Abstract
Gradients of humidity on tropical high mountains are reflected by shifts of vegetation belts and changes in species composition and vegetation structure on the wind- and leeward sides. The superpáramo flora and vegetation were studied on opposite sides (west and east) of two Ecuadorian mountains, Chimborazo and Antisana. Zonal vegetation was studied in vegetation samples set out in a semirandom way, and the data were complemented by species occurring in azonal habitats. Ordination and clustering techniques were used to analyze the vegetation samples, while two different measures of the floristic similarity (index of quantitative floristic similarity and comparison of the observed and expected numbers of species shared between the mountain pairs) were employed to evaluate the floristic relationships between the study sites. The superpáramo belt occurs asymmetrically, with regard to altitude, on the two mountains, being generally lower on the eastern side. Slopes with corresponding aspects between the mountains were more similar than were the opposite slopes of each mountain. The observed floristic pattern is interpreted in the context of the precipitation gradient leading to a rain-shadow on the western side of the mountains. The occurrence of the desert-like area, the so-called Arenal Grande, and the unique páramo vegetation on the western side of Chimborazo is discussed.
Introduction
Tropical high mountains usually fall under the influence of trade winds. Because of this, there is an uneven distribution of precipitation on the opposite mountain slopes (see e.g., CitationCoe, 1967; CitationMahaney, 1989; CitationBendix and Lauer, 1992; CitationMiehe and Miehe, 1994; CitationRundel, 1994), often resulting in a distinct rain-shadow on the leeward side (CitationPérez Preciado, 1984; CitationSarmiento, 1986; but cf. CitationSmith, 1986). In some cases, the rain-shadow of the mountains can be so strong that semidesert areas develop even near the equator (CitationWalter, 1971). Direct consequences are patterns in plant distribution, changes in vegetation structure, and the altitudinally asymmetrical position of vegetation belts. In general, the vegetation belts, and also the snowlines, tend to occur at lower elevations on the atmospherically humid slopes, which has been documented for the East African volcanoes (CitationHedberg, 1951; CitationCoe, 1967; CitationSchmitt, 1991) and the South American Andes (CitationTroll, 1968; CitationLauer, 1979; CitationCleef, 1981; Citationvan der Hammen and Cleef, 1986).
The effect of uneven distribution of precipitation can also be observed in the Andes of Ecuador. Most humidity brought to the Ecuadorian Andes originates in the Amazon basin, from where it is driven by the predominant eastern and northeastern winds. Upon meeting the Andes, the air masses ascend, cool, and lose a substantial portion of their humidity (orographic rain), while on the opposite side of the mountain range the air warms and tends to absorb available humidity from the environment. This results in a distinct humidity gradient across the Andean ranges (CitationHastenrath, 1981; CitationBendix and Lauer, 1992; CitationJørgensen and Ulloa, 1994). Although generalization cannot be made for the whole of Ecuador, where two parallel cordilleras span more than 400 km in length, and allow for many locally modified climatic situations, the vegetation belts and snowlines are usually found lower on the humid sides of the mountains (e.g., CitationLægaard, 1992). Traces of older glaciations usually occur lower on the eastern sides of the Andes as well, suggesting that a similar pattern likely occurred through at least the late Pleistocene (CitationHastenrath, 1981; CitationClapperton, 1987, Citation1990; CitationBendix and Lauer, 1992).
The Ecuadorian páramos, i.e., the lands above the treeline, are generally rather humid (CitationJørgensen and Ulloa, 1994). A remarkable exception is Volcán Chimborazo. Its western side hosts a desert-like vegetation which contrasts greatly with other páramos in the country, including páramos on the opposite side of this mountain (CitationCerón, 1994; CitationSklenář, 2000). A very sparse and patchy vegetation has developed in the area of the Arenal Grande, which is a sandy, flat or gently sloping land at altitudes above 4100 m (CitationAcosta-Solís, 1985; CitationRamsay, 1992). The bare, desert-like landscape was already known to the first travelers in Ecuador (e.g., CitationBouguer, 1749; Citationde la Condamine, 1751), and its flora was explored by many naturalists (CitationMeyer, 1907; CitationDiels, 1937; CitationHarling, 1979). Since the area was covered with ice during the late phase of the last glaciation (CitationClapperton, 1987, Citation1993), and the last volcanic events are dated ca. 11,000 BP (CitationHall, 1977; CitationClapperton, 1990), the Arenal Grande apparently cannot be older than that.
Vegetation of western and eastern sides of two volcanoes, Chimborazo and Antisana, was studied as part of a survey of Ecuadorian superpáramos (CitationSklenář, 2000). Our first visit to Antisana revealed several remarkable similarities with Chimborazo. Although a large-scale desert-like landscape comparable to the Arenal of Chimborazo is not present, patches of eroded, sandy land and at some places a vegetation physiognomically resembling that of the Arenal can be found on the western side of Antisana . It was observed that the two volcanoes act as an obstacle for westward-moving air masses, forcing the clouds to flow around the mountain bases, which often leaves the adjacent western sides cloudless. Moreover, upon meeting warmer air masses above the elevated western plateaus of both mountains, clouds were unable to penetrate there (Chimborazo) or if so, they eventually disappeared without providing any precipitation (, ). A search of the literature revealed that the föhn-like phenomenon on Chimborazo had already been noted by CitationMeyer (1907), while CitationBlack (1982) observed similar wind-patterns on Antisana. The observations from the western side of Antisana encouraged us to make an additional trip to the eastern side of the mountain, the purpose of which was to document the expected analogy with Chimborazo, i.e., the remarkable asymmetry in species composition and vegetation structure on the opposite mountain sides.
This paper analyzes the composition of the superpáramo vegetation on the eastern and western sides of the two mountains. The observed patterns are put into the context of available climatic data.
Study Area
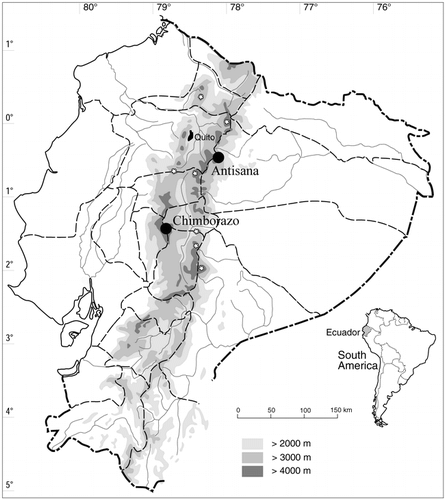
Chimborazo (6310 m) lies in the Ecuadorian western cordillera and is the highest mountain in the country. It is a heavily glaciated double volcano which has been extinct for ca. 11,000 yr (CitationSauer, 1971; CitationHall, 1977). The huge mountain massif runs west-east and has a base diameter of ca. 20 km. Recent glaciers descended to 4700–4800 m in the east and 4900–5000 m in the west; however, traces of older glaciations have been found as low as 3400 m (CitationHastenrath, 1981; CitationClapperton, 1987, Citation1990). In the vicinity of Chimborazo, only a few kilometers to the northeast, across the west-east-running Abraspungo Pass, there is another glaciated volcano, Carihuairazo (5020 m) .
Antisana (5704 m), the other studied mountain, is an active volcano in the Ecuadorian eastern cordillera , with latest activity reported for the beginning of the 19th century (CitationMeyer, 1907; CitationHall, 1977). Antisana's base diameter is ca. 14 km, running north-south (CitationSauer, 1971). There, recent glaciers descended to approximately 4600 m (eastern side) and 4800 m (western side), while older moraines indicate glaciers about 1200 m lower on the east (CitationHastenrath, 1981; CitationClapperton et al., 1997). The western sides of both mountains form extensive high-altitude plateaus above 4000 m of altitude . On Chimborazo, this plateau supports the poor Arenal vegetation, while most of the plateau on Antisana is covered by grass páramo.
Methods
We visited Chimborazo five times during 1995, 1997, and 1999 and visited Antisana twice in 1997. The mountains were studied from their opposite sides, west and east (northeast in the case of Antisana), yielding four study sites, each representing an independent data set and, for simplicity, each called a mountain when referring to the respective study sites. Zonal superpáramo was delimited as the vegetation replacing grass páramo at its lower range (generally 4200 m) and by plant cover of less than ca. 5% at the upper range. We studied the vegetation by stratified random sampling (CitationMueller-Dombois and Ellenberg, 1974) using 100-m altitudinal intervals as predefined strata. At each altitudinal level we surveyed three randomly selected vegetation plots. The plots were mostly 25 m2, which is generally a sufficient sample area for páramo vegetation studies (e.g., CitationRamsay, 1992). However, in the upper superpáramo belt the “minimal-area” condition (CitationMueller-Dombois and Ellenberg, 1974) is not met by this sample size (P. Sklenář, unpubl. data), so at the highest elevations we used larger plots of 100 m2 instead. Since the vegetation of the Arenal on Chimborazo is very patchy, thus corresponding to the structure of the upper superpáramo, the larger plots were also used there except at the lowest elevation. We estimated species cover within the vegetation plots using an 8-grade semiquantitative abundance scale. In further analysis the estimates were converted to scores using their midpoint values (r = 0.05%, + = 0.5%, 1 = 2.5%, 2a = 10%, 2b = 20%, 3 = 37.5%, 4 = 62.5%, 5 = 87.5%). We then surveyed the surrounding vegetation for additional species that had not been found within the sample plots and for species occurring in azonal habitats such as lake shores, boggy depressions, and rocky escarpments.
We analyzed the vegetation samples by means of the polythetic divisive clustering method using TWINSPAN (CitationHill, 1979). We used six pseudospecies cut levels by assigning an equal weight to the two lowest and two highest species score categories, respectively. We excluded species with uncertain determination from the list of possible indicators. Clustering was terminated at the third division level. Ordination analyses of the vegetation samples were done afterwards employing the statistical package CANOCO (Citationter Braak and Šmilauer, 1998). Detrended correspondence analysis (DCA) was run first to demonstrate the position of the samples in the ordination space. Explanatory power of two categorical variables, volcano (Chimborazo vs. Antisana) and aspect (west vs. east), was tested by means of canonical correspondence analysis (CCA) after the effect of altitude was partialed out. CCA was run first with the three variables to test their overall effect. After this, partial CCA was performed (with data after effect of altitude was removed) to evaluate the significance of each categorical variable, while controlling for the effect of the other categorical variable by keeping it as covariable. The significance of the results was estimated by the Monte Carlo permutation test (199 permutations in blocks defined by covariable). Species with uncertain determination were excluded from the ordination analysis.
We estimated degree of floristic similarity between the mountains from pooled vegetation samples for each mountain, where the species were given their average score from the vegetation samples (i.e., sum of species' scores divided by the number of samples). Species found outside the plots and species from azonal habitats were added to the pooled samples and assigned a value of 0.001, which was equivalent to the minimum average score of species in the pooled samples. We calculated an index of quantitative floristic similarity for all mountain pairs from these pooled samples as:
where ai refers to species scores in the sample A, bi refers to species scores in the sample B, and ci refers to scores of species shared between the two samples (CitationGleason, 1920).
We then expressed floristic similarities in an alternative way, i.e., by comparing the observed and expected numbers of shared species between the mountain pairs. Expected numbers, estimated according to CitationConnor and Simberloff (1978), were obtained by randomly generating pairs of species samples from a total species pool (i.e., all species in the study), where the species were given adjusted probabilities of being sampled (null hypothesis II of CitationConnor and Simberloff, 1978). Probabilities of species from the vegetation plots were their number of occurrences (frequency) in the original vegetation samples, while probabilities of species from outside the plots and azonal habitats were their occurrences on the mountains (thus ranging from 1, i.e., species found on a single mountain, to 4, i.e., species found on all mountains). The random species samples were drawn with the constraint that the number of species be equal to the actual number of species found on a particular mountain. Expected numbers were estimated repeatedly, 999 times via computer simulation (CitationSklenář, 2000). The χ2 value was calculated between the expected and observed numbers of species held in common, providing a standardized measure of floristic distance between the mountains, independent of the sample size (i.e., number of species per mountain).
Results
Fifteen vegetation plots were sampled on both sides of Antisana and on the eastern side of Chimborazo, and 21 plots were studied on the western side of Chimborazo, giving a total of 66 vegetation plots. Chimborazo was surveyed from 4200 m to 4600 m on the eastern side, but as high as 4800 m on the western side . On Antisana, there was also an altitudinal asymmetry in the location of the transects; it was studied between 4200–4600 m on the eastern side and between 4300–4700 m on the western side. A total of 247 vascular plant species were found, including two subspecies in Eudema nubigena (the species list is provided by CitationSklenář, 2000). From the total number, 186 species were found in the vegetation plots, while an additional 61 species were found outside the samples and in azonal habitats. Western Chimborazo had the lowest number of species (103), while the highest number (140 species) was found on western Antisana . Western Chimborazo and eastern Antisana shared the lowest number of species (33). On the other hand, the highest number, 87 species in common, was found in the pair east Chimborazo–west Antisana.
On the eastern side of Chimborazo lush vegetation occurs with a conspicuous abundance of cushion plants (Azorella corymbosa, Distichia muscoides, Plantago rigida, Xenophyllum humile), at lower altitudes sharing dominance with tussock grasses. Acaulescent rosulate herbs, e.g., Oreomyrrhis andicola and Uncinia macrolepis, are present at the ground level, as well as sclerophyllous shrubs of Loricaria thuyoides and Diplostephium rupestre locally. Numerous small lakes and water-filled depressions are conspicuously scattered throughout the landscape. Cushion plants are absent from the Arenal vegetation on the western side of Chimborazo, with only Xenophyllum rigidum representing this life form at the highest altitudes there. Chuquiraga jussieui and Loricaria ilinissae represent the sclerophyllous shrubs, while the prostrate subshrubs, Astragalus geminiflorus and Baccharis caespitosa, the ascending subshrub Valeriana alypifolia, and short-stemmed grasses Calamagrostis mollis and Agrostis tolucensis are characteristic of the herb layer.
Lower-altitude vegetation on east Antisana is characterized by shrubs of Loricaria antisanensis and Valeriana microphylla, those sharing dominance with the cushion plants (Azorella aretioides, Plantago rigida, and Xenophyllum humile), and locally also tussock grasses . Disterigma empetrifolium, an ericoid prostrate subshrub, and several herbs or small grasses (e.g., Lachemilla nivalis, Oritrophium peruvianum, Agrostis foliata) were also abundant, but usually at higher elevations. On the western side of Antisana, tussocks of Calamagrostis intermedia were usually abundant at lower altitudes (this species was also present on the eastern slope but at much lower densities), together with rosulate herbs (Valeriana rigida, Werneria nubigena, Uncinia macrolepis), and prostrate subshrubs Baccharis caespitosa and Lupinus microphyllus. From the cushion plants, only Azorella pedunculata was present more often at lower and Xenophyllum rigidum at higher elevations, respectively.
Cluster analysis of the vegetation samples resulted in seven final groups . The majority of high-altitude samples, above (4500–)4600 m, occur in one-half of the dendrogram together with the western Chimborazo samples from 4200–4600 (–4800) m. The western Antisana samples are separated next in the high-altitude group, leaving samples from both sides of Chimborazo and a single 4500 m east Antisana sample together. In the western Chimborazo group, two 4200 m samples are separated from the remainder in the third division step. The second half of the dendrogram comprises mostly lower-elevation samples (except western Chimborazo) and four high-altitude samples (4500–4600 m) from east Antisana, which are separated in the second division. In the remaining group, east Antisana 4200–4400 m samples are paired with (4300–)4400–4500 m samples from east Chimborazo, while west Antisana 4300–4500 m samples are grouped with east Chimborazo samples from 4200–4300 m. One sample from east Antisana (4600 m) remained unclassified.
The position of the vegetation samples in the ordination space obtained by the DCA can be seen in . The samples of the same aspect occur in opposite halves of the diagram (with regards to the first ordination axis) and tend to be grouped by the mountain. Altitude is responsible for the scatter along the second ordination axis with high-altitude samples, as these delimited by cluster analysis, occurring in the lower part of the diagram. The three measured variables together, i.e., aspect, mountain, and altitude, explained 17.3% of the total data variability (CCA: λ1 = 0.751, λ2 = 0.557, λ3 = 0.392, total inertia = 9.831). This indicates that even though the variables are significant (Monte Carlo permutation test of all canonical axes: F = 4.323, P = 0.005, 199 permutations), other untreated variables are responsible for a substantial amount of the variability. Partial CCA and subsequent permutation tests, measuring individual effects of the categorical variables (and while controlling for altitude), confirmed their significance for the observed pattern (aspect: 7.7% explained variability, F = 5.155, P = 0.005; mountain: 6.7% explained variability, F = 4.460, P = 0.005; 199 permutations).
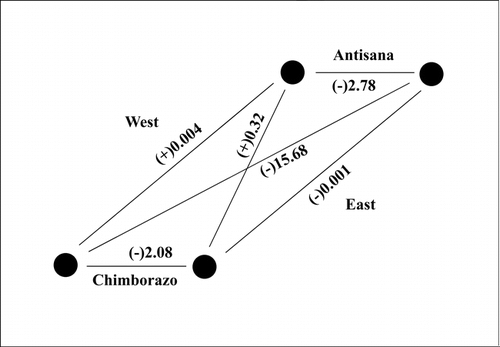
The floristic similarities between the mountain pairs are consistent with ordination and clustering results . CitationGleason's (1920) index demonstrates high values for the corresponding aspects, i.e., 71.7 for the western and 68 for the eastern slopes, respectively, and for the pair east Chimborazo–west Antisana (71.3). On the other hand, the opposite aspects and the pair west Chimborazo–east Antisana resulted in remarkably lower values. Distance between the observed and expected values, expressed as the χ2, is provided in . Highest similarity is found between slopes facing the inter-Andean valley (east Chimborazo–west Antisana), indicated by the highest “positive” (i.e., more observed species than expected) value of 0.32. Corresponding aspects were also relatively similar, which is indicated by χ2 values near zero. Conversely, much lower similarity (high “negative” χ2 values) was found for opposite mountain slopes [(–)2.08 for Chimborazo, (–)2.78 for Antisana], and the lowest similarity was between the outer mountain sides, i.e., west Chimborazo–east Antisana [(–)15.68].
Discussion
The data from Chimborazo and Antisana demonstrate the asymmetrical position of superpáramo, with the belt occurring at lower altitudes on the eastern slopes. This is seen especially well on Antisana, where both the lower and upper limits of superpáramo are shifted by about 100–200 m. Although the lower superpáramo limit was found at the same altitude on both sides of Chimborazo (but see below discussion regarding the origin of Arenal), the upper vegetation limit was higher by about 200 m on the western slope, consistent with Antisana. These findings conform to the general pattern in the tropical high Andes. Although the degree of asymmetry may differ, the superpáramo belt is altitudinally shifted on the opposite mountain slopes, occurring lower on the humid slopes in Colombia (CitationCleef et al., 1983; CitationCleef and Rangel-Ch., 1984), while higher on the humid side in Venezuela (CitationMonasterio and Reyes, 1980). Since environmental conditions vary between the páramo regions of Ecuador, being modified by wind and precipitation patterns, topography, mountain size, and/or presence of glaciers (e.g., CitationWitte, 1995), superpáramo asymmetry need not be distinct on all mountains.
Altitude is a very important factor in the superpáramo, delimiting the lower and upper superpáramo belts (CitationCleef, 1981; CitationRamsay, 1992; CitationSklenář, 2000). Two high-altitude groups, corresponding to upper superpáramo vegetation, were recognized by the cluster analysis, and the effect of altitude was evident also in the ordinations. Unlike the high-altitude samples, which contain few but generally more widespread species (CitationSklenář, 2000), aspect and mountain are much more important variables in the lower superpáramo samples. Due to the generally lower species richness (see below) and distinct species composition, the western side of Chimborazo stands apart from other low-altitude samples and is instead clustered with the species-poor high-altitude samples. While the position of the opposite Antisana slopes in the final dendrogram is clear , the east Chimborazo low-altitude samples are split. The 4200–4300 m samples are clustered with west Antisana, while the (4300–)4400–4500 m samples are grouped with east Antisana. Due to the dominance of tussock grasses, often shared with the cushion plants, CitationSklenář (2000) considered the lower-altitude samples from west Antisana and east Chimborazo transitional between the grass páramo and superpáramo vegetation, and consistently the corresponding samples are grouped together. The two slopes facing the inter-Andean valley also share the highest number of species and demonstrate high(est) similarity values (Gleason index, χ2). As the tussock grasses decline in favor of cushion plants and sclerophyllous shrubs at 4400–4500 m on east Chimborazo, the vegetation structure and species composition becomes closer to that of the lower superpáramo of east Antisana. As a result, those samples are clustered together and also the similarity values between the eastern mountain slopes are high.
The results confirm our expectation that, similar to Chimborazo, species composition and vegetation structure on opposite slopes of Antisana are very distinct. Moreover, despite the distance of ca. 130 km and occurrence in the other Andean ranges, there is a higher similarity between the two western and two eastern mountain sides, respectively, than between the opposite sides of each mountain. This pattern was demonstrated consistently by the ordination and cluster analyses, based on the semirandomly recorded vegetation samples, and the two similarity measures, based on species data including both zonal and azonal habitats.
We attribute these within-mountain patterns primarily to humidity conditions. There is a strong precipitation decline from 990 mm on the eastern side of Chimborazo (climatic station Urbina: 3619 m, ) to ca. 145 mm on the western side (CitationAcosta-Solís, 1985; CitationInstituto Nacional de Meteorología e Hidrología, 1974–1996). Although CitationClapperton (1990) indicated higher sums (2000 mm vs. 500 mm, respectively), his data are consistent by showing the strong precipitation gradient. The situation on Antisana seems to be similar, although the available data do not provide as good a picture and the gradient may be less. Short-term precipitation measurements resulted in 605–990 mm on the western slopes (CitationSémiond et al., 1998; CitationBontron et al., 1999), while CitationBlack (1982) measured 722 mm during his one-year recording. Moreover, Black interviewed local people and concluded that significant decrease in precipitation and an overall land desiccation due to construction of drainage canals (acequias) occurred in the area over preceding decades. There are no exact data from the eastern side of Antisana and so we used the climatic station at Papallacta (3160 m), located ca. 10 km to the north of Antisana , as a reference to the precipitation regime experienced by the eastern Andean slopes of that region. That station indicated a yearly sum of 1668 mm (CitationJørgensen and Ulloa, 1994), although CitationLauer (1979) and CitationClapperton et al. (1997) reported lower values (1292 mm and 1325 mm, respectively).
Distribution of many vascular plants and bryophytes clearly follow the above precipitation pattern. Several species, which we found only on the western sides (e.g., Astragalus geminiflorus, Calamagrostis mollis, Cerastium imbricatum, Conyza cardaminifolia, Festuca vaginalis, Plantago nubigena, and Silene thysanodes) characterize other relatively dry superpáramos of central Ecuador, such as Cotopaxi and Iliniza (CitationSklenář, 2000). On the other hand, species like Cortaderia sericantha, Diplostephium rupestre, Lachemilla holosericea, Pentacalia arbutifolia, and the fern genus Jamesonia, common in humid páramos elsewhere, were exclusively found on the eastern slopes. Bryophytes, and especially hepatics, which are very good indicators of humidity conditions (CitationGradstein, 1998), were almost absent from the western mountain slopes.
With regard to the remarkable dryness of Arenal on the western side of Chimborazo, CitationAcosta-Solís (1985) noted that water melted from glaciers does not emerge in the area above 4000 m. There are indeed several small or larger depressions along the western edge of Arenal (ca. 4000–4100 m) which apparently provide sufficient water supply. These depressions are vegetated by cushions of Plantago rigida and Distichia muscoides, accompanied by other species characterizing humid Ecuadorian páramos, e.g., Cuatrecasasiella isernii, Gentianella limoselloides, Hypsela reniformis, Caltha sagittata, Lachemilla mandonii, and Werneria pygmaea (CitationSklenář, 2000). However, we believe that the primary factor there is the shadowing effect of the volcano. This was recognized also by CitationRamsay (1992), who classified the vegetation of Arenal as rain-shadow desert páramo.
Winds play an important role in the climate of Chimborazo and Antisana. They are almost a constant feature in the high-altitude plateaus of the two mountains (CitationBouguer, 1749; Citationde la Condamine, 1751; CitationWhymper, 1892; CitationMeyer, 1907; CitationBlack, 1982). Northern winds, directed by the valley between Chimborazo and Igualatoa, occurred in 80% of the 1002 observations, while only 9% were calms (CitationInstituto Nacional de Meteorología e Hidrología, 1996). On the Arenal, winds are reportedly strong enough to move small stones, and formation of sand dunes can be observed (CitationMeyer, 1907; CitationAcosta-Solís, 1985; Citationde Noni et al., 1986; CitationPodwojewski and Poulenard, 2000). From Antisana only short-term wind measurements made on a glacier surface (4870–5100 m) are available from the northwestern side (CitationBontron et al., 1999). There were almost no calm observations there, although the conditions on the plateau several hundred meters below may differ. Winds not only determine the uneven distribution of precipitation described above, but also tend to sweep away the limited amount of rain clouds reaching above the western plateaus and thus further contribute to the outlined rain-shadow. They also accelerate soil desiccation, which further promotes deflation (CitationPodwojewski and Poulenard, 2000). Due to deflation, plant roots are exposed to drying winds, which eventually leads to the plant's death (see CitationMeyer, 1907: Photo 24), thus closing the cycle: deflation → death of plants → reduction of plant cover → increased deflation.
Within the páramos of Ecuador, this study stressed the peculiarity of Chimborazo's western side. Despite the longest altitudinal gradient surveyed, the lowest species richness was found there, a fact already noticed by Humboldt (CitationKletke, 1859), and later documented by others (CitationRamsay, 1992; CitationCerón, 1994; CitationSklenář and Jørgensen, 1999). In the explored part of western Chimborazo we found 20% less species than on the opposite side, and 26% less species than on western Antisana. However, despite relatively low species richness, the western side of Chimborazo belongs to the few Ecuadorian superpáramos which host locally (or nearly so) endemic species (CitationSklenář, 2000, Citation2001).
CitationAcosta-Solís (1985) compared the western side of Chimborazo to dry and patchy puna vegetation of the central Andes, with which Arenal indeed shares several floristic elements, e.g., Werneria apiculata, Cerastium cf. crassipes, Nototriche spp., Stipa spp. The desert-like appearance of Arenal resembling puna may be a relatively recent situation, however. Important indicators for this are scattered relicts of soil, which can be found in some marginal parts of Arenal (CitationAcosta-Solís, 1985; CitationBrandbyge and Holm-Nielsen, 1986: ; CitationLuteyn, 1999: ). These soil relicts, i.e., erosional forms sometimes called camel backs (CitationRubín and Balatka, 1986), are up to 3 m high and several square meters in areal extent, and bear individual tussocks or larger fragments of grass páramo, which by species composition does not differ from the grass páramo around Arenal Grande. These forms indicate that, at least in parts, Arenal Grande was once covered by a closed páramo vegetation, which has gradually disappeared due to soil erosion.
CitationPérez (1993) suggested that cattle activity may be the primary factor needed to open turf in the páramos, thus starting land degradation, which may further be enhanced by natural factors. Similarly, CitationGrubb (1970) connected the occurrence of bare grounds in the páramos of Antisana with heavy grazing and trampling by sheep. Importance of animal activity in the process of vegetation degradation and resulting erosion has been recently stressed also in various alpine or tundra regions (e.g., CitationHall et al., 1999; CitationRost, 1999). Disturbance in a form of burning and overgrazing of the grasslands and depletion of shrubs occurs on the western side of Chimborazo (CitationAcosta-Solís, 1985; CitationRamsay, 1992). Since it is questionable that natural factors alone could have triggered the soil erosion of the original, presumably closed, plant cover there, we suggest that human activity has been a very important factor for the development of the Arenal. Once initiated by the man-induced disturbance, openings in the vegetation would be further enlarged by wind and water erosion, needle-ice, and solifluction. In addition to the strong rain-shadow of the mountain, soils of heavily burned and grazed páramos have a decreased water retention capacity (CitationHofstede, 1995; CitationPodwojewski and Poulenard, 2000), which together with the desiccating winds would decrease soil resistance to erosion and contribute further to the overall “desertification” and possibly irreversible land degradation. Primary phases of this process can be observed along the western and southwestern edges of the Arenal, where the grass páramo contains numerous patches of open, eroded soil that is bare of vegetation . Centuries ago, similarly eroded patches in any part of the western side of Chimborazo may have been precursors of today's large-scale Arenal. Research using permanent transects was established at the Arenal/grass páramo boundary in 1999 to study the processes involved (CitationKovář, 2001).
We document the altitudinal asymmetry of the superpáramo belt on Chimborazo and Antisana, and we attribute the observed floristic and vegetation patterns primarily to unequal distribution of precipitation on opposite mountain sides, resulting in a rain-shadow on the western slopes. We suggest that the occurrence of the desert-like páramo vegetation on the western flanks of Chimborazo is due to the combined effect of (1) rain-shadow of the volcano, (2) human-induced disturbance of the vegetation, and (3) resulting erosion. On the western side of Antisana, the rain-shadow is presumably less pronounced, due to the higher precipitation in the area, and the vast sandy area has not developed. However, patches of eroded soil and observed deflation indicate, that, like Chimborazo, the western side of Antisana is a fragile area under serious threat of land degradation (CitationBlack, 1982).
FIGURE 1. Western side of Volcán Chimborazo with the sparse, desert-like páramo vegetation of the Arenal Grande (4200 m). The clouds coming from the east apparently cannot penetrate above the Arenal and form a “climatic” border.

FIGURE 2. Patchy vegetation on the western base of Volcán Antisana resembling the Arenal of Chimborazo (4200 m). The clouds coming from the east flow around the mountain and leave its western side cloudless.

FIGURE 4. Contour maps of the studied areas, A. Chimborazo, B. Antisana. Wind directions are indicated by solid arrows (partly based on CitationClapperton, 1990 and CitationBlack, 1982); black squares indicate the closest climatic stations referred to in the text.
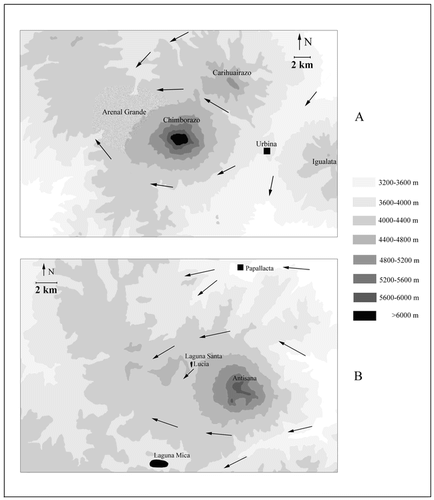
FIGURE 5. Shrubby vegetation with dominant Loricaria antisanensis on the eastern, humid side of Volcán Antisana (ca. 4200 m).

FIGURE 6. Resulting TWINSPAN dendrogram of the 65 vegetation samples (one sample remained unclustered) with corresponding eigenvalues for each division level.
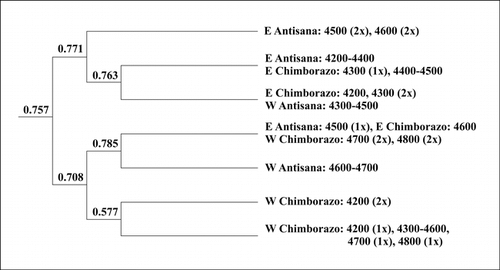
FIGURE 7. DCA ordination diagram of the vegetation samples, ▪ = E Chimborazo, • = E Antisana, □ = W Chimborazo, ○ = W Antisana, * = high-altitude samples; λ1 = 0.796, λ2 = 0.559, total inertia 9.831.
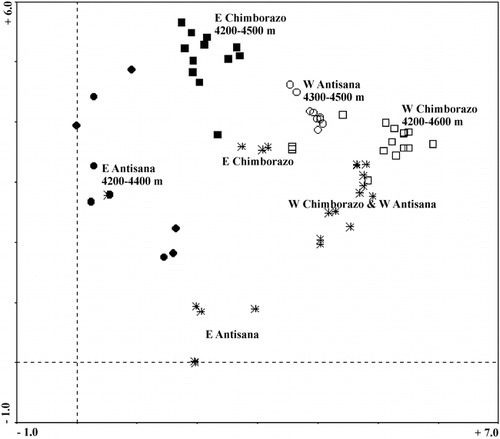
TABLE 1 Number of species for each examined mountain (bold diagonal), values of the Gleason's index of quantitative floristic similarity (above diagonal), and the observed (roman)/expected (italic) numbers of species held in common for the mountain pairs (below diagonal)a
Acknowledgments
The research of P. S. was supported by a fellowship from the Danish Research Academy and by the Grant Agency of the Czech Republic (grant nos. 206/97/0336 and 206/98/1447). The research of S. L. was supported by a grant from Danida/ENRECA project. INEFAN (Quito) is acknowledged for issuing research permits, INAMHI (Quito) for providing the climatic data. We would like to thank H. Balslev and R. Valencia for general support during the work in Ecuador and V. Sklenářová for assistance with data collection. Comments from H. Balslev, P. Kovář, D. Stančík, and two anonymous reviewers significantly improved the quality of the paper; T. Herben is thanked for advice with the statistics; and J. Luteyn kindly revised the English.
References Cited
- Acosta-Solís, M. 1985. El Arenal del Chimborazo, ejemplo de puna en el Ecuador. Revista Geográfica 22:115–122.
- Bendix, J. and W. Lauer . 1992. Die Niedeschlagsjahreszeiten in Ecuador und ihre klimadynamische Interpretation. Erdkunde 46:18–134.
- Black, J. M. 1982. Los páramos del Antisana. Revista Geográfica 17:25–52.
- Bontron, G. , B. Francou , E. Ayabaca , B. Cáceres , L. Maisincho , R. Chango , A. de la Cruz , L. A. Garzón , and D. Neubert . 1999. El Glaciar 15 del Antizana (Ecuador) Mediciones Glaciológicas, Hidrométricas, Meteorológicas y Topográficas (Años 1997 y 1998). Quito: IRD—INAMHI—EMAAP-Quito. 142. pp.
- Bouguer, M. 1749. La Figure de la Terre. Paris: Jombert. 396. pp.
- Brandbyge, J. and L. B. Holm-Nielsen . 1986. Reforestation of the high Andes with local species. Reports from the Botanical Institute, University of Aarhus 13:1–114.
- Cerón, C. E. M. 1994. Vegetación y diversidad en la Reserva de producción faunística del Chimborazo—Ecuador. Revista Geográfica 33:19–42.
- Clapperton, C. M. 1987. Glacial geomorphology, Quaternary glacial sequence and palaeoclimatic inferences in the Ecuadorian Andes. In Gardiner, V. (ed.), International Geomorphology 1986. Part II. London: Wiley and Sons. 843–870.
- Clapperton, C. M. 1990. Glacial and volcanic geomorphology of the Chimborazo-Carihuairazo massif, Ecuadorian Andes. Transactions of the Royal Society of Edinburgh: Earth Sciences 81:91–116.
- Clapperton, C. M. 1993. Glacier readvances in the Andes at 12,500–10,000 yr. BP: implications for mechanism of Late-glacial climatic change. Journal of Quaternary Science 8:197–215.
- Clapperton, C. M. , M. Hall , P. Mothes , M. J. Hole , J. W. Still , K. F. Helmens , P. Kuhry , and A. M. D. Gemmell . 1997. A Younger Dryas icecap in the equatorial Andes. Quaternary Research 47:13–28.
- Cleef, A. M. 1981. The Vegetation of the Páramos of the Colombian Cordillera Oriental. Dissertationes Botanicæ 61. Vaduz: Cramer. 320. pp.
- Cleef, A. M. and O. Rangel-Ch . 1984. La vegetación del páramo del noroeste de la Sierra Nevada de Santa Marta. In van der Hammen, T. and Ruiz, P.M. (eds.), La Sierra Nevada de Santa Marta (Colombia) Transecto Buritaca—La Cumbre. Berlin: Cramer. 203–266.
- Cleef, A. M. , O. Rangel-Ch , and S. V. Salamanca . 1983. Reconocimiento de la vegetación de la parte alta del transecto Parque los Nevados. In van der Hammen, T., Pérez Preciado, A. and Pinto, P.E. (eds.), La Cordillera Central Colombiana Transecto Parque los Nevados (Introducción y Datos Iniciales). Berlin: Cramer. 150–173.
- Coe, M. J. 1967. The Ecology of the Alpine Zone of Mount Kenya. Monographiae Biologicae 17. The Hague: Junk Publisher. 136. pp.
- Connor, E. F. and D. Simberloff . 1978. Species number and compositional similarity of the Galápagos flora and avifauna. Ecological Monographs 48:219–248.
- de la Condamine, M. 1751. Journal du Voyage Fait par Ordre du Roi, a L'Équateur, Servant d'Introduction Historique a la Mesure der Trois Premiers Degrés du Méridien. Paris: Imprimerie Royal. 266. pp.
- de Noni, G. , G. Trujillo , and M. Viennot . 1986. L'erosion et la conservation de sols en Equateur. Cahiér ORSTOM, série Pédologie 22:235–245.
- Diels, L. 1937. Beiträge zur Kenntnis der Vegetation und Flora von Ecuador. Bibliotheca Botanica 116, Stuttgart. 190. pp.
- Gleason, H. A. 1920. Some application of the quadrate method. Bulletin of the Torrey Botanical Club 47:21–33.
- Gradstein, S. R. 1998. Hepatic diversity in the neotropical páramos. In Fortunato, R. and Bacigalupo, N. (eds.), Proceedings of the VI Congreso Latinoamericano de Botánica. St. Louis: Missouri Botanical Garden Press. 69–85.
- Grubb, P. J. 1970. The impact of man on the Páramo of Cerro Antisana. Journal of Applied Ecology 7:7p–8p.
- Hall, K. , J. Boelhouwers , and K. Driscoll . 1999. Animals as erosion agents in the alpine zone: some data and observations from Canada, Lesotho, and Tibet. Arctic, Antarctic, and Alpine Research 31:436–446.
- Hall, M. 1977. El Volcanismo en el Ecuador. Biblioteca Ecuador, Quito. 120. pp.
- Harling, G. 1979. The vegetation types of Ecuador—a brief survey. In Larsen, K. and Holm-Nielsen, L.B. (eds.), Tropical Botany. London: Academic Press. 165–174.
- Hastenrath, S. 1981. The Glaciation of the Ecuadorian Andes. Rotterdam: Balkema. 174. pp.
- Hedberg, O. 1951. Vegetation belts of the East African mountains. Svensk Botanisk Tidskrift 45:140–202.
- Hill, M. O. 1979. TWINSPAN: a FORTRAN Program for Arranging Multivariate Data in an Ordered Two-way Table by Classification of the Individuals and Attributes. Cornell University, Ithaca, New York.
- Hofstede, R. G. M. 1995. Effects of Burning and Grazing on a Colombian Páramo Ecosystem. Ph.D. dissertation, University of Amsterdam, Amsterdam. 185. pp.
- Instituto Nacional de Meteorología e Hidrología 1974–1996. Anuário Meteorológico, Vols. 12–34. Quito, Ecuador.
- Jørgensen, P. M. and C. U. Ulloa . 1994. Seed plants of the high Andes of Ecuador—a checklist. AAU Reports 34:1–443.
- Kletke, H. 1859. Alexander von Humboldt's Reisen in Amerika und Asien. Vol. 2, 4th Edn. Berlin: Hasselberg. 656. pp.
- Kovář, P. (ed.). 2001. Srovnávací Studie Biodiversity v Andských Ekosystémech Ekvádoru Ovlivněných Lidskou a Vulkanickou Činností [Comparative Study of Biodiversity in Human- and Volcano-Influenced Ecosystems in the Ecuadorian Andes] (in Czech). Research report, GA ČR 206/98/1194, Prague.
- Lægaard, S. 1992. Influence of fire in the grass páramo vegetation of Ecuador. In Balslev, H. and Luteyn, J.L. (eds.) Páramo: An Andean Ecosystem under Human Influence. London: Academic Press. 151–170.
- Lauer, W. 1979. Die hypsometrische Asymmetrie der Páramo Höhenstufe in der nördlichen Anden. Innsbrucker Geografische Studien 5:115–130.
- Luteyn, J. L. 1999. Páramos: A Checklist of Plant Diversity, Geographical Distribution, and Botanical Literature. Memoirs of the New York Botanical Garden 84:1–278.
- Mahaney, W. C. (ed.). 1989. Quaternary and Environmental Research on East African Mountains. Rotterdam: Balkema. 483. pp.
- Meyer, H. 1907. In den Hoch-Anden von Ecuador. Berlin: Sietrich Reimer–Ernst Vohsen. 522. pp.
- Miehe, S. and G. Miehe . 1994. Ericaceous Forests and Heathlands in the Bale Mountains of South Ethiopia: Ecology and Man's Impact. Reinbeck: Traute Warnke Verlag. 206. pp.
- Monasterio, M. and S. Reyes . 1980. Diversidad ambiental y variación de la vegetación en los páramos de los Andes venezolanos. In Monasterio, M. (ed.), Estudios Ecológicos en los Páramos Andinos. Mérida: Ediciones Universidad de Los Andes. 47–91.
- Mueller-Dombois, D. and H. Ellenberg . 1974. Aims and Methods of Vegetation Ecology. New York: Wiley and Sons. 547. pp.
- Pérez, F. L. 1993. Turf destruction by cattle in the high equatorial Andes. Mountain Research and Development 13:107–110.
- Pérez Preciado, A. 1984. Aspectos climáticos de la Sierra Nevada de Santa Marta. In van der Hammen, T. and Ruiz, P.M. (eds.) La Sierra Nevada de Santa Marta (Colombia) Transecto Buritaca—La Cumbre. Berlin: Cramer. 33–44.
- Podwojewski, P. and J. Poulenard . 2000. La degradación de los suelos en los páramos. In Mena, P.A., Josse, C. and Medina, G. (eds.) Los Suelos del Páramo. Quito: Serie Páramo 5, Abya Yala. 27–36.
- Ramsay, P. M. 1992. The Páramo Vegetation of Ecuador: the Community Ecology, Dynamics and Productivity of Tropical Grasslands in the Andes. Ph.D. dissertation, University of Wales, Bangor. 274. pp.
- Rost, K. T. 1999. Observations on deforestation and alpine turf destruction in the Central Wutai Mountains, Shanxi province, China. Mountain Research and Development 19:31–40.
- Rubín, J. and B. Balatka . (eds.). 1986. Atlas Skalních, Zemních a Půdních Tvarů [Atlas of Rock, Ground and Soil Forms]. (in Czech). Prague: Academia. 385. pp.
- Rundel, P. W. 1994. Tropical alpine climates. In Rundel, P.W., Smith, A.P., and Meinzer, F.C. (eds.), Tropical Alpine Environments. Cambridge: Cambridge University Press. 21–44.
- Sarmiento, G. 1986. Ecological features of climates in high tropical mountains. In Vuilleumier, F. and Monasterio, M. (eds.), High Altitude Tropical Biogeography. Oxford: Oxford University Press. 11–45.
- Sauer, W. 1971. Geologie von Ecuador. Berlin: Gebrüder Borntræger. 316. pp.
- Schmitt, K. 1991. The Vegetation of the Aberdare National Park Kenya. Innsbruck: Universitätsverlag Wagner. 259. pp.
- Sémiond, H. , B. Francou , E. Ayabaca , A. de la Cruz , and R. Chango . 1998. El Glaciar 15 del Antizana (Ecuador) Investigaciones Glaciológicas 1994–1997. Quito: ORSTOM—IFEA—EMAAP-Quito—INAMHI. 87. pp.
- Sklenář, P. 2000. Vegetation Ecology and Phytogeography of Ecuadorian Superpáramos. Ph.D. dissertation, Charles University, Prague. 188. pp.
- Sklenář, P. 2001. Notes on taxonomy, distribution and ecology of some Ecuadorian high páramo Asteraceae, tribe Senecioneae. Compositae Newsletter 36:1–8.
- Sklenář, P. and P. M. Jørgensen . 1999. Distribution patterns of páramo plants in Ecuador. Journal of Biogeography 26:681–692.
- Smith, J. M. B. 1986. Origins and history of the Malesian high mountain flora. In Vuilleumier, F. and Monasterio, M. (eds.), High Altitude Tropical Biogeography. Oxford: Oxford University Press. 469–477.
- ter Braak, C. J. F. and P. Šmilauer . 1998. CANOCO Reference Manual and User's Guide to Canoco for Windows. Wageningen: Centre of Biometry. 353. pp.
- Troll, C. 1968. The cordilleras of the tropical Americas. Aspects of climatic, phytogeographical and agrarian ecology. In Troll, C. (ed.), Geo-ecology of the Tropical Mountainous Regions of the Tropical Americas. Colloquium Geographicum 9, Bonn. 15–56.
- van der Hammen, T. and A. M. Cleef . 1986. Development of the high Andean páramo flora and vegetation. In Vuilleumier, F. and Monasterio, M. (eds.), High Altitude Tropical Biogeography. Oxford: Oxford University Press. 153–201.
- Walter, H. 1971. Ecology of Tropical and Subtropical Vegetation. Edinburgh: Oliver and Boid. 539. pp.
- Whymper, E. 1892. Travels amongst the Great Andes of Ecuador. New York: Charles Scribner's Sons. 456. pp.
- Witte, H. J. L. 1995. Seasonal and altitudinal distribution of precipitation, temperature and humidity in the Parque los Nevados transect (Central Cordillera, Colombia). In van der Hammen, T. and Dos Santos, A.G. (eds.), La Cordillera Central Colombiana Transecto Parque los Nevados (Tercera Parte). Berlin: Cramer. 279–328.