Abstract
We surveyed 14 ponds in 1999 and 28 ponds in 2000 to better understand the basic limnology of alpine ponds and to predict how the planktonic and epipelic (sediment-living) algal communities may respond to nutrient deposition and climate change. Based on nitrogen to phosphorus ratios, nitrogen limitation is likely common in these alpine ponds, which makes them particularly susceptible to current increases in atmospheric N deposition. Regression and redundancy analysis (RDA) showed phytoplankton abundance and community composition was best explained by total phosphorus (TP), pH, and conductivity. Epipelon abundance was best explained by nitrite plus nitrate and community composition was best explained by TP and dissolved organic carbon (DOC) in addition to nitrite plus nitrate. Some of these chemical variables, DOC, pH, and conductivity, have been linked to climate in alpine ponds and lakes and in low elevation lakes, which suggests alpine ponds may be sensitive to climate change. However, because we found interannual variability in the environmental-algal relationships, several years of study may be required in order to make realistic predictions on how algal communities will respond to increasing nutrient deposition and climate change.
Introduction
Mountain ecosystems may be particularly sensitive to environmental change (CitationHauer et al., 1997; CitationBowman, 2000). Alpine aquatic ecosystems, for example, are sensitive to increases in atmospheric nitrogen (N) deposition because their steep, sparsely vegetated watersheds retain little nitrogen (CitationBaron et al., 2000). Increased nitrogen loading has been linked to eutrophication in some subalpine and alpine lakes in the western United States (CitationBaron et al., 2000). Furthermore, alpine ecosystems may be sensitive to global climate change because snow-albedo feedback in high elevation sites is predicted to decrease, causing faster warming than at low elevation sites (CitationFyfe and Flato, 1999). Climate-sensitive variables, such as conductivity, dissolved organic carbon, and the elevation of treeline, have been identified as predictors of algal communities in mountain lakes and ponds (CitationVinebrooke and Leavitt, 1999). Although we are beginning to understand the impacts of environmental change on high elevation lakes, little is known regarding how alpine ponds will respond.
Alpine ponds are numerous, with over 3000 located in Banff National Park (6641 km2), Canada. Despite their abundance, only five studies have been published since North American alpine ponds were described and classified by Ives in 1941. In the Colorado Rocky Mountains, very basic seasonal limnological changes were described in one large (CitationNeldner and Pennak, 1955) and two small (CitationSchmitz, 1959) alpine ponds. In the Canadian Rocky Mountains, planktonic crustaceans were surveyed in 14 alpine ponds (CitationAnderson, 1971; CitationAnderson, 1974), and planktonic and benthic algal communities were surveyed in 2 alpine ponds (CitationVinebrooke and Leavitt, 1999). A comprehensive description of the physical and chemical features and algal communities that characterize several alpine ponds has yet to be completed. However, a recent study of five alpine ponds in Banff National Park found that length of the ice-free season, water temperature, maximum depth, pH, and conductivity respond rapidly to changes in temperature and precipitation (CitationMcMaster, 2003).
Multivariate gradient techniques such as redundancy analysis have been applied to survey data to clarify relationships between environmental variables and algal communities (CitationVinebrooke and Leavitt, 1999; CitationLafrançois, 2002). These methods allow prediction of the impact of future environmental change on algal communities (CitationVinebrooke and Leavitt, 1999; CitationLafrançois, 2002) and deduction of past environmental conditions based on current algal-environmental relationships (CitationSmol and Cumming, 2000). However, most studies base their predictions and deductions on surveys from one year, even though environmental conditions (CitationBennion and Smith, 2000; CitationMcMaster, 2003) and the controls on the algal communities (CitationVillar-Argaiz et al., 2001; CitationMcMaster, 2003) may vary over time. Consequently, predictions and deductions may change depending on which year was sampled.
The aim of this study was to better understand the limnology of alpine ponds and predict how their algal communities may respond to environmental change. The main objectives were to identify the physical and chemical variables that best explain phytoplankton and epipelon abundance and community composition in alpine ponds, and to determine if the physical and chemical variables that best explain algal abundance and community composition differ between two summers with different temperature and precipitation. In the process, we (1) describe the physical and chemical limnology of alpine ponds; (2) assess nutrient limitation with nitrogen to phosphorus ratios; and (3) describe the phytoplankton and epipelon communities across 28 alpine ponds in Banff National Park. To address these objectives we surveyed 14 ponds in 1999 and 28 ponds in 2000. We recorded 15 physical and chemical variables and sampled planktonic and epipelic (sediment-living) algal communities at each pond, then analyzed the data using multivariate gradient techniques. Phytoplankton and epipelon abundance and community composition were inferred from pigment concentrations.
Methods
STUDY SITE
The survey ponds are located in the alpine ecoregion (2150–2570 m a.s.l.; 51°27′–51°44′N, 115°39′–115°56′W) in the eastern front ranges of the Rocky Mountains in Banff National Park, Alberta, Canada (). Alpine ponds in this area typically have drainage basins consisting of a mosaic of small patches of exposed sedimentary rock, including limestone, shale, and sandstone, and sparsely vegetated pockets on orthic eutric brunisols (CitationHolland and Coen, 1982). Vegetation on sites with soils include white heather, yellow heather, arctic willow, white mountain avens, bladder locoweed, sedges, lichens, kruppelholz Engelmann spruce, and kruppelholz alpine fir.
Long-term climate records are not available for high elevation sites in the Canadian Rockies. The nearest long-term climate record is for the Banff townsite (1383 m a.s.l.; 51°11′N, 115°34′W), 29–64 km south and 813–1173 m lower than the survey ponds (; CitationEnvironment Canada, 2002). Mean annual air temperature for the last 100 years at Banff townsite was 2.5°C. Mean summer (June–September) air temperature was 12.1°C, but daily temperatures ranged from −1.4°C to 26.6°C. Of the last 100 years, the summer of 1999 was the 11th coolest (mean = 11.0°C), and the summer of 2000 was the 16th coolest (mean = 11.4°C). Mean annual precipitation for the last 100 years at Banff townsite was 467 mm, with 267 mm as rain and 200 mm as snow. Of the last 100 years, the year leading up to and including the ice-free season of 1999 (October 1998–September 1999) was the 37th wettest (495 mm), and the year leading up to and including the ice-free season of 2000 (October 1999–September 2000) was the 10th driest (362 mm). Therefore, in the vicinity of the study ponds the summer of 1999 was slightly cooler and much wetter than the summer of 2000.
SAMPLING PROTOCOL AND ANALYSES
We sampled 14 ponds once during the period of 23–27 July 1999. These 14 ponds and an additional 14 ponds were sampled once during the period of 25–30 July 2000 (). Ponds were selected to cover a range of elevations, drainage basin characteristics, sizes, depths, and bottom types.
Latitude (LAT), longitude (LONG), and elevation (ELV) were determined with a Global Position System (GPS) and 1:50,000 maps. Approximate surface area (AREA) of each pond was determined by visual estimation. Approximate maximum water depth (DEPTH) of each pond was determined with a depth line. We categorized the bottom (BOTT) as sediment (BTS), sediment and rock (BTSR), or rock (BTR), and the drainage basin (DRBN) as meadow (DBM), meadow and rock (DBMR), or rock (DBR). Water temperature (WTEMP) was measured with a handheld thermometer 0.2 m below the surface at the time of sampling. Conductivity (COND) was measured in situ with a Cole-Parmer® handheld TDSTestr 40 with automatic temperature correction.
Water was sampled by plunging pre-rinsed 500 ml polyethylene bottles below the water surface 0.5 m from each pond's edge. Half of the water was filtered in the field through Whatman® GF/F filters. Samples were analyzed for ammonium (NH4-N), nitrite plus nitrate (NO2-N+NO3-N), total phosphorus (TP), soluble reactive phosphorus (SRP), dissolved organic carbon (DOC), silica (Si), alkalinity (ALK), and pH by the University of Alberta's Water Laboratory (CitationMcMaster, 2003).
Algal nutrient limitation was assessed with DIN:TP ratios from the water column. DIN:TP, rather than TN:TP or DIN:SRP, agrees most strongly with nutrient limitation bioassays in other mountain lakes and ponds (CitationMorris and Lewis, 1988; CitationSickman, 2001). Ratios less than 1 indicate N-limitation, and greater than 4 indicate P-limitation. Ratios between 1 and 4 indicate intermediate limitation, i.e., reciprocal or co-limitation by N and P (CitationMorris and Lewis, 1988; CitationSickman, 2001).
Phytoplankton were sampled by filtering 0.4–1 L of pond water through Whatman GF/F filters, and epipelon were sampled by pooling three sediment surface samples collected with plastic Petri dishes (4.7 cm diameter × 0.5 cm depth) (CitationVinebrooke and Leavitt, 1999). Filters and Petri dishes were wrapped in aluminium foil and stored in the dark at −20°C until further analysis. Samples were freeze-dried for 24 h then extracted in a 10 ml mixture of acetone, methanol, and water (80:15:5 by volume) for 24 h at 10°C in the dark. Extracts were filtered (0.22 μm Acropore Nylon membrane), dried under nitrogen gas and stored in the dark at −30°C under nitrogen gas until analysis. Dried extracts were dissolved in a known volume of injection solvent of acetone, ion pairing reagent, and methanol (70:25:5 by volume) containing 3.2 mg L−1 of the internal standard Sudan II. Pigments were separated by high performance liquid chromatography (HPLC) with a Hewlett Packard® Model 1100 and a Rainin Model 200 C18 column (CitationVinebrooke et al., 2002).
Algal abundance and composition were inferred from concentrations of taxonomically diagnostic chlorophylls and carotenoids derived from high performance liquid chromatography (HPLC) (CitationMillie et al., 1993; CitationJeffrey et al., 1997; CitationSchmidt et al., 1998). Major algal groups identified with HPLC included cryptophytes (alloxanthin [All]), chlorophytes (chlorophyll b [Chlb], lutein [Lut], violaxanthin [Viol]), chromophytes (diadinoxanthin [Diad], fucoxanthin [Fuc], chlorophyll c [Chlc]), including diatoms (diatoxanthin [Diat]) and Cyanobacteria (echinenone [Ech], canthaxanthin [Can], zeaxanthin [Zea]), including colonial forms (myxoxanthophyll [Myx]).
STATISTICAL ANALYSIS
Physical and chemical variables were either log10 or square root transformed, and chlorophyll a concentrations were log10 transformed to approximate normal distribution before statistical analysis (CitationZar, 1999). Two-tailed t-tests were used to assess significant differences in the physical and chemical variables between 1999 and 2000. Univariate linear regressions followed by a stepwise backward multiple regression were used to determine significant relationships between DIN:TP ratios and physical and chemical variables in the 28 ponds sampled in 2000 using STATISTICA (CitationStatSoft, 1998).
To address the main objectives, the data were grouped into three sets: all 28 ponds sampled in 2000; the 14 ponds sampled in 1999, and the same 14 ponds resampled in 2000. The first dataset was used to identify the best physical and chemical predictors of patterns in phytoplankton and epipelon abundance and community composition across alpine ponds. Comparison of the remaining two datasets test whether these best predictors differed between the two summers. First, Pearson product-moment correlations with Bonferroni-adjusted probabilities were used to detect significant (P ≤ 0.05) correlations among physical and chemical variables in each of the three datasets using STATISTICA. Second, univariate linear regressions followed by a stepwise backward multiple regression were used to identify the physical and chemical variables that best explain phytoplankton and epipelon abundance (chlorophyll a) in each of the three datasets. Finally, physical and chemical variables that best explain patterns in the phytoplankton and epipelon community composition in each of the three datasets were identified with ordination analysis (CANOCO version 4; Citationter Braak and Šmilauer, 1998).
Detrended correspondence analysis (DCA) established that pigment variation was best explained by the linear response model, redundancy analysis (RDA) (Citationter Braak and Šmilauer, 1998). Pigment concentrations were log10(x+1) transformed to reduce the influence of rare or dominant pigments, which might otherwise dominate the analysis, and to increase normality of the data. Si was excluded from the RDA because it was not measured in all ponds. Nominal variables (BOTT and DRBN) were included as binary codes (value 0 or 1). Environmental datasets were screened to eliminate physical and chemical covariables and insignificant variables with a correlation matrix, forward selection, and a series of partially constrained RDAs (CitationVinebrooke and Graham, 1997). The final RDA models for each dataset and algal community included just independent and significant (P < 0.1) variables. The significance of the first two ordination axes was tested against 1000 Monte Carlo permutations. Variation partitioning was used to estimate how much of the variation in pigment concentrations can be explained by each physical and chemical variable in the final RDA (CitationAnderson and Gribble, 1998).
Results
PHYSICAL AND CHEMICAL FEATURES
Elevation of the 28 ponds ranged from 2150 to 2570 m a.s.l. (). Most ponds (68%) were located in alpine meadows, and only three ponds were located in rock basins. Ponds were small, with surface areas ranging from 50 to 6000 m2, and shallow, with depths ranging from 10 to 200 cm (). Most ponds (86%) were less than 2000 m2 in area and 90 cm deep. Ponds with large surface areas tended to be deep. Ponds ranged in shape from rectangular to circular, and most had steep-sided, U-shaped basins. Most ponds were found in interconnected groups of 2 to 4, and the remaining ponds lacked inflows and outflows. Most ponds (62%) had bottoms consisting of dark, highly organic unconsolidated sediment and only one (HS) had a bottom consisting of rock and no sediment. Higher elevation ponds were more likely to have bottoms and drainage basins containing rock whereas lower elevation ponds were more likely to have bottoms consisting of sediment and drainage basins consisting of alpine meadows. Water temperatures ranged from 0 to 24°C across the 28 ponds in 2000 (). In the 14 ponds sampled in both years, surface areas were smaller (P < 0.1) in 2000 than in 1999 ().
DOC concentrations were fairly high in comparison to alpine lakes and streams (). Ponds with the lowest DOC concentrations were typically found at higher elevations and had rocky bottoms, rocky drainage basins, and large surface areas. Ponds were relatively dilute (conductivity < 190 μS cm−1), suggesting that seepage rather than evaporation was a predominant water output. Low conductivity also suggests that precipitation rather than seepage was an important water input. Alkalinity was fairly low, and most ponds had close to neutral pH. Nitrogen and phosphorus were typically above detection (1 μg L−1; ). Ponds with higher nitrogen concentrations typically had lower phosphorus concentrations, larger surface areas, and rocky bottoms and were found at higher elevations. Silica was below 2.4 mg L−1 but always above detection (). In the 14 ponds sampled in both 1999 and 2000, pH, NH4-N, DIN, and TP were higher (P < 0.1) in 2000 than in 1999 ().
Correlation analysis of the physical and chemical variables measured in the 28 ponds in 2000 showed that TP and DIN:TP ratios, and alkalinity and pH were significantly correlated. Correlation analysis of the physical and chemical variables measured in the 14 ponds in 1999 and 2000 showed that alkalinity and conductivity were significantly correlated.
NUTRIENT LIMITATION
Based on DIN:TP ratios, 6 ponds were likely P-limited, 11 ponds were likely N-limited, 5 ponds likely had intermediate limitation, and 6 ponds were likely N-limited one year and had intermediate limitation the other year (). Ponds with high DIN:TP ratios tended to have deep water (), large surface areas (>2000 m2), rocky bottoms, and rocky drainage basins, and were found at higher elevations (>2300 m). Multiple regression analysis identified elevation and water temperature as the strongest predictors of DIN:TP ratios in the 28 ponds sampled in 2000 (log10DIN:TP = −7.31 + 0.0036ELV − 0.29WTEMP; P < 0.001; r 2 = 0.56).
PHYTOPLANKTON ABUNDANCE—ENVIRONMENTAL RELATIONSHIPS
In the 28 ponds sampled in 2000, phytoplankton abundance (chlorophyll a) ranged from close to detection (0.1 μg L−1) to 27.9 μg L−1 (). Based on univariate linear regressions, phytoplankton abundance significantly (P < 0.05) decreased with pond surface area, NO2-N+NO3–N, DIN:TP ratios, and pH, and increased with water temperature, TP, SRP, and DOC. Multiple regression analysis identified TP, pH, and conductivity as the strongest predictors of phytoplankton abundance in the 28 ponds sampled in 2000 ().
In the 14 ponds sampled in both years, phytoplankton abundance was only slightly higher in 2000 than in 1999 (). In 1999, phytoplankton abundance significantly increased with TP and decreased with DIN:TP ratios and pH, and multiple regression analysis identified pH and surface area as the strongest predictors (). In 2000, TP was identified as the only significant predictor of phytoplankton abundance in univariate and multiple regression analyses ().
PHYTOPLANKTON COMMUNITY COMPOSITION—ENVIRONMENTAL RELATIONSHIPS
Based on carotenoid analysis, phytoplankton typically consisted of non-diatom chromophytes (chrysophytes and dinoflagellates; diadinoxanthin, fucoxanthin, and chl c) and/or chlorophytes (lutein, chl b, and violaxanthin; ). Cyanobacteria (echinenone, canthaxanthin, zeaxanthin, and myxoxanthophyll) were usually detectable but were dominant in only two ponds (BML and TP). Diatoms (diatoxanthin) and cryptophytes (alloxanthin) were scarce in the phytoplankton.
RDA of the 28 ponds sampled in 2000 showed that phytoplankton community composition was best explained by TP, pH, and conductivity (). Together these three variables accounted for 32% of the total variance in the pigment concentrations in the first two RDA axes. The first axis (λ1 = 0.29) was significant (P < 0.01) and represented a gradient of TP and pH, each of which accounted for 9% of the explained variation. The first axis contrasted ponds with high TP concentrations and low pH, which consisted of a mixed community of cryptophytes (alloxanthin), chlorophytes (chl b, violaxanthin), chromophytes (chl c), diatoms (diatoxanthin), and colonial Cyanobacteria (myxoxanthophyll). Ponds with low TP concentrations and high pH contained higher proportions of chromophytes (diadinoxanthin, fucoxanthin) and Cyanobacteria (echinenone, canthaxanthin) (). Although insignificant, the second axis (λ1 = 0.03) represented a conductivity gradient, which captured 3% of the explained variation. Ponds with high conductivity generally had more non-diatom chromophytes (diadinoxanthin, fucoxanthin) and fewer Cyanobacteria (canthaxanthin, echinenone) and diatoms (diatoxanthin) than ponds with low conductivity ().
RDA of the phytoplankton data from 14 ponds sampled in both 1999 and 2000 indicated that the environmental variables that best explain phytoplankton community composition differed between the two years (). In 1999, TP, pH, and water temperature accounted for 66% of the total variance in the phytoplankton community composition (). In 2000, none of the environmental variables produced a significant primary axis. The most “important” (but nonsignificant) variables did not include any of the three significant variables from 1999 ().
EPIPELON ABUNDANCE—ENVIRONMENTAL RELATIONSHIPS
In the 28 ponds sampled in 2000, epipelon abundances (chlorophyll a) ranged from 0.67 to 39.31 μg cm−2 (). NO2-N+NO3–N was identified as the only significant predictor of epipelon abundance in univariate and multiple regression analyses ().
In the 14 ponds sampled in both years, epipelon abundance was over four times higher in 2000 than in 1999 (). In 1999, epipelon abundance was not related to NO2-N+NO3–N (P = 0.20) nor to any other environmental feature in univariate or multiple regression analysis (). In 2000, epipelon abundance significantly increased with DOC and decreased with maximum depth and conductivity. Multiple regression analysis identified maximum depth, NO2-N+NO3–N, and conductivity as the strongest predictors of epipelon abundance in 2000 ().
EPIPELON COMMUNITY COMPOSITION—ENVIRONMENTAL RELATIONSHIPS
Epipelon consisted mainly of non-diatom chromophytes (chrysophytes and dinoflagellates) (). Cyanobacteria and chlorophytes were usually present but in low concentrations. However, Cyanobacteria were dominant in one pond (SR299). Diatoms and cryptophytes made up a larger proportion of the epipelon communities than the phytoplankton communities but were still in low concentrations.
RDA of the 28 ponds sampled in 2000 showed that epipelon community composition was best explained by NO2-N+NO3–N, TP, and DOC (). Together these three variables accounted for 31% of the total variance in the pigment concentrations in the first two RDA axes. The first axis (λ1 = 0.26) was significant (P < 0.02) and represented a gradient of NO2-N+NO3–N and TP, which accounted for 13% and 9% of the explained variation, respectively. The first axis contrasted ponds with high NO2-N+NO3–N concentrations and low TP concentrations, which consisted of chromophytes (fucoxanthin) and colonial Cyanobacteria (myxoxanthophyll), from ponds with high TP and low NO2-N+NO3–N concentrations, which contained a higher proportion of noncolonial Cyanobacteria (echinenone) and chlorophytes (chlorophyll b) (). Although insignificant, the second axis (λ1 = 0.05) represented a DOC concentration gradient, which captured 4% of the explained variation. Ponds with high DOC concentrations generally had more diatoms (diatoxanthin), cryptophytes (alloxanthin), and chlorophytes (chlorophyll b) and fewer Cyanobacteria (myxoxanthophyll, echinenone, canthaxanthin) and non-diatom chromophytes (fucoxanthin, diatoxanthin, chlorophyll c) than ponds with low DOC concentration ().
RDA of the epipelon data from 14 ponds sampled in both 1999 and 2000 identified that the environmental variables that best explain epipelon community composition differed between the two years (). In 1999, elevation, pH, and conductivity accounted for 40% of the total variance in the epipelon community composition (). In 2000, water temperature and DOC accounted for 57% of the total variance in the epipelon community composition ().
Discussion
PHYSICAL AND CHEMICAL LIMNOLOGY
Based on their basin morphology, the majority of alpine ponds examined in this study represent thrust ponds (CitationIves, 1941). Thrust ponds are typically found in meadows and are characterized by bottoms of fine flocculent organic silt and steep-sided, U-shaped basins formed as a result of ice thrust (CitationIves, 1941). Thrust ponds are also prevalent in the Arctic but are called ice-wedge polygons (CitationHobbie, 1980). Dynamic freeze-thaw cycles and water budgets make the basins of thrust ponds quite stable over time (CitationIves, 1941; CitationHobbie, 1980). Thrust ponds may be interconnected but typically lack inflows and outflows, and consequently their water budgets depend on precipitation, evaporation, and seepage. This dependence makes alpine ponds particularly vulnerable to climate change, similar to arctic ponds (CitationDouglas and Smol, 1994).
Ponds sampled in this study had fairly high DOC concentrations (mean 5.4 mg L−1) in comparison to nearby lakes (<2.5 mg L−1) (CitationVinebrooke and Leavitt, 1999), probably because the ponds had organic bottoms and higher bottom area to water volume ratios and therefore more autochthonous DOC than lakes. Furthermore, pond catchments had more vegetation and therefore more allochthonous DOC inputs than lakes. DOC concentrations in these alpine ponds generally decreased at higher elevations as the amount of vegetation in their catchments decreased, similar to mountain lakes (CitationVinebrooke and Leavitt, 1999). DOC concentrations were lower and likely less aromatic, due to photodegradation, than in low elevation lakes. Low DOC concentrations combined with the intense ultraviolet radiation in the alpine altitudes (CitationBlumthaler et al., 1997) and shallow depths of ponds, likely exposes the communities in alpine ponds to high biologically damaging ultraviolet radiation.
NUTRIENT LIMITATION
Based on DIN:TP ratios, the majority of alpine ponds sampled in Banff National Park are likely N-limited. This is the first time nutrient limitation has been assessed in North American alpine ponds. In contrast, nutrient limitation has been assessed in numerous mountain lakes throughout the western United States and N-limitation is common (CitationMorris and Lewis, 1988; CitationSickman, 2001; CitationLafrançois, 2002). Similar to alpine lakes, N-limitation makes alpine ponds particularly susceptible to current increases in atmospheric N deposition (CitationBaron et al., 2000).
Although N-limitation was prevalent, P-limitation did occur, particularly in ponds at high elevations with rock drainage basins. This agrees with another study that found mountain lakes with steep, rocky watersheds have high N concentrations and N:P ratios, indicative of P-limitation, and lakes with gently sloping, vegetated watersheds have high P concentrations and low N:P ratios, indicative of N-limitation (CitationKamenik et al., 2001). If climate warming stimulates upward treeline migration and vegetation development, the number of P-limited alpine ponds may decrease.
ALGAL COMMUNITY COMPOSITION
Phytoplankton community composition varied greatly among the 28 alpine ponds surveyed, whereas community composition was quite similar among 20 mountain lakes and ponds surveyed using similar methods (CitationVinebrooke and Leavitt, 1999). The opposite was expected, because CitationVinebrooke and Leavitt's (1999) lakes and ponds were surveyed along an elevation gradient over four weeks, and some systems contained fish, whereas these ponds were surveyed all in the alpine over five days, and lacked fish. The variability in phytoplankton community composition may reflect the considerable spatial and temporal variability of the physical and chemical features in alpine ponds (CitationMcMaster, 2003).
Similar to other mountain lakes and ponds (CitationVinebrooke and Leavitt, 1999), algal communities in alpine ponds consisted mainly of non-diatom chromophytes and chlorophytes. However, Cyanobacteria were more important and diatoms were less important in alpine ponds than in other mountain lakes and ponds (CitationVinebrooke and Leavitt, 1999). The dominance of non-diatom chromophytes and chlorophytes in alpine ponds is likely due to the abundance of small taxa in these groups that typically do extremely well in harsh environments, such as the alpine (CitationReche et al., 1994; CitationLafrançois, 2002). The occurrence of Cyanobacteria in alpine ponds likely reflects their ability to live in nitrogen-deficient waters that undergo periods of desiccation and intense ultraviolet radiation. However, the sensitivity of Cyanobacteria to rapid shifts in environmental conditions, which occur often in alpine ponds (CitationMcMaster, 2003), may limit their dominance. The scarcity of diatoms in alpine ponds is likely due to their sensitivity to desiccation (CitationMosisch, 2001) and grazing (CitationMcMaster, 2003), and to silica being below or close to limiting concentrations (<0.5 mg L−1).
PHYTOPLANKTON ABUNDANCE AND COMMUNITY COMPOSITION—ENVIRONMENTAL RELATIONSHIPS
TP, pH, and conductivity were the main environmental gradients that controlled phytoplankton abundance and community composition in the 28 alpine ponds surveyed in 2000. These findings contrast with those of CitationVinebrooke and Leavitt (1999), who sampled mountain lakes and ponds in Banff National Park using similar methods. They found phytoplankton abundance was explained by none of the environmental variables measured, and phytoplankton community composition was best explained by zooplankton biomass, elevation, and DOC (CitationVinebrooke and Leavitt, 1999). However, similar to this study they found that conductivity was an important environmental gradient (CitationVinebrooke and Leavitt, 1999). The differences between results probably occurred because we did not sample zooplankton, and all our ponds were located in the alpine rather than along an elevation gradient. Although similar DOC concentration gradients were sampled in both studies, montane and subalpine lakes sampled by CitationVinebrooke and Leavitt (1999) likely had more colored allochthonous DOC than alpine ponds. Therefore, their DOC concentration gradient likely represents a larger ultraviolet radiation attenuation gradient than ours.
Phytoplankton abundance and the abundance of most algal groups in alpine ponds were positively related to water column TP. This is consistent with results from low elevation lakes (CitationMcCauley et al., 1989; CitationWatson et al., 1997). Non-diatom chromophytes, such as chrysophytes, were unrelated to water column TP in alpine ponds. Others have also found that the abundance of chrysophytes was unrelated to TP and that chrysophytes typically do well in low nutrient environments with high N:P ratios (CitationSandgren, 1988; CitationWatson et al., 1997).
Water column pH was also a significant predictor of phytoplankton abundance and community composition in alpine ponds. As pH decreased, overall abundance and abundance of most phytoplankton groups increased. It is important to note that our pH gradient was neutral to alkaline (6.4–8.4); therefore, the community composition shifts relative to pH should be different from community shifts expected along an acidic pH gradient. The negative relationship between pigment abundances and pH is likely related to the negative relationship between pH and TP, and to the effect of pH on nitrogen availability. Since algae in alpine ponds are likely N-limited, and NH4 + is likely their preferred N source, it was expected that phytoplankton abundance would decrease as pH increased.
Consistent with results from other montane ponds and lakes (CitationVinebrooke and Leavitt, 1999), conductivity was a significant predictor of phytoplankton abundance and community composition in alpine ponds. In another study the relationship between conductivity and algal abundance has been attributed to conductivity being a surrogate measure of inorganic nutrient availability (CitationVinebrooke and Leavitt, 1999), but we have no evidence to confirm this.
EPIPELON ABUNDANCE AND COMMUNITY COMPOSITION—ENVIRONMENTAL RELATIONSHIPS
In the 28 alpine ponds surveyed, epipelon abundance was best explained by NO2-N+NO3–N, and epipelon community composition was best explained by TP and DOC in addition to NO2-N+NO3–N. In contrast, CitationVinebrooke and Leavitt (1999) found that epipelon abundance in mountain lakes and ponds was best explained by DOC, and community composition was explained by none of the environmental variables measured. They attributed their weak epipelon-environmental relationship to the importance of substratum or algal mat chemistry instead of water column chemistry in regulating nutrient availability in montane lakes (CitationVinebrooke and Leavitt, 1999). Conversely, our strong epipelon-environmental relationship likely reflects the importance of water column chemistry in addition to sediment chemistry for epipelon in these shallow alpine ponds. The boundary between the water column and sediment is usually not distinct in shallow lakes and ponds, because sediment and epipelon are being constantly suspended into the water column (CitationMcMaster, 2003; CitationHobbie, 1980).
Similar to other N-limited systems (CitationLafrançois, 2002), epipelon abundance in alpine ponds increased significantly with water column NO2-N+NO3–N. This further suggests that epipelon rely on water column nutrients in addition to nutrients from the sediment. Both N and P were significant predictors of epipelon community composition in alpine ponds. N and P were inversely related; therefore, shifts in epipelon communities can be related to changes in the N:P ratios as well as individual nutrient concentrations. Based on the resource-ratio theory (CitationSmith and Bennett, 1999) and results from nutrient enrichment experiments in alpine ponds (CitationMcMaster, 2003), we expected N-fixing Cyanobacteria to thrive in low N and high P environments (low N:P), and chlorophytes and chromophytes to thrive in high N and low P environments (high N:P). We did find that noncolonial Cyanobacteria were common at low N:P, and chromophytes were common at high N:P but, in contrast to what was expected, chlorophytes were common at low N:P, and colonial Cyanobacteria were common at high N:P. Chlorophytes usually have a high N optimum (CitationSandgren, 1988) and flourish at high N:P; however, many of the chlorophytes species present in alpine ponds are small with large surface area:volume ratios (McMaster, unpublished data) thus do well in low nutrient environments (CitationSandgren, 1988). Most colonial Cyanobacteria taxa found in these ponds are not capable of N-fixing and therefore likely to be N-limited (CitationMcMaster, 2003).
Water column DOC was a significant predictor of epipelon community composition in alpine ponds. Much of the response to DOC can be explained by its correlation to TP and NO2-N+NO3–N, and by the ability of DOC to attenuate photosynthetically active radiation (PAR) and biologically damaging ultraviolet radiation (UVR). Epipelon communities in alpine ponds with higher DOC concentrations and therefore low PAR and UVR generally had more diatoms (diatoxanthin), cryptophytes (alloxanthin), and chlorophytes (chlorophyll b), and fewer Cyanobacteria (myxoxanthophyll, echinenone, canthaxanthin) and non-diatom chromophytes (fucoxanthin, diatoxanthin, chlorophyll c) than ponds with lower DOC concentrations and therefore high PAR and UVR. Most diatom taxa are sensitive to UVR because they are unable to efficiently produce photoprotective pigments (CitationRoy, 2000), and they have a poor capacity for repair after UVR damage (CitationQuesada and Vincent, 1997). In contrast, most Cyanobacteria are able to withstand high UVR because they are able to produce photoprotective pigments (CitationRoy, 2000), and have a good repair capacity (CitationQuesada and Vincent, 1997). Cryptophytes and many forms of chlorophytes do very well in low PAR conditions and therefore high DOC concentrations (CitationKlug and Cottingham, 2001). Non-diatom chromophytes, such as chrysophytes and dinoflagellates, can actively avoid UVR and consequently flourish in low DOC systems (CitationVinebrooke and Leavitt, 1999). In other respects, our results are inconsistent with previous work. CitationVinebrooke and Leavitt (1998) found epipelon in alpine lakes were unresponsive to DOC because most taxa were capable of active avoidance of UVR. Furthermore, they found that epilithic Cyanobacteria in mountain ponds and lakes increase with DOC, and they attributed this to Cyanobacteria's heterotrophic metabolism (CitationVinebrooke and Leavitt, 1999). In these alpine ponds, bottom sediment rather than the water column is probably the main carbon source for the epipelon.
INTERANNUAL VARIABILITY IN THE ALGAL—ENVIRONMENTAL RELATIONSHIPS
The physical and chemical variables that best explain phytoplankton and epipelon abundance and community composition in 14 alpine ponds differed between two summers. Interannual variability in phytoplankton-environmental relationships was also documented in high mountain lakes in Spain (CitationVillar-Argaiz et al., 2001). As we found, their interannual variability in relationships coincided with significant changes in the environmental variables between years (CitationVillar-Argaiz et al., 2001). In our ponds, surface area decreased and NH4-N, DIN, TP, and pH increased significantly as precipitation amounts decreased significantly and temperatures increased slightly in 2000. Similarly, interannual fluctuations of primary production in subalpine lakes were linked to precipitation amounts (CitationGoldman et al., 1989). Interannual variability in algal-environmental relationships may also have resulted from changes in the environmental variables not measured, such as grazing. Grazers can alter community composition and impact available nutrient stoichiometry and, therefore, changes in grazer abundance or composition could have direct and indirect effects on the algal-environmental relationships. However, because our results are correlation based, experiments are needed to confirm if the algal-environmental relationships actually change interannually and determine the drivers of these changes.
Summary
These results suggest that algal communities in alpine ponds are controlled by different physical and chemical variables than in nearby mountain lakes and ponds (CitationVinebrooke and Leavitt, 1999). Yet similar to CitationVinebrooke and Leavitt's (1999) results, our results suggest that algal communities in alpine ponds may be sensitive to climate change because the chemical variables that we found to best explain patterns in abundance and community composition, such as pH, conductivity, and DOC, have been linked to changes in climate in alpine ponds (CitationMcMaster, 2003) or other systems (CitationPsenner and Schmidt, 1992; CitationSchindler et al., 1996). However, because we found major interannual variability in the environmental-algal relationships, we will not make predictions on how the algal communities may respond to environmental change. We believe that it is necessary to have several years of data to generate realistic predictive models for the future or deductive models about the past.
FIGURE 1. Study ponds are located north of Banff townsite (51°27′–51°44′N, 115°39′–115°56′W) in Banff National Park, Alberta, Canada. Ponds are numbered alphabetically, and the full name and abbreviations are given. Ponds sampled in both 1999 and 2000 have a star (*) next to their abbreviation
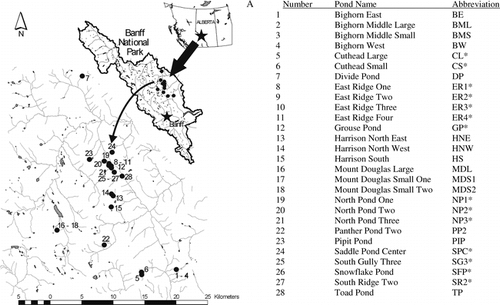
FIGURE 2. DIN:TP ratios measured from the water column of 28 alpine ponds in Banff National Park at end of July 1999 and/or 2000. Ponds are ordered in increasing depth to the right. Ponds sampled in both 1999 and 2000 are grouped together. Dashed lines define nutrient limitation cut-offs as N-limited, DIN:TP ratios ≤1, and P-limited, DIN:TP ratios ≥4 (CitationMorris and Lewis 1988; CitationSickman 2001). Ponds with ratios between one and four were considered to have intermediate limitation, i.e., reciprocal or co-limitation by N and P. Gray bars signify ponds that were N-limited in epilithon nutrient enrichment experiments (CitationMcMaster, 2003)
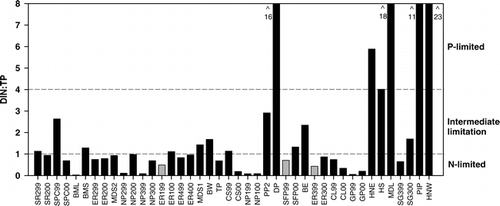
FIGURE 3. Phytoplankton abundance and community composition inferred from pigments from the water column of 28 alpine ponds in Banff National Park at end of July 1999 and/or 2000. Ponds are ordered in increasing depth to the right
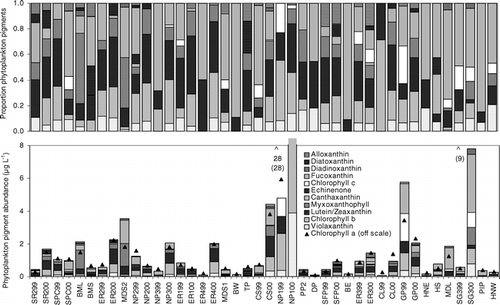
FIGURE 4. Correlation triplot based on partially constrained redundancy analysis representing the influence of total phosphorus, pH, and conductivity on phytoplankton community composition as inferred from pigments from 28 ponds sampled in 2000. The first two axes explain 31.9% of the variation in the pigment concentrations. The relative length of an environmental arrow corresponds to the relative importance of that variable explaining the observed variance in the pigment data. The length of a pigment arrow corresponds to its relative importance in the differences across ponds. The placement of arrows relative to other arrows represents their approximate correlation to each other
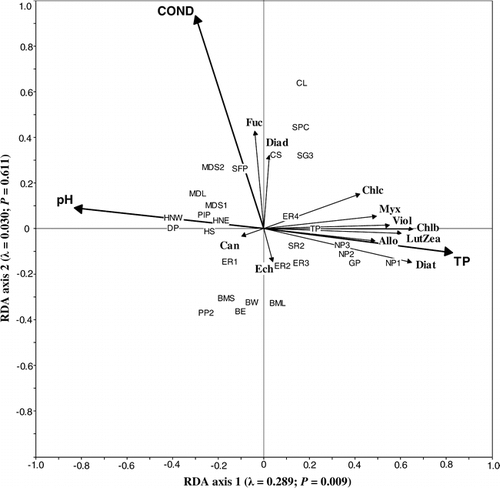
FIGURE 5. Correlation triplots based on partially constrained redundancy analysis on phytoplankton community composition as inferred from pigments from 14 ponds sampled in 1999 (a) and 2000 (b). RDA on 1999 data represents the influence of total phosphorus, pH, and water temperature. The first two axes explain 66.2% of the variation in pigment concentrations. RDA on 2000 data was not significant but we include the four most important variables, maximum depth, conductivity, surface area, and ammonium concentration. Each axis is labelled with its eigenvalue and P-value
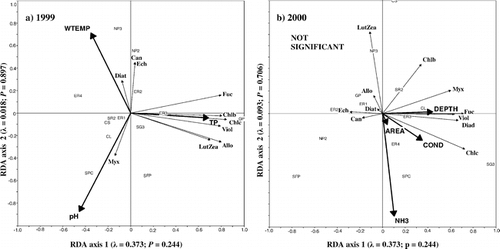
FIGURE 6. Epipelon abundance and community composition inferred from pigments from the sediment of 28 alpine ponds in Banff National Park at end of July 1999 and/or 2000. Ponds are ordered in increasing depth to the right
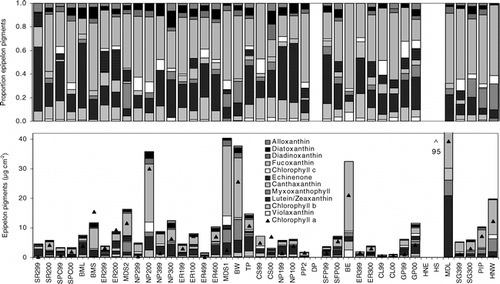
FIGURE 7. Correlation triplot based on partially constrained redundancy analysis representing the influence of nitrite plus nitrate, total phosphorus, and dissolved organic carbon concentrations on epipelon community composition as inferred from pigments from 28 ponds sampled in 2000. The first two axes explain 31.0% of the variation in the pigment concentrations. Each axis is labelled with its eigenvalue and P-value
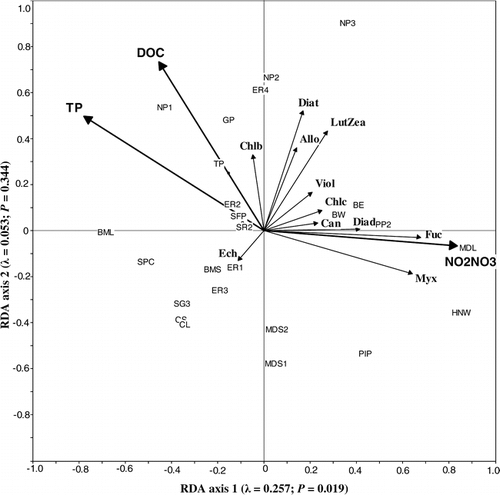
FIGURE 8. Correlation triplots based on partially constrained redundancy analysis on epipelon community composition as inferred from pigments from 14 ponds sampled in 1999 (a) and 2000 (b). RDA on 1999 data represents the influence of pH, conductivity, and elevation. The first two axes explain 40.5% of the variation in pigment concentrations. RDA on 2000 data represents the influence of dissolved organic carbon concentration and water temperature. The first two axes explain 57.1% of the variation in pigment concentrations. Each axis is labelled with its eigenvalue and P-value
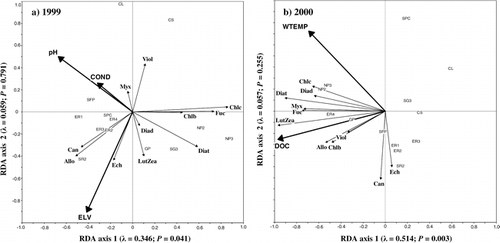
TABLE 1 Mean (standard deviation), minimum, and maximum values for physical, chemical, biological variables measured in 28 alpine ponds in 2000 in Banff National Park, Canada. Means (standard deviation) are also given for 14 ponds sampled in both 1999 and 2000. Significant differences between 1999 and 2000 data are indicated (* = P < 0.1, ** = P < 0.05, *** = P < 0.01)
TABLE 2 Stepwise backward multiple regression results showing the physical and chemical variables that best explain phytoplankton (ChlaP) or epipelon (ChlaE) abundance in alpine ponds in Banff National Park, Canada. All reported equations and parameters were significant (P < 0.05)
Acknowledgments
Funding for this study was provided by the Biodiversity Grant Program (University of Alberta and Alberta Conservation Association), Circumpolar Boreal Alberta Research grant, and Friends of Banff National Park Research grant to McMaster and discovery grant from the Natural Sciences and Engineering Council of Canada (NSERC 89673) to Schindler. McMaster was supported by a NSERC postgraduate scholarship and a University of Alberta teaching assistantship. Field assistance was provided by D. Oickle and W. Pruitt. J. Brzustowski and S. Tank provided critical reviews of an early draft of this manuscript.
Notes
Revised ms submitted December 2004
References Cited
- Anderson, M. J. and N. A. Gribble . 1998. Partitioning the variation among spatial, temporal and environmental components in a multivariate dataset. Australian Journal of Ecology 23:158–167.
- Anderson, R. S. 1971. Crustacean plankton of 146 alpine and subalpine lakes and ponds in Western Canada. Journal of Fisheries Research Board of Canada 28:311–321.
- Anderson, R. S. 1974. Crustacean plankton communities of 340 lakes and ponds in and near the national parks of the Canadian Rocky Mountains. Journal of the Fisheries Research Board of Canada 31:855–869.
- Baron, J. A. , H. M. Rueth , A. M. Wolfe , K. R. Nydick , E. J. Allstott , J. T. Minear , and B. Moraska . 2000. Ecosystem response to nitrogen deposition in the Colorado Front Range. Ecosystems 3:352–368.
- Bennion, H. and M. A. Smith . 2000. Variability in the water chemistry of shallow ponds in southeast England, with special reference to the seasonality of nutrients and implications for modeling trophic status. Hydrobiologia 436:145–158.
- Blumthaler, M. W. , W. Ambach , and R. Ellinger . 1997. Increase in solar UV radiation with altitude. Journal of Photochemistry and Photobiology 39:130–134.
- Bowman, W. D. 2000. Biotic controls over ecosystem response to environmental change in alpine tundra of the Rocky Mountains. Ambio 29:396–400.
- Douglas, M. S V. and J. P. Smol . 1994. Limnology of high arctic ponds (Cape Herschel, Ellesmere Island, N.W.T). Archiv für Hydrobiologie 131:401–434.
- Environment Canada, 2002. Database for Banff Town Site available through Environment Canada's National Climate Data and Information Archive. (http://climate.weatheroffice.ec.gc.ca/climateData/canada_e.html) Site last accessed 24 May 2005.
- Fyfe, J. C. and G. M. Flato . 1999. Enhanced climate change and its detection over the Rocky Mountains. Journal of Climate 12:230–243.
- Goldman, C. R. , A. Jassby , and T. Powell . 1989. Interannual fluctuations in primary production: Meteorological forcing at two subalpine lakes. Limnology and Oceanography 34:310–323.
- Hauer, F. R. , J. S. Baron , D. H. Campbell , K. D. Fausch , S. W. Hostetler , S. W. Leavesley , P. R. Leavitt , D. M. McKnight , and J. A. Stanford . 1997. Assessment of climate change and freshwater ecosystems of the Rocky Mountains, US and Canada. Hydrological Processes 11:903–924.
- Hobbie, J. E. 1980. Limnology of Tundra Ponds: Barrow Alaska. Stroudsburg, Pennsylvania: Dowden, Hutchinson and Ross, Inc., 514 pp.
- Holland, W. D. and G. M. Coen . 1982. Ecological (biophysical) land classification of Banff and Jasper National Parks. Volume II: Soil and Vegetation Resources and Map Supplement. Alberta Institute of Pedology.
- Ives, R. L. 1941. Tundra Ponds. Journal of Geomorphology 4:285–296.
- Jeffrey, S. W. , R. F C. Mantoura , and S. W. Wright . 1997. Phytoplankton pigments in oceanography. Paris: UNESCO Publishing.
- Kamenik, C. , R. Schmidt , G. Kum , and R. Psenner . 2001. The influence of catchment characteristics of the water chemistry of mountain lakes. Arctic, Antarctic, and Alpine Research 33:404–409.
- Klug, J. L. and K. L. Cottingham . 2001. Interactions among environmental drivers: Community responses to changing nutrients and dissolved organic carbon. Ecology 82:3390–3403.
- Lafrançois, B. M. 2002. Algal and invertebrate response to atmospheric nitrogen deposition in Rocky Mountain lakes. Ph.D. thesis, Colorado State University, Fort Collins, Colorado, U.S.A.
- McCauley, E. , S. B. Watson , and J. A. Downing . 1989. Sigmoid relationships between nutrients and chlorophyll among lakes. Canadian Journal of Fisheries and Aquatic Sciences 46:1171–1175.
- McMaster, N. L. 2003. The effects of climate change and nitrogen deposition on the limnology of alpine ponds. M.Sc. Thesis, University of Alberta, Edmonton, Alberta, Canada.
- Millie, D. F. , H. W. Paerl , and J. P. Hurley . 1993. Microalgal pigment assessments using HPLC: A synopsis of organismal and ecological applications. Canadian Journal of Fisheries and Aquatic Sciences 505:2513–2526.
- Morris, D. P. and W. M. Lewis . 1988. Phytoplankton nutrient limitation in Colorado mountain lakes. Freshwater Biology 20:315–327.
- Mosisch, T. D. 2001. Effects of desiccation on stream epilithic algae. New Zealand Journal of Marine and Freshwater Research 35:173–179.
- Neldner, K. N. and R. W. Pennak . 1955. Seasonal faunal variations in a Colorado alpine pond. American Midland Naturalist 53:419–430.
- Psenner, R. and R. Schmidt . 1992. Climate-driven pH control of remote alpine lakes and effects of acid deposition. Nature 356:781–783.
- Quesada, A. and W. F. Vincent . 1997. Strategies of adaptation by Antarctic Cyanobacteria to ultraviolet radiation. European Journal of Phycology 32:335–342.
- Reche, I. , P. Sachez-Castillo , and P. Carrillo . 1994. The principal regulating mechanism on the structure of the phytoplankton community in a high mountain lake. Archiv für Hydrobiologie 130:163–178.
- Roy, S. 2000. Strategies for the minimization of UV-induced damage. In: deMora, S., Demers, S., and Vernet, M. (eds.), The Effects of UV Radiation in Marine Ecosystems. Cambridge: Cambridge University Press, 177–205.
- Sandgren, C. D. 1988. The ecology of chrysophyte flagellates: Their growth and perennation strategies as freshwater phytoplankton. In: Sandgren, C. D. (ed.), Growth and reproductive strategies of freshwater phytoplankton. Cambridge: Cambridge University Press, 9–104.
- Schindler, D. W. , S. E. Bayley , and B. R. Parker . 1996. The effects of climatic warming on the properties of boreal lakes and streams at the Experimental Lakes Area, northwestern Ontario. Limnology and Oceanography 41:1004–1017.
- Schmidt, H. , F. Bauer , and B. Berndstich . 1998. Determination of algal biomass with HPLC pigment analysis from lakes of different tropic state in comparison to microscopically measured biomass. Journal pf Plankton Research 20:1651–1661.
- Schmitz, E. H. 1959. Seasonal biotic events in two Colorado alpine tundra ponds. American Midland Naturalist 61:424–445.
- Sickman, J. O. 2001. Comparative analysis of nitrogen biogeochemistry in high elevation ecosystems. Ph.D. thesis, University of California, Santa Barbara, California, U.S.A.
- Smith, V. H. and S. J. Bennett . 1999. Nitrogen:phosphorus supply ratios and phytoplankton community structure in lakes. Archiv für Hydrobiologie 146:37–53.
- Smol, J. P. and B. F. Cumming . 2000. Tracking long-term changes in climate using algal indicators in lake sediments. Journal of Phycology 36:986–1011.
- StatSoft, 1998. STATISTICA. Tulsa, Oklahoma: StatSoft, Inc.
- ter Braak, C. J F. and P. Šmilauer . 1998. CANOCO reference manual and user's guide to CANOCO for Windows: Software for Canonical community ordination (version 4). New York: Microcomputer Power.
- Villar-Argaiz, M. , J. M. Medina-Sanchez , and L. Cruz-Pizarro . 2001. Inter- and intra-annual variability in the phytoplankton community of a high mountain lake: the influence of external (atmospheric) and internal (recycled) sources of phosphorus. Freshwater Biology 46:1017–1034.
- Vinebrooke, R. D. and M. D. Graham . 1997. Periphyton assemblages as indicators of recovery in acidified Canadian Shield lakes. Canadian Journal of Fisheries and Aquatic Sciences 54:1557–1568.
- Vinebrooke, R. D. and P. R. Leavitt . 1998. Direct and interactive effects of allochthonous dissolved organic matter, inorganic nutrients, and ultraviolet radiation on an alpine littoral food web. Limnology and Oceanography 43:1065–1081.
- Vinebrooke, R. D. and P. R. Leavitt . 1999. Phytobenthos and phytoplankton as potential indicators of climate change in mountain lakes and ponds: a HPLC-based pigment approach. Journal of North American Benthological Society 18:14–32.
- Vinebrooke, R. D. , S. S. Dixit , M. D. Graham , J. M. Gunn , Y. Chen , and N. Belzile . 2002. Whole-lake algal responses to a century of acidic industrial deposition on the Canadian Shield. Canadian Journal of Fisheries and Aquatic Sciences 59:483–493.
- Watson, S. B. , E. McCauley , and J. A. Downing . 1997. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnology and Oceanography 42:487–495.
- Zar, J. H. 1999. Biostatistical Analysis. New Jersey: Prentice Hall, 663 pp.