Abstract
Nitrogen availability is considered limiting for plant growth at the forest-tundra ecotone, and it might modulate ecosystem response to climate warming. The aim of this research was to compare the impact of climate, vegetation cover, and soil organic matter (SOM) chemistry on N mineralization rates at the forest-tundra ecotone. We therefore estimated N mineralization in mountain birch (Betula pubescens Ehrh. ssp. czerepanovii) forest and tundra soil across a broad-scale latitudinal gradient in Fennoscandia, which incorporated 4 research sites (Dovrefjell, Vassijaure, Abisko, and Joatka). During the summer period, ammonium was the dominant form of mineralized nitrogen in forest soils, while nitrate mineralization rates were higher at tundra sites during the winter. A negative regression relationship between an index of climatic continentality and N mineralization was found. Further, summer NH4 + mineralization rates increased with total N content in soils, while NO3 − mineralization seemed to be associated with C availability. Our study showed markedly contrasting inorganic N release in forest and tundra soil, and that, although mineralization rates differed between the summer and winter period, the winter activity was relatively high and should not be ignored. We conclude that a shift in the forest-tundra ecotone in response to climate warming will have stronger effects on nitrogen availability at these sites than the direct effects of warming.
Introduction
Biologically available nitrogen (N) is closely associated with the quantity and quality of soil organic matter (SOM), particularly in high-latitude ecosystems where decomposition is slow and large amounts of N are stored in SOM (CitationRobinson and Wookey, 1997; CitationJonasson et al., 1999). Nitrogen is generally considered the most limiting nutrient in high-latitude arctic/subarctic ecosystems, and N availability (of both inorganic and organic forms) is therefore likely to modulate ecosystem response to climate warming (CitationSveinbjörnsson et al., 1996; CitationPress et al., 1998; CitationArft et al., 1999; CitationJonasson et al., 1999; CitationRustad et al., 2001; CitationHobbie et al., 2002). Since decomposition of SOM and the related release of N are sensitive to increased temperature (CitationNadelhoffer et al., 1991; CitationLloyd and Taylor, 1994; CitationPeterjohn et al., 1994; CitationRustad et al., 2001; CitationFang and Moncrieff, 2001; CitationSjögersten and Wookey, 2002), the warming predicted at high latitudes (CitationHagen et al., 2001) will likely increase the bioavailable N. Increased N availability is likely to increase tree growth at the tree line in subarctic Fennoscandia (CitationSveinbjörnsson et al., 1996) and current predictions are indeed for an expansion of trees into tundra areas (CitationEmanuel et al., 1985; CitationKittel et al., 2000; CitationWhite et al., 2000).
In addition to climatic controls, the storage and release of nutrients are strongly linked both to the quantity and quality of litter inputs, and to the nature of the decomposer community (CitationSwift et al., 1979). SOM quality is therefore expected to vary among areas with contrasting litter inputs and climate (CitationSwift et al., 1979; CitationDyer et al., 1990; CitationRaich and Schlesinger, 1992; CitationCoûteaux et al., 1995). Indeed, many studies carried out in subarctic and tundra environments have noted differences in respiration and net mineralization rates over short distances between contrasting plant communities (e.g., CitationGiblin et al., 1991; CitationNadelhoffer et al., 1991; CitationHobbie, 1996; CitationWeih, 1998; CitationRustad et al., 2001; CitationSjögersten and Wookey, 2002). Contrasting decomposition, for example, and carbon and nutrient storage, have been observed across the mountain birch forest-tundra ecotone in the Fennoscandian mountains (CitationSjögersten et al., 2003; CitationSjögersten and Wookey, 2004). Greater storage of labile carbon in soils was found in mesic tundra heath areas compared to nearby mountain birch forest, which is attributed to lower decomposition rates in tundra areas (CitationSjögersten et al., 2003). There are also indications of higher SOM quality in forest soils (CitationSjögersten and Wookey, 2002; CitationSjögersten et al., 2003), including lower C:N ratios, that might be related to the higher quality and quantity of litter inputs that these soils receive compared to nearby tundra heaths dominated by evergreen ericaceous dwarf shrubs (CitationCornelissen, 1996; CitationQuested et al., 2002). Indeed, CitationSveinbjörnsson et al., (1996) found 10 times lower ammonium concentrations in soils above the treeline. Thus, we hypothesize that the amount of N mineralized will be greater in mountain birch forest soils compared with tundra heaths, in response either to more favorable climatic conditions within the forest canopy, or to higher litter quality. The decomposer community would likely be responsive to alteration in the above-ground vegetation, together with the associated change in microclimate and substrate quality (CitationRobinson, 2001). Although we focus here on the release of inorganic N from SOM, we acknowledge the demonstrated role of direct uptake of organic N by several boreal and tundra plant species (CitationKielland, 1994; CitationNäsholm et al., 1998).
There is growing evidence of significant microbial activity beneath the snow cover in high-latitude and alpine areas during the winter period. This activity is fuelled by plant litter inputs at the end of the growing season, as well as by rapid recycling of microbial residuals (CitationSchmidt and Lipson, 2004; CitationSchimel et al., 2004). There is, however, still a need for information on how variation in latitude and SOM and litter quality between contrasting vegetation communities influence the winter activity. In subarctic and arctic environments, the long duration of the winter period, compared with a much shorter growing season for plants, makes research on winter processes imperative.
The aim of the current study was to answer the following specific questions: Does N-mineralization differ between forest and tundra soils, and is the difference consistent over similar vegetation communities along the Fennoscandian mountain range? If so, can the difference be linked to climate or SOM quality? Does significant mineralization occur during the winter period beneath the snowpack?
Materials and Methods
FIELD SITES
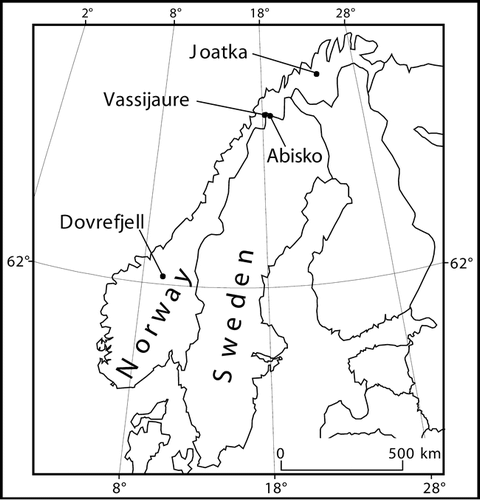
The three main study areas were Dovrefjell (Sør-Tröndelag, Norway), Abisko (Norrbotten, Sweden), and Joatka (Finnmark, Norway). In addition, a fourth supplementary site was established at Vassijaure, Sweden, as a more oceanic latitudinal parallel to Abisko (). These areas in the Fennoscandian mountain range form a gradient in both latitude and continentality; Dovrefjell is the most southerly and maritime, and Joatka is the most northerly and continental (), based upon Gorzinski's continentality index (Barry and Chorley, 1998). The study sites are situated in the mountain birch [Betula pubescens Ehrh. ssp. czerepanovii (Orlova) Hämet-Ahti]-tundra ecotone region. The main species in the tundra heath areas are Empetrum hermaphroditum, Vaccinium uliginosum, V. vitis-idea, and Betula nana, lichens and bryophytes (and additionally, in Dovrefjell, Arctostaphylos uva-ursi). Mesic areas within the mountain birch forest have comparable understory vegetation, tending toward a greater cover of V. myrtillus. The structure of the mountain birch forest at the four sites differs considerably, with denser forests at the two most maritime sites, especially at Dovrefjell. Also the understory vegetation is higher within the forest sites at Dovrefjell and Vassijaure. The sites are mesic and the soils are predominantly thin spodosols (principally orthods) developed within medium- to coarse-grained till deposits. For detailed soil profile descriptions see CitationSjögersten et al. (2003).
EXPERIMENTAL DESIGN
At Abisko and Vassijaure, summer and winter N mineralization rates were measured from June 1999 to June 2000. At Dovrefjell and Vassijaure, the experiment was initiated in September 1999 and continued to the following autumn. At the main study areas (Dovrefjell, Abisko, and Joatka), we established four experimental sites, spanning the mountain birch-tundra ecotone. Two of these sites have a mountain birch canopy (one at lower altitude and one at higher, denoted “Lower Forest” and “Upper Forest,” respectively) and two sites represent open tundra at different altitudes (subsequently denoted “Lower Tundra” and “Upper Tundra”). At Vassijaure one tundra and one mountain birch forest site were chosen. Five experimental plots were established at each site.
In addition to the basic setup, we established a soil transplant experiment to evaluate the impact on N-mineralization of the physical environment compared to the SOM quality. Soils were transplanted both between forest and tundra sites at Abisko and Vassijaure, as well as between sites, to study both the altitudinal ecotone gradient and the precipitation gradient between Vassijaure and Abisko (see ). The incubation period was from June 1999 to September 1999.
We measured surface air and soil temperature (50 mm depth) year-round with TinyTag data loggers [Gemini Data Loggers (UK) Ltd., Chichester]. Due to problems with rodents destroying the soil temperature cables, and some water leakage into the loggers, the data set is not, however, complete.
SEASONAL N MINERALIZATION RATES
In this paper we define N mineralization as the conversion of organic forms of N to inorganic (NH4 + and NO3 −) as a result of decomposition by the soil decomposer community. More specifically, we use the term “ammonification” to refer to the biochemical process whereby ammoniacal nitrogen is released from nitrogen-containing organic compounds, while “nitrification” refers to the biochemical oxidation of ammonium to nitrate (CitationBrady and Weil, 1999).
Extractable N is defined as “the N displaced from soil exchange sites by an excess of a replacement ion” (CitationRobinson et al., 1995). We used the “buried-bag” method (CitationEno, 1960; CitationRobinson et al., 1995; CitationHartley et al., 1999) as an in situ incubation technique to measure N-mineralization rates, whereby extractable N is measured before and after a period of incubation in situ in the field in the absence of root uptake of inorganic N forms. Mineralization is defined as the end-of-period extractable N minus the initial figure prior to incubation: A negative figure is possible if nutrients are immobilized (assimilated) by decomposer organisms. The technique integrates the effects of temperature during in situ incubation, while soil moisture content varies less than it would in natural conditions due to incubation in a polyethylene bag (note, however, that polyethylene is permeable to CO2 and O2, as well as to water vapor).
This technique was chosen because it is robust, applicable across widely distributed (and inaccessible) field sites with small N pools, and can be used during both winter and summer seasons, unattended. The buried-bag incubation method is not, however, free from constraints (CitationAdams et al., 1989; CitationDavidson et al., 1992), and we therefore used it as a “bioassay” for comparing N mineralization in contrasting environments, rather than for measuring actual N-mineralization rates.
Five replicate-paired soil cores, 50 mm in diameter and ≥50 mm deep, were taken from each site (pairs taken as close as possible to one another). The vegetation was immediately clipped from these, and larger roots/stolons removed. One of each of the paired cores was placed in a polyethylene bag and replaced in the soil, to be collected at a later date (after 3–3.5 months or 8–8.5 months, for summer and winter measurements, respectively). The other core was returned to the laboratory and stored at 2°C prior to extraction to quantify initial mineral nitrogen pools shortly after sampling. With both initial and final samples, loose litter material was removed from the top of the soil cores prior to extraction, and the cores were cut to 50 mm depth and passed through a 2 mm sieve. Five grams of fresh soil were dried to determine soil moisture content, and a further 5 g of fresh soil were then extracted in 50 ml 6 % KCl solution (shaken for 1 h) and filtered through Whatman No. 2 filter papers. Extracts were stored immediately at −20°C prior to analyses. NH4 +-N and NO3 −-N were analyzed simultaneously on a segmented flow auto-analyzer (SANplus ANALYSER).
DATA ANALYSIS
Effects of site, subsite, and season were tested by ANOVA for both NH4 + and NO3 − mineralization, separately. The data were tested for normality, and two extreme values were removed from the NH4 + data set. Since no significant difference between the higher and low altitude sites within each vegetation community (e.g., Upper Tundra, UT, and Lower Tundra, LT) was found, they were combined for subsequent analysis.
The tranplantation study was analyzed by one-way ANOVA and post hoc analysis (Tukey HSD) to enable comparisons between control soils and the transplanted soils. The p-values given in the text are from the post hoc analysis.
We tested the relationship between the physical environment (as summarized in ) and the mean mineralization rates at each main site. Refining this further, regression analyses were conducted with January and July soil temperatures at forest and tundra sites () at Dovrefjell, Abisko, and Joatka.
To address the links between N mineralization and SOM quality vs. climate, we performed regression analysis between the N-mineralization data obtained in this paper and SOM chemistry data (i.e., total C, N, and P content and concentrations of alkyls, O-alkyls, aromatics, and carboxyl compounds in the soils) from previous work performed at these sites (CitationSjögersten et al., 2003). For this work the more labile organic functional groups (N-alkyls, O-alkyls, and acetals) were combined in the term “O-alkyls.” Similarly, aromatics and phenolics were combined in the term “aromatics.” For the analysis, we used mean mineralization rates for summer and winter, at forest and tundra sites, at each location. No SOM or detailed temperature data were available from the Vassijaure site.
Results
Surface air and soil temperatures at 50 mm depth were generally higher farther south, and forest sites were warmer than tundra, with the largest difference during the winter period due to the deeper snow cover at forest sites ( and ). The period with subzero soil temperatures (50 mm depth) differs between areas, and between forest and tundra sites. During the winter of 1999/2000, the period with subzero temperatures lasted 23, 27, and 30 weeks at Dovrefjell, Abisko, and Joatka, respectively. The duration of below-zero temperatures was 3, 1, and 2 weeks longer at tundra sites than at forest sites at Dovrefjell, Abisko, and Joatka, respectively. Forest soils were drier than tundra soils (F = 14.8, p < 0.001) with 51.4% and 63.1% gravimetrical water content, respectively, except at Dovrefjell, where no difference was found (). Soils at Joatka were drier than at the other sites (F = 11.8, p < 0.001) (). Despite these differences in moisture content, regression analysis between soil moisture content and nitrification and ammonification rates, respectively, explained none of the variance in mineralization rates.
We measured the highest rates of NH4 + release at Dovrefjell and Abisko. Ammonification rates were also higher at forest sites (, and and ). We recorded immobilization of NH4 + at Joatka during the winter period. The significant interaction between site and season for NH4 + reflect the reduced mineralization rates during the winter period at Joatka and Dovre fjell, while mineralization actually tended to increase during the winter period at Abisko and Vassijaure.
Tundra soils had generally higher rates of NO3 − release (nitrification), and the rates were higher during the winter period. However, the pattern of higher mineralization during winter was not repeated at Dovrefjell; here the mineralization rates were highest in the summer. It is also worth noting that we recorded immobilization of nitrate in the summer period at Joatka.
We found a significant negative relationship between N-mineralization and the degree of continentality at each site (). No significant regressions were found between N-mineralization rates and summer and winter soil temperature or pH.
Further, we found that significant variation in N-mineralization could be explained by the C and N content in the soil: Positive regressions were detected between summer ammonification rates and total N content in the soils (R2 = 0.71; p = 0.03), and between winter nitrification rates and total C content (R2 = 0.76; p = 0.02). In addition, nitrification rates also tended to be positively associated with the concentration of labile carbon (N-alkyls + O-alkyls + acetals) in the soil (R2 = 0.57; p = 0.08).
The transplantation of soils between sites generally increased immobilization of both NH4 + and NO3 − irrespective of soil origin, and no effect of incubation (recipient) site was detected (). This response was significant for nitrate in all transplanted soils, while for ammonium this pattern was only significant for soils originating from tundra at Vassijaure and forest in Abisko. Furthermore, significantly higher NO3 − immobilization occurred if the recipient site was warmer than the original site (i.e., from tundra to forest), both at Vassijaure (p = 0.007) and Abisko (p = 0.002) ().
Discussion
Ammonification (release of NH4 +) was consistently higher in forest soil than tundra across the ca. 8° latitudinal gradient. Similar variation in ammonification rates between forest (ca. 400 mg m−2) and tundra (ca. 100 mg m−2) soils was noted in the Abisko region by CitationRobinson et al. (1995) and CitationSchmidt et al. (1999), respectively. The values from the current study are, however, lower than those previously reported: ca. 135 and 12 mg m−2 (in the upper 50 mm of soil), at Lower Forest and Upper Tundra sites, respectively. By way of explanation, it is possible that the soils investigated by CitationRobinson et al. (1995) which were frozen before extraction, may have increased the release of extractable constituents. The difference between tundra sites is probably linked to the higher total N content in the soils studied by Schmidt et al. (22.8 mg g−1, compared to 13.3 mg g−1 at the Abisko Upper Tundra site in the current study), and the higher pH (7.1, compared to 3.9 at our tundra site). The higher ammonification in forest soils compared with tundra heaths is probably associated with the higher N content in forest soils. Increased N availability in forest soils could be explained by a combination of factors: (1) higher litter quality in the birch forest (CitationCornelissen, 1996; CitationSveinbjornsson et al., 1996; CitationWeih and Karlsson, 2001), and (2) the deeper snow accumulation at forest sites, which increases both winter soil temperatures and the flux of airborne N-containing compounds (CitationWeih, 1998) compared with nearby tundra heaths. The greater difference in ammonification rates between forest and tundra sites at Dovrefjell, compared to Joatka, could indeed be related to the higher snowfall at the southerly site, and possibly also linked to increased N deposition in precipitation at Dovrefjell. Tissue N concentrations in mountain birch trees have been shown to increase as a physiological response to decreased temperature at higher altitudes (CitationWeih and Karlsson, 2001). This provides an interesting link with the higher N-mineralization rates found at forest sites in this study, probably partly explaining the significant difference in ammonification rates between forest and tundra.
Although temperature has been shown to determine the position of the treeline on a macroscale (CitationKörner and Paulsen, 2004), small-scale variation is likely to relate to topography, snow cover, hydrology, and soil nutrient status (CitationWalker et al., 1993; CitationKjällgren and Kullman, 1998). This study suggests that a positive feedback could be possible whereby the establishment of birch trees on former tundra heath has the capacity to ameliorate the local physicochemical environment (as discussed above) by increasing N availability. Mountain birch is most likely, nonetheless, to become established at sites that are topographically predisposed to accumulate snow, and hence both additional N fluxes and also eolian inputs of inorganic and organic material.
The apparent relationship between summer N-mineralization rates and continentality () suggests that climate exerts some form of control on the overall rates of summer N mineralization. This relationship is not readily explained, however, on the basis of our directly measured climatic variables: We saw no clear differences, for example, in mean July temperatures between the main study sites over the measurement periods. It is also important to note that a regression relationship based on 4 sites (two of which with the same Gorzinski continentality index—Abisko and Vassijaure—but with markedly contrasting precipitation; , and ) should be interpreted with a degree of caution. If, nonetheless, a climatic control on N mineralization is accepted, it appears likely that it is a long-term effect of climate, or one exerted indirectly. This might be linked, for example, with a microbial community that is increasingly conservative of assimilated N further at more continental sites, but mineralization rates could also be affected by indirect factors such as quality and quantity of litter input. The total N content in the soils is, however, inversely related to continentality, which makes the two potential drivers difficult to separate. The relatively high mineralization rates at the tundra site at Dovrefjell could be linked to the higher pH in the organic horizon (6.2 at Dovrefjell, compared with 3.9–5.2 at the other sites), but we found no significant regression relationship between pH and either ammonium or nitrate fluxes. It is also worth noting, however, the large difference in the deposition of airborne N-containing pollutants between these areas. N-deposition rates reported from the Dovrefjell region (Osen; 61°15′N, 11°47′E) and Finnmarksvidda (Karasjokk; 69°28′N, 25°13′E) show three times higher N deposition at the more southerly site (325.3 compared to 104.3 mg N m−2, average from 1998, 2000, and 2001) (CitationHjellbrekke, 2002). Thus, our continentality/latitude gradient has, superimposed upon it, a further gradient in terms of N inputs to the ecosystems.
Nitrification rates were highest in tundra soils and during the winter period. This is in agreement (but see comments in paragraph below) with a gradient study by CitationIneson et al. (1998) in the Pennines in the U.K., where the highest NO3 −-N leaching was found at the higher elevations sites, and suggests increased nitrification at colder sites. The significant regression relationship between winter nitrification rates and both total C and labile C content in the soils suggest that the nitrifiers depend upon the amount of available C for growth in these soils. It is, however, surprising that bacteria in these C-rich soils should be generally C limited, and we therefore propose that it is the presence of labile C that is of importance to the nitrifying bacteria (if we assume that these are of greater significance than autotrophic nitrifiers; see CitationHart et al., 1994). The regression is, however, driven strongly by the immobilization rates measured at the forest site in Abisko, and should therefore be interpreted cautiously. CitationSchimel et al. (2004) and CitationSchmidt and Lipson (2004) suggest that nitrification in tundra soil during the winter period is due to the heterotrophic microbial community becoming C-limited during the winter, leading to increased NH4 + availability and hence nitrification. This mechanism could explain the higher nitrification rates at the tundra site in the current study, where temperatures are lower during the winter period: In addition, litter quality could also be important here.
The transplant experiment () showed that sudden alterations in the physical environment have the potential to shift the system from net N mineralization to immobilization, but it did not provide a clear answer as to the importance of temperature vs. SOM for N mineralization. The greater increase in NO3 − immobilization in response to a move to a warmer environment suggests that the increased immobilization is related to temperature, but the transplanted soils did not adjust to the mineralization rates of native soils at the recipient site. Similar results were found in the transplant study by CitationIneson et al. (1998), in which transplanting soil down an altitudinal gradient (to a warmer environment) reduced nitrate leaching from lysimeter cores. The study by CitationIneson et al. (1998), however, involved the use of intact lysimeter cores, so that leachate chemistry was the net product of mineralization and assimilation, both by the decomposer community and the vegetation. Direct comparison with the present study is not, therefore, straightforward.
Since the methodology used in the present study (soil coring, and incubation in polyethylene bags) will undoubtedly kill fungal hyphae and plant roots, the technique produces several artifacts (CitationAdams et al., 1989; CitationRobinson et al., 1995; CitationHartley et al., 1999). In a nearby tundra heath area, ca. 5% of the total N in SOM was contained within the microbial biomass (CitationSchmidt et al., 1999). Specifically, decaying hyphae and fine roots will provide labile resource to the surviving microbial community during the incubation period, nutrient and water uptake by plants is prevented, and root exudates and fresh litter inputs are excluded. Our cores are therefore not likely to reflect the natural rates of N mineralization and should only be used for relative comparisons; additionally, the results will inevitably reflect initial responses to disturbance.
N mineralization/immobilization proved rather tolerant of low temperatures. Although net NH4 + release was lower during the winter period at some sites, NO3 − release rates were actually higher during the winter period ( and ). This activity could have occurred mainly during the late autumn, when soil temperatures were generally above zero (soils froze first in mid–late October at tundra sites in Joatka and Abisko, and last in early–mid December at forest sites in Dovrefjell). However, there is growing evidence that soil microbial activity can continue during the winter period despite below-zero temperatures (CitationZimov et al., 1996; CitationFahnestock et al., 1998; CitationSchmidt and Lipson, 2004; CitationSchimel et al., 2004), and the January temperature range at the research sites in this study suggests that this could be the case at both tundra and forest sites across the mountain range. The higher winter ammonification rates at forest sites at Dovrefjell and Abisko are likely related to the longer active periods, as well as to the factors discussed above.
Finally, we want to stress that the links between above-ground vegetation and N mineralization presented here have important implications in a changing environment. We suggest that an expansion of the mountain birch forest in response to a warmer climate would alter the N cycling in these soils more drastically than the actual temperature increase itself, both due to the input of higher quality litter to the soils and to the changes in microclimate that are associated with the mountain birch forest, especially snow accumulation and higher winter soil temperatures.
FIGURE 2. Schematic diagram showing the transplant procedure between forest, and tundra soil, and between Abisko and Vassijaure, during the summer of 1999. The transplants involved gradients in altitude, vegetation type, and precipitation. At Abisko the sites involved were the Upper Tundra and the Lower Forest sites
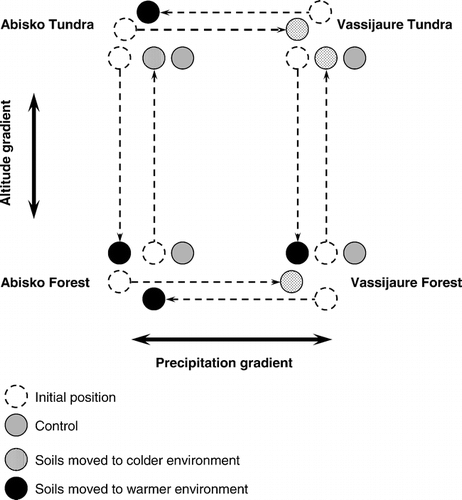
FIGURE 3. Net mineralization (and immobilization) of ammonium (NH4 +) during the summer and winter season of forest (F) and tundra (T) soils at Dovrefjell, Vassijaure, Abisko, and Joatka. Negative values indicate that N is immobilized by the decomposer organisms. Mean values and Standard Error (SE) of the mean are shown
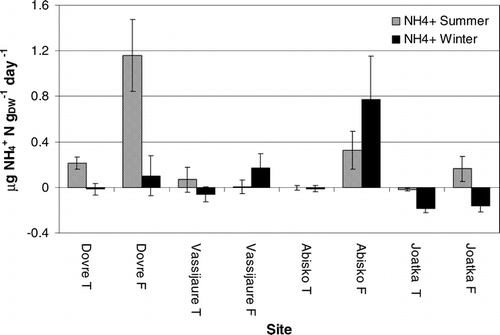
FIGURE 4. Net mineralization (and immobilization) of nitrate (NO3 −) during the summer and winter season at forest (F) and tundra (T) soils at Dovrefjell, Vassijaure, Abisko, and Joatka. Negative values indicate that N is immobilized by the decomposer organisms. Mean values and SE are shown
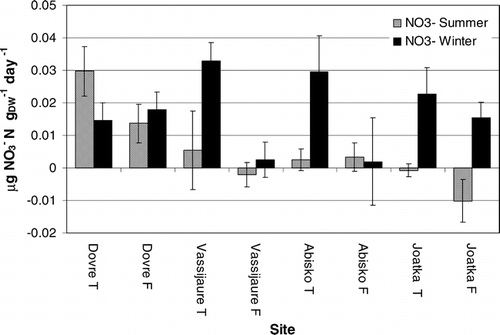
FIGURE 5. Regressions between mean summer N mineralization rates at both forest and tundra sites and the Gorzinski continentality index at the main sites (Dovre fjell, Vassijaure, Abisko, and Joatka). Note that Vassijaure and Abisko share the same continentality index, although they have markedly contrasting precipitation totals: 848 and 304 mm, respectively
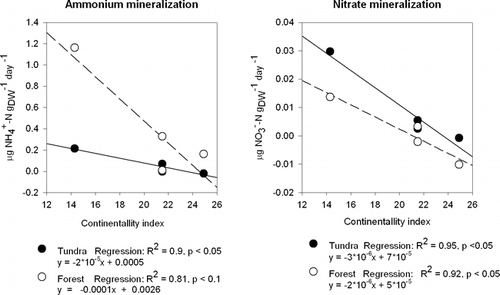
FIGURE 6. N-mineralization rates for control and transplanted soils from the Upper Tundra and the Lower Forest sites at Abisko, and from one tundra and one forest site at Vassijaure (see for a description of the transplant approach). “Control” is where native soil remains in situ at the site of origin. Mean values and SE are shown. Significant differences in mineralization rates compared to the control data are indicated in each graph, * indicates that the difference is significant at p < 0.05; **, p < 0.01; ***, p < 0.001
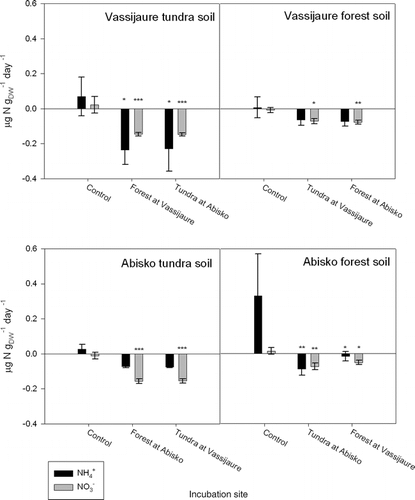
TABLE 1 Summary environmental information on the four sites in the Fennoscandian mountains
Table 2 Micrometerological data, unshielded surface temperature data and soil temperature at 50 mm (where soil is indicated in the column head) presented as mean annual temperatures (MAT) from 1999 and 2000, and January and July average temperatures from 2000 (calculated from hourly means). At Vassijaure only mean July temperature was available. nd = no data
TABLE 3 Gravimetric soil moisture content (gH2O gsoil −1) in forest and tundra soils at the four study areas at the start of the summer and winter incubation period
TABLE 4 Summary of statistical analysis of nitrogen mineralization rates at the four study sites with contrasting vegetation (forest vs tundra) over the summer and winter season. Data presented are from Type 1 test ANOVAs for (A) NH4 + and (B) NO3 −
Acknowledgments
We thank the European Commission IVth Environment and Climate Framework Programme, the Swedish Natural Environment Research Council (Naturvetenskapliga Forskningsrådet—NFR), the Swedish Royal Academy of Sciences, Svenska Sällskapet för Antropologi och Geografi, Uppsala University Linné Fonden, and Sernanders Resestipendium for funding this work. We are grateful for laboratory and field assistance from Katrin Sjögersten, Marie Nilsson, Karin Luthbom, Per Thermaenius, Pär Eriksson, Dr. Robert Baxter, Dr. Mathew Davy and Dr. Neil Ellwood. We also thank Dr. Benjamin Turner for constructive comments on the manuscript.
References Cited
- Adams, M. A. , P. J. Polglase , P. M. Attiwill , and C. J. Weston . 1989. In situ studies of nitrogen mineralization and uptake in forest soils; some comments on methodology. Soil Biology & Biochemistry 21:423–429.
- Arft, A. M. , M. D. Walker , J. Gurevitch , J. M. Alatalo , M. S. Bret-Harte , M. Dale , M. Diemer , F. Gugerli , G. H R. Henry , M. H. Jones , R. D. Hollister , I. S. Jónsdóttir , K. Laine , E. Lévesque , G. M. Marion , U. Molau , P. Molgard , U. Nordenhäll , V. Raszhivin , C. H. Robinson , G. Starr , A. Stenström , M. Stenström , Ö Totland , P. L. Turner , L. J. Walker , P. J. Webber , J. M. Welker , and P. A. Wookey . 1999. Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecological Monographs 69:491–511.
- Barry, R. G. and R. J. Chorley . 1995. Atmosphere, Weather & Climate. Sixth Edition. New York, Routledge, 392 pp.
- Brady, N. C. and R. R. Weil . 1999. The Nature and Properties of Soils. Prentice Hall, 740 pp.
- Cornelissen, J. H C. 1996. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. Journal of Ecology 84:573–582.
- Coûteaux, M. , P. Bottner , and B. Berg . 1995. Litter decomposition, climate and litter quality. Tree 10:63–66.
- Davidson, E. A. , S. C. Hart , and M. K. Firestone . 1992. Internal cycling of nitrate in soils of a mature coniferous forest. Ecology 73:1148–1156.
- Dyer, M. L. , V. Meentemeyer , and B. Berg . 1990. Apparent controls of mass loss rates of leaf litter on a regional scale: litter quality vs. climate. Scandinavian Journal of Forest Research 5:311–323.
- Emanuel, W. R. , H. H. Shugart , and M. P. Stevenson . 1985. Climate change and broad-scale distribution of terrestrial ecosystem complexes. Climatic Change 7:29–43.
- Eno, C. F. 1960. Nitrate production in the field by incubating the soil in polyethylene bags. Soil Science Society of America Proceedings 24:277–279.
- Fahnestock, J. T. , M. H. Jones , P. D. Brooks , D. A. Walker , and J. M. Welker . 1998. Winter and early spring CO2 efflux from tundra communities of northern Alaska. Journal of Geophysical Research—Atmospheres 103:D22 29023–29027.
- Fang, C. and J. B. Moncrieff . 2001. The dependence of soil CO2 efflux on temperature. Soil Biology & Biochemistry 33:155–165.
- Giblin, A. E. , K. J. Nadelhoffer , G. R. Shaver , J. A. Laundre , and A. J. McKerrow . 1991. Biogeochemical diversity along a riverside toposequence in arctic Alaska. Ecological Monographs 61:415–435.
- Hagen, J. O. , R. Jefferies , H. Marchant , F. Nelson , T. Prowse , and D. G. Vaughan . 2001. Polar regions (Arctic and Antarctic). In: McCarthy, J. J., Canziani, O. F., Leary, N. A., Dokken, D. J., and White, K. S., (eds.), Climate Change 2001. Impacts, Adaptation, and Vulnerability. Cambridge, Cambridge University Press, 801–841.
- Hart, S. C. , G. E. Nason , D. D. Myrold , and D. A. Perry . 1994. Dynamics of gross nitrogen transformations in an old-growth forest—the carbon connection. Ecology 75:880–891.
- Hartley, A. E. , C. Neill , J. M. Melillo , R. Crabtree , and F. P. Bowles . 1999. Plant performance and soil nitrogen mineralization in response to simulated climate change in subarctic dwarf shrub heath. Oikos 86:331–343.
- Hjellbrekke, A-G. 2002. Data Report 2000 Acidifying and eutrophying compounds. Part 1: Annual summaries. Kjeller, Norway, Norwegian Institute for Air Research (NILU), Co-operative Programme for Monitoring and Evaluation of the Long-Range Transmission of Air pollutants in Europe, EMEP/CCC-Report 6/2002, 93 pp. (http://www.nilu.no/projects/ccc/reports/#2002, last accessed February 2005).
- Hobbie, S. E. 1996. Temperature and plant species control over litter decomposition in Alaskan tundra. Ecological Monographs 66:503–522.
- Hobbie, S. E. , K. J. Nadelhoffer , and P. Hogberg . 2002. A synthesis: the role of nutrients as constraints on carbon balances in boreal and arctic regions. Plant and Soil 242:163–170.
- Ineson, P. , K. Taylor , A. F. Harrison , J. Poskitt , D. G. Benham , E. Tipping , and C. Woof . 1998. Effects of climate change on nitrogen dynamics in upland soils. 1. A transplant approach. Global Change Biology 4:143–152.
- Jonasson, S. , A. Michelsen , and I. K. Schmidt . 1999. Coupling of nutrient cycling and carbon dynamics in the Arctic, integration of soil microbial and plant processes. Applied Soil Ecology 11:135–146.
- Kielland, K. 1994. Amino acid absorption by arctic plants: Implications for plant nutrition and nitrogen cycling. Ecology 75:2373–2383.
- Kittel, T. G F. , W. L. Steffen , and F. S. Chapin III . 2000. Global and regional modelling of Arctic-boreal vegetation distribution and its sensitivity to altered forcing. Global Change Biology 6:1–18.
- Kjällgren, L. and L. Kullman . 1998. Spatial patterns and structure of the mountain birch tree-limit in the southern Swedish Scandes—a regional perspective. Geografiska Annaler—Physical Geography 80A:1–16.
- Körner, C. and J. Paulsen . 2004. A world-wide study of high altitude treeline temperatures. Journal of Biogeography 31:713–732.
- Lloyd, J. and J. A. Taylor . 1994. On the temperature-dependence of soil respiration. Functional Ecology 8:315–323.
- Nadelhoffer, K. J. , A. E. Giblin , G. R. Shaver , and J. A. Laundre . 1991. Effects of temperature and substrate quality on element mineralization in six Arctic soils. Ecology 72:242–253.
- Näsholm, T. , A. Ekblad , A. Nordin , R. Giesler , M. Högberg , and P. Högberg . 1998. Boreal forest plants take up organic nitrogen. Nature 392:914–916.
- Peterjohn, W. T. , J. M. Melillo , P. A. Steudler , K. M. Newkirk , F. P. Bowles , and J. D. Aber . 1994. Responses of trace gas fluxes and N availability to experimentally elevated soil temperatures. Ecological Application 4:617–625.
- Press, M. C. , J. A. Potter , M. J W. Burke , T. V. Callaghan , and J. A. Lee . 1998. Responses of a subarctic dwarf shrub heath community to simulated environmental change. Journal of Ecology 86:315–327.
- Quested, H. M. , M. C. Press , T. V. Callaghan , and J. H C. Cornlissen . 2002. The hemiparasitic angiosperm Bartsia alpina has the potential to accelerate decomposition in sub-arctic communities. Oecologia 130:88–95.
- Raich, J. W. and W. H. Schlesinger . 1992. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44B:81–91.
- Robinson, C. H. 2001. Cold adaptation in Arctic and Antarctic fungi. New Phytologist 151:341–353.
- Robinson, C. H. and P. A. Wookey . 1997. Microbial ecology, decomposition and nutrient cycling. In: Woodin, S. J., and Marquiss, M. (eds.), Ecology of Arctic Environments. British Ecological Society Special Publication 13: 41–68.
- Robinson, C. H. , P. A. Wookey , A. N. Parsons , J. A. Potter , T. V. Callaghan , J. A. Lee , M. C. Press , and J. M. Welker . 1995. Response of plant litter decomposition and nitrogen mineralisation to simulated environmental change in a high arctic polar semi-desert and a subarctic dwarf shrub heath. Oikos 74:503–512.
- Rustad, L. E. , J. L. Campbell , G. M. Marion , R. J. Norby , M. J. Mitchell , A. E. Hartley , J. H C. Cornelissen , and J. Gurevitch . 2001. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562.
- Schimel, J. P. , C. Bilbrough , and J. A. Welker . 2004. Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biology & Biochemistry 36:217–227.
- Schmidt, I. K. , S. Jonasson , and A. Michelsen . 1999. Mineralization and microbial immobilization of N and P in arctic soils in relation to season, temperature and nutrient amendment. Applied Soil Ecology 11:147–160.
- Schmidt, S. K. and D. A. Lipson . 2004. Microbial growth under the snow: implications for nutrient and allelochemical availability in temperate soils. Plant and Soil 25:1–7.
- Sjögersten, S. and P. A. Wookey . 2002. Climatic and resource quality controls on soil respiration across a forest-tundra ecotone in Swedish Lapland. Soil Biology & Biochemistry 34:1633–1646.
- Sjögersten, S. and P. A. Wookey . 2004. Decomposition of mountain birch leaf litter at the forest-tundra ecotone in the Fennoscandian Mountains in relation to climate and soil conditions. Plant and Soil 262:215–227.
- Sjögersten, S. , B. L. Turner , N. Mahieu , L. M. Condron , and P. A. Wookey . 2003. Soil organic matter biochemistry and potential susceptibility to climatic change across the forest-tundra ecotone in the Fennoscandian mountains. Global Change Biology 8:885–894.
- Sveinbjörnsson, B. , H. Kauhanen , and O. Nordell . 1996. Tree-line ecology of mountain birch in the Torneträsk region. Ecological Bulletins 45:65–70.
- Swift, M. J. , O. W. Heal , and J. M. Anderson . 1979. Decomposition in Terrestrial Ecosystems. Berkeley, University of California Press, 362 pp.
- Walker, D. A. , J. C. Halfpenny , M. D. Walker , and C. A. Wessman . 1993. Long-term studies of snow-vegetation interactions. Bioscience 43:287–301.
- Weih, M. 1998. Seasonality of nutrient availability in soils of subarctic mountain birch woodlands, Swedish Lapland. Arctic and Alpine Research 30:19–25.
- Weih, M. and P. S. Karlsson . 2001. Growth response of mountain birch to air and soil temperature: Is increasing leaf-nitrogen content an acclimation to lower air temperature?. New Phytologist 150:147–155.
- White, A. , M. G R. Canell , and A. D. Friend . 2000. The high-latitude terrestrial carbon sink: a model analysis. Global Change Biology 6:227–245.
- Zimov, S. A. , S. P. Davidov , Y. V. Voropaev , S. F. Prosiannikov , I. P. Semiletov , M. C. Chapin , and F. S. Chapin . 1996. Siberian CO2 efflux in winter as a CO2 source and cause of seasonality in atmospheric CO2. Climatic Change 33:111–120.