Abstract
We quantitatively investigated a snow algal community on Tyndall Glacier of the Southern Patagonia Icefield, Chile, at an elevation from 300 to 1500 m a.s.l. We observed 7 species of snow and ice algae (Chlorophyta and cyanobacteria) on the glacier. These species were Mesotaenium (M.) berggrenii, Cylindrocystis (Cyl.) brébissonii, Ancylonema sp., Closterium sp., Chloromonas sp., Oscillatoriaceae cyanobacterium, and an unknown alga. The spatial distribution of these algae differed among the species. M. berggrenii, Cyl. brébissonii, Ancylonema sp., and Closterium sp. appeared mainly in the lower-elevation area (370–770 m a.s.l.), the unknown alga in the higher-elevation area (900–1500 m a.s.l.), and Chloromonas sp. and Oscillatoriaceae cyanobacterium in the middle part of the glacier. The mean cell concentration and total cell volume biomass ranged from 0 to 9.2 × 104 (mean: 1.8 × 104) cells mL−1, from 0 to 327 (mean: 63) μL m−2, respectively. The cell volume biomass generally decreased with altitude. The community structure showed that M. berggrenii was dominant in the ice area (65–100% of total cell volume) and lower snow area (50–70%) and that the unknown alga was dominant in the higher snow area (100%). The Simpson’s species diversity index was significantly different among the study sites but was generally low (less than 1.9) at all sites. The cell volume biomass and diversity index are relatively smaller on the Patagonian glacier than those in algal communities on Alaskan and Himalayan glaciers. Lower nutrient levels in precipitation are likely to cause the smaller algal biomass on the glacier.
Introduction
Glaciers have been reported to be habitats for diverse organisms. The glacial organisms are cold-tolerant special species that form simple and relatively closed ecosystems (e.g., CitationKohshima, 1994, Citation1987; CitationHoham and Duval, 2001). Snow and ice algae usually play a role as primary producers in the ecosystems. They sustain heterotrophic communities such as insects, ice worms, yeast, and bacteria (e.g., CitationGoodman, 1971; CitationKohshima, 1984; CitationKikuchi, 1994; CitationAitchison, 2001; CitationHoham and Duval, 2001). The community of these organisms has particular characteristics associated with the physical and chemical condition of the glaciers. For example, a community of the snow algae on glaciers usually consists of several species and has a unique structure and distribution on the glaciers. The algal biomass and community structure changes with altitude due to a drastic change of the surface condition from ablation area (ice) to accumulation area (snow) (e.g., CitationTakeuchi, 2001; CitationYoshimura et al., 1997). However, information on the glacial ecology is still limited. Especially little quantitative study of the communities has been done.
Ecology of snow algae is important in terms of the effect on surface albedo and ice core studies on glaciers. Blooms of snow algae can reduce the surface albedo of snow and ice and significantly affect their melting (CitationKohshima et al., 1993; CitationThomas and Duval, 1995; CitationHoham and Duval, 2001; CitationTakeuchi et al., 2001a). For example, some glaciers in the Himalayas are covered with a large amount of dark-colored biogenic material (cryoconite) derived from snow algae and bacteria (CitationKohshima et al., 1993; CitationTakeuchi et al., 2001a). The surface albedo of these glaciers is significantly reduced by the cryoconite. Consequently, the melting rates of the surfaces were 3 times greater than that of surfaces without cryoconite on the Himalayan glaciers. Thus, the snow algal activity can affect glacial fluctuation. Ice cores, which provide well-preserved proxies of the paleoenvironment, are usually analyzed by physical and chemical methods. The snow algae can be used in ice core analyses as indicators of the paleoenvironment (CitationYoshimura et al., 1997, Citation2000) because their biomass and community structure in a layer of the ice core may reflect the environment at the time when the algae grew on the surface of the glacier. Thus, it is important to understand the ecology of snow algae in studies of glacial variations and ice cores. However, ecological information on snow algae is available only on a limited number of glaciers.
The Patagonia Icefield is located in the southern part of South America. It covers an area of 13,000 km2 and after the Antarctic Ice Sheet is the largest icefield in the Southern Hemisphere. A diverse animal community has been reported on Patagonian glaciers (e.g., CitationKohshima, 1985). Stoneflies and collembola (snow fleas, springtails) can be commonly observed on the surfaces, and snow algae may be an important food source for the animal community. However, little information about snow algae is available for Patagonian glaciers. During our research expedition in November and December 1999, we drilled an ice core in an accumulation area of the icefield in order to estimate the annual accumulation rate and to reconstruct the paleoenvironment (CitationShiraiwa et al., 2002). The snow algal community in the ice core was expected to be a useful indicator of dating and paleoenvironment reconstruction. Thus, ecological information about the snow algae was necessary to interpret the information in the ice core.
In this paper we aim to describe a snow algal community on a Patagonian glacier, Tyndall Glacier in the Southern Patagonia Icefield, Chile. The spatial distribution of the snow algal community was qualitatively analyzed and is discussed along with environmental conditions on the glacier. The results are compared with those from studies of Alaskan and Himalayan glaciers, which are the only glaciers upon which algal communities have ever been quantified (CitationYoshimura et al., 1997; CitationTakeuchi, 2001). The differences are discussed in terms of geographical differences among the regions.
Study Site and Methods
Tyndall Glacier is an outlet glacier of the Southern Patagonia Icefield (). The glacier is located in Torres del Paine National Park, Chile, and flows southward from the icefield (top approximately 1700 m a.s.l.) down to the terminus in a proglacial lake at an elevation of about 50 m a.s.l. The length and area of the glacier are approximately 40 km and 355 km2, respectively. Tyndall Glacier has been glaciologically studied recently and shows a significant retreat in the last 50 yr (e.g., CitationAniya, 1997). Sampling of the algae was carried out at the 10 sites in the area between 370 and 1500 m a.s.l. (S1–S10, ). The surface condition of the sites was ice at sites S1–S6 and snow at sites S7–S10. pH and electrical conductivity on the glacial ice have been reported as 5.26–6.38 (mean 5.92) and 1.66–5.37 (mean 2.49) μS cm−2, respectively (CitationSatow, 1995).
Surface ice and snow including algae were collected with a stainless-steel scoop at depths of 1–2 cm. The surface of the collected area was measured to calculate the algal volume biomass per unit area. For statistical analysis, 5 samples were collected at each site. At sites S8, S9, and S10, only 1 sample was collected and the collected area was not measured due to logistical constraints. The collected samples were melted and preserved as a 3% formalin solution in clean 100-mL polyethylene bottles. These formalin samples were used for cell counting. Although the fine structure of the algae might be lost in formalin, the algae could still be counted with a microscope. For identification of algal species, other samples were collected and preserved with Lugol's solution. All samples were transported to the biological laboratory of Tokyo Institute of Technology in Japan for analysis.
The algal biomass of each site was represented by the cell number per unit water volume and algal volume per unit area. Cell counts and estimations of cell volume were conducted with an optical microscope (Nikon E600). The samples were stained with 0.5% erythrosine (0.1 mL was added to 3 mL of the sample) and ultrasonicated for 5 min to loosen sedimented particles. We filtered 50–1000 μL of the sample water through a hydrophilized PTFE membrane filter (pore size 0.5 μm, Millipore FHLC01300), which became transparent with water, and counted the number of algae on the filter (1–3 lines on the filter). The counting was conducted 3–6 times on each sample. From the mean results and the filtered volume, we obtained the cell concentration (cells mL−1) of the sample. Mean cell volume was estimated by measuring the size of 50–100 cells for each species. The total algal biomass was estimated by multiplying algal concentrations by mean cell volume for each species at each site. To obtain spatial biomass at each site, the total biomass was represent as cell volume per square meter of glacial surface (μL m−2). Community structure was represented by the mean proportion of each species in 5 samples to the total algal volume at each sample.
Species diversity was calculated using Simpson's diversity index D (CitationBegon et al., 1990):
where S is the total number of species in the community, and Pi is the proportion of the ith species to the total algal biomass.
Results
Seven species of snow algae (Chlorophyta and cyanobacteria) were observed on the glacier surface (). The descriptions of the seven species are as follows:
Chlorophyta (green algae): Mesotaenium berggrenii (Wittrock) Lagerheim (): cells single or paired, cylindrical with rounded apices; 1 to 2 chloroplasts, with 1 pyrenoid; 12.1 ± 3.0 μm (mean ± SD) in length, 8.7 ± 1.0 μm in width; cell sap dark brownish.
Cylindrocystis brébissonii (Ralfs) De Bary f. cryophila Kol (): Cells cylindrical with rounded apices; axial chloroplast usually 2, with 1 pyrenoid; cells 24.9 ± 5.7 μm in length, 14.4 ± 1.5 μm in width.
Ancylonema sp. (): Filaments straight or slightly curved, consisting of 2–8 cells; cells 51.7 ± 7.9 μm in length, 10.5 ± 1.0 μm in width; chloroplast with 1–4 pyrenoids; cell sap usually dark brownish. The alga closely resembled A. nordenskioldii, a common species on glaciers in the Northern Hemisphere (e.g., CitationYoshimura et al., 1997; CitationTakeuchi et al., 2001a; CitationTakeuchi, 2001), but the cell length is significantly longer.
Closterium sp. (): Cells solitary, 2 after cell division, straight or slightly curved, with tapered apices; chloroplast with 1–3 pyrenoids; 59.9 ± 5.4 μm in length, 12.9 ± 1.0 in width.
Chloromonas sp. (): Cells round, red-orange pigmented; a chloroplast without pyrenoid; round cells 11.7 ± 1.7 μm (mean ± SD) in diameter; smooth outer envelopes 1–2 μm in thickness. Most of the cells were the round-shaped resting cells. The algae caused visible red blooms on patchy remaining snow around site S3.
Cyanobacteria (blue-green algae): Oscillatriaceae cyanobacterium (): trichomes 1.46 ± 0.14 μm in width, 2.91 ± 0.36 μm in length; cell length about 2 times longer than the width.
Unknown alga (): Cells spherical, 6.10 ± 0.9 μm (mean ± SD) in diameter with thick outer envelopes.
Each species showed a different pattern of spatial distribution (). M. berggrenii, Ancylonema sp., and Closterium sp. were observed on the ice area (sites S1–S6) and appeared in greatest numbers at the lowest-elevation site (site S1, 340 m a.s.l.). Small amounts of M. berggrenii were also observed on the snow surface (sites S7, S8). Cyl. brébissonii was observed on the ice area, with the greatest number at site S3 (640 m a.s.l.). Oscillatoriaceae cyanobacterium appeared at 1 site on the ice (site S2) and at 3 sites on the snow (sites S7, S8, S9). Chloromonas sp. was observed on middle-elevation sites, including both ice and snow surfaces (sites S3, S6, S7, S8). Its highest cell concentration occurred at site S8. The unknown alga appeared on the higher-elevation sites (sites S6–S10) and in the greatest number at the highest site (S10). Few algae were observed at site S5 (ice, 900 m a.s.l.), which is located near snowline and the right bank of the glacier.
The algal biomass generally decreased with altitude (). The mean total cell concentration and total cell volume biomass ranged from 0 to 9.2 × 104 cells mL−1, and from 0 to 327 μL m−2, respectively. The lack of data for S8–S10 is due to sampling procedure (the sampled area was not measured). The largest biomass was 202 μL m−2 at the lowest-elevation site (S1). The biomass gradually decreased with altitude up to the highest site on the ice area (S6), except site S5, where few algae were observed. The biomass at the site on the snow surface (S7) was significantly smaller than that at any site on the ice surface except site S5.
The community structure showed that M. berggrenii was dominant in the ice area and lower snow area and that the unknown alga was dominant at the highest 2 snow sites (100% of total biomass, ). The percentage of M. berggrenii dominance at each site ranged from 65 to 100% of total biomass in the ice area and from 0 to 70% in the snow area. The percentages did not show clear altitudinal trends. Cyl. brébissonii was the secondary dominant species in the ice area. Its percentage ranged from 0 to 32. Chloromonas sp. was little dominant in the ice area and appeared at the lower snow area (sites S7 and S8). Its percentage reached 22% at site S8. The other species, Closterium sp., Ancylonema sp., and Oscillatriaceae cyanobacterium, were minor algae in the community of the glacier in terms of cell volume biomass (less than 5%).
The species diversity index varied among the study sites but was generally small at all of the sites (<1.9, ). The diversity index ranged from 1.00 to 1.89 in the ice area and was 1.86 in the snow area. The relatively higher index occurred at the middle of the ice area (site S3) and lower snow area (sites S7 and S8). The index did not show a clear altitudinal trend.
Discussion
Our results showed an abundant algal community on this Patagonian glacier. During the field investigation, insect communities were also observed on this glacier, as reported by CitationKohshima (1985). Predatory insects, stoneflies (Plecoptera), were walking on the ice surface and staying at the bottom of cryoconite holes. Collembola (snow fleas, springtails) were walking and jumping on the ice surface and on the water surface of cryoconite holes. At least 3 species of collembola were observed on this glacier. One was semiaquatic; these animals stayed at the bottom of cryoconite holes. This semiaquatic insect may be eaten by the stoneflies, which also often stayed at the bottom of cryoconite holes. Rotifers were also observed in the collected ice samples. Microscopy revealed abundant bacteria in the glacial ice samples, which may decompose the dead bodies of animals and algae on the glacier. These heterotrophic organisms are likely sustained by the snow algal primary production. Food webs consisting of snow algae, animals, and bacteria have been reported in various glaciers of the world. CitationKohshima (1987) reported cold-tolerant insects (midges) and copepods living on a Himalayan glacier by feeding on the algae and bacteria. Ice worms and collembola are common to certain glaciers in Alaska and on the west coast of North America (e.g., CitationShain et al., 2001). Stoneflies have been reported only on Patagonian glaciers at present. The findings suggest the existence of an ecosystem based on the primary production of the snow algae on this Patagonian glacier, as on the glaciers in the Himalayas, Alaska, and other parts of North America.
The algal species observed on this glacier do not coincide with the species that have been reported in snow- and icefields in the Southern Hemisphere. Taxonomical studies for snow algae in the Southern Hemisphere have been carried out mainly in Antarctica and New Zealand (e.g., CitationLing, 1996; CitationMataloni and Tesolín, 1997; CitationThomas and Broady, 1997). M. berggrenii has been reported in the Windmill Island region in Antarctica (CitationLing and Seppelt, 1990). In the Antarctic Peninsula, which is the antarctic location closest to Patagonia, more than 60 species have been reported on the snow and ice surfaces (CitationMataloni and Tesolín, 1997). However, only 2 of the 7 genera reported here have been included in the algae list of the Antarctic Peninsula (Chloromonas sp. and Oscillatoriaceae alga). Cylindrocystis, Ancylonema, and Closterium have been reported nowhere else in the Southern Hemisphere at present. These may be locally distributed species within the Patagonia region.
M. berggrenii reported in Windmill Island, Antarctica, corresponds well with that on this Patagonian glacier in terms of cell size, pigmentation, and life stages. The cell size in the Windmill Island has been reported as 7.3–10 μm wide and 13–22 μm long, whereas that on the Patagonian glacier was 8.7 ± 1.0 μm wide and 12.1 ± 3.0 μm long. The pigmentation of the algal cells was reddish-brown in both regions. The life stages in Windmill Island were mainly vegetative and dividing cells and were the same on this Patagonian glacier. However, habitat conditions and population size differ between the two regions. Electrical conductivity is higher in the Windmill Island samples than in the Patagonian (6–33 versus 1.66–5.37 μS cm−1). The pH is lower in the Windmill Island samples than in the Patagonian (4.5–5.7 versus 5.26–6.38). The population size in Windmill Island has been reported as up to 100,000 cells mL−1, much larger than that in Patagonia (12,000 cells mL−1, mean). The smaller population size in Patagonia may be due to lower nutrients, also suggested by the lower electrical conductivity.
According to four categories for snow algae on the basis of their habitats suggested by CitationYoshimura et al. (1997), the species on this glacier can be classified as follows: M. berggrenii, Cyl. brébissonii, Ancylonema sp., and Closterium sp. are ice-environment specialists; unknown alga is a snow-environment specialist; and Chloromonas sp. and Oscillatoriaceae cyanobacterium are opportunists. This spatial distribution may reflect preferable growth conditions for each species. The classification of the species mostly agrees with previous studies. M. berggrenii and Cyl. brébissonii have been reported as ice-environment specialists in Alaska (CitationKol, 1942; CitationTakeuchi, 2001). Ancylonema nordenskioldii, a similar alga to Ancylonema sp. of the Patagonian glacier, has also been reported as an ice-environment specialist (CitationKol, 1942; CitationTakeuchi, 2001). Chloromonas algae have been widely reported from snowfields in both the Northern and the Southern Hemisphere (e.g., CitationHoham et al., 1979; CitationLing, 1996). One of the algae, Chloromonas sp. in a Himalayan glacier, was categorized as an opportunist. Oscillatoriaceae cyanobacteria have been reported as opportunists in Alaska. A Closterium alga (Closterium exile) has been noted on an Alaskan glacier as a generalist (thriving both on snow and ice: Kol, 1942); this observation does not agree with the alga on the Patagonian glacier, probably due to a species difference.
The decrease of algal biomass with altitude is probably due to light conditions in the algal habitat. This altitudinal trend is the same as that of the algal community on a Himalayan glacier (CitationTakeuchi et al., 1998; CitationYoshimura et al. 1997). On the Himalayan glacier, the biomass decreased continuously with altitude, which was explained by an increase of snow-cover frequency with altitude reducing the light intensity of the algal habitats (CitationRichardson and Salisbury, 1977). Also, winter snow cover on the ice surface disappears from lower elevations to higher; thus, the algal habitat at the ice surface can be exposed to sun for a longer time in the lower-elevation area. The biomass distribution on the Patagonian glacier is likely due to the same reason as that in the Himalayas. However, the algal community on an Alaskan glacier showed an opposite trend: the biomass increased with altitude in the ice area (CitationTakeuchi, 2001). This trend was explained by the amount of running meltwater on the glacier surface, which can wash the snow algae out of the glacier. Since the amount of meltwater is larger at lower altitude, the algal biomass would decrease as altitude decreases. On the Patagonian glacier, the running meltwater on the surface seemed to have less effecte on the algal biomass distribution. Because this glacier flows for a long distance along a flat valley and its surface slope is much gentler than that of the Alaskan glacier, the running water flows more slowly on the surface. Therefore, on the Patagonian glacier, the effect of meltwater on the algae would be less effective than changes in light conditions. The low number of algae at site S5 may be due to frequent avalanches from a nunatak at the west side of the glacier.
Community structure in the ice area differs from that on Alaskan and Himalayan glaciers (CitationTakeuchi, 2001; CitationYoshimura et al., 1997), although the major algal genera are similar on the three glaciers. Cylindrocystis, Ancylonema, and Mesotaenium all appeared on the ice area of the glaciers in Patagonia, the Himalayas, and Alaska. However, the dominant genus is different. Mesotaenium was dominant on the Patagonian glacier, while Cylindrocystis was dominant on the Himalayan glacier and Ancylonema on the Alaskan glacier. This difference probably results from environmental factors affecting the species competition. However, such factors are uncertain. Cylindrocystis and Ancylonema seem to be related to latitude, which may affect the radiation conditions for algal habitats. The latitudes are 28°14′N for the Himalayan glacier (the lowest of the three), 51°15′S for the Patagonian, and 63°15′N for the Alaskan. Cylindrocystis may become dominant on glaciers at lower latitudes, and Ancylonema may become dominant on glaciers at higher latitudes. M. berggrenii was dominant in the ice area on the Patagonian glacier, and was the secondary dominant species on the Himalayan and Alaskan glaciers. It was dominant in the area near the snow line on the Himalayan glacier. These observations suggest that this species is a generalist, which is able to grow in various environmental conditions.
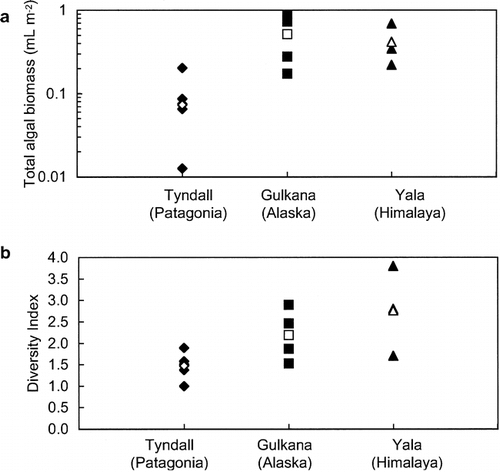
The cell volume biomass and diversity index are relatively smaller on the Patagonian glacier compared to these on the Alaskan and Himalayan glaciers (CitationTakeuchi, 2001; CitationYoshimura et al., 1997). shows a comparison of snow algal biomass and diversity index on the ice surface among the three glaciers. Both the biomass and the diversity index are significantly different among the glaciers (ANOVA one-way analysis, F = 5.35, P = 0.026 < 0.05 for the biomass; F = 4.78, P = 0.035 < 0.05 for the diversity index). The mean algal biomasses on the Alaskan and Himalayan glaciers were 0.52 and 0.42 mL m−2, respectively, approximately 7 and 6 times larger than that on the Patagonian glacier (0.073 mL m−2). The smaller biomass suggests less algal activity on the Patagonian glacier. The mean diversity indexes for the Alaskan and Himalayan glaciers were 2.19 and 2.77, respectively, larger than that for the Patagonian glacier (1.47). According to CitationYoshimura et al. (1997), the diversity index for glacial algae is associated with the stability of the snow cover. The snow cover could be a great disturbance for algal growth and could maintain a high diversity of the algal community. Hence, the smaller diversity index suggests a more stable environment for the algal growth on the Patagonian glacier.
A lower nutrient level may be the most likely cause of the smaller biomass on the Patagonian glacier. shows a comparison of chemical conditions related to algal growth between the Patagonian, Alaskan, and Himalayan glaciers. Although pH and electrical conductivity do not show clear differences among the glaciers, nitrate concentration, an important nutrient for snow algae (e.g., CitationJones, 1991), is remarkably lower on the Patagonian glacier than on the Alaskan and Himalayan glaciers. CitationAristarain and Delmas (1993) noted low chemical solutes levels in snow, especially nitrate (0.32 μ eq L−1) on Moreno Glacier of the Southern Patagonia Icefield. CitationSatow (1995) also reported low nitrate concentration in precipitation on Tyndall Glacier (0.44 μ eq L−1, mean). The nitrate concentrations of the ice-core samples drilled at the accumulation area of Tyndall Glacier in 1999 were all below the detection level of the ion chromatograph used (CitationShiraiwa et al., 2002). In contrast, nitrate concentration in precipitation in Alaska was 2.0 μ eq L−1 for snow (1999–2000 mean, National Atmospheric Deposition Program) and 2.1 μ eq L−1 in the Himalayas (Yala Glacier) (CitationWatanabe et al., 1984). These levels are larger than those of the Patagonian glacier. According to CitationHoham et al. (1989), nitrate depletion induces a shift in the life cycle of snow algae from resting stage to sexual reproduction. The dominant alga, M. berggrenii, was mostly observed as vegetative or dividing cells, which is consistent with that observation. CitationLing and Seppelt (1990) suggested that M. berggrenii adapts to low nutrient status, which may be the reason for the dominance of M. berggrenii on this glacier. CitationAristarain and Delmas (1993) suggested that the low solute level of the Patagonian glacier is due to its remote location, far from large populated, industrial, or agricultural centers. The large annual snow accumulation on the glacier, which is a characteristic of the Patagonian Icefield, may also be a possible cause for the low algal biomass. A frequent and large snow cover may hamper algal photosynthesis on the glacier surface. Although latitudinal location may also affect the algal production in terms of intensity of solar radiation, the latitude cannot explain the biomass difference among the glaciers because the Alaskan glacier has a larger algal biomass. Therefore, the low concentration of nutrients in precipitation is most likely to limit the algal growth on the Patagonian glacier.
The smaller algal biomass on the Patagonian glacier may result in a high albedo of the glacier surface. Surface albedo of glaciers is one of the important factors affecting glacier heat budget and mass balance. The surface albedo is affected by impurities on the glacier surface (e.g., CitationWarren and Wiscombe, 1980; CitationWarren, 1982). Biogenic impurities, called cryoconite, derived from algal production can reduce the surface albedo and then accelerate melting of the glacial surface. This effect may be especially significant on the Himalayan glaciers (mean albedo 0.05, CitationTakeuchi et al., 2001a; CitationKohshima et al., 1993). In contrast with the Himalayan glaciers, the surface albedo of this Patagonian glacier has been reported as generally high (0.45 mean in the ice area), almost equivalent to albedo of a clean, bare ice surface (CitationTakeuchi et al., 2001b). This high surface albedo is due to a small amount of cryoconite (38 g m−2 mean, CitationTakeuchi et al., 2001b). The organic matter content in the cryoconite (decrease in cryoconite weight upon ashing) is also smaller on the Patagonian glacier (0.6–2.7%, CitationTakeuchi et al., 2001b) as compared to the Himalayan (5.4–9.9%, CitationTakeuchi et al., 2001a), suggesting smaller algal production on the Patagonian glacier. Thus, the small amount of cryoconite on the Patagonian glacier may be due to small algal activity on the glacier surface.
FIGURE 1. Location of Tyndall Glacier in the Southern Patagonia Icefield (SPI) and map of the glacier showing the sampling sites (S1–S10)
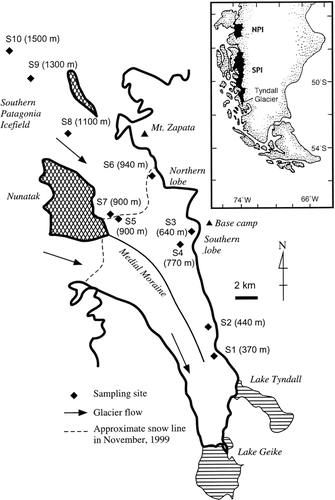
FIGURE 2. Pictures of the snow algae observed on Tyndall Glacier: (a) Mesotaenium berggrenii, (b) unknown algae, (c) Chloromonas sp., (d) Ancylonema sp., (e) Cylindrocystis brébissonii, (f) Oscillatoriaceae cyanobacterium, (g) Closterium sp. All pictures were taken with a normal optical microscope. All samples were fixed with Lugol's solution before being photographed
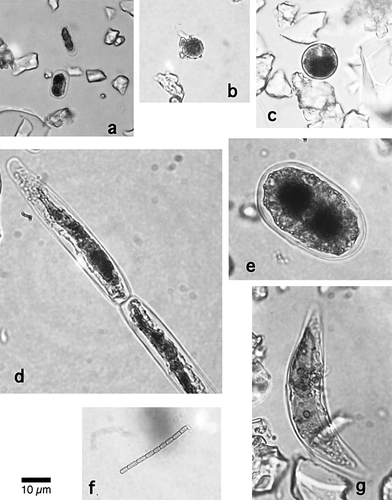
FIGURE 3. Variation of the cell number concentration (cells mL−1) of each snow alga among the collection sites on Tyndall Glacier. The numbers in brackets show the altitude of each site (m a.s.l)
FIGURE 4. Variation of the total cell volume biomass among the collection sites on Tyndall Glacier (1200–1800 m). Error bar = standard deviation. The numbers in brackets show the altitude of each site (m a.s.l.). The data of S5 is out of range (0.000014). The lack of data for S8–S10 is due to sampling procedure (sampled area was not measured)
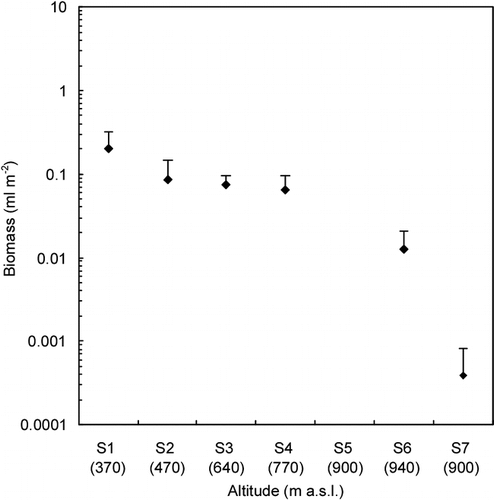
FIGURE 5. Variation of the community structure of snow algae among the collection sites on Tyndall Glacier (proportion of cell volume biomass). Error bar = standard deviation. The numbers in brackets show the altitude of each site (m a.s.l.). The lack of error bar for S8–S10 is due to sampling number (only 1 sample was available)
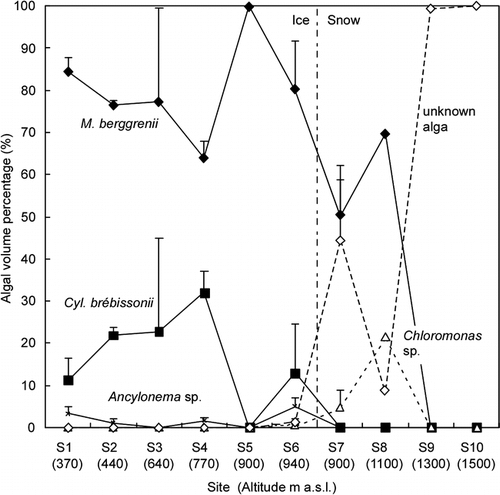
FIGURE 6. Variation of Simpson's diversity index of the snow algal community among the collection sites on Tyndall Glaicer. Error bar = standard deviation. The numbers in brackets show the altitude of each site (m a.s.l.). The lack of error bar for S8–S10 is due to sampling number (only 1 sample was available)
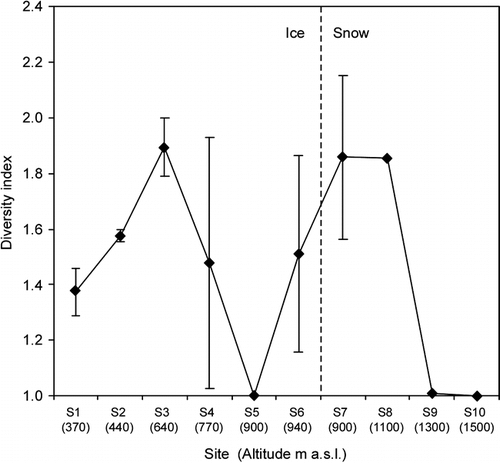
Table 1 Comparison of chemical conditions related to algal growth between Patagonian, Alaskan, and Himalayan glaciers. (EC = electrical conductivity)
Acknowledgments
We thank the staff of the Headquarters of Torres del Paine National Park; Dr. Gino Casassa; and Mr. Franco, Universidad de Magallanes, for supporting our fieldwork on Tyndall Glacier. We also thank Dr. T. Shiraiwa and Mr. K. Kubota for cooperation in the fieldwork on the glacier, Drs. A. Tsuji and M. Nakanishi for suggestions for algal species identification, and Dr. K. Sato for ion solutes data for Tyndall Glacier. We also thank W. H. Thomas and an anonymous reviewer for helpful suggestions on the manuscript. This study was supported by Grants-in-Aid for Scientific Research (No. 10041105, 11640422 for fieldwork, No. 13480154 for laboratory analysis) from the Ministry of Education, Science, Sports, and Culture, Japanese government.
References Cited
- Aitchison, C. W. 2001. The effect of snow cover on small animals. In Jones, H. G., Pomeroy, J. W., Walker, D. A., and Hoham, R. W. (eds.), Snow Ecology. Cambridge: Cambridge University Press, 229–265.
- Aniya, M. , H. Sato , R. Naruse , P. Skvarca , and G. Casassa . 1997. Recent glacier variations in the Southern Patagonia Icefield, South America. Arctic and Alpine Research 29:1–12.
- Aristarain, A. J. and R. J. Delmas . 1993. Firn-core study from the southern Patagonia ice cap, South America. Journal of Glaciology 39:249–254.
- Begon, M. , J. L. Harper , and C. R. Townsend . 1990. The nature of the community. In Begon, M., Harper, J. L., and Townsend, C. R (eds.), Ecology. Oxford: Blackwell Scientific Publications, 613–647.
- Goodman, D. 1971. Ecological investigations of ice worms on Casement Glacier, Southeastern Alaska. The Ohio State University Research Foundation, Institute of Polar Studies Report No. 39: 59.
- Hoham, R. W. and B. Duval . 2001. Microbial ecology of snow and freshwater ice. In Jones, H. G., Pomeroy, J. W., Walker, D. A., and Hoham, R. W. (eds.), Snow Ecology. Cambridge: Cambridge University Press, 168–228.
- Hoham, R. W. , S. C. Roemer , and J. E. Mullet . 1979. The life history and ecology of the snow algae Chloromonas brevispina comb. Nov. (Chlorophyta, Volvocales). Phycologia 18:55–70.
- Hoham, R. W. , C. P. Yatsko , L. Germain , and H. G. Jones . 1989. Recent discoveries of snow algae in upstate New York and Québec Province and preliminary reports on related snow chemistry. Proceedings 46th Annual Eastern Snow Conference: 196–200.
- Jones, H. G. 1991. Snow chemistry and biological activity: a particular perspective on nutrient cycling. In Davis, T. D., Tranter, M., and Jones, H. G. (eds.), NATO ASI series G: Ecol. Sci., Vol. 28, Seasonal Snowpacks, Processes of Compositional Change. Berlin: Springer-Verlag, 173–228.
- Kikuchi, Y. 1994. Glaciella , a new genus of freshwater Canthocampyidae (Copepoda Harpacticoida) from a glacier in Nepal, Himalayas. Hydrobiologia 192–193:59–66.
- Kohshima, S. 1984. A novel cold-tolerant insect found in a Himalayan glacier. Nature 310:225–227.
- Kohshima, S. 1985. Patagonian glaciers as insect habitats. In Nakajima, K. (ed.), Glaciological studies in Patagonia Northern Iccefield 1982–1984. Kyoto: Data Center for Glacier Research, Japanese Society of Snow and Ice, 94–99.
- Kohshima, S. 1987. Glacial biology and biotic communities. In Kawano, S., Connell, J. H., and Hidaka, T. (eds.), Evolution and Coadaptation in Biotic Communities. Kyoto: Faculty of Science, Kyoto University, 77–92.
- Kohshima, S. 1994. Ecological characteristics of the glacier ecosystem. Japanese Journal of Ecology 44:93–98. (In Japanese).
- Kohshima, S. , K. Seko , and Y. Yoshimura . 1993. Biotic acceleration of glacier melting in Yala Glacier, Langtang region, Nepal Himalaya. Snow and Glacier Hydrology (Proceeding of the Kathumandu Symposium, November 1992), IAHS Publication 218: 309–316.
- Kol, E. 1942. The snow and ice algae of Alaska. Smithsonian Miscellaneous Collections 101:1–36.
- Ling, H. U. 1996. Snow algae of the Windmill Island region, Antarctica. Hydrobiologia 336:99–106.
- Ling, H. U. and R. D. Seppelt . 1990. Snow algae of the Windmill Islands, continental Antarctica: Mesotaenium berggreniii (Zygnematales, Chlorophyta), the alga of grey snow. Antarctic Science 2:143–148.
- Mataloni, G. and G. Tesolín . 1997. A preliminary survey of cryobiontic algal communities from Cierva Point (Antarctic Peninsula). Antarctic Science 9:250–258.
- Richardson, S. G. and F. B. Salisbury . 1977. Plant responses to the light penetrating snow. Ecology 58:1152–1158.
- Satow, K. 1995. Chemical features of the precipitation in summer at Tyndall Glacier, Patagonia. Bulletin of Glaciological Research 13:57–61.
- Shain, D. , T. Mason , A. Farrell , and L. Michalewicz . 2001. Distribution and behavior of ice worms ( Mesenchytraeus solifugus ) in south-central Alaska. Canadian Journal of Zoology 79:1813–1821.
- Shiraiwa, S. , S. Kohshima , R. Uemura , N. Yoshida , S. Matoba , J. Uetake , and M. A. Godoi . 2002. High net accumulation rates at the Southern Patagonia Icefield revealed by analysis of a 45.97-m-long ice core. Annals of Glaciology,. 35:85–90.
- Takeuchi, N. 2001. The altitudinal distribution of snow algae on an Alaska glacier (Gulkana Glacier in the Alaska Range). Hydrological Processes 15:3447–3459.
- Takeuchi, N. , S. Kohshima , and K. Fujita . 1998. Snow algae community on a Himalayan glacier, Glacier AX010 East Nepal: relationship with glacier summer mass balance. Bulletin of Glacier Research 16:43–50.
- Takeuchi, N. , S. Kohshima , and K. Seko . 2001a. Structure, formation, and darkening process of albedo reducing material (cryoconite) on a Himalayan glacier: a granular algal mat growing on the glacier. Arctic, Antarctic, and Alpine Research 33:115–122.
- Takeuchi, N. , S. Kohshima , T. Shiraiwa , and K. Kubota . 2001b. Characteristics of cryoconite (surface dust on glaciers) and surface albedo of a Patagonian glacier, Tyndall Glacier, Southern Patagonia Icefield,. Bulletin of Glaciological Research 18:65–69.
- Thomas, W. H. and B. Duval . 1995. Sierra Nevada, California, U.S.A., snow algae: snow albedo changes, algal-bacterial interrelationships, and ultraviolet radiation effects. Arctic and Alpine Research 27:389–399.
- Thomas, W. H. and P. A. Broady . 1997. Distribution of coloured snow and associated algal genera in New Zealand. New Zealand Journal of Botany 35:113–117.
- Warren, S. G. 1982. Optical properties of snow. Review of Geophysics and Space Physics 20:67–89.
- Warren, S. G. and W. J. Wiscombe . 1980. A model for the spectral albedo of snow. II. Snow containing atmospheric aerosols. Journal of Atomspheric Science 37:2734–2745.
- Watanabe, O. , S. Takenaka , H. Iida , K. Kamiyama , K. B. Thapa , and D. D. Mulmi . 1984. First results from Himalayan Glacier Boring Project in 1981–1982. Bulletin of Glaciological Research 2:7–23.
- Yoshimura, Y. , S. Kohshima , and S. Ohtani . 1997. A community of snow algae on a Himalayan glacier: change of algal biomass and community structure with altitude. Arctic and Alpine Research 29:126–137.
- Yoshimura, Y. , S. Kohshima , N. Takeuchi , K. Seko , and K. Fujita . 2000. Himalayan ice-core dating with snow algae. Journal of Glaciology 46:335–340.