Abstract
Arctic foxes are evaluated as seed dispersal vectors for Greenlandic plant species through a feeding experiment with subsequent scat analysis and germination test. Seeds of 22 common species with different morphology were tested. Passage time ranged between 4 and 48 h. No significant differences were detected in passage time for seeds with different morphology. Cerastium alpinum and Stellaria longipes had higher germination after passage through the fox’s digestive tract when compared to controls. Sibbaldia procumbens, Oxyria digyna, and Silene acaulis were favored by passage when shorter than 10 h. Salix glauca ssp. callicarpaea, Veronica alpina, Gnaphalium norvegicum, Papaver radicatum, Ranunculus hyperboreus, Chamaenerion latifolium, Luzula parviflora, and bulbils of Polygonum viviparum and Saxifraga cernua were inhibited by passage, whereas the remaining species had germination percentage too low to allow for evaluation. Species with adaptations to wind dispersal seemed particularly vulnerable to gut passage. Arctic foxes are able to provide long-distance dispersal of seeds lacking morphological adaptations to dispersal, but for most species seeds need to be defecated within 12 h to remain viable.
Introduction
During the early Holocene there was a rapid migration of plants and animals toward the newly deglaciated regions in the north. Seed dispersal must have been very efficient and long ranging, yet the rapid migration is poorly explained by our present knowledge of dispersal. CitationClark et al. (1998) argued that occasional long-distance seed dispersal events must have been very important in recolonization after glaciation.
Similarly, most plant species in Greenland have spread thousands of kilometers during the first couple of millennia of the Holocene to reach their current distribution, and in steps to hundreds of kilometers to pass over the sea (CitationIversen, 1954; CitationFredskild, 1973, Citation1996; CitationFunder, 1989; CitationBennike, 1999). Seeds of most arctic plant species are small and often have adaptations for wind dispersal, and CitationPorsild (1910) suggested that most species were dispersed over long distances by wind. However, wind dispersal over long distances may cause enormous losses of seeds, particularly where dispersal routes cross great distances of water or ice. CitationWarming (1887), CitationIversen (1954), and CitationBennike (1999) suggested that chance dispersal by birds is important, but other animals have not been considered, and so far no experiments have been conducted to identify seed dispersal vectors in Greenland.
CitationClark et al. (1998) described frugivorous mammals, such as foxes and bears, as underappreciated agents of long-distance seed dispersal. Canids have been shown to act as seed dispersal agents of native plant species in many ecosystems, e.g. tropical savanna grasslands (CitationMotta-Junior et al., 1994; CitationMotta-Junior and Martins, 2002), mediterranean shrublands (CitationBustamante et al., 1992; CitationCastro et al., 1994; CitationLeon Lobos and Kalin Arroyo, 1994; CitationAronne and Russo, 1997; CitationWilson and Thomas, 1999), temperate grasslands (CitationJaeger, 1950; CitationCypher and Cypher, 1999), and boreal forest and tundra (CitationWillson, 1993), as well as of invasive species (CitationBrunner et al., 1976; CitationMeek, 1998). Almost all of these studies have focused on frugivory, and seed dispersal of fleshy-fruited species by carnivores has been an area of focus recently (CitationTraveset, 1998; CitationTraveset and Verdú, 2002).
The arctic fox (Alopex lagopus) is known to consume fleshy fruits (CitationBirks and Penford, 1990; CitationNielsen, 1994; CitationFrafjord, 1995; CitationKapel, 1999). Only a few arctic plant species develop fleshy fruits, but other seed types may be consumed unintentionally with prey items handled on the ground or buried in caches, because soil would be ingested in these processes. Alternatively, they may originate from the guts of prey items, namely indirect or secondary seed dispersal, a phenomenon shown for carnivorous birds (CitationDarwin, 1859; CitationDean and Milton, 1988; CitationNogales et al., 1998, Citation2002) and feral cats (CitationNogales et al., 1996). Berries and vegetative plant parts, as well as soil, have been frequently reported from arctic fox scats and stomach samples (e.g., CitationGarrott et al., 1983; CitationPrestrud, 1992; CitationHersteinsson and Macdonald, 1996; CitationKapel, 1999; CitationElmhagen et al., 2000), and though we do not have data on the actual seed and bulbil content in fox scats yet, it is very likely that seeds and bulbils (henceforth both called seeds) are unintentionally eaten by foxes from time to time and that foxes can provide rare but long-distance seed dispersal events for species without morphological adaptations to endozoochory.
The summer home range of arctic foxes is in the magnitude of 20 to 60 km2, but varies between locations and years (CitationAngerbjörn et al., 1997; CitationZakrzewski et al., 1999). Arctic foxes have been recorded to occasionally move between 800 and 2000 km during the period from October to May (CitationBraestrup, 1941; CitationWrigley and Hatch, 1976; CitationEberhardt et al., 1983; CitationBennike, 1999). This implies minimum mean daily movements of up to 10 km, and potentially much farther.
Depending on the gut passage time, the animal may disperse seeds over long distances to new suitable sites. Arctic foxes' gut retention time seems to be relatively short compared to that of seeds observed in animals of comparable sizes (CitationHickey et al., 1999; CitationWilliams et al., 2000). CitationSzuman and Skrzydlewski (1962) found the defecation patterns of caged arctic foxes to be highly variable. In general, the first defecation after a meal took place after a minimum of 3 to 4 h, but normally after 9 h, and the last defecation took place within 27 h. However, after complete gut evacuation, particles might rest in the appendix. Seeds may therefore be dispersed over at least 10 km if they stay in the gut for 24 h, and even by staying just 9 h within the animal, a fox may often have moved several kilometers.
Passage of seeds through animal digestive tracts may enhance or inhibit germination. In a meta-analysis, CitationTraveset (1998) found that 8 out of 28 studies showed germination to be significantly enhanced, the rest finding no significant effect. CitationTraveset and Willson (1997) reported that most species showed enhancement, many showed no significant effect, and fewer studies observed species to envisage considerable losses in germinability during passage. For canids, studies by CitationCypher and Cypher (1999), CitationMotta-Junior and Martins (2002), and CitationTraveset et al. (2001) have found some plant species to suffer considerable losses during gut passage.
In this study, we experimentally fed arctic foxes with seeds from both fleshy- and nonfleshy-fruited plant species from Greenland to investigate (a) the passage time, once ingested, of seeds through the digestive tract of the fox, and (b) how passage through the fox affects the germination ability of the plant species.
Methods
Diaspores of 21 species and bulbils of 2 species () were collected on Disko, Western Greenland, in August, September, and October 2001. The species were selected for seeds with as much different morphology and size as we could find. However, the species with the smallest seeds, Cassiope tetragona, was excluded from the experiment because of difficulties with handling it. The seeds were dried and stored at room temperature until the feeding experiment, except for berries of Vaccinium uliginosum and Empetrum nigrum ssp. hermaphroditum, which were kept frozen until the experiment. In the feeding experiment, the seeds were given within intact berries, and seed number per berry was calculated by dissecting 20 berries of each species and counting number of seeds within them to calculate an average number per species. For convenience, seeds of the two fleshy-fruited species, bulbils of two species, and diaspores (including achenes and similar seeds) of the remaining 18 species are henceforth all called seeds. Species will be identified by their generic names in the following, except Saxifraga tricuspidata and S. cernua. Nomenclature follows CitationBöcher et al. (1978).
Ten arctic foxes (three males and seven females) kept at the Royal Agricultural University in Copenhagen were included in the experiment. The foxes were held in separate standard farm cages (0.8 × 1.2 × 0.95 m) with the net bottom elevated about 1 m above the ground. A wooden tray beneath each cage collected the scats from each fox separately, while letting the urine run off. Foxes were fed on a dog plate within the cage once a day in the late afternoon. Leftovers from the meal, such as pieces of meat or bones that had fallen through the net bottom, were put back on the plate in the cage after each collection event.
To simulate a natural diet in Greenland (CitationNielsen, 1991), the foxes were fed daily 125 g of poultry (newly hatched but dead chicken) and 125 g of mixed raw fish (cod, Gadus morhua; herring, Culpea harengus; flounder, Platichthys flesus; and whiting, Merlangius merlangius). All parts of the chickens and fish were included. After 72 h on this diet, seeds were mixed into some raw liver and given before a meal. All the liver with seeds was consumed readily, and in general the foxes ate all the food they were given during the experiment. After the meal containing seeds, scats were collected every 4 h during the following 52 h.
The scats were washed through a 250 μm sieve. The resulting samples were analyzed under a dissecting microscope. Seeds were counted and put in Petri dishes lined with moist filter paper for subsequent germination. The procedure involved soaking the seeds in water in the Petri dishes for about 24 h at room temperature, followed by 24 h in a refrigerator. Thereafter, the seeds were kept on wet filter paper at room temperature (18–23°C) in a southeast-facing window for 8 weeks. Three controls were made for each species with seeds that had not passed through the fox guts, but which had otherwise been treated similarly. Number of seeds in the controls varied due to shortage of seeds from some species (see ). Germination in the controls was noted after 2, 3, 4, 5, 6, and 7 weeks.
Germination of the seeds (radicle or shoot emergence) was noted for each species at each sampling time after 3, 5, 7 and 8 weeks until there was no further germination for any species since last recording. After 3 weeks all the seeds infected with fungi were removed from the dishes.
Differences in means in passage time were analyzed with a Z-test. Differences between germination percentage in scats and controls was analyzed by a Chi square test with Yates correction, and correlations between passage-time and germination percentages were calculated with Spearman Rank correlation after excluding passage times with less than 5 seeds (CitationFowler and Cohen 1990).
Results
For most species the percentage of seeds recovered was relatively high (): 11 of the 22 species had percentages of more than 75%, 6 species had percentages between 50% and 75%, and only Gnaphalium and Veronica had percentages lower than 40%. The mean passage time of the seeds varied between 16.2 and 25.5 h for the individual species, but the standard deviation was quite high, indicating a considerable variation in passage time within species. Some foxes passed many seeds after 4 h, whereas others did not produce any scats with seeds until after 20 h. After 44 h there were still seeds of all species in the scats except for Saxifraga cernua, which had its last bulbil recovered in a scat after 40 h. Eighteen seeds of 12 different species were found after 48 h but none after 52 h.
The fleshy-fruited species and the species with appendages had the longest mean passage time, but due to the large intraspecific variation the differences in mean passage time were not statistically different, neither between individual species nor between groups of species with different morphology ().
In , the average germination percentage over time is shown together with the average germination percentage in the three controls (with curves). The bars show number of seeds found at each time (for the seeds that appeared unaffected by fungi after 3 weeks) together with the number of germinated seeds. Only the 8 species with more than 20 germinated seeds each in the scats are included in the figure. Spearman rank correlations between passage time and germination ability is given for each species (passage times with less than 5 seeds are excluded). The chance of germination decreased significantly with passage time for all species except Stellaria, Cerastium, and Luzula, but Luzula was marginally significant. For most species, there was no germination after 24 h. The seeds of Stellaria were either favored or unaffected when passed within 40 h. The curves show how germination percentages for Stellaria and Cerastium are higher than controls at any time of passage (Cerastium control germination = 0%). Oxyria, Sibbaldia, and Silene seem to be favored by passage when passage time was short (<12–16 h), whereas Ranunculus, Luzula, and Polygonum had lower or equivalent chance of germinating after passage, even when passage time was short. Luzula had a peak at 40 h, but this was caused by a single seed, as indicated by the seed and germination columns.
Seeds of 3 species did not germinate at all, neither in the controls nor in the samples recovered from the scats. Five species had small germination percentages (<10%) in both control and after passage. For the remaining 14 species, gives average germination percentages in the 3 controls and the germination percentage for all seeds of each of the species in the scats. Nine species had significantly higher germination in the control than in the treatment. The germination of bulbils of Saxifraga cernua and of seeds of Salix, Veronica, Gnaphalium, Papaver, and Chamaenerion had hardly any germination after passage. Two species had higher germination after passage through fox guts as compared to the controls. Germination percentages of Oxyria, Sibbaldia, and Silene were not significantly different but as shows, this is due to the higher germination percentage if passage time was short, whereas the germination percentage decreased drastically for the slow passing seeds.
Discussion
In the present study we have demonstrated that all the investigated species are able to be carried in fox guts for 44–48 h. This passage time is of intermediate length among mammals of comparable body size. It is longer than that reported for berries fed to Martes americana (4–5 h; CitationHickey et al., 1999), but shorter than that reported for opossums (many days; CitationWilliams et al. 2000). CitationSzuman and Skrydlewski (1962) reported passage times of entire meals to be 27 h in Arctic foxes. Seeds seem to pass through the digestive tract of the foxes at the same speed as other food items, with no significant differences for different seed morphology. This disagrees with what has been reported by CitationDeVlaming and Proctor (1968) for waterbirds, in which species of Cyperaceae pass slowly, whereas species of Asteraceae rarely retain for more than a few hours.
Slow passage increases the potential dispersal distance, but need not increase the efficiency of the endozoochorous seed disperser. Sibbaldia, Oxyria, and Silene were only favored if passage was fast, and for most species the germination ability decreased with time, meaning that the seeds of most species need to pass within 12–24 h to remain viable. This limits the dispersal distance that the seeds can obtain with the arctic fox as a dispersal vector. However, an arctic fox can run several kilometers within a few hours, and may therefore increase the normal dispersal distances for these species many-fold compared to unassisted dispersal.
Among species favored by the treatment, Cerastium alpinum is the most remarkable. No germination experiments have been carried out so far on this species, according to CitationBaskin and Baskin (1998). Immersion in water during germination may increase germination in this species, as is the case for Cerastium arcticum (CitationBell and Bliss, 1980), but passage through the fox also broke the dormancy. Stellaria was also favored by passage. This is remarkable, as there are reports from the study region that this species is considerably more abundant around dens of arctic foxes than in the near surroundings (CitationNielsen et al., 1994). Other Caryophyllaceae, Cerastium alpinum and Stellaria calycantha, have been found to behave similarly near dens of arctic foxes in Scandinavia (CitationBruun et al., 2005).
Studies of fleshy-fruited species eaten by foxes have shown germination percentages up to 50–70% (CitationAronne and Russo 1997; CitationMeek, 1998). However, the 2 species in the present study showed no germination, neither after passage nor in controls. Unfortunately, we did not stratify any of the seeds before the experiment. However, Betula nana, Empetrum nigrum ssp. hermaphroditum, and Vaccinium uliginosum have physiological dormancy according to CitationVander Kloet and Hill (1994), CitationBaskin and Baskin (1998), and CitationBaskin et al. (2000, Citation2002). CitationVander Kloet and Hill (2000) showed high germination percentages for Vaccinium uliginosum after stratification of seeds in scats as well as seeds cleaned from fruit (59–69%), whereas seeds without stratification only obtained germination percentages of 0–5%. The results for these 3 species, and the other species with little germination therefore have no value with respect to the germination question of this study.
Species with long appendages and Saxifraga cernua, Veronica, and Papaver were strongly inhibited by passage. The seeds of these species all imbibed after passage and seemed to be more infected by fungi than most other species. This may be due to the thin seed coats of these species or, for bulbils of Saxifraga cernua, no seed coat. CitationDeVlaming and Proctor (1968) showed that only seeds with hard integuments or small size were able to survive passage through the avian intestinal tract. Species with adaptations to wind dispersal do not have the same need for animals as long-distance dispersal vectors as do others. How long-distance dispersal of Saxifraga cernua, Veronica, and Papaver might take place still remains unclear, but the present study does not support endozoochorous dispersal for these species. Saxifraga cernua is widespread and a rapid colonizer in the region (CitationGraae et al., unpublished). Papaver is also widespread and a ruderal species, but this may relate to seed bank as well as dispersal (CitationHansen, 2003). Both these species were probably early immigrants (CitationFredskild, 1973). Veronica is at its northern limit in the region but is widespread in southern Greenland. It arrived late in Greenland (CitationFredskild, 1973), probably because it needed higher temperatures before it could colonize.
In conclusion, the present study indicates that seeds of many arctic plant species, once ingested, can survive passage through the guts of the arctic fox and thereby become dispersed over considerable distances. This result calls for quantitative fields studies on which species actually become ingested and eventually defecated by arctic foxes, and on the effect upon seed germination and plant recruitment of seeds ending up in fox scats.
FIGURE 1. Germination percentages for the 8 most efficiently germinating species in the experiment. Horizontal curve (-×-) gives germination percent for control. The other curve (-▴-) gives germination percentage for scats collected at the given passage time. The bars give number of seeds passed in scats at that passage time (white bars), and number of these seeds that germinated (dark bars)
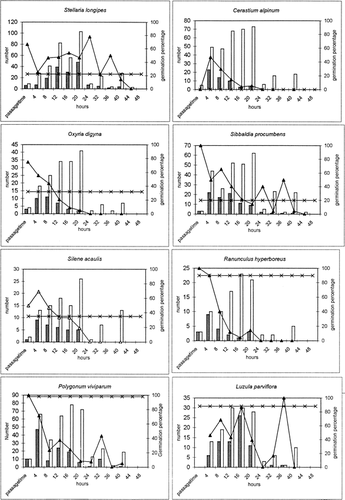
TABLE 1 Number of seeds recovered in the scats and recover percentage of total given for the studied species. Seed morphology is given and the mean passage time with std dev. for each species
TABLE 2 Mean passage time and std dev. for the species categorized after morphological adaptations to seed dispersal
TABLE 3 Number of seeds in germination samples and germination percentages as means of the three controls and of all samples collected from the scats (unaffected by fungy after three weeks, see text)
Acknowledgments
The authors thank the staff at the Royal Veterinary and Agricultural University's experimental fur farm, Rørrendegård, especially manager Boye Pedersen, for admittance and a lot of practical help. Jim Latham, Countryside Council of Wales, kindly improved the English and Nick Hill and Anders Angerbjörn gave valuable comments during the review process.
References
- Angerbjörn, A. , J. Ströman , and D. Becker . 1997. Home range pattern in arctic foxes in Sweden. Journal of Wildlife Research 2:9–14.
- Aronne, G. and D. Russo . 1997. Carnivorous mammals as seed dispersers of Myrtus communis (Myrtaceae) in the mediterranean shrublands. Plant Biosystems 131:189–195.
- Baskin, C. and J. M. Baskin . 1998. Seeds: ecology, biogeography and evolution of dormancy and germination. New York, Academic Press.
- Baskin, C. C. , P. Milberg , L. Andersson , and J. M. Baskin . 2000. Germination studies on three dwarf shrubs ( Vaccinium , Ericaceae) of northern hemisphere coniferous forests. Canadian Journal of Botany 78:1552–1560.
- Baskin, C. C. , O. Zackrisson , and J. M. Baskin . 2002. Role of warm stratification in promoting germination of seeds of Empetrum hermaphroditum (Empetraceae), a circumboreal species with a stony endocarp. American Journal of Botany 89:486–493.
- Bell, K. L. and L. C. Bliss . 1980. Plant reproduction in a high arctic environment. Arctic and Alpine Research 12:1–10.
- Bennike, O. 1999. Colonisation of Greenland by plants and animals after the last ice age: a review. Polar Record 35:323–336.
- Birks, J. D S. and N. Penford . 1990. Observations on the ecology of arctic foxes Alopex lagopus in Eqalummiut Nunaat, West Greenland. Meddelelser om Grønland: Bioscience 32:1–26.
- Böcher, T. W. , B. Fredskild , K. Holmen , and K. Jakobsen . 1978. The flora of Greenland. P. Haase and Sons, Copenhagen, Denmark, 327 pp.
- Braestrup, F. W. 1941. A study of the arctic fox in Greenland: immigrations, fluctuations in numbers, based mainly on trading statistics. Meddelelser om Grønland 131:1–101.
- Brunner, H. , R. V. Harris , and R. L. Amor . 1976. A note on the dispersal of seeds of blackberry ( Rubus procerus P.J. Muell.) by foxes and emus. Weed Research 16:171–173.
- Bruun, H. H. , S. Österdahl , J. Moen , and A. Angerbjörn . 2005. The plant community on dens of arctic fox in a subarctic-alpine landscape. Ecography 28:81–87.
- Bustamante, R. O. , J. A. Simonetti , and J. E. Mella . 1992. Are foxes legitimate and efficient seed dispersers? A field test. Acta Oecologica 13:203–208.
- Castro, S. A. , S. I. Silva , P. L. Meserve , J. R. Gutierrez , L. C. Contreras , and F. M. Jaksic . 1994. Frugivoría y dispersión de semillas de pimiento ( Schinus molle ) por el zorro culpeo ( Pseudalopex culpaeus ) en el Parque Nacional Fray Jorge (IV Región, Chile). Revista Chilena de Historia Natural 67:169–176.
- Clark, J. S. , C. Fastie , G. Hurtt , S. T. Jackson , C. Johnson , G. A. King , M. Lewis , J. Lynch , S. Pacala , C. Prentice , E. W. Schupp , T. Webb III , and P. Wyckoff . 1998. Reid's Paradox of Rapid Plant Migration. Dispersal theory and interpretation of paleoecological records. BioScience 48:13–24.
- Cypher, B. L. and E. A. Cypher . 1999. Germination rates of tree seeds ingested by coyotes and raccoons. American Midland Naturalist 142:71–76.
- Darwin, C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Muray, London, 502 pp.
- Dean, W. R J. and S. J. Milton . 1988. Dispersal of seeds by raptors. African Journal of Ecology 26:173–176.
- DeVlaming, V. and V. W. Proctor . 1968. Dispersal of aquatic organisms: viability of seeds recovered from the droppings of captive killdeer and mallard ducks. American Journal of Botany 55:20–26.
- Eberhardt, L. E. , R. A. Garrot , and W. C. Hanson . 1983. Winter movements of arctic foxes, Alopex lagopus , in a petroleum development area. Canadian Field Naturalist 97:66–70.
- Elmhagen, B. , M. Tannerfeldt , P. Verucci , and A. Angerbjörn . 2000. The arctic fox ( Alopex lagopus ): an opportunistic specialist. Journal of Zoology 251:139–149.
- Frafjord, K. 1995. Summer food-habits of arctic foxes in the alpine region of southern Scandinavia, with a note on sympatric red foxes. Annales Zoologici Fennici 32:111–116.
- Fredskild, B. 1973. Studies in the vegetational history of Greenland. Palaeobotanical investigations of some Holocene lake and bog deposits. Meddelelser om Grønland, 198: 247 pp.
- Fredskild, B. 1996. A phytogeographical study of the vascular plants of West Greenland (62°20′–74°00′N). Meddelelser om Grønland Bioscience, 45: 157 pp.
- Fowler, J. and L. Cohen . 1990. Practical Statistics for field biology. Wiley & Sons, Chicester, U.K., 227 pp.
- Funder, S. 1989. Quaternary geology of West Greenland. In Fulton, R. J. (ed.), Quaternary geology of Canada and Greenland. Geological Survey of Canada: 749–756.
- Garrott, R. A. , L. E. Eberhardt , and W. C. Hanson . 1983. Summer food habits of juvenile arctic foxes in northern Alaska. Journal of Wildlife Management 47:540–544.
- Graae, B. J. , R. H. Økland , B. Strandberg , G. B. Linde , and E. A. Fischer . in review. Relative importance of seed dispersal, germination and seedling establishment for colonization success in arctic bush vegetation.
- Hansen, K. 2003. Arctic seed banks. M.sc. thesis. University of Oslo, Norway, 83 pp.
- Hersteinsson, P. and D. W. Macdonald . 1996. Diet of arctic foxes ( Alopex lagopus ) in Iceland. Journal of Zoology 240:457–474.
- Hickey, J. R. , R. W. Flynn , S. W. Buskirk , K. G. Gerow , and M. F. Willson . 1999. An evaluation of a mammalian predator, Martes americana , as a disperser of seeds. Oikos 87:499–508.
- Iversen, J. 1954. Origin of the flora of Western Greenland in the light of pollen analysis. Oikos 4:85–103.
- Jaeger, E. C. 1950. The coyote as a seed distributor. Journal of Mammalogy 31:452–453.
- Kapel, C. M O. 1999. Diet of arctic foxes ( Alopex lagopus ) in Greenland. Arctic 52:289–293.
- Leon Lobos, P. M. and M. T. Kalin Arroyo . 1994. Germinación de semillas de Lithrea caustica (Mol.) H. et A. (Anacardiacea) dispersadas por Pseudalopex sp. (Canidae) en el bosque esclerófilo de Chile Central. Revista Chilena de Historia Natural 67:59–64.
- Meek, P. D. 1998. ‘Weed seeds and whoopsie daisies': viability of bitou bush Chrysanthemoides monilifera seeds in fox ( Vulpes vulpes ) scats. Plant Protection Quarterly 13:21–24.
- Motta-Junior, J. C. and K. Martins . 2002. Frugivorous diet of the maned wolf, Chrysocyon brachyurus , in Brazil: ecology and conservation. In: Levey, D. J., Silva, W. R., and Galetti, M. (eds.), Seed dispersal and frugivory: ecology, evolution and conservation. Wallingford, U.K., CAB International, 291–303.
- Motta-Junior, J. C. , J. A. Lombardi , and S. A. Talamoni . 1994. Notes on crab-eating fox ( Dusicyon thous ): seed dispersal and food-habits in southeastern Brazil. Mammalia 58:156–159.
- Nielsen, S. M. 1991. Fishing arctic foxes Alopex lagopus on a rocky island in West Greenland. Polar Research 9:211–213.
- Nielsen, S. M. 1994. The marine canine. Feeding ecology and behaviour of coastal arctic foxes Alopex lagopus in West Greenland. Ph.D. thesis. University of Copenhagen, Denmark.
- Nielsen, S. M. , V. Pedersen , and B. B. Klitgaard . 1994. Arctic fox ( Alopex lagopus ) dens in the Disko Bay area, West Greenland. Arctic 47:327–333.
- Nogales, M. , F. M. Medina , and A. Valido . 1996. Indirect seed dispersal by the feral cats Felis catus in island ecosystems (Canary Islands). Ecography 19:3–6.
- Nogales, M. , J. D. Delgado , and F. M. Medina . 1998. Shrikes, lizards and Lycium intricatum (Solanaceae) fruits: a case of indirect seed dispersal on an oceanic island (Alegranza, Canary Islands). Journal of Ecology 86:866–871.
- Nogales, M. , V. Quilis , F. M. Medina , J. L. Mora , and L. S. Trigo . 2002. Are predatory birds effective secondary seed dispersers?. Biological Journal of the Linnean Society 75:345–352.
- Porsild, M. 1910. The plant life of Hare Island off the coast of West Greenland. Meddelelser om Grønland 47:252–274.
- Prestrud, P. 1992. Food habits and observations of the hunting behaviour of Arctic foxes, Alopex lagopus , in Svalbard. Canadian Field Naturalist 106:225–236.
- Szuman, J. and A. Skrzydlewski . 1962. Über die Durchgangszeit des Futters durch den Magen-Darm-Kanal beim Blaufuchs. Archiv für Tierernährung 12:1–4.
- Traveset, A. 1998. Effect of seed passage through vertebrate frugivores' guts on germination: a review. Perspectives on Plant Ecology Evolution and Systematics 1:151–190.
- Traveset, A. and M. Verdú . 2002. A meta-analysis of the effect of gut treatment on seed germination. In: Levey, D. J., Silva, W. R. and Galetti, M. (eds.), Seed dispersal and frugivory: ecology, evolution and conservation. Wallingford, U.K., CAB International, 339–350.
- Traveset, A. and M. F. Willson . 1997. Effect of birds and bears on seed germination of fleshy-fruited plants in temperate rainforests of southeast Alaska. Oikos 80:89–95.
- Traveset, A. , N. Riera , and R. E. Mas . 2001. Passage through bird guts causes interspecific differences in seed germination characteristics. Functional Ecology 15:669–675.
- Vander Kloet, S. P. and N. M. Hill . 1994. The paradox of berry production in temperate species of Vaccinium . Canadian Journal of Botany 72:52–58.
- Vander Kloet, S. P. and N. M. Hill . 2000. Bacca quo vadis: regeneration niche differences among seven sympatric Vaccinium species on headlands of Newfoundland. Seed Science Research 10:89–97.
- Warming, E. 1887. Om Grønlands vegetation. Meddelelser om Grønland 12:1–245.
- Williams, P. A. , B. J. Karl , P. Bannister , and W. G. Lee . 2000. Small mammals as potential seed dispersers in New Zealand. Austral Ecology 25:523–532.
- Willson, M. F. 1993. Mammals as seed-dispersal mutualists in North America. Oikos 67:159–176.
- Wilson, J. A. and B. Thomas . 1999. Diet and seed dispersal efficiency of the gray fox ( Urocyon cinereoargenteus ) in chaparral. Bulletin Southern California Academy of Sciences 98:119–126.
- Wrigley, R. E. and D. R M. Hatch . 1976. Arctic fox migrations in Manitoba. Arctic 29:147–158.
- Zakrzewski, M. , M. Lieser , and B. Sittler . 1999. Zur Raumnutzung eines Polarfuchspaares ( Alopex lagopus ) in zwei aufeinanderfolgenden Sommern in Nordost-Grönland [Summer home ranges of an arctic fox pair ( Alopex lagopus ) in two consecutive years]. Zeitschrift für Jagdwissenschaft 45:134–138.