which was associated with treatments. The response to phosphorus enrichment was taxon-specific, and the two chlorophyte species which became more abundant, Chlamydomonas sp. and Scenedesmus sp., were strongly weighted on the secondary axis. These results indicate that the productivity of this phytoplankton community is phosphorus-limited throughout the summer. Therefore, additional inputs of nitrogen are not expected to directly alter the productivity of the phytoplankton community.
Introduction
Mobilization of reactive nitrogen by human activities has increased nitrogen availability over large regions (CitationVitousek et al., 1997). Although the recent increase in anthropogenic nitrogen deposition is especially severe in the northeastern United States, high elevation alpine systems in the Colorado Front Range have moderately high deposition rates, which are increasing steadily (CitationLewis and Grant, 1980; CitationFenn et al., 1998, Citation2003; CitationBaron et al., 2000; CitationWilliams and Tonnessen, 2000; CitationBurns, 2003). By the 1980s, ambient concentrations of anthropogenically fixed atmospheric nitrogen were 30 times greater than pre-industrial concentrations (CitationFahey et al., 1986). Studies of the spatial distribution of atmospheric nitrogen deposition in Colorado have shown relatively high deposition rates in the Front Range (CitationLewis et al., 1984; National Atmospheric Deposition Program: http://nadp.sws.uiuc.edu/). For example, Niwot Ridge, located 6 km east of the Continental Divide in northern Colorado, has experienced an increase in deposition of inorganic nitrogen as wetfall at a rate of 0.32 kg ha−1 yr−1 over the last 20 years; and annual rates of deposition approximately doubled between 1984 and 1996 (CitationWilliams and Tonnessen, 2000). This increase is a function of both increasing precipitation and increasing concentrations of nitrogen in wet deposition (CitationBaron et al., 2000). Because alpine watersheds have extensive areas of exposed bedrock, limited vegetation, and thin soils, and because they accumulate deep snowpacks in winter, the inorganic nitrogen deposited in winter is mobilized during snowmelt and rapidly flushed into alpine aquatic ecosystems, making these ecosystems particularly vulnerable to changes in atmospheric nitrogen deposition (CitationLewis and Grant, 1979; CitationWilliams et al., 1996; CitationSeastedt et al., 2004).
Unlike terrestrial alpine plant communities, phytoplankton growth rates are on the scale of days and respond rapidly to changes in nutrient availability and other environmental conditions. In addition, some members of the phytoplankton community (the diatoms, division Bacillariophyta) leave a fossil record in sediments of lakes. Shifts in diatom communities from oligotrophic to mesotrophic diatoms have been documented in alpine lakes in the Colorado Front Range (CitationWolfe et al., 2001). A recent study of a sediment core in the Green Lakes Valley of Colorado indicates that there have been significant shifts in diatom species composition with planktonic species becoming less abundant than benthic species in Green Lake 4 since the 1940s, coincident with the introduction of nitrogen fertilizers for agriculture (CitationWaters, 1999).
Although phosphorus availability limits primary production in many temperate lakes, there are many examples of temperate freshwater ecosystems that are limited by nitrogen (CitationElser et al., 1990; CitationAxler et al., 1994; CitationKilham et al., 1996; CitationLafrancois et al., 2003a). CitationMorris and Lewis (1988) showed that phytoplankton in Colorado mountain lakes can be limited by either nitrogen or phosphorus, and the limiting nutrient can change seasonally. Atmospheric nitrogen deposition could cause a shift from nitrogen limitation to phosphorus limitation of phytoplankton production. CitationJassby et al. (1994) found that in Lake Tahoe there was a shift from colimitation by nitrogen and phosphorus to phosphorus limitation in response to atmospheric nitrogen deposition. Alternatively, in historically phosphorus-limited systems, increases in atmospheric nitrogen deposition would not be expected to alter the status of the system. The ratio of dissolved inorganic nitrogen (DIN) to total phosphorus (TP) is shown to be a good predictor of the nutrient limitation status in lakes (CitationMorris and Lewis, 1988; CitationAxler et al., 1994). In Green Lake 4, average DIN:TP ratios from 1995 to 2000 during late June through early August were 7.14 ± 1.46 (NWT-LTER database: http://www.culter.colorado.edu). DIN:TP ratios greater than 4 are indicative of phosphorus limitation (CitationAxler et al., 1994).
Nutrient limitation of phytoplankton growth commonly has been studied using short-term mesocosm experiments to determine changes in biomass in response to nutrient additions, and taxon-specific responses have been investigated in a few studies. CitationNydick et al. (2004a) conducted enclosure experiments in two small alpine lakes in the Rocky Mountains and observed increases in cyanobacteria and chlorophytes in response to nitrogen and nitrogen plus phosphorus enrichments. Diatom species composition shifts with nutrient enrichment have been shown to occur in alpine lakes in the Yellowstone region (CitationInterlandi and Kilham, 1998). Comparable shifts in the total algal community composition have also been observed in several warm monomictic lakes (CitationGonzalez, 2000).
The goals of the research presented here were to evaluate the potential influence of nutrient limitation on the density and species composition of phytoplankton in an alpine lake during the summer ice-free period. We hypothesized that the growth of the phytoplankton community in the lake is limited by phosphorus. We also hypothesized that phosphorus limitation persists through the temporal changes in community composition during the summer. Moreover, based on the changes in diatom species distribution observed in lake sediments (CitationWaters, 1999), we anticipated that there would be a taxon-specific shift in response to nutrient enrichment. We addressed these hypotheses by weekly sampling of the water column and by employing in situ nutrient enrichment experiments in the epilimnion of Green Lake 4, a well-studied alpine lake in the Front Range of the Colorado Rocky Mountains.
Methods
Site Description
The Green Lakes Valley (GLV) within the Colorado Front Range is located about 6 km east of the Continental Divide and is one of many alpine basins located west of large agricultural and urban areas between Fort Collins and Colorado Springs, Colorado. GLV is located within the Silver Lake Watershed, which provides approximately 40% of the water supply for the City of Boulder, Colorado. Public access is prohibited throughout the watershed. GLV has been studied as part of the Niwot Ridge Long Term Ecological Research (NWT-LTER) project. Niwot Ridge has been the location of a continuous climate record since 1951, and data on atmospheric chemistry have been collected since the 1970s (CitationLewis and Grant, 1980; CitationGrant and Lewis, 1982; CitationSievering et al., 1996). In addition, long-term hydrological and surface water chemistry data, including water temperature, snow depth, ice thickness on lakes, and discharge have been collected for over 30 years by CitationCaine (1995).
The GLV watershed consists of two catchments (). The upper catchment is an alpine ecosystem of about 2 km2 located above treeline, and includes Green Lakes 4 and 5. Green Lake 4, which is indirectly fed by the Arikaree Glacier, is an oligotrophic, alpine lake with low annual primary production (mean chlorophyll a: 2.37 ± 0.28 µg L−1; Gardner, unpublished data). The current study is focused on Green Lake 4, which has been studied previously (CitationMcNeely, 1983; CitationToetz and Windell, 1984, Citation1993; CitationWaters, 1999). provides morphometric information for Green Lake 4. The GLV watershed receives most of its precipitation in the form of winter and spring snowfall. Green Lakes 4 and 5 are ice-free only during the short growing season from late June or early July until early to mid October. Residence time of the lake is as low as one week during snowmelt, and by mid October it rises to around 40 days (CitationWaters, 1999). This study took place in the summer of 2002, which was a time of severe drought throughout most of Colorado. Streamflow at the outlet to Green Lake 4 during the summer of 2002 was 60% (±15%) of recent historical averages (1983–1999).
Figure 1 Map of study site. Inset shows a bathymetric map of Green Lake 4. Location of nutrient enrichment experiments is marked with a black star.
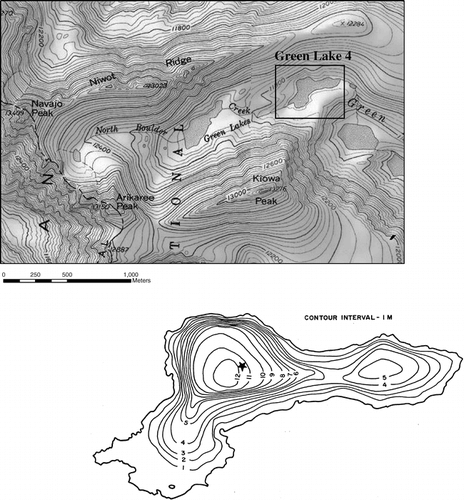
Table 1 Morphometric characteristics of Green Lake 4.
Monitoring of seasonal changes in community composition of phytoplankton within the water column has shown that diatoms comprise the largest portion of biovolume in the lake and that there are seasonal shifts within the diatom community (CitationWaters, 1999; Gardner, unpublished data). Historically, the upper two lakes were fishless, but due to accidental stocking in 1998, Yellowstone cutthroat trout now inhabit both Green Lakes 4 and 5.
Monitoring Study
Discharge was measured at a gauge located at the outlet of Green Lake 4 (NWT-LTER database: http://www.culter.colorado.edu). Water samples were collected from the water column for chemical analysis and algal biomass on 25 June; 2, 9, 16, 23, and 30 July; and 6 August 2002. The first sampling date occurred shortly after the ice had melted on Green Lake 4. Samples were collected with a Van Dorn sampler from the deepest part of the lake at the surface and at depths of 3 and 9 m. Subsamples were taken for phytoplankton biomass, community composition, and chemical analysis. All samples were collected between 09:00 and 11:00 MST. Dissolved oxygen (DO), temperature, and solar irradiance were measured at the three depths.
Samples for chemical analysis were filtered within 4–8 hours of collection with 47 mm Gelman A/E glass-fiber filters (effective pore size of approximately 1 µm) in a syringe filtration system, and then refrigerated in the dark until analysis. Samples were analyzed for total dissolved phosphorus (TDP), TP, and ammonium by colorimetric autoanalyzer and nitrate (NO3 −) by ion chromatography. Chlorophyll a samples were filtered within 12 hours through Whatman glass fiber filters (pore size 1.2 µm) with a hand-pump filtration system, and filters were frozen in aluminum foil until they were processed. Chlorophyll was removed from the filters by hot ethanol extraction, and quantified by the spectrophotometric method of CitationMarker et al. (1980) and CitationNusch (1980), and included a phaeopigment correction. Phytoplankton samples were preserved with Lugol's solution (1%) within 6 hours of collection. A subsample (5–50 mL depending on concentration of cells) was settled in Utermohl settling chambers overnight and identified to the genus level, or the species level when possible, at 1000× with an inverted microscope. Phytoplankton identification guides (CitationSmith, 1950; CitationPatrick and Reimer, 1966; CitationJohn et al., 2002), were the basis of identifications. A minimum of 400 cells were identified and counted in each sample. Community composition data are archived in the NWT-LTER database. Biovolume estimates for specific algal taxa were determined by Flanagan (unpublished data). Specifically, biovolumes were determined by measuring mean cell dimensions (Sick-Goad et al., 1977).
Nutrient Enrichment Experiments
Nutrient enrichment experiments were performed four times for a duration of five days each throughout the summer of 2002 (11–16 July, 18–23 July, 25–30 July, and 1–6 Aug) following methods similar to those outlined in CitationLewis et al. (1984). Briefly, water was pumped from the depth of incubation (2 m) into 20-L polyethylene carboys, spiked with nitrogen (N), phosphorus (P), both (N + P), or neither, and suspended from an incubation raft at a depth of 2 m for five days. Two replicates of each treatment were incubated with the exception of the ‘N + P’ treatment in incubation 3. Sample water was not filtered to remove grazers. Nitrogen was added as potassium nitrate (KNO3) dissolved in deionized water. Phosphorus was added as monobasic potassium phosphate (KH2PO4) dissolved in deionized water. Target concentrations for the spike additions were 930 µg L−1 NO3 − (double background concentrations) and 93 µg L−1 TDP (approximately 10× background concentrations) (following target concentrations used by CitationMorris and Lewis, 1988).
Samples were taken from the carboys directly after spiking with nutrients and after incubations for chemical analysis in three of the four incubations. Only post-incubation samples and an initial sample from the water column were taken during the first incubation for chemical analysis. Samples for chemical analyses were collected in 250 mL Nalgene bottles, and were processed using the methods described for the monitoring project.
At the beginning of each incubation period, separate 500-mL samples were taken from the water column at a depth of 2 m for chlorophyll analysis and phytoplankton identification. After each five-day incubation, samples for chlorophyll analysis and phytoplankton analysis were taken from each of the eight carboys. Chlorophyll samples and phytoplankton counts were processed as described above.
Principal components analysis (PCA) was used to assess the importance of incubation (time) and treatment (control, N, P, or N + P) in determining variance in the phytoplankton community. Given that using Euclidian distance can be problematic for species abundance data, chord distance was used as a metric to examine the relationship between incubations (CitationLegendre and Gallagher, 2001). The data were fourth root transformed prior to the analysis to account for large differences in species abundance (CitationClarke and Warwick, 1994). The Matlab code provided by CitationLegendre and Gallagher (2001) was used to run the PCA. The PCA scores of the variables were plotted with respect to the primary and secondary axes as a means to analyze the differences in community composition between incubations.
Results
Limnological Conditions
In 2002, ice-out occurred on 9 June, almost three weeks earlier than the previous two summers, and surface temperatures in the lake reached a maximum of 14°C in mid July (). Discharge reached an initial peak during snowmelt and then decreased throughout the summer. Two lesser discharge peaks occurred in late July and early August, coincident with large rainfall events (). The lake was thermally stratified throughout the summer (). Both the surface and 3 m sampling depths were in the epilimnion; there was no marked difference in temperature between these depths on any sampling date. The 9 m sampling depth had consistently lower temperatures than the surface and 3 m sampling depths. Epilimnetic chlorophyll a concentrations peaked at 4 µg L−1 in late July (). Chlorophyll a concentrations were consistently higher at 9 m than at the surface or 3 m. There was no difference in TDP or nitrate concentrations across depths. Average TDP concentrations from three depths in the water column were consistently low during the summer indicating oligotrophic conditions (). Nitrate concentrations decreased as the summer progressed but were on the upper end of concentrations reported in a survey of high elevation lakes in the Colorado Front Range (CitationLafrancois et al., 2003b). The peak chlorophyll a concentration on 23 July occurred shortly before the peak in the average TDP concentration on 30 July. The ratio of DIN:TP in the epilimnion over the course of the study was consistently above 4 and averaged 16.3 ± 2.76.
Figure 2 Surface temperature and discharge as a function of time in Green Lake 4. Note that ice out occurred on 9 June. Time periods for incubations 1–4 are indicated by brackets on the x-axis.
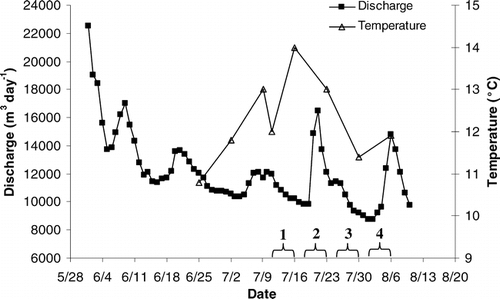
Figure 3 (a) Water temperature and (b) chlorophyll a concentrations with depth and time. Time periods for incubations 1–4 are indicated by brackets on the x-axis.
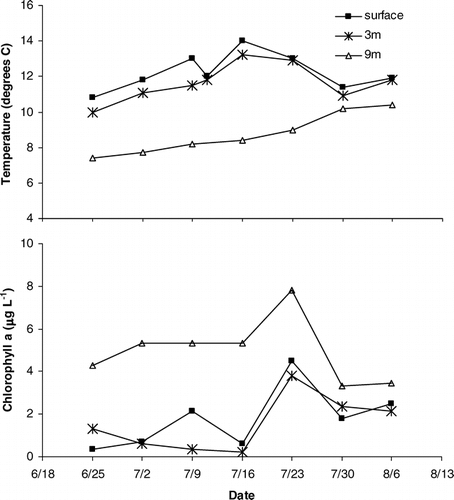
Table 2 Nitrate (NO3 −) and total dissolved phosphorus (TDP) concentrations in the water column of Green Lake 4 (average of 3 depths).
The algal taxa found in Green Lake 4 during the summer of 2002 are listed in . The shifts in the dominant taxa typically occurred at all three depths sampled, despite the greater chlorophyll a concentrations at depth (NWT-LTER database: http://www.culter.colorado.edu). The relative importance of Bacillariophyta decreased steadily throughout the summer primarily due to the decrease in the abundance of Synedra sp. However, it is worth noting that biovolumes of the species of Bacillariophyta are greater than most other taxa; ranging from 58 to 115 µm3. One of the diatom species found by CitationWolfe et al. (2001) to be an indicator of atmospheric nitrogen deposition, Asterionella formosa, was only found early in the summer and at a depth of 9 m. The Chrysophyta, Cryptophyta, and Haptophyta all reached maximum abundances later in the summer, including increases in Chromulina sp. (Chrysophyta), Plagioselmis sp. (Cryptophyta), and Chrysochromulina sp. (Haptophyta). Chrysococcus sp. (Chrysophyta) was the taxa with the greatest biovolume found in this study (523 µm3); while other taxa from the Chrysophyta, Cryptophyta, and Haptophyta range in biovolume from 6 to 46 µm3. The Cyanophyta and Chlorophyta consistently accounted for a large percentage of the phytoplankton based on cell numbers. There was a change in the relative abundance of species within the Chlorophyta. For example, Ankyra sp., Raphidocelis microscopica, and Chlamydomonas sp. decreased, while Chlorella minutissima remained abundant throughout the summer. With the exception of Chlamydomonas sp. (Chlorophyta) (70 µm3), the Cyanophyta and Chlorophyta tend to have slightly smaller biovolumes than species from other divisions.
Table 3 Species of algae found in Green Lake 4. The abundance of each taxa found at the end of each incubation as well as the ambient abundance in the epilimnion of the lake is denoted by ‘++++’ for taxa with total cell concentrations greater than 100,000 cells mL−1, taxa with cell concentrations from 10,000 to 100,000 cells mL−1 are indicated by ‘+++,’ taxa with cell concentrations ranging from 1000 to 10,000 are indicated by ‘++,’ taxa with concentrations from 100 to 1000 are denoted by ‘+,’ and rare taxa with concentrations less than 100 cells mL−1 are denoted by a ‘—’ symbol. The incubations are separated by time. Concentrations in replicate incubations were averaged. Given that control incubations were grouped with N-only treatments, and P treatments were grouped with N + P treatments in the PCA output, concentrations in the controls were combined with those in the N-only treatments and concentrations in the P-only treatments were combined with those in the N + P treatments. The PCA weightings for each taxa are also shown.
Enrichment Incubations
At the beginning of each incubation nitrogen treatments had an average of 1240 µg NO3 − L−1 and phosphorus treatments had an average of 93 µg L−1 TDP. The concentrations were double the background nitrate concentrations and 10 times the background TDP concentrations. Nitrate concentrations were consistently lower at the end of each incubation, indicating a net uptake by the plankton. The percentage change in nitrate concentrations ranged from −20% to −81%, and were highest in P-treatment samples (). The percent change in TDP concentrations during each incubation varied from −100% (net uptake of all available TDP) to +88% (net release of TDP).
Table 4 Nitrate (NO3 −) and total dissolved phosphorus (TDP) concentrations for background (water column), control group, and three treatment groups. Background concentrations are an average of three depths and were taken at the end of the date range listed. Incubation concentrations are an average of two replicates and are displayed here as concentrations at the beginning of the incubation, with percent change over the 5-day period in parentheses. A positive percent change indicates release by the plankton community, and a negative percent change indicates uptake by the plankton community.
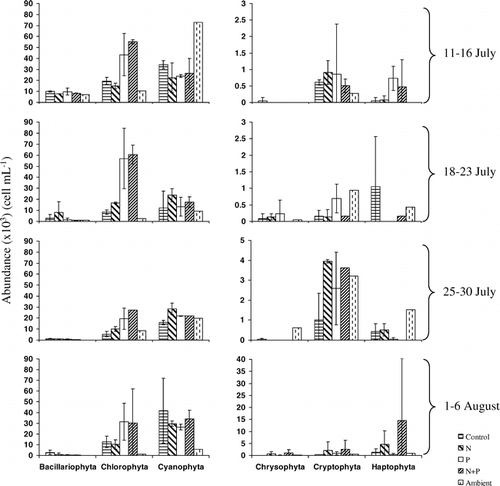
shows the chlorophyll a concentrations for each of the treatments in all four incubations. Average chlorophyll a concentrations plus or minus two standard deviations are presented to allow for easier determination of significant differences. In all four incubations, controls showed a decrease in algal biomass, as measured by chlorophyll a concentration, compared to the initial in-lake ambient concentrations. The in-lake ambient concentrations measured at the end of each incubation do not show a consistent trend with respect to the control concentrations measured at the same time. Algal biomass did not increase significantly in comparison with the control when nitrate alone was added. In contrast, there was a significant increase in algal biomass in comparison with the control when phosphate was added, either alone or with nitrogen (three of four incubations).
Figure 4 Average chlorophyll a concentrations of duplicates (± two standard deviations) at the end of 5 day incubations in each treatment group for four incubations performed in Green Lake 4 during the summer of 2002. Enrichment incubation samples without error bars (except for the N + P treatment in incubation 3) indicate that there was no discernable difference between the duplicates. Also shown are the in-lake ambient chlorophyll a concentrations measured in the epilimnion at the beginning and end of the incubations.
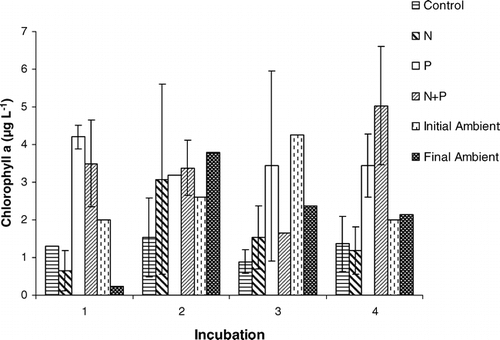
Chlorophyte abundance increased in the P and the N + P treatments in three of four incubations (). For the most part, the abundances of the ambient in-lake phytoplankton were similar to the abundances in the control treatments at the end of the incubations. The species within the Chlorophyta that increased the most consistently in response to the P and N + P treatments were Chlorella minutissima, Scenedesmus sp., and Chlamydomonas sp. (). In the first incubation, the treatments did not change the abundance of the dominant diatom species. However, in all three subsequent experiments, in the P and the N + P treatments the abundance of Synedra sp. decreased and the abundance of Navicula sp. increased. The abundance of Fragilaria sp. decreased in the P and the N + P treatments in the latter two experiments. Asterionella formosa was not found in any incubation during the course of the study. Among the chrysophytes, the abundance of Dinobryon sp. increased from rare to moderately abundant in the P and the N + P treatments for the second and third experiments. The species or taxonomic groups in the other divisions did not respond in a consistent manner to the nutrient additions. With a few exceptions, taxa in the epilimnion at the end of the incubations were slightly lower in abundance than were the same taxa in the control and N-alone treatments.
Principal Components Analysis
The first and second axes of the PCA accounted for 34% and 21%, respectively, of the variance in the community composition among the samples. Axis 1 clearly separated the samples by incubation, a proxy of time (). All samples from incubation 1 had low scores on Axis 1, while subsequent incubations had higher scores on this axis. The species which were strongly weighted on Axis 1 are from several divisions. The species that was most positively weighted on Axis 1 was the diatom Navicula sp. (). Further, the diatom Synedra sp. and the chlorophytes Raphidocelis microscopica and Ankyra sp. were most negatively weighted on Axis 1.
Figure 6 PCA scores for all phytoplankton species counts. Points are labeled by treatment and incubation date, 1–4.
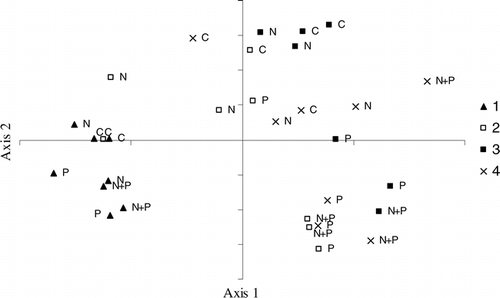
Similarly, Axis 2 separated the samples based on treatment. With only a few exceptions, samples from the control and N-alone treatments had positive scores on Axis 2, whereas samples from P and N + P treatments had negative scores on Axis 2 (). The results of a two-sample t-test for the two-tailed hypothesis show that the samples from the control and N-alone treatments had a significantly greater score on Axis 2 than did the P and N + P treatments (p < 0.01). Two of the three species with strong negative weightings on Axis 2 were species of Chlorophyta. The chlorophyte Chlamydomonas sp. had the strongest negative weighting on Axis 2, followed by the chrysophyte Dinobryon sp. and the chlorophyte Scenedesmus sp.
Discussion
Phosphorus Limitation of Phytoplankton Growth in Green Lake 4
The results of this study as a whole indicate that the phytoplankton community in Green Lake 4 is nitrogen insensitive, even well into August when nitrate concentrations approach annual minima. The high epilimnetic DIN:TP ratios found throughout the summer indicate consistent phosphorus limitation. These results suggest that if nitrate concentrations continue to increase in this watershed over time, a current trend (CitationWilliams and Tonnessen, 2000), this increase alone would not drive increases in phytoplankton productivity within the lakes. In order for the lakes to become eutrophic (or even mesotrophic), an increase in phosphorus availability would be necessary. The results of this study cannot identify the historic limitation status of Green Lake 4 phytoplankton. However, if the growth of the Green Lake 4 phytoplankton was historically nitrogen-limited, as is the current status of some other alpine lakes in the western U.S. (e.g. CitationFenn et al., 2003; CitationLafrancois et al., 2003a), it is possible that a shift from nitrogen to phosphorus limitation of phytoplankton growth has occurred.
The results of the nutrient enrichment experiments in Green Lake 4, showing an effect of addition of P and N + P throughout the summer, contrast with the results reported by CitationNydick et al. (2004a) for two alpine lakes in Wyoming. Nydick et al. observed no response to phosphorus addition but consistent increases in productivity in response to N and N + P additions. The differences in the response may reflect differences in the level of nitrogen enrichment from anthropogenic sources, differences in phosphorus availability in the lake systems, as well as differences in the dominant phytoplankton species in the lakes. In a study of high elevation lakes in Colorado, CitationMorris and Lewis (1988) found that of the eight lakes studied, the lake closest to Green Lake 4 was the only lake with a primarily phosphorus-limited phytoplankton community. CitationLafrancois et al. (2004) found that in Shelf Lake 4, an alpine lake with low nitrate concentrations, that phytoplankton chlorophyll a and productivity increased in response to additions of nitrogen, nitrogen plus acid, and nitrogen plus acid and phosphorus, but did not respond to additions of phosphorus alone. In contrast, CitationLafrancois et al. (2004) found that in the Loch, a high nitrate lake with nitrate concentrations more similar to those measured in Green Lake 4, phytoplankton productivity increased slightly in response to phosphorus additions as well as nitrogen plus acid plus phosphorus additions. In the Wyoming lakes studied by CitationNydick et al. (2004a), species of chrysophytes were abundant in the controls and phosphorus additions throughout the summer, and cyanobacteria and chlorophytes became more abundant with N and N + P additions; whereas in Green Lake 4 chlorophytes and diatoms were dominant in the controls and nitrogen additions. In both lakes studied by CitationLafrancois et al. (2004) there was a shift in community composition in response to the nitrogen plus acid and nitrogen plus acid plus phosphorus additions. Shelf Lake 4 showed a shift in community composition toward increased chlorophyte abundance; whereas there were increases in chlorophytes, chrysophytes, and a dinoflagellate species in the Loch.
Phosphorus limitation of phytoplankton growth in Green Lake 4 may be partly a function of the fact that Green Lake 4 drains an area comprised mainly of rock and talus. In a study of three subalpine lakes in the Colorado Front Range that are impacted by high levels of atmospheric nitrogen deposition, the limiting nutrient for phytoplankton growth was largely dependent upon watershed characteristics (CitationNydick et al., 2003). Specifically, phytoplankton phosphorus limitation was greatest in lakes that drain watersheds dominated by rock and talus.
Taxon-specific Responses to Nutrient Additions
The temporally sequential separation of the samples along Axis 1 of the PCA reflects the seasonal change in phytoplankton species in the Green Lake 4 ecosystem. The finding that for a given experiment there is little spread in the samples along Axis 1 provides further evidence that seasonal effects are important in determining community composition. Temporal variation of various species within the phytoplankton community is commonly observed in lakes (CitationReynolds, 1984; CitationSoylu and Gonulol, 2006), and the results of recent monitoring of phytoplankton in Green Lake 4 suggest that these seasonal changes are driven primarily by hydrological changes throughout the summer. In snowmelt-dominated catchments such as the Green Lakes Valley, peak discharge occurs in the late spring and early summer during snowmelt. As the summer progresses, discharge decreases to baseflow conditions. Given the large number of species observed in Green Lake 4 in concert with the small number of potentially limiting resources, the temporal shifts in species composition observed in this study may be a result of competition between species (CitationHuismann and Weissing, 1999) as well as hydrological changes.
The second axis of the PCA for our enrichment study corresponds to a separation based on treatment, leading us to further hypothesize that there is taxon-specific phosphorus limitation, and refuting the null hypothesis that nutrient enrichment will not result in changes in the productivity or community composition of the phytoplankton. The observation that the changes in species distribution are well represented by Axis 2 for all four experiments indicates that the chlorophyte species with the potential to grow rapidly in response to increases in phosphorus availability are present in the water column throughout the summer. Others have also observed taxon-specific responses to nutrient addition in studies of alpine lakes (e.g. CitationLafrancois et al., 2004; CitationNydick et al., 2004a), and the results for Green Lake 4 further indicate that assumptions regarding an equal limitation among different divisions of the phytoplankton community may not be valid.
Taxa within the Chlorophyta showed a much larger and more consistent response to phosphorus additions than taxa from other divisions, which has been shown in other nutrient enrichment experiments (e.g. CitationPollingher et al., 1988; CitationGonzalez, 2000). The observation of differences in response among the diatom species suggests that the diatom record in alpine lake sediments may provide some indication for changing nutrient limitation in alpine lakes along with other documented effects, such as exposure to ultraviolet radiation (CitationSaros et al., 2005). Moreover, the importance of a given taxa in lakes with similar morphometric characteristics is likely to vary between basins (CitationKolesar et al., 2002). This finding provides further support for examining taxon-specific responses in investigations of nutrient limitation of phytoplankton.
Consequences of Increasing Nitrate for Phytoplankton
The two most common consequences of nitrogen saturation of surface waters are eutrophication and acidification (CitationStoddard, 1994). Given that low phosphorus availability may constrain eutrophication of the water column, acidification due to increasing concentrations of nitrate (a strong acid), may be a more likely consequence in the Green Lakes Valley. Episodic acidification has been documented in the headwaters of this catchment (CitationCaine, 1995; CitationWilliams and Tonnessen, 2000), and the buffering capacity of weakly buffered mountain lakes in Colorado declined significantly between the 1940s and 1980s (CitationLewis, 1982). Potential acidification of Green Lake 4 may result in shifts in phytoplankton productivity and community composition. In an in situ acidification experiment in a nearby alpine lake, CitationMcKnight et al. (1990) found that photosynthetic rates decreased in the late summer during a cyanobacteria bloom but remained elevated when diatoms were the dominant taxa in early summer. In a nutrient enrichment and acidification experiment in two alpine lakes, phytoplankton community composition shifted in response to both nutrient enrichment and acidification (CitationLafrancois et al., 2004). However, it is also possible that benthic uptake of nitrate in Green Lake 4 and subsequent denitrification may increase the alkalinity of the system and act as a buffer against any potential acidification. It has been observed that in shallow oligotrophic lakes, the benthic community was responsible for more uptake of nitrate than the phytoplankton (CitationNydick et al., 2004a, Citation2004b).
While it is evident that nitrogen saturation is occurring in many high-elevation aquatic systems in the Rocky Mountains (e.g. CitationWilliams et al., 1996), it is unclear whether the growth of Green Lake 4 phytoplankton has been historically phosphorus-limited, or if there has been a shift from nitrogen limitation or colimitation of phosphorus and nitrogen to phosphorus limitation. It is possible that a shift in phytoplankton species composition has already occurred in response to changing chemical conditions, as indicated for diatom assemblages by sediment core studies in the area (CitationWaters, 1999; CitationWolfe, et al., 2001). Some locations, particularly on the Western Slope of the Continental Divide, have not shown evidence of biological nitrogen saturation (CitationKaushal and Lewis, 2003). However, for lakes in Rocky Mountain National Park, on the Eastern Slope, CitationBaron (2006) suggested that the ecological critical load for nitrogen deposition has been exceeded. Research comparing the Eastern and Western Slope of the Colorado Rockies indicates that there is a regional pattern of greater nitrogen concentrations in surface waters east of the Continental Divide (CitationBaron et al., 2000), as also indicated by deposition maps (CitationLewis et al., 1984; National Atmospheric Deposition Program data). This pattern suggests that regional biological nitrogen saturation due to anthropogenic nitrogen deposition may be underway on the Eastern Slope. The results of this study show that the Green Lake 4 phytoplankton community is nitrogen insensitive and that an increase in phosphorus concentrations would be necessary to increase phytoplankton productivity. Furthermore, taxon specific responses within the Green Lake 4 phytoplankton have been observed in response to phosphorus enrichment. However, it is possible that increasing nitrate concentrations could act to acidify the system resulting in potential shifts in phytoplankton community composition.
Acknowledgments
We would like to thank William Bowman and Alan Townsend, as well as the staff of the Center for Limnology at the University of Colorado, for all of their helpful comments and suggestions regarding both the project design and the presentation of this research.
Dr. Richard Dufford identified the dominant algal species in Green Lake 4, and those identifications were the basis for the cell counts. Christine Seibold, director of the Kiowa Chemistry Laboratory at the University of Colorado's Mountain Research Station, performed a large portion of the chemical analysis of the water samples from Green Lake 4. Daniel Liptzin provided assistance with statistical methods.
This work was made possible through the financial support of the National Science Foundation's Long Term Ecological Research Program (grants DEB-9810218 and DEB-0423662), the University of Colorado's Department of Ecology and Evolutionary Biology, and Colorado's Ocean Journey.
References Cited
- Axler, R. P. , C. Rose , and C. A. Tikkanen . 1994. Phytoplankton nutrient deficiency as related to atmospheric nitrogen deposition in northern Minnesota acid-sensitive lakes. Canadian Journal of Fisheries and Aquatic Sciences 51:1281–1296.
- Baron, J. S. 2006. Hindcasting nitrogen deposition to determine an ecological critical load. Ecological Applications 16:2 433–439.
- Baron, J. S. , H. M. Rueth , A. M. Wolfe , K. R. Nydick , E. J. Allstott , J. T. Minear , and B. Moraska . 2000. Ecosystem responses to nitrogen deposition in the Colorado Front Range. Ecosystems 3:352–368.
- Burns, D. A. 2003. Atmospheric nitrogen deposition in the Rocky Mountains of Colorado and southern Wyoming—A review and new analysis of past study results. Atmospheric Environment 37:921–932.
- Caine, N. 1995. Snowpack influences on geomorphic processes in Green Lakes Valley, Colorado Front Range. Geographical Journal 161:55–68.
- Clarke, K. R. and R. M. Warwick . 1994. Similarity-based testing for community patter—The 2-way layout with no replication. Marine Biology 118:1 167–176.
- Elser, J. J. , E. R. Marzolf , and C. R. Goldman . 1990. Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a review and critique of experimental enrichments. Canadian Journal of Fisheries and Aquatic Sciences 47:1468–1477.
- Fahey, D. W. , G. Hubler , D. D. Parish , E. J. Williams , R. B. Norton , B. S. Ridley , H. B. Singh , S. C. Liu , and F. C. Fehsenfeld . 1986. Reactive nitrogen species in the troposphere: measurements of NO, NO2, HNO3, particulate nitrate, peroxyacetyl nitrate (PAN), O3, and total reactive odd nitrogen (NO4) at Niwot Ridge, Colorado. Journal of Geophysical Research 91:9781–9793.
- Fenn, M. E. , M. A. Poth , J. D. Aber , J. S. Baron , B. T. Bormann , D. W. Johnson , A. D. Lemly , S. G. McNulty , D. F. Ryan , and R. Stottlemyer . 1998. Nitrogen excess in North American ecosystems: predisposing factors, ecosystem responses, and management strategies. Ecological Applications 8:706–733.
- Fenn, M. E. , J. S. Baron , E. B. Allen , H. M. Rueth , K. R. Nydick , L. Geiser , W. D. Bowman , J. O. Sickman , T. Meixner , D. W. Johnson , and P. Neitlich . 2003. Ecological effects of nitrogen deposition in the western United States. Bioscience 53:4 404–420.
- Gonzalez, E. J. 2000. Nutrient enrichment and zooplankton effects on the phytoplankton community in microcosms from El Andino reservoir (Venezuela). Hydrobiologia 434:81–96.
- Grant, M. C. and W. M. Lewis . 1982. Chemical loading rates from the precipitation in the Colorado Rockies. Tellus 34:74–88.
- Huismann, J. and F. J. Weissing . 1999. Biodiversity of plankton by species oscillations and chaos. Nature 42:407–410.
- Interlandi, S. J. and S. S. Kilham . 1998. Assessing the effects of nitrogen deposition on mountain waters: a study of phytoplankton community dynamics. Water Science and Technology 38:139–146.
- Jassby, A. D. , J. E. Reuter , R. P. Axler , C. R. Goldman , and S. H. Hackley . 1994. Atmospheric deposition of nitrogen and phosphorus in the annual nutrient load of Lake Tahoe (California–Nevada). Water Resources Research 30:2207–2216.
- John, D. M. , B. A. Whitton , and A. J. Brook . 2002. The Freshwater Algal Flora of the British Isles: an Identification Guide to Freshwater and Terrestrial Algae New York Cambridge University Press. 702.
- Kaushal, S. and W. M. Lewis . 2003. Patterns in the chemical fractionation of organic nitrogen in Rocky Mountain streams. Ecosystems 6:483–492.
- Kilham, S. S. , E. C. Theriot , and S. C. Fritz . 1996. Linking planktonic diatoms and climate change in the large lakes of the Yellowstone ecosystem using resource theory. Limnology and Oceanography 41:1052–1062.
- Kolesar, S. E. , D. M. McKnight , and S. B. Waters . 2002. Late fall phytoplankton dynamics in three lakes, Rocky Mountain National Park. Hydrobiologia 472:249–263.
- Lafrancois, B. M. , K. R. Nydick , and B. Caruso . 2003a. Influence of nitrogen on phytoplankton biomass and community composition in fifteen Snowy Range lakes (Wyoming, USA). Arctic, Antarctic, and Alpine Research 35:4 499–508.
- Lafrancois, B. M. , D. M. Carlisle , K. R. Nydick , B. M. Johnson , and J. S. Baron . 2003b. Environmental characteristics and benthic invertebrate assemblages in Colorado mountain lakes. Western North American Naturalist 63:137–154.
- Lafrancois, B. M. , K. R. Nydick , B. M. Johnson , and J. S. Baron . 2004. Cumulative effects of nutrients and pH on the plankton of two mountain lakes. Canadian Journal of Fisheries and Aquatic Sciences 61:1153–1165.
- Legendre, P. and E. D. Gallagher . 2001. Ecologically meaningful transformations of ordinations of species data. Oecologia 129:271–280.
- Lewis, W. M. 1982. Changes in pH and buffering capacity of lakes in the Colorado Rockies. Limnology and Oceanography 27:167–172.
- Lewis, W. M. and M. C. Grant . 1979. Relationships between stream discharge and yield of dissolved substances from a Colorado mountain watershed. Soil Science 128:6 353–363.
- Lewis, W. M. and M. C. Grant . 1980. Effect of urban sources on acid precipitation in the western United States. Science 210:1043–1043.
- Lewis, W. M. , J. F. Saunders , D. W. Crumpacker , and C. Brendecke . 1984. Eutrophication and Land Use: Lake Dillon, Colorado New York Springer-Verlag. 202.
- Marker, A. F. , E. A. Nusch , H. Rai , and B. Reimann . 1980. The measurement of photosynthetic pigments in freshwaters and standardization of methods: conclusions and recommendations. Archiv für Hydrobiologie Beiheft 14:91–106.
- McKnight, D. M. , R. L. Smith , J. P. Bradbury , J. S. Baron , and S. Spaulding . 1990. Phytoplankton dynamics in three Rocky Mountain lakes, Colorado, USA. Arctic and Alpine Research 22:3 264–274.
- McNeely, R. 1983. Preliminary physical data on Green Lakes 1–5, Front Range, Colorado. LTER Working Paper 83/4.
- Morris, D. P. and W. M. Lewis . 1988. Phytoplankton nutrient limitation in Colorado mountain lakes. Freshwater Biology 20:315–327.
- Nusch, E. A. 1980. Comparison of different methods for chlorophyll and phaeopigment determination. Archiv für Hydrobiologie Beiheft 14:14–36.
- Nydick, K. R. , B. M. Lafrancois , J. S. Baron , and B. M. Johnson . 2003. Lake specific responses to elevated atmospheric nitrogen deposition in the Colorado Rocky Mountains, U.S.A. Hydrobiologia 510:103–114.
- Nydick, K. R. , B. M. Lafrancois , J. S. Baron , and B. M. Johnson . 2004a. Nitrogen regulation of algal biomass, productivity, and composition in shallow mountain lakes, Snowy Range, Wyoming, USA. Canadian Journal of Fisheries and Aquatic Sciences 61:7 1256–1268.
- Nydick, K. R. , B. M. Lafrancois , and J. S. Baron . 2004b. NO3 uptake in shallow, oligotrophic, mountain lakes: the influence of elevated NO3 concentrations. Journal of the North American Benthological Society 23:3 397–415.
- Patrick, R. and C. W. Reimer . 1966. The Diatoms of the United States: Vol. 1 13:688. Monographic Series of Academy of Natural Sciences of Philadelphia,.
- Pollingher, U. , T. Berman , B. Kaplan , and D. Scharf . 1988. Lake Kinneret phytoplankton: response to N and P enrichments in experiments and nature. Hydrobiologia 166:65–75.
- Reynolds, C. S. 1984. Phytoplankton periodicity—The interactions of form, function, and environmental variability. Freshwater Biology 14:111–142.
- Saros, J. E. , S. J. Interlandi , S. Doyle , T. J. Michel , and C. E. Williamson . 2005. Are the deep chlorophyll maxima in alpine lakes primarily induced by nutrient availability, not UV avoidance. Arctic, Antarctic, and Alpine Research 37:4 557–563.
- Seastedt, T. R. , W. D. Bowman , T. M. Caine , D. McKnight , A. Townsend , and M. W. Williams . 2004. The landscape continuum: a model for high-elevation ecosystems. Bioscience 54:2 111–121.
- Sievering, H. , D. Rusch , and L. Marquez . 1996. Nitric acid, particulate nitrate and ammonium in the continental free troposphere: nitrogen deposition to an alpine tundra ecosystem. Atmospheric Environment 30:2527–2537.
- Smith, G. M. 1950. The Freshwater Algae of the United States New York McGraw-Hill. 719.
- Soylu, E. N. and A. Gonulol . 2006. Seasonal variation in the diversity, species richness and composition of the phytoplankton assemblages in a shallow lake. Cryptogamie: Algologie 27:1 85–101.
- Stoddard, J. L. 1994. Long-term changes in watershed retention of nitrogen: its causes and aquatic consequences. In Baker, L. A. , editor. Environmental Chemistry of Lakes and Reservoirs Washington, D.C American Chemical Society, Advances in Chemistry Series. 237:223–284.
- Toetz, D. and J. Windell . 1984. Lake productivity in the Green Lakes Valley, Front Range, Colorado—1983. LTER Data Report 84/5.
- Toetz, D. and J. Windell . 1993. Phytoplankton in a high-elevation lake, Colorado Front Range: application to lake acidification. Great Basin Naturalist 53:350–357.
- Vitousek, P. M. , J. D. Aber , R. W. Howarth , G. E. Likens , P. A. Matson , D. W. Schindler , W. H. Schlesinger , and D. G. Tilman . 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecological Applications 7:737–750.
- Waters, S. B. 1999. Responses of algal communities to environmental change in an alpine lake, Green Lakes Valley, Colorado. Master's thesis. University of Colorado, Boulder.
- Williams, M. W. and K. A. Tonnessen . 2000. Critical loads for inorganic nitrogen deposition in the Colorado Front Range, USA. Ecological Applications 10:1648–1665.
- Williams, M. W. , J. S. Baron , N. Caine , R. Sommerfeld , and R. Sanford . 1996. Nitrogen saturation in the Rocky Mountains. Environmental Science and Technology 30:640–646.
- Wolfe, A. P. , J. S. Baron , and R. J. Cornett . 2001. Anthropogenic nitrogen deposition induces rapid ecological changes in alpine lakes of the Colorado Front Range (USA). Journal of Paleolimnology 25:1–7.