ABSTRACT
Snow properties such as snow density will likely change in a warmer climate. Changes in depth and extent of snow cover have been shown to affect soil nutrient dynamics and plant growth; however, effects of a changed snow density have so far not been explicitly tested. We altered snow properties (especially depth and density according to those found on ski runs) and investigated effects on soil temperatures, soil nitrogen mineralization, plant phenology, and productivity. A denser, thinner snow cover led to reduced soil insulation and lower soil temperatures, which consequently increased net N mineralization. A denser snow cover furthermore resulted in a delay in plant phenology of up to five weeks after melt-out. The results suggest that changes in snow density, which have been largely neglected in the global change discussion until now, can cause significant changes in soil and vegetation processes.
Introduction
Winter snow cover represents a crucial environmental factor in alpine ecosystems. Its duration and extent determine the beginning and duration of the growing period, and meltwater provides water and nutrients that are crucial for plant growth (CitationJones et al., 2001). The snow cover, therefore, determines the distribution of characteristic alpine plant communities (e.g. CitationKörner, 2003). Besides the timing of formation and melt of the snow cover, its temperature insulation capacity is one of the most critical characteristics from an ecosystem perspective, as it defines the temperatures that soil and plants experience during winter (CitationSturm et al., 1997). A thinner and denser snow cover has lower insulation capacity and hence leads to colder and more variable winter soil temperatures (CitationSturm et al., 1997).
Whereas the insulation by a deep snow cover usually results in stable soil temperatures of 0 °C at the soil surface (CitationPomeroy and Brun, 2001), insufficient insulation by the snow cover in winter can lead to soil freezing (CitationKeller et al., 2004; CitationRixen et al., 2004b), which represents a critical step for the ecosystem functioning. Soil freezing alters soil properties and processes such as soil aggregate formation (CitationEdwards and Cresser, 1992), size and composition of the soil microbial community (CitationSchimel and Clein, 1996), microbial activity (CitationGrogan et al., 2004), litter decomposition (CitationAerts, 2006), and nitrogen mineralization rates (CitationSchimel and Mikan, 2005; CitationFreppaz et al., 2007a). Moreover, soil freezing can affect plant performance through freeze-thaw related damage of fine roots (CitationTierney et al., 2001). Although these studies have successfully demonstrated that many ecosystem processes depend on the amount and duration of snow cover, the influence of other snow properties, such as density, remained largely unaddressed.
Climate change may affect the seasonal snow cover in several ways. Higher temperatures result in a higher percentage of the precipitation falling as rain instead of snow, causing snow covers to be thinner and to melt earlier, a phenomenon already visible in many mountain ranges (e.g. CitationLaternser and Schneebeli, 2003). Snow density is likely to increase in a warmer climate, as higher temperatures may cause wetter snow and increase rain-on-snow events (e.g. CitationRasmus et al., 2004). Interestingly, a warmer climate will not necessarily result in warmer soils: a thinner and denser snow cover will reduce the insulation of the soil. Consequently, alpine ecosystems might face the counterintuitive situation that soils could become colder in winter in a warmer climate (CitationVenalainen et al., 2001; CitationEdwards et al., 2007).
Another consequence of global warming is that the altitudinal boundary, above which snow conditions are sufficiently reliable for winter sport in the Alps, is predicted to rise from 1200 m to 1500 m a.s.l. within the next 50 years (CitationElsasser and Messerli, 2001). To guarantee skiing despite unfavorable weather conditions, snowmaking facilities have been installed in many ski resorts. In 1984, almost 60% of the U.S. ski resorts already relied on snowmaking (CitationKocak and van Gemert, 1988). In Switzerland, more than 19% of the entire area with ski runs is covered with artificial snow, and this number is rapidly growing (CitationCIPRA, 2004). To enhance ice crystal formation under warmer temperatures, ice nucleation active additives are increasingly used in snowmaking (CitationRixen et al., 2003). Most widespread is Snomax®, which is made from sterilized bacteria of the species Pseudomonas syringae (CitationBrown, 1997). General aspects of skiing and ski run leveling have been studied since the 1980s (CitationMosimann, 1985; CitationHaeberli, 1992), but the impacts of artificial snow on the environment only became an object of investigation 15 years ago (CitationKammer and Hegg, 1990; CitationNewesely, 1997; CitationRixen et al., 2004a; CitationRixen et al., 2004b; CitationWipf et al., 2005). Today, despite the increasing use of artificial snow and snow additives, many of the environmental consequences of these techniques still remain unclear.
In this field experiment, we manipulated snow characteristics, measured the changes in winter soil temperatures and nutrient dynamics, and investigated the responses in plant phenology and productivity in the following summer. This study is the first to independently manipulate multiple snow characteristics in a controlled experimental set-up. Although the experimental treatments closely simulated the snow conditions on ski runs, they also enabled analysis of correlations between different snow cover characteristics in general. Hence, the results of this experiment have implications beyond ski run conditions and can contribute to an understanding of conditions expected under climate change.
Methods
Field Site and Experimental Design
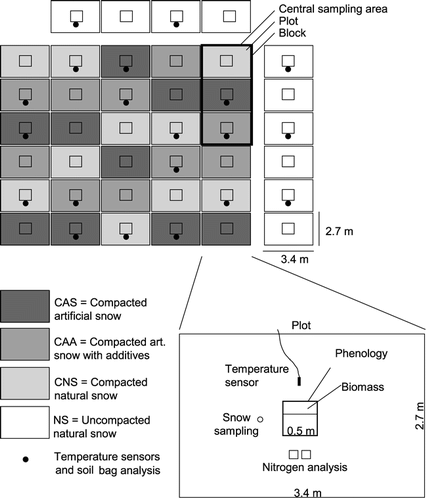
The experimental field consisted of 40 plots (), each with a size of 3.4 × 2.7 m, on a subalpine meadow near Davos, SE Switzerland (1530 m a.s.l.; 46°47′N, 9°49′E; annual precipitation: 959 mm; yearly mean temperature: 2.7 °C). The plant community of the grassland was Trisetetum flavescentis, a typical plant association of utilized subalpine meadows (CitationEllenberg, 1988) growing on a cambisol (CitationIUSS Working Group WRB, 2006) with a 10-cm-deep organic-rich surface horizon (Corg = 13.5%; C/N ratio: 16; average bulk density: 1.0 Mg m−3). In the winter of 1999/2000, one of four snow cover types was assigned to each plot (): (1) uncompacted natural snow (NS), (2) compacted natural snow (CNS), (3) compacted artificial snow with snow additives (CAA), and (4) compacted artificial snow (CAS). These snow types were chosen for two reasons. First, these four snow types cover a wide range of different snow densities that occur in nature and that are likely to affect soil and vegetation. Densities of natural snowpacks in mid and late winter can range between ∼250 and 670 kg m−3 (CitationMolotch et al., 2005), while those of groomed snow on ski runs range between 400 and 600 kg m−3 (CitationFauve et al., 2002). Second, with increasing use of artificial snow on ski runs, it is highly relevant to understand impacts of compacted natural snow vs. compacted artificial snow, as well as artificial snow with vs. without additives.
The plots with compacted snow were arranged in a randomized complete block design, but the plots with uncompacted natural snow were distributed around the compacted part of the experimental field (), as it was impossible to leave single plots unaffected between the compacted plots. Although the NS plots were not randomized, we considered them as real replicates because no natural gradients or spatial patterns (which would have been indicated by significant differences between blocks) were apparent within the experimental field (see statistical analysis).
Snow Treatments and Analysis
Artificial snow for the experiment was produced at the adjacent Jakobshorn ski resort during a cold night (minimum temperatures: −15 °C) from 21 to 22 January 2000 with water from the river Landwasser (chemical data from regular survey on water quality: NO3 −: <1–2.5 mg L−1; NH4 +: <0.01–0.04 mg L−1; SO4 2−: 4.9–11.9 mg L−1; Ca2+: 6.2–16.8 mg L−1; Mg2+: 3–7.1 mg L−1). The artificial snow with ice nucleation additives contained a standard concentration of 0.8 g of Snomax® per m3 water. On the artificial snow plots, the natural snow cover of 50 cm depth at that time was manually removed and replaced with artificial snow on the same day. Thereafter, all but the NS plots were compacted with a grooming vehicle as used on ski runs, which resulted in an overall snow depth of approx. 30 cm on all compacted plots and 50 cm on uncompacted NS plots. The snow compaction was repeated after each natural snow fall of more than 30 cm (two occurrences before March 2000).
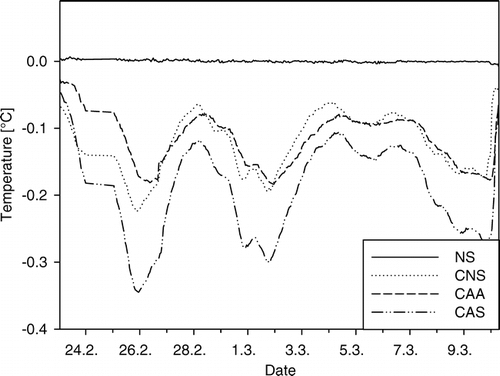
On 14 March 2000 (before spring melt-out) we recorded the depth of the snow cover on each plot and measured its density by sampling and weighing 30-cm sections of the snow column with a SIPRE corer (Snow, Ice, Permafrost Research Establishment; CitationHorner, 1990). As an estimate for snow mass, the water equivalent was calculated as the product of snow depth and density. To measure the ionic contents of the snow, the samples were melted and the electrical conductivity of the water measured with a conductivity meter. As a measure of the relative heat transfer through the snow, we calculated k/ΔZ where k is the effective thermal conductivity of the snow cover (in W m−1 K−1) and ΔZ the snow depth (CitationRixen et al., 2004b). The effective conductivity was calculated as k = 0.138–1.01 ρ + 3.233 ρ2 (where ρ is density; CitationSturm et al., 1997). The timing of melt-out of each plot was determined visually. Soil temperatures were recorded at the soil surface and at 5 cm depth every hour with temperature sensors (107-L, Campbell; accuracy ± 0.2 °C) in every other block (5 replicates per treatment; ). The sensors were placed perpendicular to the surface and were connected to a central data logger outside of the experimental field. Prior to the experiment, the sensors were calibrated at 0 °C, and at the end of the experiment they were still highly precise at that temperature (see constant 0 °C temperatures under natural snow, ).
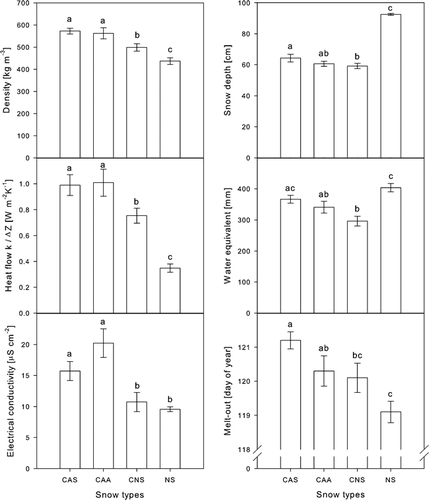
Figure 2 Snow properties, i.e. snow density, snow depth, heat flow, water equivalent, electrical conductivity, and melt-out date, in response to the four snow types: compacted artificial snow (CAS), compacted artificial snow with snow additives (CAA), compacted natural snow (CNS), and uncompacted natural snow (NS). Different letters indicate statistically significant differences in LSD–post hoc tests (P < 0.05). Shown are means ± 1 SE.
Soil Nitrogen Dynamics
Net ammonification and net nitrification were measured in the field using the buried-bag technique (CitationNadelhoffer et al., 1984; CitationSchmidt et al., 1999), which enables measurements that account for fluctuations in soil temperature while the water content is kept constant. The technique prevents plant uptake of mineralized nutrients but allows uptake by microbes and denitrification (CitationSchmidt et al., 1999). From each plot, one soil core (10 cm deep) from the topsoil was collected, totaling 40 samples. Half of the samples were analyzed in the laboratory for extractable inorganic N. The other half were carefully placed in polyethylene bags, tightly sealed, replaced in the hole and covered with the organic layer (CitationSchmidt et al., 1999). The incubation period lasted from 1 December 1999 to immediately after melt-out when the bags were removed.
The seasonal net ammonification and nitrification were expressed as the difference in soil NH4-N and NO3-N, respectively, before and after the incubation period. Net N mineralization was calculated as the sum of net ammonification and net nitrification on a g N m–2 (to 10 cm depth) basis by multiplying the inorganic N content (per gram dry soil) by the mass of dry soil per m2. In addition, unbagged (undisturbed) soil samples were collected at the plots with uncompacted and compacted natural snow in April 2000.
The soil samples were stored at + 4 °C for at maximum 24 h before analysis. Soil extracts were obtained by shaking 5 g of soil in 50 mL of 1N potassium chloride (KCl) at 250 rpm for 1 h (CitationKeeney and Nelson, 1982). Ammonium (NH4 +) and nitrate (NO3 −) concentrations in the solution were determined by the indophenol-blue method (CitationBonmati et al., 1985) and by the salicylic method (CitationKeeney and Nelson, 1982), respectively.
Vegetation Analysis
To assess flowering phenology in spring and summer 2000, we visited the experimental field daily and noted the date when easily recognizable stages were reached by three of the most common meadow species: Crocus albiflorus Kit. (main flowering), Taraxacum officinale Weber sensu latissimo agg. (withering of flowers), and Polygonum bistorta L (start of flowering). On 18 July 2000 we assessed productivity on each plot by harvesting the biomass on 20 × 50 cm of the central sampling area 3 cm above the ground, drying it for 48 h at 70 °C and weighing it.
Statistical Analysis
The effects of the four snow treatments on response variables (snow density; depth; heat flow; water equivalent; electrical conductivity of meltwater; day of melt-out; minimum soil surface temperature; ammonification; nitrification; mineralization; phenology of C. albiflorus, T. officinale, and P. bistorta; biomass) were tested with one-way ANOVA and subsequent post-hoc LSD (Least Significant Difference) tests. The factor “block” was pooled with the error term as it did not explain significant variation in any of the analyses. Residuals were visually checked for normality and homoscedasticity. Differences in ammonification between bagged and unbagged soil samples were tested with a t-test.
To analyze correlations between variables, we employed path analysis (CitationWright, 1934) with a subsample of all 20 plots that were equipped with temperature sensors. Within a given multivariate path model the magnitude of a path coefficient (standardized regression coefficients) indicates the strength of the partial effect of an explanatory variable upon the response variable. Single correlations between variables were analyzed with the Pearson correlation coefficient. Path analyses were carried out using CitationAMOS (2004), and all other analyses with SPSS 13 (CitationSPSS, 2003).
Results
Snow Properties and Soil Temperatures
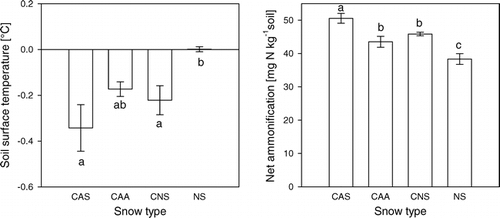
The snow density was on average highest in compacted artificial snow (CAS), followed by compacted artificial snow with snow additives (CAA), compacted natural snow (CNS), and uncompacted natural snow (NS) (F 3,36 = 12.227, P < 0.001; ). On 14 March 2000, the depth of the natural snow cover was 92 ± 0.6 cm and that of the compacted snow types between 60 and 65 cm (F 3,36 = 82.06, P < 0.001). The effective thermal conductivity through the snow cover k/ΔZ showed a similar pattern as the density with highest conductivities in CAS and CAA, lower values in CNS, and lowest ones in NS (F 3,36 = 17.389, P < 0.001). These patterns in effective thermal conductivity led to significant changes in soil temperatures between snow types. The high thermal insulation by natural snow caused stable soil surface temperatures at 0 °C (). Under the compacted snow types, temperatures fluctuated between 0 and –0.35 °C. The higher the fluctuations, the lower were absolute minimum soil surface temperatures. Consequently, soil surface minimum temperatures were significantly influenced by the snow types (F 3,15 = 5.069, P < 0.05). They were lowest under CAS (–0.34 ± 0.1 °C), followed by CNS (–0.22 ± 0.06 °C), CAA (–0.17 ± 0.03 °C), and NS (0.00 ± 0.01 °C; and ). No soil freezing was detected by the sensors installed at 5 cm depth, and minimum temperatures at 5 cm depth differed significantly between snow treatments in the same order as at the soil surface (between 0 and 0.25 °C, F 3,15 = 16.522, P < 0.001). The water equivalent was highest in NS, lowest in CNS, and intermediate in the two artificial snow types (F 3,36 = 8.766, P < 0.001; ).
Figure 4 Minimum soil surface temperatures and net ammonification in response to the four different snow types (means ± 1 SE). Different letters indicate statistically significant differences in LSD–post hoc tests (P < 0.05).
Melt-out of the snow cover showed the same pattern as snow density: the densest snow types melted last (F 3,36 = 5.4, P < 0.01; ). The melt-out date showed a highly significant correlation with snow density, but not with water equivalent (Pearson correlation: P > 0.5, not shown). The average difference in melt-out date between the plots with artificial snow and uncompacted natural snow was two days (LSD: P < 0.001).
The electrical conductivity of the meltwater comprised between 9 and 24 µS cm–2 (F 3,36 = 9.485, P < 0.001; ), and the values derived from the artificial snow types were significantly higher than those from the natural snow types (LSD: P < 0.05). Within the artificial snow types, electrical conductivity was higher when the snow contained ice nucleators (LSD: P = 0.053; ).
Nitrogen Mineralization
The values of net ammonification over winter showed the same pattern as the soil temperatures and were highest under CAS (50.6 ± 1.5 mg N kg−1), intermediate under CNS and CAA (45.9 ± 0.5 and 43.5 ± 1.6 mg N kg−1, respectively), and lowest under NS (38.4 ± 1.6 mg N kg−1; F 3,15 = 14.5, P < 0.001; ). Net nitrification was not significantly influenced by the snow types (P = 0.39). Winter net N mineralization was highest under CAS (7.9 g N m–2) followed by 6.9 g N m–2 under CAA, 6.8 g N m–2 under CNS, and 6.1 g N m–2 under NS (F 3,15 = 5.96, P < 0.01). The NH4 + increase in the soil (comparing before and after the snow-treatment period) was significantly higher in bagged soils than in unbagged ones (under uncompacted snow: 38.4 ± 1.6 vs. 15.3 ± 2.7 mg N kg−1; under compacted snow: 45.9 ± 0.5 vs. 22.5 ± 1.6 mg N kg−1, both P < 0.001).
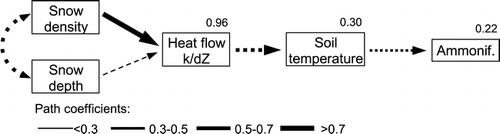
Path analysis revealed the relationships between snow characteristics and below-ground processes (). Snow depth and density both affected the effective thermal conductivity through the snow cover, however, density was the more important factor: denser snow corresponded to higher thermal conductivity (path coefficient 0.80), and greater snow depths corresponded to lower conductivity (path coefficient –0.29). Increased thermal conductivity resulted in colder soil temperatures, which subsequently led to enhanced net ammonification (and net N mineralization; not shown).
Figure 5 Results of path analysis for the relationships between physical parameters of the snowpack, minimum soil surface temperatures, and net ammonification. All path relationships shown are statistically significant (P < 0.05). Line width indicates the magnitude of the path coefficients. Negative correlations are denoted by dotted, positive by solid lines. Model R 2 values are indicated for heat flow, soil temperatures, and net ammonification.
Plant Phenology and Biomass Production
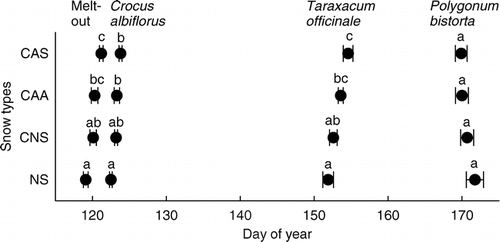
The flowering phenology was significantly affected by the snow treatments: Crocus albiflorus, the first species to flower at the study site, flowered earliest in the NS and CNS plots and latest in the CAS plots (). Flower senescence of Taraxacum officinale, which occurred 33 days after melt-out, was also delayed in the densest snow treatment plots. Six weeks after melt-out, however, when Polygonum bistorta started flowering, the treatment effects on phenology had disappeared.
Figure 6 Phenology of the meadow plants Crocus albiflorus (flowering), Taraxacum officinale agg. (flower senescence), and Polygonum bistorta (flowering) on plots with different snow treatments (means ± 1 SE). Different letters indicate statistically significant differences in LSD–post hoc tests (P < 0.05).
Path analysis revealed that the strongest influence on the flowering phenology of C. albiflorus was exerted by the compaction of the snow, which led to delayed melt-out and delayed flowering (). To a lesser degree, C. albiflorus was also influenced by soil surface temperatures: cold soil temperatures that were fostered by high snow density and low snow depth also delayed flowering. The phenology of T. officinalis was only influenced by snow density and melt-out, not by soil temperatures.
Figure 7 Path diagram showing influence of environmental parameters on plant responses. (A) Hypothesized relationships, (B) flowering of Crocus albiflorus, (C) flower senescence of Taraxacum officinale, (D) above-ground biomass production. All path relationships shown in (B), (C), and (D) are statistically significant (P < 0.05). Line width indicates the magnitude of the path coefficients. Negative correlations are denoted by dotted, positive by solid lines. Model R 2 values are indicated for soil temperatures, melt-out, phenology, and biomass.
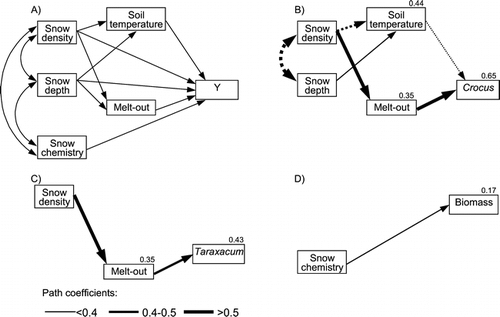
Total above-ground biomass production was not affected by any of the physical characteristics of the snow cover, soil temperatures, melt-out, or plant phenology (all Pearson correlations: P > 0.1). However, biomass production did respond positively to the input of chemical compounds from the snow cover, resulting in higher production in plots that had received meltwater with higher electrical conductivity ().
Discussion
This study is one of the first to simultaneously test the effects of multiple snow characteristics, i.e. snow density, depth, and chemistry, on soil nitrogen cycling and vegetation processes. These snow properties shaped the conditions that soils and plants experienced over winter in various ways. A denser snow cover led to reduced soil insulation and lower soil temperatures, which consequently increased nitrogen mineralization in the soil. A denser snow cover furthermore resulted in a delay in plant development, which persisted several weeks after melt-out.
Many dependent variables (heat flow, melt-out date, soil temperatures, and net ammonification) responded to the treatments in approximately the same order as the snow density, i.e. compacted artificial snow, compacted artificial snow with snow additives, compacted natural snow and uncompacted natural snow. This general pattern was consistent, although the significance between treatments varied between the variables. In our experiment, snow density had a relatively higher relevance for heat flow and melt-out date than snow depth or water equivalent. The surprisingly higher correlation of the melt-out date with density than with the water equivalent may have been due to differences in soil temperatures: plots with natural uncompacted snow showed the highest water equivalent but also the warmest soil temperatures, which may have caused more rapid melting.
Our results are in line with numerous previous studies showing that winter soil processes are strongly controlled by soil temperatures and highly sensitive to even slight temperature changes. For instance, winter soil temperatures have a considerable influence on microbial processes. Microbes can remain active below 0 °C as long as liquid water is available, typically down to –5 °C (CitationCoxson and Parkinson, 1987; CitationSchimel and Clein, 1996). The freezing of soil can have considerable effects on the below-ground ecosystem by curtailing the microbial community (CitationDe Luca et al., 1992) and reducing microbial activity (CitationGrogan et al., 2004), and also through the mechanical impact on soil aggregates (CitationEdwards and Cresser, 1992) and on fine roots of plants (CitationTierney et al., 2001). These factors can affect several key processes of nutrient cycling including decomposition (CitationAerts, 2006) and nitrogen mineralization (CitationSchimel and Mikan, 2005; CitationFreppaz et al., 2007a, Citation2007b). The sum of these studies support our result that changes in soil temperature may significantly alter winter soil processes.
The recorded net N mineralization rates under the snow types in our experiment are in accordance with those reported by several authors in seasonally snow covered soils in the Alaskan and Scandinavian Arctic (CitationGiblin et al., 1991; CitationSchmidt et al., 1999) and the North American alpine (CitationBrooks et al., 1998), where inorganic N accumulates in the soil under the winter snowpack.
The enhanced net ammonification found in the treatments with colder soils may have resulted either from increased ammonium production or from decreased ammonium depletion. Ammonium production may be enhanced by two likely pathways: (1) The volumetric changes of water upon freezing may lead to the disintegration of soil aggregates, which could increase microbial activity and ammonium production due to the exposure of formerly unavailable soil surfaces (CitationEdwards and Cresser, 1992; CitationHerrmann and Witter, 2002). (2) Microbes have been found to use different carbon sources at different soil temperatures over winter (CitationSchimel and Mikan, 2005). Since microbes immobilize N when they are N limited and mineralize N when they are C limited (CitationSchimel et al., 2004), microbes might shift from using C-rich organic substrates to more N-rich compounds under colder conditions (CitationSchimel et al., 2004). Possible reasons are either a relative decrease in the availability of C-rich substrates, e.g. plant litter, or a relative increase in the availability of N-rich substrates, e.g. microbial products (CitationSchimel and Mikan, 2005).
Enhanced net ammonification in colder soils might also result from reduced ammonium depletion, which could be explained by two mechanisms: (1) Less of the ammonium produced may be immobilized by microbes (CitationBrooks and Williams, 1999). (2) Ammonium losses, e.g. due to denitrification, leaching or plant uptake, may be decreased. In bagged soils, ammonium depletion is limited to nitrification, immobilization, and gaseous losses of N2O, NO, or N2 via nitrification and denitrification (CitationBrooks et al., 1997). Winter denitrification rates can be considerable, particularly in wet soils (up to 0.65 g N m–2 y−1, CitationGroffman et al., 2001). In unbagged soil, ammonium may be leached with percolating water (CitationBrooks et al., 1998). Leaching after increased mineralization was often found in winters with little snow and, subsequently, cold soils (CitationBoutin and Robitaille, 1995; CitationGroffman et al., 2001). Ammonium may, furthermore, be taken up by plants (CitationBilbrough et al., 2000). Winter and early spring nitrogen uptake by alpine plants was estimated at 0.5 g N m–2 y−1 (CitationBilbrough et al., 2000), which represented a considerable percentage of the annual N uptake of these plants. Ammonium contents in our experiment were indeed lower in unbagged than in bagged soil, which suggests leaching and/or plant uptake of ammonium.
Plant phenology in our study was strongly controlled by the timing of melt-out, but to a lesser degree was also influenced by soil temperatures during winter. Short-term reactions in plant phenology to changes in melt-out have been reported in several studies (e.g. CitationWalker et al., 1999; CitationWipf et al., 2006). Like in our study, early-flowering species usually responded more strongly to changes in melt-out, while later-flowering species often showed no reaction. Biomass production was not affected by delayed flowering phenology, which supports the view that alpine plants show high phenological plasticity towards interannual variation in development (CitationStinson, 2004).
Neither the delayed phenology nor the increased ammonification in plots with manipulated snow cover had any significant impact on the above-ground biomass production in our experiment. A positive impact of increased N availability might have been balanced by negative effects of soil freezing on plants, such as disruption of fine roots (CitationTierney et al., 2001). Still, plant growth was sensitive to an ionic input, as seen from the positive response of plant productivity to addition of snow with higher electrical conductivity of the meltwater. The positive relationship between ionic input through meltwater and biomass production further supports the view that mountain plants are able to take up nutrients from meltwater in early spring and that this uptake has direct impacts on their fitness (CitationBilbrough et al., 2000).
Our experiment demonstrates that even small changes in snow properties, such as snow density, snow depth, or snow chemistry can alter soil and vegetation processes. On ski runs, changes of the snow cover are much more dramatic than those applied in our experiment. The snow mass on ski runs is as much as doubled due to addition of artificial snow, and melt-out subsequently occurs with a delay of two to three weeks (CitationRixen et al., 2004b). The heat flow through the compacted snow cover on ski runs is enhanced such that soil temperatures can fall below −15 °C, which is many times colder than in our experiment (CitationRixen et al., 2004b). The ionic input from meltwater of artificial snow was eightfold that of natural snow covers (CitationRixen et al., 2004b). Therefore, we expect consequences for soil and ecosystem processes to be much greater on ski runs than in our experiment. Indeed, changes in the vegetation composition on ski runs indicate that ecosystems do rapidly respond to continuous changes of the snow cover such as the shortened vegetation period and increased nutrient input imposed by ski run use (CitationWipf et al., 2005; CitationBarni et al., 2007; CitationRixen et al., 2008). The artificial snow produced with snow additives contained a relatively low concentration of nitrogen (0.8 g additives m−3 water, resulting in an input of 0.1 g N m−3 water; CitationBUWAL, 1997). Previous experiments with higher concentrations of the same additive resulted in a moderate, but by no means negligible impact on the growth of potted plants (CitationRixen et al., 2003). Nevertheless, our results demonstrate that even a low input of ions may influence growth of sensitive alpine plants.
Conclusions
Our data show that changes in snow characteristics, which have been largely neglected in the global change discussion until now, can cause significant changes in soil and vegetation processes. Due to its insulation capacity, the snow cover controlled the soil temperatures. The snow chemistry furthermore determined ion and nutrient influx during melt-out. The different snow properties had important consequences on several important ecosystem processes. A denser snow cover reduced soil insulation and enhanced soil temperature variation, which increased net N mineralization in the soil. A denser snow cover furthermore resulted in a delay in plant phenology still visible several weeks after melt-out. This shift in phenology did not affect biomass production, but snow chemistry did.
Acknowledgments
We are grateful to the Swiss Federal Office for the Environment FOEN and the cantons of Grisons and Valais for financial support. We also want to thank Tony Edwards, Melissa Martin, and two anonymous referees for comments on an earlier version of this manuscript.
References Cited
- Aerts, R. 2006. The freezer defrosting: global warming and litter decomposition rates in cold biomes. Journal of Ecology 94:713–724.
- AMOS 2004. AMOS 6 for Windows. Chicago SPSS Inc.
- Barni, E. , M. Freppaz , and C. Siniscalco . 2007. Interactions between vegetation, roots, and soil stability in restored high-altitude ski runs in the Alps. Arctic, Antarctic, and Alpine Research 39:25–33.
- Bilbrough, C. J. , J. M. Welker , and W. D. Bowman . 2000. Early spring nitrogen uptake by snow-covered plants: a comparison of arctic and alpine plant function under the snowpack. Arctic, Antarctic, and Alpine Research 32:404–411.
- Bonmati, M. , M. Pujola , J. Sana , and M. Soliva . 1985. Chemical properties of nitrite oxidizers, urease and phosphate activities in sewage sludge–amended soils. Plant and Soil 84:79–91.
- Boutin, R. and G. Robitaille . 1995. Increased soil nitrate losses under mature sugar maple trees affected by experimentally-induced deep frost. Canadian Journal of Forest Research–Revue Canadienne De Recherche Forestiere 25:588–602.
- Brooks, P. D. and M. W. Williams . 1999. Snowpack controls on nitrogen cycling and export in seasonally snow-covered catchments. Hydrological Processes 13:2177–2190.
- Brooks, P. D. , S. K. Schmidt , and M. W. Williams . 1997. Winter production of CO2 and N2O from alpine tundra: environmental controls and relationship to inter-system C and N fluxes. Oecologia 110:403–413.
- Brooks, P. D. , M. W. Williams , and S. K. Schmidt . 1998. Inorganic nitrogen and microbial biomass dynamics before and during spring snowmelt. Biogeochemistry 43:1–15.
- Brown, R. 1997. Man-made snow. Scientific American 1997:1 100.
- BUWAL 1997. SnomaxTM Bundesamt für Umwelt, Wald und Landschaft (BUWAL). Bern.
- CIPRA 2004. Künstliche Beschneiung im Alpenraum—Ein Hintergrundbericht.
- Coxson, D. S. and D. Parkinson . 1987. Winter respiratory activity in aspen woodland forest floor litter and soils. Soil Biology & Biochemistry 19:49–59.
- De Luca, T. H. , D. R. Keeney , and G. W. McCarty . 1992. Effect of freeze-thaw events on mineralization of soil nitrogen. Biology and Fertility of Soils 14:116–120.
- Edwards, A. C. and M. S. Cresser . 1992. Freezing and its effect on chemical and biological properties of soil. Advances in Soil Science 18:59–95.
- Edwards, A. C. , R. Scalenghe , and M. Freppaz . 2007. Changes in the seasonal snow cover of alpine regions and its effects on soil processes: a review. Quaternary International 162–163:172–181.
- Ellenberg, H. 1988. Vegetation ecology of Central Europe. 4th edition Cambridge Cambridge University Press. 731 pp.
- Elsasser, H. and P. Messerli . 2001. The vulnerability of the snow industry in the Swiss Alps. Mountain Research and Development 21:335–339.
- Fauve, M. , H. Rhyner , and M. Schneebeli . 2002. Preparation and maintenance of pistes—Handbook for practitioners: Eidgenössisches Institut für Schnee- und Lawinenforschung, SLF, 134 pp.
- Freppaz, M. , B. L. Williams , A. C. Edwards , R. Scalenghe , and E. Zanini . 2007a. Simulating soil freeze/thaw cycles typical of winter alpine conditions: Implications for N and P availability. Applied Soil Ecology 35:247–255.
- Freppaz, M. , B. L. Williams , A. C. Edwards , R. Scalenghe , and E. Zanini . 2007b. Labile nitrogen, carbon, and phosphorus pools and nitrogen mineralization and immobilization rates at low temperatures in seasonally snow-covered soils. Biology and Fertility of Soils 43:519–529.
- Giblin, A. E. , K. J. Nadelhoffer , G. R. Shaver , J. A. Laundre , and A. J. McKerrow . 1991. Biogeochemical diversity along a riverside toposequence in Arctic Alaska. Ecological Monographs 61:415–435.
- Groffman, P. M. , C. T. Driscoll , T. J. Fahey , J. P. Hardy , R. D. Fitzhugh , and G. L. Tierney . 2001. Effects of mild winter freezing on soil nitrogen and carbon dynamics in a northern hardwood forest. Biogeochemistry 56:191–213.
- Grogan, P. , A. Michelsen , P. Ambus , and S. Jonasson . 2004. Freeze-thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biology & Biochemistry 36:641–654.
- Haeberli, W. 1992. Construction, environmental problems and natural hazards in periglacial mountain belts. Permafrost and Periglacial Processes 3:111–124.
- Herrmann, A. and E. Witter . 2002. Sources of C and N contributing to the flush in mineralization upon freeze-thaw cycles in soils. Soil Biology & Biochemistry 34:1495–1505.
- Horner, R. A. 1990. Techniques for sampling sea-ice algae. In Medlin, L. K. and J. Priddle , editors. Polar marine diatoms Cambridge, England British Antarctic Survey. 19–23.
- IUSS Working Group WRB 2006. World reference base for soil resources 2006. Second edition World Soil Resources Reports No. 103, FAO, Rome.
- Jones, H. G. , J. W. Pomeroy , D. A. Walker , and R. W. Hoham , editors. 2001. Snow ecology. 1st edition Cambridge University Press: Press Syndicate of the University of Cambridge. 378 pp.
- Kammer, P. and O. Hegg . 1990. Auswirkungen von Kunstschnee auf subalpine Rasenvegetation. Verhandlungen der Gesellschaft für Ökologie 19:758–767.
- Keeney, D. R. and D. W. Nelson . 1982. Nitrogen-inorganic forms. In Page, A. L. , D. R. Miller , and D. R. Keeney , editors. Methods of soil analysis, part 2. Chemical and microbiological methods Madison, Wisconsin ASA, SSSA. 643–698.
- Keller, T. , C. Pielmeier , C. Rixen , F. Gadient , D. Gustafsson , and M. Staehli . 2004. Impact of artificial snow and ski-slope grooming on snowpack properties and soil thermal regime in a sub-alpine ski area. Annals of Glaciology 38:314–318.
- Kocak, R. and H. van Gemert . 1988. Man-made snow: biotechnology assisting the skiing industry. Australian Journal of Biotechnology 2:37–38.
- Körner, C. 2003. Alpine plant life. 2nd edition Berlin Springer Verlag. 344 pp.
- Laternser, M. and M. Schneebeli . 2003. Long-term snow climate trends of the Swiss Alps (1931–99). International Journal of Climatology 23:733–750.
- Molotch, N. P. , M. T. Colee , R. C. Bales , and J. Dozier . 2005. Estimating the spatial distribution of snow water equivalent in an alpine basin using binary regression tree models: the impact of digital elevation data and independent variable selection. Hydrological Processes 19:1459–1479.
- Mosimann, T. 1985. Geo-ecological impacts of ski piste construction in the Swiss Alps. Applied Geography 5:29–37.
- Nadelhoffer, K. J. , J. D. Aber , and J. M. Melillo . 1984. Seasonal patterns of ammonium and nitrate uptake in nine temperate forest ecosystems. Plant and Soil 80:321–335.
- Newesely, C. 1997. Auswirkungen der künstlichen Beschneiung von Schipisten auf Aufbau, Struktur und Gasdurchlässigkeit der Schneedecke, sowie auf den Verlauf der Bodentemperatur und das Auftreten von Bodenfrost. PhD thesis. Naturwissenschaftliche Fakultät, Leopold Franzens Universität, Innsbruck, Austria.
- Pomeroy, J. W. and E. Brun . 2001. Physical properties of snow. In Jones, H. G. , J. W. Pomeroy , D. A. Walker , and R. W. Hoham , editors. Snow Ecology Cambridge Cambridge University Press. 45–126.
- Rasmus, S. , J. Räisänen , and M. Lehning . 2004. Estimating snow conditions in Finland in the late 21st century using the SNOWPACK model with regional climate scenario data as input. Annals of Glaciology 38:238–244.
- Rixen, C. , V. Stoeckli , and W. Ammann . 2003. Does artificial snow production affect soil and vegetation of ski pistes? A review. Perspectives in Plant Ecology Evolution and Systematics 5:219–230.
- Rixen, C. , A. Casteller , F. H. Schweingruber , and V. Stoeckli . 2004. Age analysis helps to estimate plant performance on ski pistes. Botanica Helvetica 114:127–138.
- Rixen, C. , W. Haeberli , and V. Stoeckli . 2004. Ground temperatures under ski pistes with artificial and natural snow. Arctic, Antarctic, and Alpine Research 36:419–427.
- Rixen, C. , C. Huovinen , K. Huovinen , V. Stoeckli , and B. Schmid . 2008. A plant diversity × water chemistry experiment in subalpine grassland. Perspectives in Plant Ecology Evolution and Systematics 10:51–61.
- Schimel, J. and J. Clein . 1996. Microbial response to freeze/thaw cycles in tundra and taiga soils. Soil Biology & Biochemistry 28:1062–1066.
- Schimel, J. P. and C. Mikan . 2005. Changing microbial substrate use in Arctic tundra soils through a freeze-thaw cycle. Soil Biology & Biochemistry 37:1411–1418.
- Schimel, J. P. , C. Bilbrough , and J. A. Welker . 2004. Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biology & Biochemistry 36:217–227.
- Schmidt, I. K. , S. Jonasson , and A. Michelsen . 1999. Mineralization and microbial immobilization of N and P in arctic soils in relation to season, temperature and nutrient amendment. Applied Soil Ecology 11:147–160.
- SPSS 2003. SPSS for Windows. Chicago SPSS Inc.
- Stinson, K. A. 2004. Natural selection favors rapid reproductive phenology in Potentilla pulcherrima (Rosaceae) at opposite ends of a subalpine snowmelt gradient. American Journal of Botany 91:531–539.
- Sturm, M. , J. Holmgren , M. König , and K. Morris . 1997. The thermal conductivity of seasonal snow. Journal of Glaciology 43:26–41.
- Tierney, G. L. , T. J. Fahey , P. M. Groffman , J. P. Hardy , R. D. Fitzhugh , and C. T. Driscoll . 2001. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 56:175–190.
- Venalainen, A. , H. Tuomenvirta , M. Heikinheimo , S. Kellomaki , H. Peltola , H. Strandman , and H. Vaisanen . 2001. Impact of climate change on soil frost under snow cover in a forested landscape. Climate Research 17:63–72.
- Walker, M. D. , D. A. Walker , J. M. Welker , A. M. Arft , T. Bardsley , P. D. Brooks , J. T. Fahnestock , M. H. Jones , M. Losleben , A. N. Parsons , T. R. Seastedt , and P. L. Turner . 1999. Long-term experimental manipulation of winter snow regime and summer temperature in arctic and alpine tundra. Hydrological Processes 13:2315–2330.
- Wipf, S. , C. Rixen , M. Fischer , B. Schmid , and V. Stoeckli . 2005. Effects of ski piste preparation on alpine vegetation. Journal of Applied Ecology 42:306–316.
- Wipf, S. , C. Rixen , and C. P. H. Mulder . 2006. Advanced snowmelt causes shift towards positive neighbour interactions in a subarctic tundra community. Global Change Biology 12:1496–1506.
- Wright, S. 1934. The method of path coefficients. The Annals of Mathematical Statistics 5:161–215.