Abstract
Flight of alpine stream insects has not been well studied but is an important ecological process that ensures successful mating and allows gene flow among relatively isolated populations. In this study, we collected actively flying insects along a perpendicular transect from an alpine headwater stream in the Colorado Rocky Mountains (U.S.A.) during the summer emergence season in two consecutive years with contrasting hydrology: 2002 had minimal snowfall the previous winter, while 2003 snowfall was above average. Flight activity patterns among four common stream taxa were similar to previously reported results from streams below treeline: Ephemeroptera and Plecoptera declined as an inverse power function, Trichoptera declined as a negative exponential function, and Simuliidae did not decrease with lateral distance. Sex ratios typically were strongly biased, possibly a result of the harsh terrestrial environment negatively influencing the naturally more sedentary sex (which varies among taxa). In 2003, the majority of common species emerged approximately one month later than in 2002, and abundance and diversity were greater in 2003 than 2002, patterns potentially attributable to increased snowpack amount and duration. Late-emerging species, by contrast, were less abundant in 2003, likely because that year emergence was delayed to later in the season, when cooler air temperatures reduce flight activity. Our results suggest that alpine streams are sensitive to interannual variation in snowpack, and therefore more research will be needed to address the potential effects of climate change and associated winter snowfall trends on these unexpectedly diverse aquatic systems.
Introduction
The dominant macrofauna in alpine streams are aquatic insects (CitationWard, 1994; CitationHauer et al., 2007), many of which develop from a relatively lengthy stream-dwelling juvenile phase into a short-lived adult form in which some degree of aerial flight is possible. Despite the brevity of the adult stage, across all common taxonomic groups flight plays a major role in among-stream dispersal important to local and regional population dynamics and in successful mating and reproduction (CitationMackay, 1992; CitationPalmer et al., 1996). Insect flight also provides an important energy subsidy (sensu CitationNakano and Murakami, 2001) to various terrestrial predators such as birds, bats, and spiders.
In the alpine zone (at altitudes above treeline), little is known of the extent of stream insect flight activity, although it has been speculated that the short growing seasons, high winds, low humidity, and cool air temperatures are particularly limiting factors (e.g. CitationDownes, 1965; CitationWard, 1994; CitationFinn and Poff, 2005). The energetic cost of insect flight can be expected to be increased extensively by these local climatic factors (CitationNilsson et al., 1993). Indeed, recent genetic studies on alpine stream specialist species have shown strong among-stream genetic structure across a small geographic extent, implicating limited flight dispersal (CitationFinn and Adler, 2006; CitationFinn et al., 2006).
To date, these indirect genetic estimates provide the only insight we have towards understanding alpine stream insect flight. However, there have been several direct observational studies of flight activity in temperate streams below treeline, where climatic conditions are considerably more benign. Where trees occur, for example, the atmospheric boundary layer is thicker (e.g. CitationJackson and Resh, 1989; CitationBriers et al., 2003) and hence presumably more conducive than alpine conditions to insect flight. Nonetheless, studies below treeline have revealed that flight tends to be concentrated near the natal stream (e.g. CitationBriers et al., 2002; CitationPetersen et al., 1999), although further studies using genetic markers or stable isotopes have demonstrated low but significant levels of long-distance movement (e.g. CitationWilcock et al., 2001; CitationBriers et al., 2004). These genetic results from non-alpine streams provide an interesting contrast to those cited above for alpine insects, which did not reveal significant long-distance movement. A consequent prediction can therefore be made that direct observational studies in alpine streams should reveal minimal flight activity and flight distance from natal streams.
Due to its patchy distribution and environmental extremes, the alpine ecosystem is considered an important indicator system for global change (CitationBowman and Seastedt, 2001, and references therein). Alpine streams, at the uppermost extent of often lengthy river systems, share similar characteristics of isolation and confinement of endemic biotic assemblages suited to this extreme (CitationWard, 1994; CitationFinn and Poff, 2005; CitationHauer et al., 2007). The current climate change trajectory of warming summer temperatures (CitationIPCC, 2001) has been a growing conservation concern, and particular emphasis has been placed on the shifting hydrologic regime of alpine streams due to loss or reduction of glaciers and permanent snowfields, which provide continual meltwater supply throughout warmer months (CitationBrown et al., 2007).
Another less widely acknowledged trend is the projected increase in winter snowfall in many alpine areas (cf. CitationIPCC, 2001; CitationWelker et al., 2001; but see CitationBorgstrøm, 2001). Most annual snowfall will melt in the early summer and thus may temporarily increase but not sustain late season streamflow as permanent meltwater sources do. Potentially higher spring/summer flows following winters of increased snowfall are thought to decrease community persistence in alpine streams due to increased flow disturbance (CitationMilner et al., 2006).
In addition to differences in meltwater regime between annual snowfall and permanent sources, these streamflow sources also have distinct spatial differences that may influence aquatic biota. Glaciers and permanent snowfields occur at the head of alpine streams, whereas annual winter snowfall distributes widely throughout the landscape and in many cases completely covers small alpine streams for a large proportion of each year. Depth of annual snowpack positively influences the duration of snow cover into the warm season (CitationWalker et al., 1999; CitationBorgstrøm, 2001). Lengthier snow cover over streams and lakes can maintain colder water temperatures (CitationBorgstrøm and Museth, 2005) that may delay the emergence timing (phenology) of alpine stream insects and indirectly influence flight patterns, which are sensitive to ambient air temperature at emergence.
In this study, we address two related objectives. First, we present a novel analysis of the flight activity patterns of several alpine stream insect species. Related taxonomic details and taxon-specific expectations are provided in the following Focal Taxa section. This information is fundamental to understanding how effectively alpine stream insects are able both to disperse and colonize neighboring habitats and to sustain local populations via mating. Second, we ask whether the observed patterns, in addition to emergence phenology, vary between years with highly contrasting hydrological properties driven by differences in previous winter snowpack. This two-year comparison provides an initial assessment of how interannual climatic variation may influence insect flight activity and will contribute to developing an understanding of the potential impacts of climate change on alpine stream systems.
Focal Taxa
We collected flying adults from each of four common aquatic insect taxa: Ephemeroptera (mayflies), Plecoptera (stoneflies), and Trichoptera (caddisflies) (taken together: “EPT”), and Simuliidae (black flies, order Diptera). We anticipated a gradient of flight activity patterns among these major taxa, with Ephemeroptera and Plecoptera expected to be the least active and show the steepest rate of decline in flight activity with distance from the natal stream, Simuliidae the most active and farthest dispersing, and Trichoptera intermediate. Ephemeroptera adults have a brief life span and lack functional mouthparts, thus precluding terrestrial feeding. Below treeline, mayflies have been shown to be relatively weak in flight and typically are not observed at great distances from streams (CitationJackson and Resh, 1989; CitationPetersen et al., 2004). Similarly, adults in one of the two suborders of Plecoptera lack functional mouthparts, and there are many cases of brachyptery (wing-size reduction) in stoneflies. Similarly to mayflies, stoneflies tend to be relatively weak in flight and concentrate most activity in the vicinity of the stream (e.g. CitationKuusela and Huusko, 1996; CitationPetersen et al., 1999; CitationBriers et al., 2002).
Of the EPT, Trichoptera are thought to be the strongest and most active fliers, as they are often larger-bodied and with proportionally larger wing size (CitationKovats et al., 1996). Several temperate-climate studies have shown that while caddisfly flight activity decreases with distance from stream, the decline is less steep and average flight distance for many species is greater than what is observed for mayflies or stoneflies (CitationSvensson, 1974; CitationJackson and Resh, 1989; CitationSode and Wiberg-Larsen, 1993). Across all EPT, males are typically more active in flight than females (e.g. CitationSode and Wiberg-Larsen, 1993; CitationKuusela and Huusko, 1996), a pattern likely attributable to trade-offs associated with the high energetic cost of egg production (i.e., CitationRankin and Burchsted, 1992).
Simuliidae include both the common blood-feeding (anautogenous) black flies as well as a number of species that have lost this ability (obligately autogenous) and instead feed either on plant materials or do not feed at all as adults (CitationAdler et al., 2004). Anautogenous black flies are presumed to be very active in flight in order to locate mammalian or avian bloodmeal sources, and individuals have been collected at impressive distances (up to >30 km) from the nearest larval habitat (CitationBaldwin et al., 1975; CitationChoe et al., 1984). Because the bloodmeal is required for egg maturation, the female black fly is typically the stronger and more active flier than the male (CitationCrosskey, 1990). Little is known about flight activity of obligately autogenous black flies, but they are probably considerably less active because a blood meal is not sought (e.g. CitationRothfels, 1981; CitationSnyder and Linton, 1984). Indeed, two population genetic studies of alpine autogenous blackflies in our study region have demonstrated strong population structure across a small spatial extent, strongly suggesting limited overland dispersal by adults (CitationFinn and Adler, 2006; CitationFinn et al., 2006).
Study Site
The study took place at a first-order alpine reach of the South Fork Cache la Poudre River (SFP) near the northern boundary of Rocky Mountain National Park (RMNP), Colorado, U.S.A. The reach is dominated by riffles interspersed with small pools. Riffles had an average width of 1.6 m and bankfull depth of 34 cm. At an altitude of 3416 m, SFP lies above permanent treeline in a north-facing valley but is unusual among alpine streams in the region in that the valley it occupies is relatively wide and low-gradient with a broad riparian zone dominated by low-lying willow (Salix spp.). The annual hydrograph is snowmelt-dominated (sensu CitationPoff and Ward, 1989), and stable summer flow is maintained by meltwater from a permanent snowfield. Temperature dataloggers have revealed continuous year-round flow (winter temperature 0–2 °C: Finn, unpublished data), and the stream is covered in snow on average from late October to early June. Winter flow is likely maintained by subsurface sources (e.g. CitationSueker et al., 2000), in addition to insulation provided by the deep winter snowpack. SFP therefore would best be characterized as nivo-krenal (CitationBrown et al., 2003). The stream insect community is relatively diverse compared to other alpine streams in the region (CitationFinn and Poff, 2005). Summer water temperature averages ca. 5 °C, and mean air temperature is ca. 9 °C with a range from slightly below freezing to 20 °C. Strong winds prevail from the west and are a pervasive feature in our study area (CitationGreenland and Losleben, 2001).
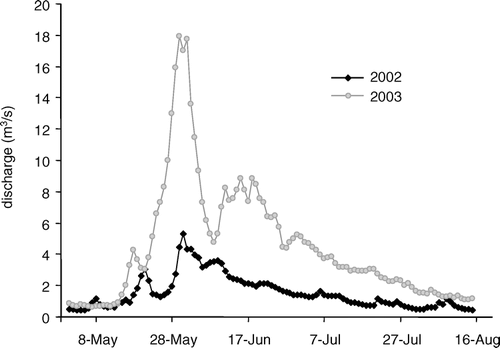
We compared hydrological data collected from nearby subalpine sites within RMNP for the emergence seasons of 2002 and 2003. Total snowfall at Bear Lake (2843 m; ∼25 km south of SFP) prior to the 2002 summer season was 3.7 m, and for 2003 it was 7.6 m. The 22-year average (years 1984–2005) total winter snowfall at Bear Lake was 5.8 m (standard deviation 1.3 m, range 3.3–8.5 m). U.S. Geological Survey gage data collected on a stream reach draining an adjacent alpine catchment reflected the substantial difference in snowpack between 2002 and 2003, revealing significantly greater peak runoff and sustained high flows continuing through a large proportion of the summer season in 2003 vs. 2002 ().
Methods
We placed four Malaise traps in an offset transect with traps at 1, 10, 30, and 60 m from the east stream bank during the emergence seasons of 2002 and 2003. Malaise traps are non-attracting, and therefore approximate natural spatial occurrences of flying insects. They are particularly effective in capturing our focal taxa. We used large two-sided traps oriented parallel to the stream, similar to those described by CitationSode and Wiberg-Larsen (1993), with openings on each side of 1.8 × 2.0 m2. Although we only were able to place a single transect, the large size of the individual traps yielded a large enough sample size of individual insects to statistically detect between-year differences and lateral patterns of flight activity. Trap roofs sloped up to single collecting heads half-filled with a 50% propylene glycol solution for specimen preservation. We monitored the traps daily during the peak of the emergence season (snowmelt to mid-August), and weekly thereafter. Collections were transferred from each trap just before heads reached capacity. Collections thus were made more frequently when insect emergence and flight activity peaked (e.g. see distribution of dates of concurrent collection from all traps in ).
Malaise traps were in continuous operation from 8 July–22 September 2002 and 23 June–21 September 2003. The earlier start date in 2003 occurred immediately following snowmelt from the stream surface. In contrast, 2002 sampling was initiated well after snowmelt: our first arrival at SFP in 2002 was 21 June, at which time the stream and riparian zone were completely snow-free. A nearby stream in RMNP at a higher elevation (3538 m) but similar aspect was snow-free on 5 June (Finn, personal observation), suggesting that full snowmelt was likely to have occurred on the order of a month earlier in 2002 than 2003.
In the laboratory, insects were transferred to 95% ethanol, separated, and species and sex determined for all EPT and Simuliidae. Females of the mayfly genus Cinygmula could not be identified to species and were therefore recorded as Cinygmula spp. (including two species: C. par Eaton and C. ramaleyi Dodds). Overall sex ratios were determined and assessed for deviations from 1:1 using G log-likelihood statistics (CitationZar, 1996) for each of the four higher-level taxa, as well as for the six most common species (having >15 individuals collected in both years; see ).
Table 1 Total numbers of males (m) and females (f) collected for each higher-level taxonomic group and common species, and total species richness within higher-level groups across all traps in 2002 and 2003. Sex ratios marked with stars are significantly different from 1:1 (***p < 0.001; **p < 0.01; *p < 0.05).
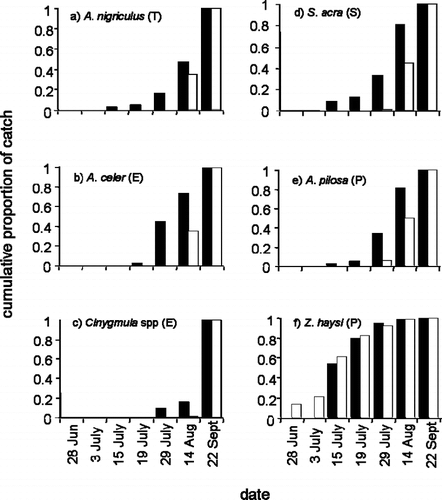
We compared emergence phenology between years for the six common species by pooling the total catch across traps for each year and plotting cumulative proportion of total annual catch by calendar date. These plots provide a visual display of phenological difference between years. We were not able to perform a robust statistical test for a directional phenological shift between years among the six common species because the relatively later start date in 2002 decreased confidence that we had captured the full distribution of emerging insects in that year. Two of the six common species, however, were late-emerging mayflies (Cinygmula spp. and Ameletus celer) for which we could be sure that the emergence period was fully sampled in both years. These in addition to two relatively common late-emerging caddisflies (Limnephilus abbreviatus and Limnephilus picturatus, recorded at ≥10 individuals per year) were included in a paired t-test of the null hypothesis that there was no mean difference in emergence timing between years. Date of first appearance in any Malaise trap was the response variable.
For each year, we also assessed relationships between distance from the stream and total abundance of individuals for each of the four taxonomic groups as well as the six most common species. For each taxon, we assessed the fit of three decreasing functions: linear, negative exponential, and inverse power. In this order, these functions model faster rates of decrease with linear distance. Each of the three models was fitted to linearly transformed data (i.e., no transformation for the linear model, log-linear for exponential, and log-log for power function) and analyzed using linear regression. The single model producing the greatest R 2 values within each taxon was selected to compare lateral flight patterns between years using analysis of covariance (ANCOVA, SAS Institute, Inc., 2003). ANCOVA used distance from stream as the covariate, year as the main factor, and total number of individuals captured as the response variable. Data were appropriately transformed according to the best-fit model to meet the ANCOVA requirement of linearity. A resulting difference in intercept would indicate a difference in total abundance between years, while an interaction with distance would indicate different slopes and therefore different rates of lateral decline in flight activity between years. In some taxa (all simuliids and the mayfly species Ameletus celer), there was no statistical evidence for decreasing flight activity with distance in either year; therefore, these taxa were not analyzed further.
We also looked for evidence of lateral variation in sex ratio for five of the six most common species and the family Simuliidae (Cinygmula was not included here because this group actually contained 2 species sensu stricto). With a similar approach as above, we used linear regression with arcsin-squareroot percent females in each year as the response variable and distance from stream as the independent variable. Regressions revealed a significant trend at α = 0.05 for only a single taxon in a single year; therefore, we did not run ANCOVA to assess differences in lateral patterns of sex ratio between years.
Results
Over the course of two alpine emergence seasons, we collected a total of 1742 individuals representing 60 species (Ephemeroptera—4, Plecoptera—17, Trichoptera—18, Simuliidae—21). All Simuliidae collected were anautogenous. Between years, we collected considerably more individuals and species in 2003 than 2002, and most of the increase in both abundance and diversity was accounted for in the stoneflies and the black flies (). In the mayflies, there was a reverse trend in abundance, which was higher in 2002 than 2003 (). Across all orders, there were six species that we considered common (range 17–373 individuals/year): Ameletus celer McDunnough and Cinygmula spp. (Ephemeroptera), Zapada haysi Ricker and Alloperla pilosa Needham and Claassen (Plecoptera), Asynarchus nigriculus Banks (Trichoptera), and Stegopterna acra Currie, Adler, and Wood (Simuliidae). Among the rarer taxa, a female and several males of an undescribed species of Prosimulium (Simuliidae) were identified in 2003. Also, several Greniera denaria Davies, Peterson, and Wood (Simuliidae) females were discovered and are a state record for Colorado and only the third record for the U.S.A. (CitationAdler et al. 2004).
For five of the six common species, we observed the progress of emergence over the full season at an approximately one-month delay in 2003 compared to 2002 (). The lone exception was the stonefly Zapada haysi, which is one of the earliest emergers from this stream and exits the water as soon as snowcover recedes. Because traps were set immediately following snowmelt in 2003 but not in 2002, data are considerably lacking for Z. haysi in early 2002; therefore, should be interpreted with caution. A paired t-test using the four most common later-emerging species revealed that timing of earliest emergence was significantly different between years (one-tailed test: p = 0.003).
Sex ratios were consistently male dominated in both collection years for all EPT and female dominated for Simuliidae (). In many cases, the unbalanced sex ratios were extreme; for example, only two females of the mayfly Ameletus celer were collected across both years, compared to 63 males (). There were no consistent trends in sex ratio with distance from stream for any of the taxa evaluated. The single instance of a significant lateral trend was for Simuliidae in 2002, in which there was an increase in percent females with distance (R2 = 0.92; p = 0.03). In 2003, when simuliid sample size increased nearly tenfold as compared to 2002, the trend was not evident.
Each of the EPT taxa demonstrated a significant lateral decline in total number of individuals collected in 2003. In 2002 each of the three decreasing functions for Trichoptera were insignificant at α = 0.05, while for Ephemeroptera and Plecoptera the 2002 decreasing function was significant and similar in shape to 2003 (, ). The best-fit model for Ephemeroptera and Plecoptera in both years was an inverse power function (). ANCOVA results confirmed a significantly greater abundance of stoneflies collected in 2003 than 2002, compared to a significantly smaller abundance of mayflies in 2003 than 2002 (, ). Power function slopes did not differ between years in either case, indicating similar lateral dispersal between years. For Trichoptera, the decreasing trend was significant only in 2003, but the negative exponential model had the best fit in both years (p = 0.10 in 2002; see , ). Simuliidae showed no evidence of decreasing abundance with distance from stream in either year. Indeed, there were far more black flies collected in the most distant trap than any other in 2003 (). Therefore, none of the three models were applicable ().
Table 2 Lateral dispersal statistics for each major taxon and common species within each, along perpendicular transects in each of two years. R 2, slope, and p-value are for the best-fit models for each order. ND = not decreasing (p-values > 0.2 for decreasing slope). P-values in bold are those significant at α = 0.05.
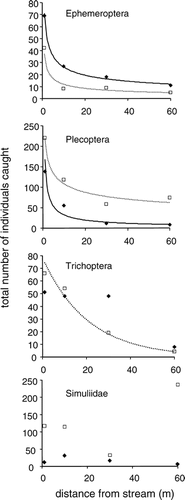
Table 3 ANCOVA results summary. Note that ANCOVA was only assessed for taxa that showed significant decrease with distance according to regression of number collected on distance from stream for either or both 2002 and 2003. See (“best model”) for transformations made to achieve linearity for the ANCOVA for each taxon. P-values in bold are those significant at α = 0.05.
Most common species showed patterns of abundance with distance inland similar to the overall pattern for each of their respective higher-level taxa (). A notable exception was the mayfly Ameletus celer, which, similarly to the black flies, showed no evidence of decreasing abundance with distance in either year ().
Discussion
Although alpine streams generally are considered depauperate relative to temperate streams (e.g. CitationWard, 1986), our detailed study of the insects emerging from the South Fork Poudre River headwaters has revealed surprisingly high species diversity. The total species count of 60 among EPT and Simuliidae captured across 2002–2003 is nearly three times the total number of taxa reported from benthic samples in the same stream (CitationFinn and Poff, 2005). This higher diversity resulted from our ability here to reveal “hidden” diversity that occurs in the aquatic larvae, many of which are more difficult to identify than the adults. Also, traps in this study integrated over all local microhabitats (likely including tiny ephemeral snowmelt tributaries, see below), while CitationFinn and Poff (2005) sampled only from shallow riffles in the main stream. Further, our discovery of a previously undescribed Prosimulium species, in addition to the state record for Greniera denaria, reflects that alpine streams in the southern Rockies have been understudied and are likely to house a higher biological diversity than previously considered.
Overall, lateral flight activity varied among major taxa as predicted from studies conducted below treeline. The tight fit of inverse power functions for Ephemeroptera and Plecoptera demonstrated a rapid decrease in flight activity with distance from stream for these taxa, as has been shown previously (e.g. CitationBriers et al., 2002; CitationPetersen et al., 2004). Trichoptera also showed decreased flight activity with distance away from the stream, but the less steep negative exponential function was the best fit for this taxon. As in some sub-treeline streams (e.g. CitationSvensson, 1974; CitationJackson and Resh, 1989), caddisflies, which at our alpine site are predominantly members of the large-bodied family Limnephilidae, appear to be stronger in flight than the other EPT. Simuliidae, conversely, showed no evidence of decreasing flight activity away from the stream. Black flies are known to be strong fliers (CitationCrosskey, 1990), and the female need for a bloodmeal in many species (including all trapped in the current study) often requires relatively distant flight.
Sex ratios of flying insects also followed expected trends, with more males in flight for most EPT and fewer males for Simuliidae. In most cases, the unbalanced sex ratios were highly significant. This result is potentially explained by the particularly harsh terrestrial flight conditions in the alpine zone, which could skew sex ratios strongly against the weaker-flying sex if there is selection for increased sedentary behavior. The unbalanced sex ratios have important implications for choosing molecular markers for population genetics of alpine stream insects. For example, CitationHughes et al. (2003) have shown that mitochondrial DNA (a maternally transmitted lineage) reveals stronger geographic structure among populations of a Rocky Mountain mayfly than does a set of nuclear markers (transmitted by both males and females), as expected if females are more sedentary than males.
Flight patterns for common species were representative of the overall patterns shown in their respective taxonomic groups, with the exception of the mayfly Ameletus celer, which did not decrease in abundance with lateral distance. The other common mayfly, Cinygmula spp., had the steepest decreasing slope of any species () and drove the overall decreasing pattern for Ephemeroptera, despite the incongruous pattern for A. celer. This interesting contrast between species within the same order supports the contention that broad generalizations about dispersal behavior in aquatic invertebrates can be unwarranted and that information about species differences is likely to be more meaningful ecologically (CitationBohonak and Jenkins, 2003). A. celer is an unusually large-bodied mayfly in which the male also has exceptionally large eyes (CitationPritchard and Zloty, 1994). Both traits may be associated with stronger flight ability (e.g. CitationCorkum, 1987), although not necessarily related to lateral dispersal as, for example, black fly males have larger eyes useful in swarming and mating but do not tend to disperse long distances relative to females (CitationCrosskey, 1990).
Among our most striking results were differences in emergence phenology, abundance, and richness between years. The major environmental difference among years at the study site was a greater than twofold increase in winter snowpack between 2002 and 2003. Increased snowpack leads to longer duration of insulating snow physically covering the stream, causes higher peak snowmelt flows (), and increases heterogeneity of local aquatic habitat by supporting ephemeral shallow water habitats after flows have peaked. These important hydrologic responses are likely to influence benthic insects directly; therefore, we interpret many of the biological differences we observed between years to the associated difference in winter snowpack, recognizing that thus far there are just two years of observation.
A generally little-appreciated influence of increased winter snowfall is the lengthier duration of snowpack covering small alpine streams, an effect that potentially has a strong influence on emergence phenology, and may also influence richness and diversity, although less directly (more below). Snowpack over the stream acts not only as a physical barrier to insect emergence and flight, but it also can directly affect water temperature via its insulating properties. Indeed, most of the small alpine streams in our study area remain covered by thick snowpack throughout the winter with no open “leads” above treeline, no matter the relative snowfall amount (Finn, personal observation). Such continuous snowcover effectively insulates streams to maintain temperatures ≥0 °C and to support permanent flow through the winter months in spite of average air temperatures well below freezing (CitationGreenland and Losleben, 2001). In contrast, extended snow cover into the warmer months likely has the opposite insulating effect, serving to maintain lower temperatures in the water column despite warming air temperatures (CitationBorgstrøm and Museth, 2005). Snow depth covering our study site on 21 May 2003, during the first month that average maximum air temperature exceeds 0 °C, was approximately 2 m (Finn, unpublished data). Maintenance of colder water temperatures effectively delays the accumulation of degree days that provide essential developmental cues for many aquatic insects (CitationVannote and Sweeney, 1980). Our observations of both snowmelt and emergence of several species at an approximately one month delay in 2003 compared to 2002 is thus unlikely to be merely coincidence, although the short duration of our study and the lack of literature on the relationship between snowpack duration and emergence phenology in alpine streams precludes our drawing a firm conclusion (but see CitationBorgstrøm, 2001, regarding the negative effect of lengthier snowcover on growth in trout).
A second effect of increased snowpack was higher peak runoff flow (), which is expected to act as a disturbance to stream communities (CitationGibbins et al., 2001; CitationMilner et al., 2006). In Alaskan streams, CitationMilner et al. (2006) reported a negative correlation between community persistence (constancy of species presence/absence) and total snowfall in the winter previous to sampling. The authors speculated that greater flow disturbance in years with increased snowmelt runoff led to greater shifts in community composition. In the current study, we observed a 1.5× increase in total species richness following the winter with above-average snowfall. Although disturbance in some cases can be expected to increase diversity (e.g. CitationConnell, 1978), the trend we observed of a significant addition of species following the 2002–2003 winter was opposite what was expected given the results of the CitationMilner et al. (2006) study.
In addition to phenological and diversity disparities, there were notable differences in overall abundance of flying insects between 2002 and 2003. Both abundance and species richness were greater in 2003, primarily driven by increases in Plecoptera and Simuliidae. At first glance, the combination of earlier emergence timing with later study initiation in 2002 seems a likely explanation for these patterns, i.e., perhaps we simply missed several early-emerging individuals and species in 2002 due to the later starting date of trapping. This argument can at least partially explain the large differences in abundance as well as sex ratio of the stonefly Zapada haysi that we recorded between years. We did not capture the earliest-emerging Z. haysi in 2002; therefore, total individuals reported in are less than what actual annual totals would have been had we set traps that year immediately following snowmelt, as in 2003. Furthermore, Z. haysi is protandrous, with males emerging earlier than females (CitationCather and Gaufin, 1976); hence the expected male bias in sex ratio was observed in 2003 after thorough temporal sampling but not in 2002 when early emergence was unsampled ().
Although trapping start time differences between years probably affected abundance estimates of early-emerging species in 2002, there is little evidence that they influenced the strong between-year differences in species richness or the differences in abundance of the later-emerging species. Indeed, the vast majority of species had mid–late season emergence that would have either spanned or followed the 2002 study initiation date (and thus allowed inclusion in the total 2002 species count). Lower species richness revealed in 2002 vs. 2003 therefore was not due to failure on our part to trap for the full extent of the season but more likely was influenced by between-year habitat differences, again likely driven by differences in snowpack.
The greater quantity and duration (see ) of snowmelt in 2003 likely provided more extensive and diverse aquatic habitat, including the formation of smaller rills and seeps in the floodplain adjacent to the sample stream. Increased habitat area could have two primary effects on our results. First, it likely would yield an increase in total numbers of insects (directly affecting total abundance collected by the traps) and, as a simple sampling effect, would increase detection ability of rare species, thereby boosting species richness. Second, the increased diversity of habitat types in 2003, particularly the addition of tiny ephemeral tributaries fed by sustained snowmelt, likely spurred development of species with life histories specialized to these conditions. For example, several black flies that we trapped in 2003, including the state record Greniera denaria, are known to develop in tiny, ephemeral flowing-water habitats (P. H. Adler, personal communication).
Increased habitat area and diversity of habitat types may help explain the significantly greater abundance and richness of stoneflies and black flies in 2003, but Ephemeroptera showed an opposite pattern, with significantly greater abundance in 2002, although they showed no difference in richness between years. Each of the four mayfly species emerges very late in the season, and in 2003 as compared to 2002, emergence was delayed by a month, such that the flight period for mayflies did not begin until well into August. Average air temperature in late August and September in the southern Rockies alpine zone is <5 °C (CitationGreenland and Losleben, 2001), which is below the low temperature threshold for most stream insect flight (CitationWaringer, 1991; CitationBriers et al., 2003). July has the highest annual average air temperature, however, and July had a corresponding peak in flying insect density in both years of our study. Therefore, insects that begin emerging during or prior to July probably have strong potential for increased flight activity. Earlier mayfly emergence in 2002 likely allowed us to trap significantly greater numbers of individuals in flight. The caddisfly Asynarchus nigriculus, itself a particularly late emerger, was also significantly more abundant in 2002 vs. 2003.
In summary, then, these conclusions suggest that increased snowpack has a strong potential for explaining three notable observations in 2003 vs. 2002: (1) emergence timing approximately one month later; (2) increase in diversity of earlier-emerging species; and (3) decrease in abundance of later-emerging species.
In light of the predicted climate change trajectory towards increased winter snowfall (CitationIPCC, 2001; CitationWelker et al., 2001), it will be important to bolster these results with further study in alpine regions of the Rocky Mountains and elsewhere. The unexpectedly high diversity of aquatic insects collected from this single study site in an alpine stream in the southern Rockies accentuates the need to monitor these systems more closely as climate change progresses. As in the terrestrial alpine system, these high elevation streams are effectively isolated at the top of the stream's longitudinal gradient and are therefore likely to be sensitive indicators of change.
Acknowledgments
We thank the taxonomists who enthusiastically donated time for all aquatic insect identification/verification: Peter Adler (Simuliidae), Dave Ruiter (Trichoptera), and Boris Kondratieff (Ephemeroptera and Plecoptera). Thanks also to Aaron Flohrs and Adam Valenti for fieldwork help, and to Heidi Luter, Sudah Balakhani, and Nora Machuca for lab work. Pat Rastall and Bill Bertschy at the Colorado State University Pingree Park campus and Judy Visty at Rocky Mountain National Park provided logistical assistance. We also appreciate the thoughtful remarks of two anonymous reviewers of an earlier version of the manuscript. Finn was supported by a USEPA STAR graduate fellowship and a small grant from the Colorado Mountain Club.
References Cited
- Adler, P. H. , D. C. Currie , and D. M. Wood . 2004. The Black Flies (Simuliidae) of North America Ithaca, NY Comstock Publishing Associates (a division of Cornell University Press). 941 pp.
- Baldwin, W. F. , A. S. West , and J. Gomery . 1975. Dispersal patterns of black flies (Diptera: Simuliidae) tagged with 32P. The Canadian Entomologist 107:113–118.
- Bohonak, A. J. and D. G. Jenkins . 2003. Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecology Letters 6:783–796.
- Borgstrøm, R. 2001. Relationship between spring snow depth and growth of brown trout, Salmo trutta , in an alpine lake: predicting consequences of climate change. Arctic, Antarctic, and Alpine Research 33:476–480.
- Borgstrøm, R. and J. Museth . 2005. Accumulated snow and summer temperature—Critical factors for recruitment to high mountain populations of brown trout ( Salmo trutta L.). Ecology of Freshwater Fish 14:375–384.
- Bowman, W. D. and T. R. Seastedt . 2001. Structure and function of an alpine ecosystem: Niwot Ridge, Colorado New York Oxford University Press. 337 pp.
- Briers, R. A. , H. M. Cariss , and J. H. R. Gee . 2002. Dispersal of adult stoneflies (Plecoptera) from upland streams draining catchments with contrasting land-use. Archiv für Hydrobiologie 155:627–644.
- Briers, R. A. , H. M. Cariss , and J. H. R. Gee . 2003. Flight activity of adult stoneflies in relation to weather. Ecological Entomology 28:31–40.
- Briers, R. A. , J. H. R. Gee , H. M. Cariss , and R. Geoghegan . 2004. Inter-population dispersal by adult stoneflies detected by stable isotope enrichment. Freshwater Biology 49:425–431.
- Brown, L. E. , D. M. Hannah , and A. M. Milner . 2003. Alpine stream habitat classification: an alternative approach incorporating the role of dynamic water source contributions. Arctic, Antarctic, and Alpine Research 35:313–322.
- Brown, L. E. , D. M. Hannah , and A. M. Milner . 2007. Vulnerability of alpine stream biodiversity to shrinking glaciers and snowpacks. Global Change Biology 13:958–966.
- Cather, M. R. and A. R. Gaufin . 1976. Comparative ecology of three Zapada species of Mill Creek, Wasatch Mountains, Utah (Plecoptera: Nemouridae). American Midland Naturalist 95:464–471.
- Choe, J. C. , P. H. Adler , K. C. Kim , and R. A. J. Taylor . 1984. Flight patterns of Simulium jenningsi (Diptera: Simuliidae) in central Pennsylvania, USA. Journal of Medical Entomology 21:474–476.
- Connell, J. H. 1978. Diversity in tropical rain forests and coral reefs. Science 199:1302–1310.
- Corkum, L. D. 1987. Patterns in mayfly (Ephemeroptera) wing length: adaptation to dispersal. The Canadian Entomologist 119:783–790.
- Crosskey, R. W. 1990. The Natural History of Blackflies Chichester, England John Wiley & Sons. 711 pp.
- Downes, J. A. 1965. Adaptations of insects in the Arctic. Review of Entomology 10:257–274.
- Finn, D. S. and P. H. Adler . 2006. Population genetic structure of a rare high-elevation black fly, Metacnephia coloradensis , occupying Colorado lake outlet streams. Freshwater Biology 51:2240–2251.
- Finn, D. S. and N. L. Poff . 2005. Variability and convergence in benthic communities along the longitudinal gradients of four physically similar Rocky Mountain streams. Freshwater Biology 50:243–261.
- Finn, D. S. , D. M. Theobald , W. C. Black , and N. L. Poff . 2006. Spatial population genetic structure and limited dispersal in a Rocky Mountain alpine stream insect. Molecular Ecology 15:3553–3566.
- Gibbins, C. N. , C. F. Dilks , R. Malcolm , C. Soulsby , and S. Juggins . 2001. Invertebrate communities and hydrological variation in Cairngorm mountain streams. Hydrobiologia 462:205–219.
- Greenland, D. and M. Losleben . 2001. Climate. In Bowman, W. D. and T. R. Seastedt , editors. Structure and function of an alpine ecosystem: Niwot Ridge, Colorado New York Oxford University Press. 15–31.
- Hauer, F. R. , J. A. Stanford , and M. S. Lorang . 2007. Pattern and process in Northern Rocky Mountain headwaters: ecological linkages in the headwaters of the Crown of the Continent. Journal of the American Water Resources Association 43:104–117.
- Hughes, J. M. , P. B. Mather , M. J. Hillyer , C. Cleary , and B. Peckarsky . 2003. Genetic structure in a montane mayfly Baetis bicaudatus (Ephemeroptera: Baetidae), from the Rocky Mountains, Colorado. Freshwater Biology 48:2149–2162.
- IPCC Houghton, J. T. , et al , editor. 2001. Climate change 2001: impacts, adaptation, and vulnerability New York Cambridge University Press. 881 pp.
- Jackson, J. K. and V. H. Resh . 1989. Distribution and abundance of adult aquatic insects in the forest adjacent to a northern California stream. Environmental Entomology 18:278–283.
- Kovats, Z. E. , J. J. H. Ciborowski , and L. D. Corkum . 1996. Inland dispersal of adult aquatic insects. Freshwater Biology 36:265–276.
- Kuusela, K. and A. Huusko . 1996. Post-emergence migration of stoneflies (Plecoptera) into the nearby forest. Ecological Entomology 21:171–177.
- Mackay, R. J. 1992. Colonization by lotic macroinvertebrates—A review of processes and patterns. Canadian Journal of Fisheries and Aquatic Sciences 49:617–628.
- Milner, A. M. , S. C. Conn , and L. E. Brown . 2006. Persistence and stability of macroinvertebrate communities in streams of Denali National Park, Alaska: implications for biological monitoring. Freshwater Biology 51:373–387.
- Nakano, S. and M. Murakami . 2001. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Sciences 98:166–170.
- Nilsson, A. N. , R. B. Pettersson , and G. Lemdahl . 1993. Macroptery in altitudinal specialists versus brachyptery in generalists: a paradox of alpine Scandinavian carabid beetles (Coleoptera, Carabidae). Journal of Biogeography 20:227–234.
- Palmer, M. A. , J. D. Allan , and C. A. Butman . 1996. Dispersal as a regional process affecting the local dynamics of marine and stream benthic invertebrates. Trends in Ecology & Evolution 11:322–326.
- Petersen, I. , J. H. Winterbottom , S. Orton , N. Friberg , A. G. Hildrew , D. C. Spiers , and W. S. C. Gurney . 1999. Emergence and lateral dispersal of adult Plecoptera and Trichoptera from Broadstone Stream, U.K. Freshwater Biology 42:401–416.
- Petersen, I. , Z. Masters , A. G. Hildrew , and S. J. Ormerod . 2004. Dispersal of adult aquatic insects in catchments of differing land use. Journal of Applied Ecology 41:934–950.
- Poff, N. L. and J. V. Ward . 1989. Implications of streamflow variability and predictability for lotic community structure—A regional analysis of streamflow patterns. Canadian Journal of Fisheries and Aquatic Sciences 46:1805–1818.
- Pritchard, G. and J. Zloty . 1994. Life histories of two Ameletus mayflies (Ephemeroptera) in two mountain streams: the influence of temperature, body size, and parasitism. Journal of the North American Benthological Society 13:557–568.
- Rankin, M. A. and J. C. A. Burchsted . 1992. The cost of migration in insects. Annual Review of Entomology 37:533–559.
- Rothfels, K. 1981. Cytological approaches to the study of blackfly systematics and evolution. In Stock, M. W. , editor. Application of Genetics and Cytology in Insect Systematics and Evolution Moscow, ID Forest, Wildlife and Range Experiment Station, University of Idaho. 67–83.
- Snyder, T. P. and M. C. Linton . 1984. Population structure in black flies: allozymic and morphological estimates for Prosimulium mixtum and P. fuscum (Diptera: Simuliidae). Evolution 38:942–956.
- Sode, A. and P. Wiberg-Larsen . 1993. Dispersal of adult Trichoptera at a Danish forest brook. Freshwater Biology 30:439–446.
- Sueker, J. K. , J. N. Ryan , C. Kendall , and R. D. Jarrett . 2000. Determination of hydrologic pathways during snowmelt for alpine/subalpine basins, Rocky Mountain National Park, Colorado. Water Resources Research 36:63–75.
- Svensson, B. W. 1974. Population movements of adult Trichoptera at a south Swedish stream. Oikos 25:157–175.
- Vannote, R. L. and B. W. Sweeney . 1980. Geographic analysis of thermal equilibria: a conceptual model for evaluating the effect of natural and modified thermal regimes on aquatic insect communities. American Naturalist 115:667–695.
- Walker, M. D. , D. A. Walker , J. M. Welker , A. M. Arft , T. Bardsley , P. D. Brooks , J. T. Fahnestock , M. H. Jones , M. Losleben , A. N. Parsons , T. R. Seastedt , and P. L. Turner . 1999. Long-term experimental manipulation of winter snow regime and summer temperature in arctic and alpine tundra. Hydrological Processes 13:2315–2330.
- Ward, J. V. 1986. Altitudinal zonation in a Rocky Mountain stream. Archiv für Hyrdrobiologie, Supplement 74:133–199.
- Ward, J. V. 1994. Ecology of alpine streams. Freshwater Biology 32:277–294.
- Waringer, J. A. 1991. Phenology and the influence of meteorological parameters on the catching success of light-trapping for Trichoptera. Freshwater Biology 25:307–319.
- Welker, J. M. , W. D. Bowman , and T. R. Seastedt . 2001. Environmental change and future directions in alpine research. In Bowman, W. D. and T. R. Seastedt , editors. Structure and function of an alpine ecosystem: Niwot Ridge, Colorado New York Oxford University Press. 304–322.
- Wilcock, H. R. , A. G. Hildrew , and R. A. Nichols . 2001. Genetic differentiation of a European caddisfly: past and present gene flow among fragmented larval habitats. Molecular Ecology 10:1821–1834.
- Zar, J. H. 1996. Biostatistical Analysis. 3rd edition Upper Saddle River, NJ Prentice Hall. 662 pp.