Abstract
In a factorial field experiment we increased the temperature (Open Top Chambers) and nutrients (nitrogen, phosphorus, and potassium [NPK]) to simulate predicted future climate changes and studied the growth response of the acrocarpous bryophyte Pohlia wahlenbergii (Bryaceae) in a wet snowbed environment. The species shows a positive growth-length response to added nutrients and increased temperature. The stronger response to nutrients indicates a strong limitation of nutrients in the snowbed environment. There was an immediate response to nutrient treatment, whereas the temperature response was delayed. The growth response shows a clear interaction between temperature and nutrients. The immediate positive growth response is interpreted as a function of the wet habitat, since water makes the added nutrients immediately available to the plants. The growth form changed toward a more lax (loose) and desiccation-intolerant form with added nutrients. In a climate change scenario based on these results we hypothesize that bryophyte response will depend on the water availability from precipitation and from meltwater. In a drier environment we predict that bryophytes will become more constrained toward areas with a high continuity of meltwater, whereas increased precipitation may compensate for any changes in growth form, which would be positive for bryophytes.
Introduction
There has been considerable effort in the past few decade to study potential effects of climate change on vegetation, in particular vascular plants (CitationTotland, 1997; CitationArft et al., 1999; CitationSandvik and Totland, 2000). The increasing number of studies is related to the predictions of temperature increase both globally (CitationHoughton et al., 1990; CitationMaxwell, 1992) and locally (CitationFørland and Nordeng, 1999). An increase in average global surface temperature of about 1–3.5°C is predicted to occur within the next century (CitationHoughton et al., 1990). The climate change will be especially influential on ecosystems of arctic and alpine areas, as these regions include a high number of vulnerable habitats (CitationMitchell et al., 1990; CitationCallaghan et al., 1992; CitationMaxwell, 1992; CitationChapin et al., 1992, Citation2000). In addition to any effect of temperature alone (Heegaard, 2001), several important factors for plant response and distribution correlate with temperature in arctic and alpine regions, such as soil movement, snow cover, evaporation, and nutrient availability (CitationWookey and Robinson, 1997). With increasing temperature, nutrient availability will also increase (CitationBonan and Van Cleve, 1992; CitationNadelhoffer et al., 1991, Citation1992; CitationJonasson et al., 1993).
Nutrient availability is a very complex interaction of biotic and abiotic factors that can be summarized by increased temperature causing increased microbial activity so that with time the concentration of available nutrients for plants increases (CitationHenry et al., 1986; CitationChapin et al., 1995; CitationRobinson and Wookey, 1997). However, such effects are also linked to moisture availability, as increased evaporation may reduce microbial activity (CitationWookey and Robinson, 1997). The correlation of so many important factors with potential climate change emphasizes the importance of simultaneously studying the influence and interaction of several factors on the distribution and response of species and vegetation (CitationArft et al., 1999). However, most studies are based on vascular plants. Other plant groups are strongly under-represented, although cryptogams represent a major component of arctic and alpine ecosystems in both amount and importance (e.g., CitationClarke et al., 1971; CitationSmith, 1984; CitationLongton, 1988; CitationGreenfield, 1992; CitationConvey and Lewis-Smith, 1993; CitationFowbert, 1996; CitationØvstedal and Lewis-Smith, 2001). Bryophytes constitute a major part of several alpine plant communities (CitationGeissler, 1982). The mosses and liverworts in black crusts (i.e., a thin layer made up predominantly of creeping liverworts such as Marsupella spp., Anthelia juratzkana, and Pleurocladula albescens) strongly interact with microbial activity (CitationBliss et al., 1990; CitationLennihan et al., 1994) and successional development (CitationBliss et al., 1990) by stabilizing the moisture of the upper soil layer (CitationChapin et al., 1991). Hence, in many high-altitude and -latitude areas, bryophytes are important in various successional stages and may even form stable ecosystems such as extreme snowbeds that are dominated by bryophytes (CitationGeissler, 1982; CitationHeegaard, 1997, 2001).
Compared to vascular plants, bryophytes lack well-developed root systems. In bryophytes nutrients and water are transported predominantly by external capillary systems (CitationProctor, 1979, Citation1999, Citation2000), although internal transport is possible from cell to cell and within the cell walls. In addition, several mosses use endohydric transport by specialized cell structures; a classical example is the Polytrichales (CitationHébant, 1977). Although bryophytes have a seemingly loose attachment to the soil environment, it has repeatedly been shown that their distribution is dependent on both physical and chemical substrate characteristics (CitationLongton, 1988; CitationBates and Bakken, 1998). It has also been shown that nutrients for terricolous bryophytes are available from the substrate and from precipitation, but the ability for utilization and opportunistic response depends on the form of attachment to the substrate (CitationBates, 1994). Some elements provided by precipitation are more influential than other chemical compounds, as shown by the response of grassland species to ammonium deposition (Citationvan Tooren et al., 1990). It has further been shown that ammonium in precipitation has a negative effect on particular bryophytes (CitationLee et al., 1994).
The apparent differences in morphological structure and life strategies between vascular plants and bryophytes may result in differences between these plant groups in response to climate changes. In several studies of vascular plants a positive growth response has been detected in experimental manipulations designed to simulate predicted warming (CitationArft et al., 1999). Bryophytes, on the other hand, show either no response or a negative growth response to such treatments (CitationCallaghan et al., 1992; CitationPotter et al., 1995; CitationJägerbrand, 1996). Further, cover and number of bryophyte species have been shown to decrease with increased temperature and nutrients (CitationMolau and Alatalo, 1998; CitationChapin et al., 2000). In many future climate scenarios an increase in precipitation is predicted (CitationJaeger, 1988; CitationBoer et al., 1990), although these predictions are made with great uncertainty (CitationMaxwell, 1992). The predicted increase in precipitation may reduce the desiccation effect resulting from increased evaporation. Further, within alpine regions considerable areas receive moisture from snowmelt more or less continuously throughout the growing season. This continuity in meltwater may compensate for the increased evaporation resulting from increased temperatures. Therefore, our interest in this study is the relationship between growth form, temperature, and nutrients in habitats where water is not a currently limiting factor.
We focus on a common bryophyte, Pohlia wahlenbergii (Bryaceae), that is associated with intermediate to wet habitats. By measuring its growth and growth form in a factorial experimental design, with temperature and nutrients as the main variables, we attempt to answer the following questions: (1) How do temperature and/or nutrient supply influence the growth of Pohlia wahlenbergii in the field? (2) How do the treatments affect the growth form of the species? (3) Do these individual treatment factors interact in their effect? (4) Is there a different speed of response between the two treatments of temperature increase and nutrient addition?
Materials and Methods
STUDY AREA
The experiment was undertaken at Finse, Hardangervidda, southwestern Norway. The site was located near the low- to midalpine transition at approximately 1380 m a.s.l., on an east-facing slope northeast of Jomfrunut (60°36′N, 7°32′E). The bedrock is calcareous schist at and above about 1400 m and acidic granite below. The Finse region has an alpine-oceanic climate with mild, snowy winters combined with wet, relatively cold summers. The annual precipitation is about 1030 mm a year (CitationAune, 1993a), and the average temperature during June–September is 5.5°C (CitationAune, 1993b).
The study site is about 35 m × 14 m and contains intermingled bare, stony ground and more or less constantly irrigated bryophyte-dominated mats. The study site is a late-melting snowbed with Cerastium cerastoides, Deschampsia alpina, Epilobium anagallidifolium, Saxifraga rivularis, Saxifraga stellaris, and Veronica alpina as the most common vascular plant species. Snow cover disappeared extremely early in 1996 (around 23 June) and about 1 mo later in 1997. Snow normally covers the area again from about late October. The area is supplied with water throughout the summer by several small streams originating from a large snowpack situated upslope from the study site. After periods of heavy rain, the area is flooded. The study site was fenced to avoid disturbance from sheep.
STUDY SPECIES
Pohlia wahlenbergii is a very variable species; it grows predominantly in moist to wet basic soil from lowland to midalpine areas (CitationNyholm, 1993). The species is in the Bryaceae and is often associated with springs at high altitude, but it may also occur in late snowbeds with more or less continuous moisture availability, as at the study site. The leaves are relatively thin walled and may be considered to be intermediate and capable of some desiccation evasion. It is an acrocarpous moss; i.e., the apical cell is used in the production of sexual organs. Although it is known to have sporophytes, capsules are rare. Vegetative propagation occurs through gemmae. P. wahlenbergii occurs frequently among other bryophytes but is also capable of creating monospecific mats, which are often found around springs in alpine regions. P. wahlenbergii occurs throughout Fennoscandia but is more common toward the north (CitationNyholm, 1993). It also occurs elsewhere in Europe, Canada, China, North and South America, Australia, and New Zealand.
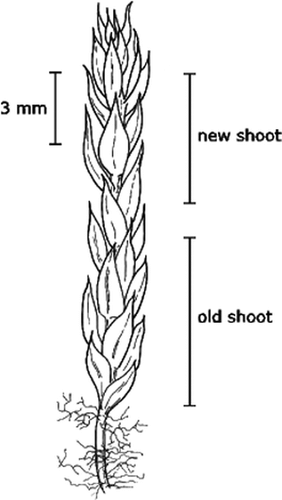
The shoots tend to change color from light green to red and black as they age. We divided each shoot into old and new sections according to this difference and measured the length of these two parts. The lower section includes the initial and early years of the experiment, whereas the upper section includes growth in the last year. The growth of P. wahlenbergii is from an apical meristematic cell (CitationSchofield and Hébant, 1984), which indicates that the elongation of the shoot occurs primarily through new growth. Thus, elongation of the old section after onset of treatment is not likely, and to reduce any difference due to variation in turgor pressure all individual shoots were analyzed in fully wet conditions. In addition, the number of leaves was counted in 3-mm stem segments. To avoid the influence of juvenile leaves, counting started just below the apical section () in order to estimate the difference in laxness between shoots from various treatments. A lax growth form is characterized by greater distance between the leaves, which causes a “loose-shoot” appearance. To study the growth form further, we divided the number of leaves by the length of the upper segment because the growth of a segment may influence the number of leaves within the 3-mm band. Hence, we may have detected differences in growth form due to treatments that are not caused by shoot elongation.
EXPERIMENTAL DESIGN
The experiment took place from 5 July 1996 until the moss shoots were collected on 5 August 1999. Open Top Chambers (OTC) made of 3-mm Plexiglas (LEXAN® Exell, UV-resistant polycarbonate), transmitting 88% of photosynthetically active radiation, caused an average air temperature increase of 1.6°C during the daytime, while mean soil temperature increased 2.6°C compared to control plots (for more details, see CitationSandvik and Totland, 2000). The chamber diameter was 92 cm at the bottom and 50 cm at the top. The chambers were 34 cm high, and the 6 panels in the OTC were inwardly inclined at 60° to achieve optimal light transmission (CitationMarion et al., 1997). The OTCs were permanently placed in the field during the experiment. Nutrients were added as 1-g sticks (4 cm long) containing 8% N, 10% P, and 13% K (Pokon plant-food sticks for flowering plants, Pokon and Chrysal, Naarden, Holland). Such nutrient sticks have been used in earlier studies (CitationSandvik and Totland, 2000; CitationSandvik, 2001). We established 20 blocks, each consisting of one OTC and one control area. The controls had the same size and forms at the ground as the OTCs. We marked two 5 × 5–cm plots in each block, one situated close to the center of the OTC/control and the other 35 cm uphill from the first plot. We prevented nutrient from leaking to the unfertilized experimental plots because of the sloping ground and the direction of the water stream (the percolating water down the hill) and by fertilizing the lowest plot.
We used solid nutrient supply to secure increased nutrient availability for a longer period. The nutrient sticks were pricked down in the soil in the same location every time, 10 cm above the lowest plot in the OTCs/controls so that the tops of the sticks were even with the soil surface. On 5 July 1996, we used 2 nutrient sticks, and thereafter we used 1 stick every 2 wk (the same place) from 5 July to 30 August 1996 and from 25 July to 10 September 1997. In total 11 sticks were added to each experimental plot during the 2 yr; for N, for example, this application constituted about 25 g N/m2. From 10 September 1997 to the harvest of the moss, no nutrients were applied. However, the nutrient may have had an effect for several years.
During the fertilization, a bright green area (15 cm wide and 20 cm long) developed around the nutrient sticks and downhill from them. We observed no gradient in moss growth across this green area. The 5 × 5–cm sample plots were situated 5 cm from the nutrient stick. Furthermore, we divided each moss shoot into a new- and old-shoot sequence based on color changes as the moss shoots grew older. Consequently, it is likely that all the new shoots represented growth after the experiment had started.
Hence, each of the 20 blocks represented four treatments: untreated control (upper plot in the control area), nutrient addition (lowest plot in the control area), temperature increase (upper plot in the OTC), and temperature increase plus nutrient addition (lowest plot in the OTC). We collected 20 moss shoots from each of the four 5 × 5–cm plots on 5 August 1999.
Because this study considers changes in the population mean, we wished to minimize pseudoreplication of the individual sampling. Therefore, the morphological parameters were averaged within each block and each treatment type. Hence, the 20 blocks lead to 80 observations.
STATISTICAL ANALYSIS
The effects of temperature and nutrient on growth and growth form of Pohlia wahlenbergii were analyzed with the use of a mixed-model Analysis of Variance (ANOVA; CitationChambers et al., 1993; CitationVenables and Ripley, 1997), with inclusion of the error layer between blocks. The error layer was included because we recognized the potential influence of differences between the various blocks, and the aim of this study was the effect of treatment on the morphological parameters independent of whether the blocks differed. For more statistical detail on error structure, see CitationZar (1984). All statistical computations were done using S-plus for Windows (CitationMathsoft, 1997).
Results
The individual moss shoots were divided into old and new sections. Although these variables are strongly correlated overall (), they provide different biological information, as shown by the correlation when only the control plots are considered (). Further, there was a significant correlation between the number of leaves per segment and the old and new shoot-length variables (). The highest overall correlation was found in the new shoot region (r = −0.86, ). The number of leaves standardized by the growth (new shoot) correlates significantly with both variables ().
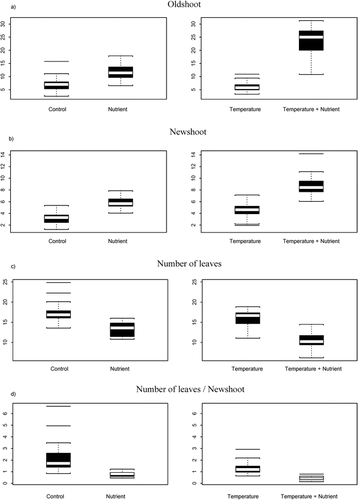
The response variables, given the various treatments, are visualized in , and the statistics of the mixed-model ANOVA are summarized in . For the shoot-length variables, there were significant interaction effects between temperature and nutrients, which suggests a dramatic elongation of the individual shoots, on average, with increased temperature and addition of nutrients in comparison with the effect of only one of the treatment types (). The average shoot length (in mm) differs from 7.22 and 3.12 (controls) to 23.52 and 8.79 in the temperature-plus-nutrient regimen for old shoots and new shoots, respectively (). Further, the significance of the interaction term for new shoots was (p < 0.02), whereas for old shoots the significance was stronger (p < 0.0001). This result suggests a greater difference between the main effect and the interaction term for old shoots than for new shoots. When we studied the main effects per se for old shoots, it became evident that the temperature effect is not significant (p > 0.1, df = 18), whereas the nutrient effect is significant (p < 0.0001, df = 18). On the other hand, the main effect of both temperature and nutrients was significant for new shoots when analyzed per se (p < 0.01 and p < 0.0001, respectively). The main effect of nutrient addition is greater than that of temperature increase for both old shoots and new shoots (). Nutrient addition induces changes in length of the shoots (mm) at a magnitude of 1.63 and 1.87 for old shoots and new shoots, respectively, whereas temperature causes changes at a magnitude of −0.86 and 1.43 for old shoots and new shoots, respectively.
Temperature and nutrient treatments do not show a significant interaction effect on the average number of leaves within the new-shoot section of the individuals (). However, both main effects, temperature and nutrients, were significant (). The nutrient treatment shows a greater effect than temperature, as seen by the residual sum of squares (479.22 and 87.91, respectively; ). This difference is also shown in , where the difference in control shoots and shoots treated with increased temperature is less than the difference between the control shoots and the shoots treated with nutrients. There is an additional reduction in the number of leaves per segment under the combined treatment, but this effect is not statistically significant. The nutrients affect the number of leaves on the plants more strongly than temperature does, so the trend is similar to that of the growth variables. However, as these response variables are strongly correlated (), the available evidence cannot be viewed as independent experiments. There may be genetic constraints on the number of leaves, and with increasing growth the number of leaves per unit of shoot length will naturally decrease. To address this problem we standardized the number of leaves by the length of the segments. The results showed the same trend as for the number of leaves. Hence, there is an effect of nutrients and temperature on the density of leaves that is independent of the induced growth. The number of leaves becomes, on average, fewer as the shoots becomes longer, but the number of leaves becomes fewer as an effect of the treatments.
Discussion
There is great variability in plant growth responses to experimental warming (e.g., CitationChapin and Shaver, 1985; CitationHavström et al., 1993; CitationWookey et al., 1993; CitationChapin et al., 1995; CitationMolau, 1996; CitationArft et al., 1999; CitationChapin et al., 2000). Most of these studies consider vascular plants, but some have gathered information about bryophytes (CitationCallaghan et al., 1978; CitationCallaghan et al., 1992; CitationPotter et al., 1995; CitationFowbert, 1996; CitationJägerbrand, 1996; CitationMolau and Alatalo, 1998; CitationChapin et al., 2000). In this study Pohlia wahlenbergii showed a positive response to both nutrients and temperature, with strikingly increased growth when these two treatments were combined. These responses are seen in both the length of shoots and the number of leaves. These responses differ from results reported for species such as Hylocomium splendens, Aulacomium turgidum, Tomentypnum nitens, and Sphagnum teres that show no response or even a negative response to experimental treatments (CitationPotter et al., 1995; CitationJägerbrand, 1996). Further, in experiments with increased temperature and nutrient concentrations, it has been shown that the cover and diversity of bryophytes decrease (CitationJonasson, 1992; CitationMolau and Alatalo, 1998). These negative responses have predominantly been associated with increased desiccation caused by increased temperature and the competitive influence of vascular plants due to increased temperature and nutrients (CitationChapin et al., 2000). The negative response to nutrient treatments is also corroborated in studies of atmospheric nutrient deposition, where added ammonium reduces the shoot density of Rhytidiadelphus squarrosus and Pleurozium schreberi after 5 yr of experiments (CitationMorecroft et al., 1994; CitationLee et al., 1998). However, the opposite, i.e., increased growth by addition of nutrients, has also been observed in field studies for species such as Polytrichum commune and Dicranum majus (CitationFurness and Grime, 1982; CitationBates, 1987, Citation1989; CitationRincon, 1988, Citation1990; CitationBakken, 1994; CitationPotter et al., 1995; CitationBates and Bakken, 1998).
Most experimental studies like ours, related to climate change scenarios, are located in intermediate wet to dry sites where bryophytes may be stressed by water shortage due to increased evaporation following increased temperatures. However, in our study area, the high water availability compensated for any potential increased evaporation resulting from increased ambient temperature, which may explain the observed minor response of Pohlia wahlenbergii to increased temperature.
Obviously, low soil-nutrient concentration is the principal constraining factor for growth in P. wahlenbergii. Considering the growth form of the control plants, these specimens are relatively short, with a short distance between leaves compared to the more lax growth form of the nutrient-treated individuals. The compact growth form is usually associated with dry habitats (CitationProctor and Smith, 1995). A lax growth form is less capable of holding water (CitationProctor, 1984, Citation2000) and will consequently, on average, be less tolerant to frequent desiccation (CitationProctor and Smith, 1995). The change from dense to lax growth forms suggests that moisture availability is adequate enough to allow individuals to respond freely to the increased nutrient concentration. These observations indicate that a dense growth form is related to the concentration of nutrients as well as to the classic water shortage (CitationProctor and Smith, 1995). Such xeromorphism due to shortage in nitrogen availability is also known to occur among vascular plants (CitationHaag, 1974).
Plants with lax growth forms generated by an increased nutrient concentration will experience a higher frequency of desiccation and rehydration. During the rehydration process, electrolytes, including nitrogen and phosphorous, are lost through leakage (CitationCoxon, 1991). This loss may be a particular problem for individuals growing in dry areas or those with intermediate water availability, as suggested by CitationBates (1987, Citation1989) and CitationBakken (1994). The water availability of the surrounding area may not be sufficient to support nutrient-induced lax growth forms. Reduced growth often follows nutrient addition (CitationJonasson, 1992; CitationPotter et al., 1995; CitationJägerbrand, 1996; CitationMolau and Alatalo, 1998), which may be explained by increased evaporation stress caused by the laxer growth forms in addition to the competitive interaction with vascular plants suggested earlier (CitationChapin et al., 2000). By such an interpretation, the seemingly opposing results in various studies for adding nutrients may not be contradictory. The various results merely suggest an interaction between nutrient concentration and available moisture. The result of both increased temperature and nutrient concentration can then be linked to similar physiological problems for bryophytes, namely, increased evaporation.
In addition to the positive response of P. wahlenbergii to nutrient treatment, we found a considerable increase in growth when temperature and nutrients were combined as a treatment. This increase suggests that temperature, although exerting only a minor influence by itself, may still have an important effect on species growth. The interaction indicates that a slight increase in temperature makes the utilization of nutrients far more efficient. The positive interaction between increased nutrient and temperature seen here has been repeatedly shown in other studies for vascular plants (CitationArft et al., 1999) but not for bryophytes (CitationCallaghan et al., 1992; CitationPotter et al., 1995; CitationJägerbrand, 1996).
Pohlia wahlenbergii shows an immediate response to increased nutrients. This result contrasts with other studies in which a delayed response to nutrients has frequently been observed (CitationShaver and Chapin, 1986). Bryophytes usually grow in close contact with the surrounding environment and have the potential for rapid response. Water and nutrients are predominantly incorporated over the leaves close to the photosynthetically active tissue and the apical growing cell. Hence, the immediate response of P. wahlenbergii to nutrients was not unexpected. In our study site, the percolating water probably dissolved the nutrient sticks more quickly, in comparison with drier sites in which a delayed response has been observed (CitationShaver and Chapin, 1986). This rapid dissolution of the nutrient stick may explain the immediate response of P. wahlenbergii, as found earlier for the vascular plant Saxifraga stellaris at the same study site (CitationSandvik and Totland, 2000). Hence, we cannot be sure that these species show a greater plasticity than plants grown in experiments under a different moisture environment. However, P. wahlenbergii shows a greater increase in cover than other species in the same plots (unpublished data), which may indicate a greater opportunistic response than in the mosses with which it grows in these particular study sites.
Conclusions
This study shows that in a warmer climate scenario, increased nutrients and temperature may favor bryophytes as long as water is freely available. The hypothesis that bryophytes will suffer in future climate change (CitationChapin et al., 2000) may be biased by moisture conditions at other experimental sites. CitationMolau and Alatalo (1998) and CitationChapin et al. (2000) show that bryophytes will suffer from desiccation when temperature is increased and from the growth of vascular plants when nutrients are increased. We show an additional effect of increased nutrient concentration: bryophytes may change their growth forms with increased nutrients, which may reduce their ability to withstand desiccation. Thus, bryophytes in alpine and arctic regions may suffer in dry and semidry areas (CitationChapin et al., 2000) but at the same time are favored in wet habitats (CitationTenhunen et al., 1992). This favorable response may lead to greater heterogeneity in future vegetation patterns. Increased future precipitation is predicted to follow increases in air temperature in some regions (Fen, 1999; CitationFørland and Nordeng, 1999). A moister climate can compensate for increased evaporation due to a temperature rise and allow increased growth when nutrients are not a limiting factor. In addition, generally higher moisture conditions may also facilitate sexual reproduction and subsequent long-distance dispersal for bryophytes (CitationLewis-Smith, 1990; CitationLongton and Schuster, 1983). Consequently, a future scenario characterized by increased precipitation may be beneficial for some bryophytes.
Table 1 Correlation matrix between the response variablesa
Table 2 Summary statistics of the mixed-model ANOVA, where site was located as a random factor introducing a variance layera
Acknowledgments
We thank Ørjan Totland and John Birks for comments and suggestions on an earlier version of the manuscript. We also thank Phillip Wookey and anonymous reviewers for valuable comments. For financial support we thank Agder University College (S. M. Sandvik) and the Norwegian Research Council (E. Heegaard) through NFR project 140874/720.
References Cited
- Arft, A. M. , M. D. Walker , J. Gurevitch , J. M. Alatalo , M. S. Bret-Harte , M. Dale , M. Diemer , F. Gugerli , G. H R. Henry , M. H. Jones , R. D. Hollister , I. S. Jónsdóttir , K. Laine , E. Lévesque , G. M. Marion , U. Molau , P. Mølgaard , U. Nordenhäll , V. Raszhivin , C. H. Robinson , G. Starr , A. Stenström , M. Stenström , Ø Totland , P. L. Turner , L. J. Walker , P. J. Webber , J. M. Welker , and P. A. Wookey . 1999. Responses of tundra plants to experimental warming: meta-analysis of the International Tundra Experiment. Ecological Monographs 69:491–511.
- Aune, B. (ed.),. 1993a. Precipitation Normals, Normal Period 1961–1990. Oslo: The Norwegian Meteorological Institute. 63 pp.
- Aune, B. (ed.),. 1993b. Air Temperature Normals, Normal Period 1961–1990. Oslo: The Norwegian Meteorological Institute. 63 pp.
- Bakken, S. 1994. Growth and nitrogen dynamics of Dicranum majus under two contrasting nitrogen deposition regimes. Lindbergia 19:63–72.
- Bates, J. W. 1987. Nutrient retention by Pseudoscleroppodium purum growing under an oak canopy. Journal of Bryology 14:565–580.
- Bates, J. W. 1989. Interception of nutrients in wet deposition by Pseudoscleropodium purum : an experimental study of uptake and retention of potassium and phosphorus. Lindbergia 15:93–98.
- Bates, J. W. 1994. Responses of the mosses Brachythecium rutabulum and Pseudoscleropodium purum to a mineral nutrient pulse. Functional Ecology 8:686–692.
- Bates, J. W. and S. Bakken . 1998. Nutrient retention, desiccation and redistribution in mosses. In Bates, J. W., Ashton, N. W., and Duckett, J. G. (eds.), Bryology for the Twenty-first Century. Leeds, UK: Maney Publishing and British Bryological Society, 293–304.
- Bliss, L. C. , D. M. Chapin , A. S. Leggett , R. Lennihan , L. G. Dickson , C. Bledsoe , and L. S. Bledsoe . 1990. Ecosystem development in a coastal lowland of the Canadian High Arctic. In Kotlyakov, V. M., and Sokolov, V. E. (eds.), Arctic Research, Advances and Prospects, Part 2. Moscow: Institute of Geography, 165–174.
- Boer, G. J. , N. A. McFarlane , J. P. Blanchet , and M. Lazare . 1990. Greenhouse gas-induced climatic simulated with the CCC second-generation GCM. In Cohen, S. J. (ed.), Application of the Canadian Climate Centre General Circulation Model Output for Regional Climate Impact Studies: Guidelines for Users. Downsview, Ontario: Atmospheric Environment Service, Canadian Climate Centre, 2–5.
- Bonan, G. B. and K. Van Cleve . 1992. Soil temperature, nitrogen mineralization, and carbon source-sink relationships in boreal forests. Canadian Journal of Forest Research 22:629–639.
- Callaghan, T. V. , N. J. Collins , and C. H. Callaghan . 1978. Photosynthesis, growth and reproduction of Hylocomium splendens and Polytrichum commune in Swedish Lapland. Oikos 31:73–88.
- Callaghan, T. V. , M. Sonesson , and L. Sømme . 1992. Responses of terrestrial plants and invertebrates to environmental change in high latitudes. Philosophical Transactions of the Royal Society of London 338:279–288.
- Chambers, J. M. , A. E. Freeny , and R. M. Heiberger . 1993. Analysis of variance; designed experiments. In Chambers, J. M., and Hastie, T. J. (eds.), Statistical Models in S New York: Chapman and Hall, 145–194.
- Chapin, D. M. , L. C. Bliss , and L. J. Bledsoe . 1991. Environmental regulation of nitrogen fixation in a high arctic lowland ecosystem. Canadian Journal of Botany 69:2744–2755.
- Chapin III., F. S. and G. R. Shaver . 1985. Individualistic growth response of tundra plant species to environmental manipulations in the field. Ecology 66:564–576.
- Chapin III., F. S. , R. L. Jefferies , J. F. Reynolds , G. R. Shaver , and J. Svoboda . 1992. Arctic plant physiological ecology: a challenge for the future. In Chapin, F. S. III., Jefferies, R. L., Reynolds, J. F., Shaver, G. R., and Svoboda, J. (eds.), Arctic Ecosystems in a Changing Climate. San Diego: Academic Press, 3–8.
- Chapin III., F. S. , G. R. Shaver , A. E. Giblin , K. J. Nadelhoffer , and J. A. Laundre . 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76:694–711.
- Chapin III., F. S. , A. D. McGuire , J. Randerson , R. Pielke Sr. , D. Baldocchi , S. E. Hobbie , N. Roulet , W. Eugster , E. Kasischke , E. B. Rastetter , S. A. Zimov , and S. W. Running . 2000. Arctic and boreal ecosystems of western North America as components of climate system. Global Change Biology 6:211–223.
- Clarke, G. C S. , S. W. Greene , and D. M. Greene . 1971. Productivity of bryophytes in polar regions. Annals of Botany 35:99–108.
- Convey, P. and R. I. Lewis-Smith . 1993. Investment in sexual reproduction by antarctic mosses. Oikos 68:293–302.
- Coxon, D. S. 1991. Nutrient release from epiphytic bryophytes in tropical montane rain forest (Guadeloupe). Canadian Journal of Botany 69:2122–2129.
- Førland, E. J. and T. E. Nordeng . 1999. Framtidig klimautvikling i Norge. Cicerone 6:21–24.
- Fowbert, J. A. 1996. An experimental study of growth in relation to morphology and shoot water content in maritime antarctic mosses. New Phytologist 133:363–373.
- Furness, S. B. and J. P. Grime . 1982. Growth rate and temperature responses in Bryophytes II: a comparative study of species of contrasting ecology. Journal of Ecology 70:525–536.
- Geissler, P. 1982. Alpine communities. In Smith, A. J. E. (ed.), Bryophyte Ecology. London: Chapman and Hall, 167–189.
- Greenfield, L. G. 1992. Retention of precipitation nitrogen by antarctic mosses, lichens and fellfield soils. Antarctic Science 4:205–206.
- Haag, R. W. 1974. Nutrient limitations to plant production in two tundra communities. Canadian Journal of Botany 52:103–116.
- Havström, M. , T. V. Callaghan , and S. Jonasson . 1993. Differential growth response of Cassiope tetragona , an arctic dwarf-shrub, to environmental perturbations among three contrasting high- and subarctic sites. Oikos 66:389–402.
- Hébant, C. 1977. The conducting tissues of bryophytes. Bryophytorum Bibliotheca 10:1–157.
- Heegaard, E. 1997. Ecology of Andreaea in western Norway. Journal of Bryology 19:527–636.
- Heegaard, E. 2002. A model of alpine species distribution in relation to snowmelt time and altitude. Journal of Vegetation Science 13:493–504.
- Henry, G. H R. , B. Freedman , and J. Svoboda . 1986. Effects of fertilization on three tundra plant communities of a polar desert oasis. Canadian Journal of Botany 64:2502–2507.
- Houghton, J. T. , G. J. Jenkins , and J. J. Ephramus . (eds.),. 1990. Climate Change: The Intergovernmental Panel on Climate Change (IPCC) Scientific Assessment. Cambridge: Cambridge University Press.
- Jaeger, J. 1988. Developing Policies for Responding to Climate Change. WMO/TD 225. Geneva: World Meteorological Organization. 53 pp.
- Jägerbrand, A. 1996. Arctic moss growth and moss communities: effects of simulated climate change. Master's thesis in plant ecology, University of Göteborg. 11pp.
- Jonasson, S. 1992. Plant responses to fertilization and species removal in tundra related to community structure and clonality. Oikos 63:420–429.
- Jonasson, S. , M. Havström , M. Jensen , and T. V. Callaghan . 1993. In situ mineralization of nitrogen and phosphorus of arctic soils after perturbations simulating climate change. Oecologia 95:179–186.
- Lennihan, R. , D. M. Chapin , and L. G. Dickson . 1994. Nitrogen fixation and photosynthesis in high arctic forms of Nostoc commune . Canadian Journal of Botany 72:940–945.
- Lee, J. A. , A. N. Parsons , and R. Baxter . 1994. Sphagnum species and polluted environments, past and future. Advances in Bryology 5:297–313.
- Lee, J. A. , S. J M. Caporn , J. Carroll , J. P. Foot , D. Johnson , L. Potter , and A. F S. Taylor . 1998. Effects of ozone and atmospheric nitrogen deposition on bryophytes. In Bates, J. W., Ashton, N. W., and Duckett, J. G. (eds.), Bryology for the Twenty-first Century. Leeds, UK: Maney Publishing and British Bryological Society, 331–341.
- Lewis-Smith, R. I. 1990. Exotic sporomorpha as indicators of potential immigrant colonists in Antarctica. Grana 30:313–324.
- Longton, R. E. 1988. The Biology of Polar Bryophytes and Lichens. Cambridge: Cambridge University Press. 391 pp.
- Longton, R. E. and R. M. Schuster . 1983. Reproductive biology. In Schuster, R. M. (ed.), New Manual of Bryology, Vol. 1. Miyazaki, Japan: The Hattori Botanical Laboratory, 386–462.
- Marion, G. M. , G. H R. Henry , D. W. Freckman , J. Johnstone , G. Jones , M. H. Jones , E. Lèvesque , U. Molau , P. Mølgaard , A. N. Parsons , J. Svoboda , and R. A. Virginia . 1997. Open-top designs for manipulating temperature in high-latitude ecosystems. Global Change Biology 3:Suppl. 1 20–32.
- Mathsoft 1997. S-PLUS 4.0 Release 2 for Windows. Seattle: Mathsoft, Inc.
- Maxwell, B. 1992. Arctic climate: potential for change under global warming. In Chapin, F. S., III., Jefferies, R. L., Reynolds, J. F., Shaver, G. R., and Svoboda, J. (eds.), Arctic Ecosystems in a Changing Climate. San Diego: Academic Press, 11–34.
- Mitchell, J. F B. , S. Manabe , V. Meleshko , and T. Tokioka . 1990. Equilibrium climatic change and its implications for the future. In Houghton, J. T., Jenkins, G. J., and Ephraums, J. J. (eds.), 1990: Climate Change: The Intergovernmental Panel on Climate Change (IPCC) Scientific Assessment. Cambridge: Cambridge University Press, 131–172.
- Molau, U. 1996. Climatic impacts on flowering, growth, and vigour in an arctic-alpine cushion plant, Diapensia lapponica , under different snow cover regimes. Ecological Bulletins 45:210–219.
- Molau, U. and J. M. Alatalo . 1998. Responses of subarctic-alpine plant communities to simulated environmental change: biodiversity of bryophytes, lichens, and vascular plants. Ambio 27:322–329.
- Morecroft, M. D. , E. K. Sellars , and J. A. Lee . 1994. An experimental investigation into the effects of atmospheric nitrogen deposition on two semi-natural grasslands. Journal of Ecology 82:473–483.
- Nadelhoffer, K. J. , A. E. Giblin , G. R. Shaver , and J. A. Laundre . 1991. Effects of temperature and substrate quality on element mineralization in six arctic soils. Ecology 72:242–253.
- Nadelhoffer, K. J. , A. E. Giblin , G. R. Shaver , and A. E. Linkins . 1992. Microbial processes and plant nutrient availability in arctic soils. In Chapin, F. S., III., Jefferies, R. L., Reynolds, J. F., Shaver, G. R., and Svoboda, J. (eds.), Arctic Ecosystems in a Changing Climate. San Diego: Academic Press, 281–300.
- Nyholm, E. 1993. Illustrated flora of Nordic mosses. Fasc. 3. Bryaceae—Rhodobryaceae—Mniaceae—Cinclidiaceae—Plagiomniaceae. Odense: AiO Print Ltd., 145–244.
- Ovstedal, D. O. and R. I. Lewis-Smith . 2001. Lichens of Antarctica and South Georgia: A Guide to Their Identification and Ecology. Cambridge: Cambridge University Press. 411 pp.
- Potter, J. A. , M. C. Press , T. V. Callaghan , and J. A. Lee . 1995. Growth response of Polytrichum commune and Hylocomium splendens to simulated environmental change in the sub-arctic. New Phytologist 131:533–541.
- Proctor, M. C F. 1979. Structure and eco-physiological adaptation in bryophytes. In Clarke, G. S., and Duckett, J. G. (eds.), Bryophyte Systematics Systematics Association special volume No. 14, London: Academic Press, 479–509.
- Proctor, M. C F. 1984. Structure and ecological adaptation. In Dyer, A. F., and Duckett, J. G. (eds.), The Experimental Biology of Bryophytes. London: Academic Press, 9–37.
- Proctor, M. C F. 1999. Water-relations parameters of some bryophytes evaluated by thermocouple psychrometry. Journal of Bryology 21:269–277.
- Proctor, M. C F. 2000. The bryophyte paradox: tolerance of desiccation, evasion of drought. Plant Ecology 151:41–49.
- Proctor, M. C F. and A. J E. Smith . 1995. Ecological and systematic implications of branching patterns in bryophytes. In Hoch, P. C., and Stephenson, A. G. (eds.), Experimental and Molecular Approaches to Plant Biosystematics. St. Louis, MO: Missouri Botanical Garden, 87–110.
- Rincon, E. 1988. The effect of herbaceous litter on bryophyte growth. Journal of Bryology 15:209–217.
- Rincon, E. 1990. Growth response of Brachythecium rutabulum to different litter arrangements. Journal of Bryology 16:120–122.
- Robinson, C. H. and P. A. Wookey . 1997. Microbial ecology, decomposition and nutrient cycling in arctic environments. In Woodin, S. J. (ed.), The Ecology of Arctic Environments. Special Symposium of the British Ecological Society. Oxford: Blackwell Scientific Publications, 41–68.
- Sandvik, S. M. and Ø Totland . 2000. Short-term effects of simulated environmental changes on phenology, reproduction, and growth in the late-flowering snowbed herb Saxifraga stellaris L. Ecoscience 7:201–213.
- Sandvik, S. M. 2001. Somatic and demographic costs under different temperature regimes in the late-flowering alpine perennial herb Saxifraga stellaris (Saxifrageae). Oikos 93:303–311.
- Schofield, W. B. and C. Hébant . 1984. The morphology and anatomy of the moss gametophyte. In Schuster, R. M. (ed.), New Manual of Bryology, Vol 2. Miyazaki, Japan: The Hattori Botanical Laboratory, 627–657.
- Shaver, G. R. and F. S. Chapin III. . 1986. Effect of fertilizer on production and biomass of tussock tundra, Alaska, U.S.A. Arctic and Alpine Research 18:261–268.
- Smith, A. J E. (ed.),. 1984. Bryophyte Ecology. London: Chapman and Hall. 511 pp.
- Tenhunen, P. , O. L. Lange , S. Hahn , R. Siegwolf , and S. F. Oberbauer . 1992. The ecosystem role of poikilohydric tundra plants. In Chapin, F. S. III., Jefferies, R. L., Reynolds, J. F., Shaver, G. R., and Svoboda, J. (eds.), Arctic Ecosystems in a Changing Climate. San Diego: Academic Press, 213–256.
- Totland, Ø 1997. Effects of flowering time and temperature on growth and reproduction in Leontodon autumnalis var. taraxaci , a late-flowering alpine plant. Arctic and Alpine Research 29:285–290.
- van Tooren, B. F. , D. van Dam , and H. J. During . 1990. The relative importance of precipitation and soil as sources of nutrients for Calliergonella cuspidata in a chalk grassland. Functional Ecology 4:101–107.
- Venables, W. N. and B. D. Ripley . 1997. Modern Applied Statistics with S-plus. New York: Springer. 548 pp.
- Wookey, P. A. , A. N. Parsons , J. M. Welker , J. A. Potter , T. V. Callaghan , J. A. Lee , and M. C. Press . 1993. Comparative responses of phenology and reproductive development to simulated environmental change in sub-arctic and high arctic plants. Oikos 67:490–502.
- Wookey, P. A. and C. H. Robinson . 1997. Responsiveness and resilience of high arctic ecosystems to environmental change. Opera Botanica 132:215–233.
- Zar, J. H. 1984. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall International. 718 pp.