ABSTRACT
We investigated vegetation structure and microenvironments on bare volcanic soil covered by scoria above the forest limit on Mt. Fuji, central Japan, to evaluate the effects of patches of a pioneer dwarf shrub (Salix reinii) on the establishment of early successional tree seedlings (Larix kaempferi). We analyzed species distribution patterns and the associations among them, and compared the performance (growth and survivorship) of Larix seedlings and the local environment (temperature, solar radiation, soil surface stability, soil moisture, and nitrogen) inside and outside Salix patches. Larix displayed significantly clumped distribution, and the clumping was apparently associated with the preferential occurrence of Larix in Salix patches. Salix patches moderated severe microenvironmental factors, such as drought, high temperature, and movement of the soil surface. Salix patches promoted increased height and decreased root∶shoot ratio, but not higher rate of biomass accumulation in Larix seedlings. Survival rate of L. kaempferi inside Salix patches was higher than that outside patches at the younger stage, but it was lower at the older stage after L. kaempferi emerged from the Salix crown. The results indicate S. reinii enhances seedling establishment and survival of young L. kaempferi, but may compete with it at later stages. The overall net effect of Salix patches on L. kaempferi is positive, and therefore S. reinii appears to accelerate succession from scoria bare land to pioneer woodland.
Introduction
The nurse-plant effect is a positive interaction in which individuals of one plant species facilitate the establishment and survival of another species by ameliorating the local environment (e.g., CitationMcAuliffe, 1988; CitationValiente-Banuet and Ezcurra, 1991; CitationFranco-Pizana et al., 1996; CitationWalker et al., 2003). Facilitation is usually considered a part of succession (CitationConnell and Slatyer, 1977), but may be especially important in harsh abiotic environments (CitationBertness and Callaway, 1994; CitationCallaway, 1995; CitationCallaway and Walker, 1997; CitationHolmgren et al., 1997; CitationFlores and Jurado, 2003). In high-elevation plant communities, positive interactions between species are more prevalent than negative interactions (CitationKikvidze, 1996; CitationCallaway, 1998; CitationKikvidze and Nakhutsrishvili, 1998; CitationNuñez et al., 1999; CitationArroyo et al., 2003; CitationOlofsson, 2004; CitationKleier and Lambrinos, 2005). In primary succession on bare volcanic soil, an early colonizing plant can provide favorable conditions for the establishment of other species (CitationVeblen et al., 1977; CitationHirose and Tateno, 1984; Citationdel Moral and Wood, 1993; CitationGrishin et al., 1996; CitationAdachi et al., 1996; CitationTitus and Tsuyuzaki, 2003; CitationUesaka and Tsuyuzaki, 2004).
In subalpine volcanic bare land on Mt. Fuji in central Japan, primary succession is still in progress, and the forest limit appears to be ascending (CitationTohyama, 1968; CitationMasuzawa, 1985). However, harsh environmental conditions such as an unstable soil-surface substratum (deposited scoria), low soil water availability (CitationMaruta, 1983, Citation1996), and low soil nutrient concentrations (CitationHirose and Tateno, 1984; CitationTateno and Hirose, 1987; CitationChiba and Hirose, 1993) are likely to delay succession by inhibiting plant establishment, growth, and survival. Facilitation is therefore potentially important in the colonization of these sites by some plant species (CitationHirose and Tateno, 1984; CitationMasuzawa, 1985; CitationAdachi et al., 1996). Larix kaempferi is the most dominant treeline species that colonizes volcanic bare land (CitationOhsawa, 1984). The seedlings and saplings of L. kaempferi occur not only on bare scoria but also in patches of the dwarf shrub Salix reinii, which dominates in bare land just above the forest limit. Salix patches promote the formation of ectomycorrhizae in tree seedlings through infection of the fungi species associated with Salix on Mt. Fuji (CitationNara and Hogetsu, 2004). On the volcanic bare land of Mt. Koma in northern Japan, Salix patches facilitate establishment of herb and grass species by ameliorating stressful environmental conditions (CitationUesaka and Tsuyuzaki, 2004). Here we hypothesize that Salix patches act as nurse plants for Larix seedlings and/or saplings during the early stages of primary succession on the subalpine volcanic bare land of Mt. Fuji.
We investigated spatial distribution patterns of species on subalpine volcanic bare land and compared the growth and survival of Larix seedlings inside and outside of Salix patches to investigate the facilitative effects of Salix on Larix seedlings. We also investigated the capacity of Salix to ameliorate climatic extremes within its patches and, in this way, to facilitate the establishment and survival of Larix seedlings.
Materials and Methods
Study Site
This study was conducted in a volcanic desert above the forest limit on the north-facing slope of Mt. Fuji (35°23′N, 138°44′E; 2400 m a.s.l.). Mt. Fuji (3776 m a.s.l.), a typical strato-volcano in central Japan, has erupted at least 10 times during recorded history, most recently in 1707. The forest limit on the north-facing slope occurs at 2300–2400 m a.s.l., which is lower than that on high non-volcanic mountains in central Japan. The mean slope inclination of the study site is ca. 18°. The ground surface of the site is covered with a thick basaltic scoria layer and is very unstable. Soil water availability is low due to high drainage in coarse textured soils. Plants are exposed to strong prevailing winds from the west.
Climatic data from the base of Mt. Fuji (860 m a.s.l.; Kawaguchiko Meteorological Observatory, 1971–2000) indicate that mean annual precipitation is 1508 mm and that monthly mean temperatures range from 21.8°C in August to −0.8°C in January. Estimated monthly mean temperatures at 2400 m a.s.l., based on a mean lapse rate of −0.6°C per 100 m increase in elevation, range from 12.6°C in August to −10.0°C in January.
There was sparse and short vegetation at the site, representing an early stage of primary succession. Several tree or shrub species (e.g., L. kaempferi, Betula ermanii, Alnus crispa ssp. maximowiczii, and S. reinii), as well as several herbs (e.g., Pleuropteropyrum weyrichii var. alpinum [syn. Polygonum weyrichii var. alpinum], Arabis serrata, and Stellaria japonica), occurred at the site. Salix reinii was the most dominant species in the vegetation and had developed into low, circular patches (ca. 10 cm in height and <3 m in diameter) by layering.
Field Measurements of Plants
A plot was established in the scoria desert just above the forest limit in the summer of 1999 (plot A, 20 m × 20 m = 400 m2). All plants in the plot were marked with numbered tape and were mapped. The height and major and minor axes of the crowns were measured for all individuals, and the basal diameter was measured for trees. Plant cover (percentage of ground area occupied) was determined for each species from the projection area of the plant crown, which was calculated as an ellipse from the major and minor axes. The ages of L. kaempferi were determined from bud-scale scars on the main stems. All measurements were performed after mid-August, when the current-year growth of plants had nearly ceased.
Another plot was established in 1999 in the neighborhood of plot A (ca. 30 m away) to provide more data for analyses of the association between S. reinii and L. kaempferi (plot B, 15 m × 25 m = 375 m2). This plot was not square to avoid gullies and large rocks on the ground. Similar measurements were made in plot B, but only for S. reinii and L. kaempferi.
In both plots, S. reinii and L. kaempferi were resurveyed in 2005 to measure the survival rates of L. kaempferi.
Sampling of L. Kaempferi
To compare growth patterns and morphology between the microhabitats, Larix individuals less than 8 years old were sampled in early September 1999, both inside and outside Salix patches around the two plots. In each habitat, eight or more individuals were collected for each age class (0-, 1-, 3-, 5-, and 6-year-old), but few 2-, 4-, and 7-year-old plants were collected because they were rare. For each plant sampled, the length and basal diameter of the trunk were measured. Subsequently, each plant was divided into leaves, stems, and roots, and the dry masses of these parts were measured after drying in an oven at 70°C for 72 h. The concentration of total nitrogen in these samples was determined using an automatic nitrogen-carbon analyzer (Sumigraph NC-900, Sumika Chemical Analysis Service, Japan).
Microenvironment Measurements
Light and temperature conditions were examined inside and outside a Salix patch in plot A. The photosynthetically active photon flux density (PPFD) was measured on the ground using photon sensors (IKS-27, Koito Industries, Japan). Temperature was measured at the soil surface and underground (5 cm deep) using copper-constantan thermocouples (type K, Hayashi-Denko, Japan). Two sensors were used for each measurement and at each site. The PPFD and temperature measurements were taken at 10-min intervals throughout the growing season in 2000, and the data were stored in a data logger (DS-36IC2, LEAP Science, Japan).
On a sunny day after several rainless days (4 August 2000), samples of surface soil (100 cm2 in area and 5 cm in depth) were collected using a steel can inside and outside of six Salix patches around the plots. Each sample was immediately sealed in a polyethylene bag to avoid evaporation and was transported in an insulated box back to the laboratory. The fresh weights of these samples were measured as quickly as possible. For a fraction of each soil sample, concentrations of NO3-N and NH4-N were determined colorimetrically after extraction with distilled water and 1.5 N KCl solution, respectively. The air-dried weight was measured after drying in the laboratory and was used to determine moisture content, and the oven-dried weight was measured after drying at 70°C for more than 72 h. The concentration of total nitrogen in these samples was determined using an automatic nitrogen-carbon analyzer (Sumigraph NC-900).
The stability of the soil-surface substratum inside and outside Salix patches was estimated by a painting method. Three circles, 15 cm in diameter, were drawn with an oil-based spray paint on the ground inside and outside of five Salix patches at the end of the growing season in 1999. The initial coverage of the painted area in each circle was defined as 100% of the total area of the circle. After one year, the percent coverage of the painted area remaining in the original circles was judged using a 1-cm-mesh frame measuring 10 × 10 cm. This value was compared between the two microhabitats as a measure of the stability of the soil-surface substratum.
Statistical Analyses
The spatial distribution patterns of each species were quantified using Ripley's K-function, K(t) (CitationRipley, 1977). To facilitate interpretation of the spatial distribution, the K-function was transformed into the L-function, L(t) = [K(t)/π]1/2 − t, where t was neighbor's distance, as suggested by CitationRipley (1977). The L-function, L(t), is plotted as a function of t. When individuals of a species are randomly distributed, the values of L(t) are expected to be zero. A departure from zero indicates a non-random distribution, with positive values indicating clumping, and negative values indicating regularity. To test the significance of departures from a random distribution, we evaluated the 95% confidence intervals from 1000 Monte Carlo simulations. Spatial pattern analyses were conducted with the Splancs Package (CitationRowlingson and Diggle, 1993) in the R-environment (R Development Core Team; http://www.R-project.org).
To evaluate species associations between S. reinii and each of the five dominant species, we looked for significant differences between the frequency with which a species occurred inside and outside Salix patches using a randomization test (CitationKikvidze et al., 2001; CitationArroyo et al., 2003). From the total frequency of a species and the area covered by S. reinii, we generated 10,000 random frequency values of that species inside and outside of Salix patches. Then we calculated the probability that the observed frequency was generated by chance. The randomization test was conducted in the R-environment (R Development Core Team; http://www.R-project.org).
We tested whether survival rates of L. kaempferi differed between microhabitats with Fisher's exact probability test in the R-environment (R Development Core Team). Significant differences in plant size and morphology, nitrogen concentration, and environmental parameters between microhabitats were tested with a one-way ANOVA or Mann-Whitney U test (StatView 5.0, SAS Institute Inc.).
Results
Plant Distribution Patterns
Four woody and five perennial herb species occurred in plot A. The abundance (number of individuals) and cover (percentage of ground area occupied) of each species are shown . In plot A, S. reinii occupied the largest ground cover of all species. The most abundant herb was P. weyrichii var. alpinum (a dominant herb above the forest limit). Larix kaempferi was the most abundant tree. Both S. reinii and L. kaempferi had higher abundance in plot B than in plot A (). Current-year seedlings of L. kaempferi occurred in small numbers in late summer, whereas seedlings of other species were rarely observed. Six species, including S. reinii and L. kaempferi, were adequately abundant for distribution pattern analyses.
TABLE 1 Abundance (number of individuals) and cover (% of ground surface area occipied by plant canopy) of species in plots A and B. All species were examined in plot A, and two dominant species in plot B.
The index of distribution pattern, L(t), was examined for the six abundant species in plot A and for S. reinii and L. kaempferi in plot B (). Three species (L. kaempferi, B. ermanii, and S. japonica) had significantly clumped distributions over all scales examined, and A. serrata also had a clumped distribution at smaller scales. In contrast, S. reinii had a random distribution over all scales in plot B and at the smaller and larger scales in plot A. Further, P. weyrichii var. alpinum also had a more random than clumped distribution at the smaller and larger scales.
FIGURE 1 Spatial analysis of the distribution pattern of six species in plot A (20 m × 20 m) and two species in plot B (25 m × 15 m). The plot of the derived statistic of Ripley's K-function [L(t) = [K(t)/π]1/2 − t] versus t reveals spatial patterns at increasing values of the neighbor's distance t. Positive values of L(t) indicate clumping and negative ones mean regularity. Dotted lines give 95% confidence intervals for complete spatial randomness from 1000 randomizations.
![FIGURE 1 Spatial analysis of the distribution pattern of six species in plot A (20 m × 20 m) and two species in plot B (25 m × 15 m). The plot of the derived statistic of Ripley's K-function [L(t) = [K(t)/π]1/2 − t] versus t reveals spatial patterns at increasing values of the neighbor's distance t. Positive values of L(t) indicate clumping and negative ones mean regularity. Dotted lines give 95% confidence intervals for complete spatial randomness from 1000 randomizations.](/cms/asset/f0f671f0-94bb-4380-bd03-498f566fbfcb/uaar_a_11957321_f0002.gif)
Statistical associations between L. kaempferi and three herb species and Salix patches are shown in . With the exception of P. weyrichii var. alpinum, each species was significantly associated with Salix patches (randomization test, P < 0.05).
TABLE 2 Density at which species were found inside and outside of Salix patches. P values are from randomization test. The test was made for four abundant species in plot A, and for L. kaempferi in plot B.
Growth, Nitrogen Content, and Survival Rate of L. Kaempferi
shows the sizes and morphology of Larix individuals of different ages collected from Salix patches and from nearby bare ground. The length of the main stem, which was nearly equivalent to plant height, was significantly greater inside than outside the patches for most size classes (ANOVA, P < 0.05 or 0.01; ). However, the basal stem diameter was smaller inside than outside the patches for 0- and 1-year-old seedlings (ANOVA, P < 0.05 or 0.01; ). The mean dry mass of individuals was not significantly different between the two microhabitats for any age examined (). In 5- and 6-year-old plants, the root∶shoot ratio, which indicates the relative allocation of dry matter to below- and aboveground parts, was significantly lower inside than outside the patches (Mann-Whitney U test, P < 0.05 or 0.01; ). The root∶shoot ratio for younger plants did not differ between the microhabitats.
FIGURE 2 Stem length (a), basal diameter (b), biomass (c), and root∶shoot ratio (d) of 0-, 1-, 3-, 5- and 6-year-old Larix individuals inside and outside Salix patches. Mean and SE are displayed in each panel (n = 8 to 20). Significant differences between microhabitats are shown by asterisks (ANOVA or Mann-Whitney U-test); *, P < 0.05; **, P < 0.01.
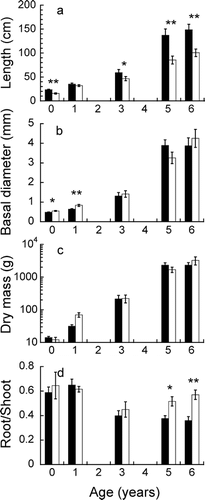
The leaf nitrogen concentration of Larix plants of each age was examined in Salix patches and bare sites. There were no significant differences in leaf nitrogen concentration between microhabitats for most of the age classes examined, except for 1-year-old plants, in which the leaf nitrogen concentration was lower inside than outside Salix patches (Mann-Whitney U test, P < 0.01; mean ± SE, 1.59 ± 0.07% in patches, 2.00 ± 0.10% in bare sites).
shows the survival rates of L. kaempferi for 6 years, from 1999 to 2005, inside and outside of Salix patches in plots A and B, where all plants were divided into two age classes (younger plants, 0- to 5-year-old; and older plants, 6-year-old and older). There were no significant differences in survival rate between microhabitats in each plot. However, when data from the two plots were pooled, significant differences were detected in both the younger and older plants; the survival rate inside patches was significantly higher than that outside of patches in younger plants, and in contrast it was significantly lower in older plants (Fisher's exact probability test, P < 0.05). Significant differences were not detected when both age classes were pooled (). As for distribution pattern of each age group of Larix, both the younger group and older group were significantly associated with the patches not only in 1999 but also in 2006 (randomization test, P < 0.01).
TABLE 3 Survivorship of L. kaempferi for 6 years inside and outside of Salix patches. Number of individuals in 1999, survivors in 2005, and survival ratio are shown for individuals in two age classes and all individuals. Differences in the survival rate between inside and outside of patches are tested by Fisher's exact probability test.
Microenvironment Inside and Outside of Salix Patches
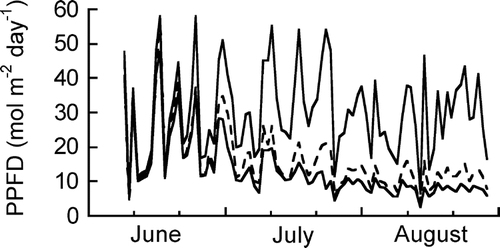
The daily cumulative PPFD inside a patch in early June was similar to that on bare ground and then decreased until the end of June, in conjunction with leaf opening and expansion in S. reinii (). The ratio of PPFD inside the patch to that on bare ground was nearly constant throughout July and August (ca. 38%). The mean PPFD between 10 and 14 h during July and August was ca. 200 µmol m−2 s−1 on cloudy days and ca. 600 µmol m−2 s−1 on sunny days on the ground in the Salix patch.
FIGURE 3 Seasonal changes in daily cumulative photosynthetically active photon flux density (PPFD) on the soil surface in the Salix patches and bare site. The upper solid line indicates the data in bare site, and the broken line and lower solid line indicate the data in the patches, respectively.
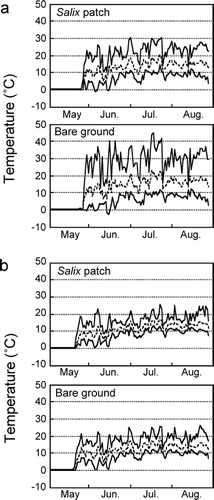
Daily changes in the soil-surface temperature inside the patch were smaller than those on bare ground during the growing period (). The daily maximum soil-surface temperature on sunny days on bare ground was occasionally more than 40°C, and averaged approximately 30°C during the growing season. Within the patch, the daily maximum temperatures were approximately 10°C lower during this period. However, there were no differences in daily minimum temperatures between sites (). Daily and seasonal changes in belowground temperatures (5 cm deep) were smaller than those in soil-surface temperatures, and differences between sites were relatively small ().
FIGURE 4 Seasonal changes in the soil-surface temperatures (a) and belowground temperatures (b) in the Salix patches and bare ground. Daily maximum and minimum temperatures are shown by upper and lower solid lines, respectively. Daily means are shown by broken lines.
On a sunny summer day, the soil-surface scoria substratum (0–5 cm depth) in the Salix patches contained significantly more moisture and total nitrogen than in bare sites, yet there were no differences in concentrations of NO3-N and NH4-N between the microhabitats (). Owing to scoria movement, the circles painted on the soil surface lost their shape both inside the patches and in bare sites. After one year, the soil surface area covered by paint in the original circles had decreased from 100% to 58.9% in patches and to 33.7% on bare ground (). The difference between the microhabitats was statistically significant (Mann-Whitney U test, P < 0.05, n = 15). Thus, the soil surface inside patches was more stable than that in bare sites.
TABLE 4 Water and nitrogen contents and stability of ground surface substratum in Salix patches and bare ground. Mean ± SE are shown (n = 6 for water and nitrogen contents and n = 15 for the stability of surface scoria).
Discussion
Distribution Patterns and Interactions Between Plants
The spatial distribution patterns of wind-dispersed pioneer plants that have colonized bare land may depend primarily on the spatial heterogeneity of the site. In early successional vegetation on Mt. Sakurajima, an active volcano in southern Japan, CitationTagawa (1965) observed a clumped distribution of pioneer plants, which he attributed to the heterogeneous rocky ground. In contrast, the two dominant pioneer species (S. reinii and P. weyrichii var. alpinum) on the bare scoria on Mt. Fuji had random distributions at some of the scales examined. These species have typical wind-dispersed seeds. The more homogeneous conditions of the scoria desert relative to rocky ground appear to allow for a random distribution. Several species other than S. reinii and P. weyrichii var. alpinum were clumped, even on scales at which S. reinii was randomly distributed. This observation is likely related to the fact that L. kaempferi, S. japonica, and A. serrata occurred more frequently in Salix patches than in bare soil (). These distribution patterns suggest that Salix patches have a facilitative effect on the establishment of later colonizers.
A similar distribution pattern has been observed on bare scoria at lower altitudes on the southeastern slope of Mt. Fuji. There, patches of Reynoutria japonica (syn. Polygonum cuspidatum), the dominant pioneer species, serve as safe sites for the establishment of later colonizers (CitationMasuzawa, 1985; CitationMasuzawa and Suzuki, 1991; CitationAdachi et al., 1996). The facilitative effect of patch-making pioneer plants may be a critical factor in the development of early successional vegetation on both the upper and lower bare scoria lands of Mt. Fuji.
Microenvironmental Modifications by S. Reinii
A nurse plant enhances the establishment and survival of other plants by altering factors such as light, moisture, and nutrient conditions in the local environment (e.g., CitationValiente-Banuet and Ezcurra, 1991; CitationUesaka and Tsuyuzaki, 2004). On the volcanic bare ground of Mt. Koma in northern Japan, Salix patches act as facilitators for herb species by improving moisture and nutrient conditions, moderating light intensity, and trapping seeds (CitationUesaka and Tsuyuzaki, 2004). We also verified that Salix patches ameliorated several microenvironmental factors (e.g., temperature, soil moisture, soil total nitrogen content, and soil-surface stability) for later-colonizing species.
On bare scoria, most seedlings of pioneer plants die from severe desiccation during mid-summer, when solar radiation is high and precipitation is low (CitationMaruta, 1976; CitationYura, 1988). The interception of solar radiation by the S. reinii crown did not depress the growth of Larix seedlings. Instead, it effectively prevented temperature increases in the patch on sunny days. The moisture content of the soil-surface stratum was significantly higher inside Salix patches than in bare sites on a sunny summer day. These temperature and moisture conditions apparently moderated water stress for L. kaempferi growing in the patches. The effect of Salix patches in lowering desiccation may enhance the survival of Larix seedlings.
Available nitrogen is the main factor limiting plant growth during primary succession in volcanic deserts (CitationHirose and Tateno, 1984; CitationTateno and Hirose, 1987; CitationChiba and Hirose, 1993). CitationHirose and Tateno (1984) suggested a facilitative effect of R. japonica patches on primary succession, demonstrating that soil organic nitrogen, ammonium, and nitrate in R. japonica patches were all higher than in surrounding bare sites. However, neither soil ammonium nor nitrate levels in Salix patches were higher than those in the surrounding sites, although total nitrogen was higher in Salix patches. Mineralization rates of organic nitrogen are highest in mid-summer in a volcanic desert (CitationHirose and Tateno, 1984). The accumulated nitrogen inside Salix patches might be mineralized and then lost immediately through absorption and leaching. Thus, more detailed investigations of soil nutrient dynamics are needed to verify that S. reinii improves the soil nutrient conditions inside the patches.
On exposed alpine gravel fields, movement of the surface substratum severely limits plant survival, and only species that develop large subterranean organs are found in these regions (CitationKoizumi, 1979; CitationChujo, 1983). The movement of scoria gravel in the volcanic desert on Mt. Fuji may frequently injure or kill small plants. Salix reinii appears to have a high tolerance for surface gravel movement, and the highly branched prostrate stems decrease substratum movement within patches of this species. This surface stabilizing capability is likely one component of the nurse-plant effect of S. reinii on L. kaempferi.
Facilitation and Competition With Nurse Plants
On occasion, nurse plants can exert a negative effect on the nursed species (CitationCallaway and Walker, 1997; CitationHolmgren et al., 1997). Desert nurse species, which protect understory plants from low temperatures or freezing, also inhibit the growth of these plants through competition for available water and solar radiation (CitationFranco and Nobel, 1988, Citation1989; CitationValiente-Banuet et al., 1991). In a glacier foreland community, negative and positive impacts of willow canopies on species occurrence cancel each other out (CitationTotland et al., 2004). The growth of L. kaempferi was not facilitated by S. reinii, even though survival was enhanced through the nurse-plant effect. Larix seedlings in Salix patches had more slender main stems and lower root∶shoot ratios than those in bare sites. Similar results were observed on another volcanic desert (CitationAkasaka and Tsuyuzaki, 2005). These morphological features clearly show etiolation in Larix seedlings, which is their response to shading by S. reinii and indicates competition for light. In addition, the leaf nitrogen concentration of L. kaempferi in Salix patches was similar to or lower than that in bare sites. This seems to indicate competition for nitrogen. The formation of ectomycorrhizae on Larix seedlings is enhanced when the seedlings are transplanted near established S. reinii (CitationNara and Hogetsu, 2004). Our data indicate that the facilitative effect on ectomycorrhizal formation does not result in higher leaf nitrogen content in Larix seedlings.
The effect of a nurse plant on the nursed species may change from positive to negative (or neutral) as the nursed plant grows (CitationKellman and Kading, 1992; CitationPugnaire et al., 1996; CitationCallaway and Walker, 1997). Although the survival rate of L. kaempferi inside Salix patches was higher than that outside of patches during younger stages, it was lower during older stages. This appears to be associated with the extent of protective effects of Salix patches on Larix seedlings against severe environmental conditions, such as strong winds. Flying gravel during high winds causes stem injuries that are substantial contributions to moisture stress in L. kaempferi on the bare lands of Mt. Fuji (CitationMaruta, 1996). The protective effects of Salix patches seems to be very important for the survival of young L. kaempferi growing under the Salix crown, but less important for the survival of older L. kaempferi emerging from the Salix crown. Therefore the competitive effects of Salix patches on Larix seedlings may be more dominant than the protective effects during the older stages, thus resulting in a lower survival rate within patches.
Roles of S. Reinii in Primary Succession
The pioneer species that accelerate primary succession vary with altitude. The forest limit on the southeastern slope of Mt. Fuji, where the most recent eruption occurred, is about 1000 m lower than on the northern slope. On the southeastern side, bare scoria spreads out below the subalpine zone. Reynoutria japonica is the dominant pioneer species on bare ground at lower altitudes but rarely occurs on the northern slope, where the bare ground is beyond the altitudinal upper limit of this species (CitationMaruta, 1994). Here, S. reinii is one of the important pioneers accelerating primary succession in the subalpine zone.
The identity of the facilitative species also appears to change throughout the successional sequence. In early successional stages, S. reinii facilitates seedling establishment of tree species that dominate the treeline vegetation. In the next successional stage, L. kaempferi develops a krummholz form and dominates (CitationMaruta, 1996). During this process, S. reinii cover decreases owing to increasing competition with L. kaempferi for some resources (nutrients, moisture, and light), in which light may be increasingly important as vegetation develops. As a cover of krummholz vegetation increases, L. kaempferi develops elect trunks to form an early successional forest. Several plant species, including later successional, shade-tolerant species such as Abies veitchii, are frequently observed under the Larix canopy. Thus, L. kaempferi may serve as a nurse plant during early stages of forest development.
Acknowledgments
We thank Emiko Maruta, Takehiro Masuzawa, Masae Shiyomi, Yoshimichi Hori, Shigeru Mariko, Hiromi Tanabe, Atsushi Ishida, and Tsuyoshi Sakata for invaluable comments on this study. We are grateful to Yoshiko Abe, Shinpei Oikawa, Hideya Ogitsu, Nozomi Isogai, and Sayano Tanaka for assistance on field works and experiments. This study was partly supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 19570013).
References Cited
- Adachi, N. , I. Terashima , and M. Takahashi . 1996. Central die-back of monoclonal stands of Reynoutria japonica in an early stage of primary succession on Mount Fuji. Annals of Botany (London) 77:477–486.
- Akasaka, M. and S. Tsuyuzaki . 2005. Tree seedling performance in microhabitats along an elevational gradient on Mount Koma, Japan. Journal of Vegetation Science 16:647–654.
- Arroyo, M. T. K. , L. A. Cavieres , A. Peñaloza , and M. A. Arroyo-Kalin . 2003. Positive associations between the cushion plant Azorella monantha (Apiaceae) and alpine species in the Chilean Patagonian Andes. Plant Ecology 169:121–129.
- Bertness, M. D. and R. M. Callaway . 1994. Positive interactions in communities. Trends in Ecology and Evolution 9:191–193.
- Callaway, R. M. 1995. Positive interactions among plants. Botanical Review 61:306–349.
- Callaway, R. M. 1998. Are positive interactions species-specific. Oikos 82:202–207.
- Callaway, R. M. and R. L. Walker . 1997. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78:1958–1965.
- Chiba, N. and T. Hirose . 1993. Nitrogen acquisition and use in three perennials in the early stage of primary succession. Functional Ecology 7:287–292.
- Chujo, H. 1983. Alpine vegetation and periglacial movement of slope materials on Mt. Ontake, central Japan: factors controlling the Cardamine nipponica community. Japanese Journal of Ecology 33:461–472. (in Japanese with English summary).
- Connell, J. H. and R. O. Slatyer . 1977. Mechanisms of succession in natural communities and their role in community stability and organization. American Naturalist 111:1119–1144.
- del Moral, R. and D. M. Wood . 1993. Early primary succession on a barren volcanic plain at Mount St. Helens, Washington. American Journal of Botany 80:981–991.
- Flores, J. and E. Jurado . 2003. Are nurse-protégé interactions more common among plants from arid environment. Journal of Vegetation Science 14:911–916.
- Franco, A. C. and P. S. Nobel . 1988. Interactions between seedlings of Agave deserti and the nurse plant Hilaria rigida . Ecology 69:1731–1740.
- Franco, A. C. and P. S. Nobel . 1989. Effects of nurse plants on the microhabitat and growth of cacti. Journal of Ecology 77:870–886.
- Franco-Pizana, J. , T. E. Fulbright , D. T. Gardiner , and A. R. Tipton . 1996. Shrub emergence and seedling growth in microenvironments created by Prosopis glandulosa . Journal of Vegetation Science 7:257–264.
- Grishin, S. Y. , R. del Moral , P. V. Krestov , and V. P. Verkholat . 1996. Succession following the catastrophic eruption of Ksudach volcano (Kamchatka, 1907). Plant Ecology 127:129–153.
- Hirose, T. and M. Tateno . 1984. Soil nitrogen patterns induced by colonization of Polygonum cuspidatum on Mt. Fuji. Oecologia 61:218–223.
- Holmgren, M. , M. Scheffer , and M. A. Huston . 1997. The interplay of facilitation and competition in plant communities. Ecology 78:1966–1975.
- Kellman, M. and M. Kading . 1992. Facilitation of tree seedling establishment in a sand dune succession. Journal of Vegetation Science 3:679–688.
- Kikvidze, Z. 1996. Neighbour interaction and stability in subalpine meadow communities. Journal of Vegetation Science 7:41–47.
- Kikvidze, Z. and G. Nakhutsrishvili . 1998. Facilitation in subnival vegetation patches. Journal of Vegetation Science 9:261–264.
- Kikvidze, Z. , L. Khetsuriani , D. Kikodze , and R. M. Callaway . 2001. Facilitation and interference in subalpine meadows of the central Caucasus. Journal of Vegetation Science 12:833–838.
- Kleier, C. and J. G. Lambrinos . 2005. The importance of nurse association for three tropical alpine life forms. Arctic, Antarctic, and Alpine Research 37:331–336.
- Koizumi, T. 1979. Periglacial processes and alpine plant communities on the high mountains in Japan, in relation to lithology I. Alpine stony desert vegetation on the windward slope of the Shirouma mountain region in the Northern Japan Alps. Japanese Journal of Ecology 29:71–81. (in Japanese with English summary).
- Maruta, E. 1976. Seedling establishment of Polygonum cuspidatum on Mt. Fuji. Japanese Journal of Ecology 26:101–105.
- Maruta, E. 1983. Growth and survival of current-year seedlings of Polygonum cuspidatum at the upper distribution limit on Mt. Fuji. Oecologia 60:316–320.
- Maruta, E. 1994. Seedling establishment of Polygonum cuspidatum and Polygonum weyrichii var. alpinum at high altitudes of Mt Fuji. Ecological Research 9:205–213.
- Maruta, E. 1996. Winter water relations of timberline larch ( Larix leptolepis ) on Mt Fuji. Trees 11:119–126.
- Masuzawa, T. 1985. Ecological studies on the timberline of Mt. Fuji I. Structure of plant community and soil development on the timberline. Botanical Magazine Tokyo 98:15–28.
- Masuzawa, T. and J. Suzuki . 1991. Structure and succession of alpine perennial community ( Polygonum cuspidatum ) on Mt. Fuji. Proceedings of NIPR Symposium on Polar Biology 4:155–160.
- McAuliffe, J. R. 1988. Markovian dynamics of simple and complex desert plant communities. American Naturalist 131:459–490.
- Nara, K. and T. Hogetsu . 2004. Ectomycorrhizal fungi on established shrubs facilitate subsequent seedling establishment of successional plant species. Ecology 85:1700–1707.
- Nuñez, C. I. , M. A. Aizen , and C. Ezcurra . 1999. Species associations and nurse plant effects in patches of high-Andean vegetation. Journal of Vegetation Science 10:357–364.
- Ohsawa, M. 1984. Differentiation of vegetation zones and species strategies in the subalpine region of Mt. Fuji. Vegetatio 57:15–52.
- Olofsson, J. 2004. Positive and negative plant-plant interactions in two contrasting arctic-alpine plant communities. Arctic, Antarctic, and Alpine Research 36:464–467.
- Pugnaire, F. I. , P. Haase , J. Puigdefabregas , M. Cueto , S. C. Clark , and L. D. Incoll . 1996. Facilitation and succession under the canopy of a leguminous shrub, Retama sphaerocarpa , in a semiarid environment in southeast Spain. Oikos 76:455–464.
- Ripley, B. D. 1977. Modeling spatial patterns. Journal of Royal Statistical Society B39:172–212.
- Rowlingson, B. and P. Diggle . 1993. Splancs: spatial point pattern analysis code in S-Plus. Computers and Geosciences 19:627–655.
- Tagawa, H. 1965. A study of the volcanic vegetation in Sakurajima, southwest Japan. II. Distribution pattern and succession. Japanese Journal of Botany 19:127–148.
- Tateno, M. and T. Hirose . 1987. Nitrification and nitrogen accumulation in the early stage of primary succession on Mt. Fuji. Ecological Research 2:113–120.
- Titus, J. H. and S. Tsuyuzaki . 2003. Influence of a non-native invasive tree on primary succession at Mt. Koma, Hokkaido, Japan. Plant Ecology 169:307–315.
- Tohyama, M. 1968. The alpine vegetation of Mt. Fuji. Journal of Faculty of Agriculture, Hokkaido University 55:459–467.
- Totland, Ø , J. A. Grytnes , and E. Heegaard . 2004. Willow canopies and plant community structure along an alpine environmental gradient. Arctic, Antarctic, and Alpine Research 36:428–435.
- Uesaka, S. and S. Tsuyuzaki . 2004. Differential establishment and survival of species in deciduous and evergreen shrub patches and on bare ground, Mt. Koma, Hokkaido, Japan. Plant Ecology 175:165–177.
- Valiente-Banuet, A. and E. Ezcurra . 1991. Shade as a cause of the association between the cactus Neobuxbaumia tetetzo and the nurse plant Mimosa luisana in the Tehuacan Valley, Mexico. Journal of Ecology 79:961–971.
- Valiente-Banuet, A. , F. Vite , and J. A. Zavala-Hurtado . 1991. Interaction between the cactus Neobuxbaumia tetetzo and the nurse shrub Mimosa luisana . Journal of Vegetation Science 2:11–14.
- Veblen, T. T. , D. H. Ashton , F. M. Schlegel , and A. T. Veblen . 1977. Plant succession in a timberline depressed by vulcanism in south-central Chile. Journal of Biogeography 4:275–294.
- Walker, L. R. , B. D. Clarkson , W. B. Silvester , and B. R. Clarkson . 2003. Colonization dynamics and facilitative impacts of a nitrogen-fixing shrub in primary succession. Journal of Vegetation Science 14:277–290.
- Yura, H. 1988. Comparative ecophysiology of Larix kaempferi (Lamb.) Carr. and Abies veitchii Lindl. I. Seedling establishment on bare ground on Mt. Fuji. Ecological Research 3:67–73.