Abstract
The modifications of humus characteristics as a result of the establishment and the increasing cover of Rhododendron ferrugineum L. (Ericaceae) in a subalpine meadow were studied in three sites in the northwestern French Alps corresponding to a west–east transect between wet and dry Alps (“Chaîne de Belledonne,” “Massif du Taillefer,” “Briançonnais”). The physical and chemical parameters of humus and biological parameters were studied during the successive studies.
As opposed to other Ericaceae heathlands on siliceous soils (Vaccinium sp., Erica sp., Calluna vulgaris), with an increasing Rhododendron cover, the humus structure changed while some other parameters remain stable or increased. The development of Rhododendron populations on calcareous soils (Briançonnais) with maintenance of a calcareous humus has never been checked before.
The modifications of the Rhododendron environment under its canopy led to an improvement in its growth conditions (positive feedback).
Introduction
The study of vegetation dynamics concerns changes in species composition, vegetation physiognomy, and the physical environment of the plants. Individuals or populations can often compete with neighboring individuals or populations in two forms (CitationMiles, 1979): a first form where the more successful species use environmental ressources (light, water, nutrients, etc.) at the expense of the lesser competitive ones. In a second form plants frequently modify their own environment and consequently the environment of neighboring species. These ideas are well known, and numerous data have allowed the formulation of a theoretical plant succession pattern (CitationLepart and Escarre, 1983). Many studies have shown how plants could affect environmental conditions, particularly soil functioning (Vinton and Burke, 1995; Hobbie, 1995; Northup, 1995; CitationBever, 1994; Gross et al., 1995; Ozinga et al., 1997; Lechowicz and Bell, 1991). Humus form, nitrogen and carbon content, microbial activity, soil pH, and cation exchange capacity are parameters subjected to changes induced by vegetation (Miles, 1985; Richter et al., 1994; Vinton and Burke, 1995; Tian et al., 1997; Groten and Bruelheide, 1997; Saetre, 1998; Watkinson, 1998). Moreover, the more a plant species concentrates biomass and nutrients under its canopy, the longer it will persist on a site and the greater its effect on the environment (Richter et al., 1994; Vinton and Burke, 1995). Hovewer, very few studies mention how a given species improves its environment for its own advantage and not only by means of directly inhibiting other species (CitationBerendse, 1994; CitationBever, 1994; CitationBever et al., 1997; CitationVan der Puten, 1997; CitationWilson and Agnew, 1992).
Ericaceous shrubs are known to generate weakly structured humus which can reduce the biological activity of soil (CitationDuchaufour, 1977; CitationBonneau, 1980; CitationPornon and Doche, 1995). Ericaceous leaves contain phenolic compounds which make phenol-protein complexes that are very resistant and able to reduce or stop nitrogen mineralization (Handley, 1961, in CitationBoullard, 1964; CitationSchvartz, 1975; CitationHaynes and Goh, 1978). These phenolic counpounds can also inhibit mycorrhizal fungi growth and decrease tree germination and seedling growth in species such as Picea abies (CitationPellissier, 1994).
Ericaceous shrubs are able to monopolize space and to structure stable plant communities with homogeneous physiognomy on barren, acidophilous, and unfertile grasslands. CitationGilot (1967) and CitationRichard and Pautou (1982) also mention the presence of Rhododendron ferrugineum on thick, brown, leached soils on calcareous bedrock, following an old decarbonation process.
These closed heathlands have biotic and mechanical resistance to colonization by trees. The maintenance of this resistance depends on the life span of individuals, on their potential for sexual and/or asexual reproduction, and on the demographic structure of their populations (CitationGimingham, 1972, Citation1978; CitationClement and Touffet, 1976; CitationMiles, 1981; CitationPrentice et al., 1987; CitationDoche et al., 1997). For example, the evolution of Calluna heathland can be cyclic (CitationBarclay-Estrup and Gimingham, 1969), gradual (CitationDoche, 1990), or regressive (CitationCoquillard and Gueugnot, 1991).
In this study, physical, chemical, and biological properties of humus were studied along with a secondary successional gradient in two acidophilous and one calcareous meadow of R. ferrugineum (Ericaceae) heathlands on three sites in the northwestern Alps (1600–2200 m). The main objectives of the study were to determine which changes in humus quality could be induced by R. ferrugineum shrubs and if R. ferrugineum heathlands have similar detrimental consequences on humus quality at low and high altitudes.
Studied Species and Study Sites
Rhododendron ferrugineum L. is an ericaceous evergreen shrub, reaching a height of 70–80 cm. R. ferrugineum forms large heathlands on the north- and northwest-facing slopes at a subalpine level (1600–2100 m) in the French Alps. This species can constitute dense populations with almost 100% of cover, and a single genotype can occupy a large surface and sometimes form a dense patch, suggesting that this species adopts a phalanx growth form (CitationLovett Doust, 1981; CitationPornon et al., 1997) with limited intermingling of some genets (CitationEscaravage et al., 1998). A clone about 300 years old (7 m in slope direction) has been investigated using molecular biology techniques (CitationEscaravage et al., 1998).
Along a successional sequence (meadow → open heathland → closed heathland), R. ferrugineum shows complementary reproductive strategies. Sexual reproduction decreases and vegetative reproduction increases. The direction toward which the clones extend is mainly determined by the topography (slopes) rather than by the need to escape unsafe sites or to reach new resources. In closed heathland (cover of 90–95%), vegetative reproduction only is maintained (CitationPornon et al., 1997).
Three study sites were selected in the northwestern French Alps (): the “Chaîne de Belledonne” (45°25′N, 6°10′E), the “Massif du Taillefer” (45°4′N, 5°58′E), and the “Briançonnais” (44°50′N, 6°50′E). A west–east transect went from wet Alps (Belledonne), favorable to Rhododendron, to dry Alps (Briançonnais), where shrub potential is reduced whatever the source rock type is. In the inner Alps (Briançonnais), the most dispersed Rhododendron heathlands grow over calcareous rocks and siliceous rocks; the Rhododendron patches (5–10 m2) do not seem to be able to monopolize meadow areas. In the external (Belledonne) and intermediate Alps (Taillefer), the closed heathlands extend over several hectares in north-facing slopes (CitationRichard and Pautou, 1982; CitationOzenda, 1981, Citation1985), although a Rhododendron population, which does not compete with other offensive woody species (shrubs, trees), needs 150 years to extend over a meadow area and to occupy 80–90% of space (CitationPornon, 1994).
The environmental features of each site are summarized in .
Methods
STRUCTURAL AND BIOLOGICAL RHODODENDRON CHARACTERISTICS
In each site (Belledonne, Taillefer, Briançonnais), a succession of three physiognomic stages was studied: a Nardus stricta meadow (Rhododendron cover: 0–5%), an open Rhododendron heathland (cover: 30–40%), and a closed Rhododendron heathland (80–96%). In each site, these three stages were 100 m apart. In this study, a synchronous approach was used (different physionomic stages were compared on the same date) because of the slow extension rates of heathlands.
At each site, the Rhododendron biomass was measured by random choice (cut and weighing 5 × 1 m2 plots at Belledonne, Taillefer) or estimation (individual number × mean height at Briançonnais). The heathland heights and covers were quantified at all sites. The colonization rates were estimated by determining the different age groups in Rhododendron populations; the age of each ramet was determined by counting growth rings after cutting the biggest stem (Belledonne, Taillefer). At Briançonnais, the age of the biggest stems found above each soil pit was analyzed in order to determine the influence periods of Rhododendron on the humus (structure, physical and chemical characteristics).
PHYSICAL AND CHEMICAL ANALYSIS OF HUMUS
Three soil profiles per physiognomic stage and per site were visually described to define the soil types. A set of 10 samples was carried out for analysis at deep horizons, under the meadows, in order to define the precise soil type of each site.
Three humus samples per physiognomic stage at each site were taken in the A1 horizon (0–10 cm; 27 samples total). In the open and closed heathland, soil pits were dug beneath Rhododendron crowns and just beside the oldest stems; in the meadow, the humus and deeper horizons were sampled under grass cover.
The chemical parameters of humus (pH, C/N, S/[capillary electrochromatography (CEC)], ions, etc.) were studied for each sample after air drying and sifting (2-mm guage). The analyses were carried out on fine-textured soil using a Carlo Erba NA 1500 for total carbon and total nitrogen, a Tacussel pHN81 pH meter for H2O and KCl pH, and the hexaminecobalt trichloride method for CEC and metallic cations (CitationAFNOR, 1985).
THIN SECTIONS OF HUMUS
Three thin sections of soil per physiognomic stage and per site (3 × 3 per site) in the A1 horizon were used in order to study the humus structure. A humus block was collected from each soil pit using metallic boxes with dimensions of 5 × 5 × 10 cm3 (CitationKubiena, 1953). The soil samples were then stored at 5°C until sent to the Soil Sciences Laboratory of the I.N.R.A. (Rennes, France) where the thin sections were prepared.
The 27 thin sections were first visually observed by depth levels (0–2 cm; 2–10 cm) and after with a standard microscope (×60 to ×250). The standardized observations of descriptive forms (different scales were defined before observations) were separately duplicated. Humus structure, aggregation degree, aggregate size, porosity types (cracks, chenals (canals), vesicles, cavities), root density, and stoniness were studied. The thin sections were then analyzed using a Karl Zeiss Jena microscope (×250 to ×630, natural and polarized light) in order to distinguish organic and mineral matter, and microarthropoda feces. The structure of sections was compared per level and the frequency, size, shape, colour, distribution pattern, and content of aggregates were recorded.
Porosity was quantified using Optimas image analyser software; for each thin section, 40–45 windows (8.5 × 6.5 mm = 55.25 mm2 per window, about 22–25 cm2 per section) were analyzed per depth level (0–3; 3–6; 6–9 cm) on the entire section.
The excrement pedofeatures were identified (intact and ageing excrements) using the Handbook for Soil Thin Section Description (CitationFedoroff et al., 1985).
HUMUS MICROFAUNA ANALYSIS
Three humus samples per physiognomic stage (meadow, open and closed heathland) from each site (Belledonne, Taillefer, Briançonnais) were taken in the litter layer and in the A1 with a bulb dibber (300 cm3 per sample, about 1200 cm3 per physiognomic stage and per site).
The sampling was carried out after one week of fine weather. The sampling dates and the soil and air temperatures for each site are as following: Belledonne (29 June 1998; mean soil temperature in A1, under meadow, open and closed heathland: 16°C, 13°C, and 11.8°C, respectively; air temperature during the sampling : 20.5°C), Taillefer (1 July 1998; soil: 16.2°C, 14°C, and 12.5°C; air: 22°C), and Briançonnais (9 July 1998; soil: 9.9°C, 7.9°C, and 6.3°C; air: 14°C).
The microfauna extraction was carried out using a Berlese apparatus (CitationVannier, 1970). In the laboratory, each humus volume (300 cm3) was put in a funnel surmounted by a 50 watt light bulb for 10 days. The microfauna migrated by negative phototropism but also by decreasing humus humidity and increasing humus temperature. The microfauna samples were collected in alcohol (70°) in test tubes.
Each microfauna sample was identified for taxons and classification according to diet (CitationPesson, 1971; CitationBachelier, 1978). Some indices and ratios were calculated (Simpson diversity, Soerensen similarity, Acariens/Collemboles) to compare the biological activities of humus between the sites and under the different physiognomic stages at each site.
Results
The structural and biological characteristics of each physiognomic stage are summarized in . These results confirmed our fields observations and the sparse literature data on the colonization potentialities of Rhododendron ferrugineum; the shrub characteristics and the dynamics of Rhododendron heathlands were very different in inner Alps as compared to intermediate and external Alps, where their potential is optimal.
PHYSICAL AND CHEMICAL ANALYSIS
The chemical parameters of humus and the main soil types under meadow of each site are presented, respectively, in and (chemical and physical parameters of depth horizons are not presented).
The ANOVA test allowed us to indicate the significant effects (“site,” “vegetation type”) for each variable studied in the humus. The Duncan's multiple comparison of means allowed us to pool the “site” and “vegetation type” data for each variable ( and ).
There was an important “site” effect for 12 variables (86%) and also an important “vegetation type” effect for 10 variables (71%) out of 14. The three sites were not individualized (83%, 75%, and 75% of variables have at least one “site” effect, respectively, for Belledonne, Taillefer, and Briançonnais), but the vegetation types were well individualized (meadow, 70%, and open and closed heathlands, 40% and 30%, respectively). Because of an increasing influence of Rhododendron, there was a proximity (50%) between open and closed heathlands (). The correlation numbers between vegetation types for all the studied variables () indicated that in Belledonne, the meadow was a differentiated type.
At the three sites, the humus pH was not modified by the Rhododendron colonization (open heathland) even when the pH values were near neutrality (calcosols in inner Alps). Under the influence of Rhododendron (closed heathland), the pH stability has been maintained for the past 150 to 300 years on siliceous rocks and for the past 60–90 years on calcareous rocks.
At two sites (Taillefer and Briançonnais), the carbon rates (and organic matter) in humus increased slightly with Rhododendron colonization (open heathland) and stabilized with increasing influence of the shrubs (closed heathland). In Belledonne, this evolution was not significant; the bioturbation was probably more important.
The sum of exchangeable bases in humus (S = Ca2+ + Mg2+ + K+ + Na+) increased with space monopolization by Rhododendron populations, except at Belledonne, where this evolution was dependant on the Ca2+ increase, and at Taillefer, where the increase was secondarily dependant on the Mg2+ increase. At the three sites, the saturation index (S/CEC) did not evolve with Rhododendron colonization but increased during the heathland aging when the cover and the biomass were high. The humus types defined under each physiognomic stage at the three sites are presented in .
THIN SECTIONS OF HUMUS
At the three sites and under each physiognomic stage, granular and crumbly structures characterized the humus between 2 and 10 cm of depth. During the Rhododendron colonization, a tendency to improve degree of aggregation (macroaggregation at Belledonne, microaggregation at Taillefer, stability at Briançonnais) was estimated by standardized observations (). The root densities tended to decrease with Rhododendron colonization at the three sites and the humus stoniness was very high (27%) under closed heathland at Taillefer.
The thin sections under the meadow (Belledonne, Briançonnais) showed high frequencies of fine pedofeatures, most of them produced by oribates and enchytraeids, whereas the structure under closed heathland was essentially made up by earthworm excrements (>2 mm, irregular shapes with mixed mineral and organic elements). The structure of the open heathland section was intermediate, but closer (more compact) under the grassland than under the closed heathland sections.
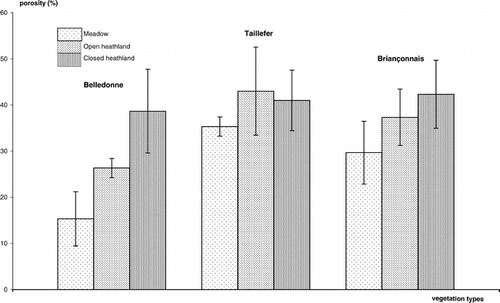
The mean porosity of meadow humus was higher at Taillefer (35%) and Briançonnais (30%) than at Belledonne (15%). Under the increasing influence of shrubs, the porosity of humus increased at the three sites from meadow to open heathland, mainly at Belledonne (). In this same site, the porosity evolution continued distinctly until closed heathland (same evolution at Briançonnais). The changes at Taillefer were less important because the humus porosity of meadows was already high; with shrubs, it increased from 15% to 25% and seemed to stabilize during the heathland aging (around 40%); in this site, the Rhododendron populations in closed heathland had also a long-term influence on humus (past 150 to 300 years) and the heathland structure was different from other closed heathlands ().
HUMUS MICROFAUNA ANALYSIS
Except at the Taillefer site, the number of individuals increased during the colonization and monopolization of meadows by Rhododendron populations (). This increment was dependant on plant biomass augmentation (abundant litter).
At the Taillefer site, the open and closed heathlands were characterized by very low quantities of microfauna organisms. In closed heathland, Rhododendron influence of humus evolution was very long (150–300 years) and important (biomass: 64 t DW/hectare; cover: 96% [1195 ± 427 shoots/m2]; CitationPornon et al., 1997). In open heathland, the cover of each Rhododendron shrub was more dense (1588 ± 469 shoots/m2) than in the two other sites (1333 ± 348 shoots/m2 in Belledonne, lower 900–1000 shoots/m2 at Briançonnais). The microclimatic conditions in shrubs could explain these low results.
At the three sites, the taxon Oribatulidae (Acarida, Oribatida) was the most abundant (40%–60% at Belledonne and Taillefer, 20%–30% at Briançonnais). These proportions tended to increase in open and closed heathlands. The taxon Nematocera (Diptera) reached 10%–26% at Briançonnais but only 0%–6% at the other two sites. The taxon Collembola arthropleona represented a 2–7% increase in closed heathland (9%, 13%, and 23%) at the three sites. Those taxa constituted the micro- and macro-phytophagous organisms.
In humus of the meadows and closed heathlands, 78% to 88% of organisms were phytophagous and 10% to 21% were carnivorous; the percentages were less important for phytophagous organisms (63% to 74%) and more important for carnivorous animals (22% to 37%) in open heathlands humus. In all sites and physiognomic stages, the percentage of organisms with other trophic diets (omnivorous, saprophytic) fluctuated between 0 and 8%.
In the closed heathland of the three sites, the ratio Acarida/Collembola indicated a defaced biocoenosis, a simplification and an imbalanced ecosystem as compared with a climax ecosystem (CitationBachelier, 1978). The similarity between meadows of the three sites was always very constant (comparison of two biotopes with Soerensen index: >84%); it decreased in closed heathlands principally between Belledonne/Taillefer (48%) and Belledonne/Briançonnais (52%). At the same site, the index was always >69% in Belledonne and Briançonnais stages, and it varied between 40% and 62% in Taillefer stages. The Simpson index decreased with colonization and monopolization of meadows by Rhododendron populations in Belledonne and Briançonnais, indicating a decrease of the standing diversity; in Taillefer, the index slightly increased in open and closed heathlands.
Discussion
Mature and degenerate heathlands on siliceous soils in the different elevation levels were associated with a moder or a raw humus (mor) showing limited biological activity (Bernier et al., 1994; CitationPellissier, 1994; CitationBernier and Ponge, 1994: Vaccinium myrtillus) (CitationDuchaufour, 1977; CitationBonneau, 1980; CitationDoche, 1986: Calluna vulgaris) (CitationGloaguen et al., 1980: Erica cinerea, E. ciliaris) (CitationAubert, 1976: Erica arborea, E scoparia), whereas meadow humus was known to be more active (CitationDuchaufour, 1977; CitationRichard and Pautou, 1982).
Our results showed that the colonization of subalpine meadows by Rhododendron ferrugineum, with an increasing cover, could improve the humus structure (porosity principally); this effect was even more obvious at Belledonne where the grassland was pastured and the humus therefore compacted.
At each site, the humus typology showed tendencies to global improvement of A1 horizons () even if, in closed heathland, litter layers got thicker (2–5 cm) with accumulation of plant biomass in plant communities. In these ecosystems, the humus functioning remained efficient. Some humus parameters increased (Ca++ concentration, individual number of microfauna), tended to remain stable (pH), or weakly increased (C/N, S/CEC).
The Ca++ accumulation under heathlands resulted in biomass growth and consequently litter growth (nutrients were taken from depth by biological raising); the acidophilous litters supplied the same quantities of Ca++ and Mg++ as the better litters. Mg++ was more labile and did not increase (or increased weakly) during the succession. Ca++ was partly stocked in humus in an inassimilable form and released very slowly (Vedy, 1973; Toutain, 1974; Messenger, 1975 in CitationDuchaufour, 1977).
The presence and the development of Rhododendron populations on calcareous mull with maintenance of the physical and chemical characteristics and improvement of porosity were unknown at Briançonnais.
This global amelioration of humus could be explained at different levels.
Earthworm's role. During our field work, some earthworms were frequently observed under the closed heathlands but for technical reasons, no quantification was realized at the three sites. However, under the closed Rhododendron plot (about 3 m2) in the subalpine level of the Northern Alps, 30 worms per m2 were counted (CitationGandoy, 2000), belonging to the genus Lumbricus. They could structure the upper layers of the soil (CitationPonge and Delhaye, 1995; CitationGrossi et al., 1997) and resurface nutriments; their feces were richer in Ca++, Mg++, NO3 −, P, and K than surrounding soil (CitationBachelier, 1978). In Vaccinium heathlands of the same level in the mountain, there were only 5 worms per m2 (CitationBernier and Ponge, 1994).
The Ca++ increase was favorable to the development of the clay humus complex and consequently to the improvement of the humus structure and humus stability (CitationDuchaufour, 1977) (from meadow to closed heathland, tendency from granular to crumbly structure with some macro-aggregates).
The modifications of humus were a direct consequence of the changes in species composition but also in the vegetation structure. At the beginning of the succession, under young Rhododendron shrubs isolated in a meadow, some other species took part in humus evolution for 50 to 100 years (Deschampsia flexuosa, Vaccinium myrtillus principally). These two species which have good capacities to colonize meadows and to structure a moder and a raw humus (CitationDuchaufour, 1989; CitationAndré, 1994; CitationPellissier, 1994; CitationPonge et al., 1994) were inhibited in their development by Rhododendron canopy growth and nutritional competition (concept of “asymmetric competition”: CitationWeiner, 1990). In closed heathlands, under the canopy, the vegetal cover and biomass (small shrubs and herbaceous plants) regressed, mainly at Taillefer (cover: 15%; biomass: <1t DW ha−1 with 0.74 t of Vaccinium sp.); concurrently, mosses (15%) and a litter layer (cover: 90%; thickness: 4 ± 1.2 cm) developed. At the two other sites, the values evolved in the same direction but under the Rhododendron canopy (less closed), the species regression was less important.
The tannin effect was more important in Vaccinium litter than in Rhododendron litter (tannin equivalent: 230 mg g−1 in Vaccinium leaves and only 120 mg g−1 in Rhododendron leaves; CitationGallet and Lebreton, 1994). This tannin effect tended to reduce or to block the nitrogen mineralization (Handley, 1961; CitationSchvartz, 1975 in CitationDuchaufour, 1977). The development of Rhododendron population in Vaccinium communities improved the humus dynamics.
The changes were also induced by modifications of microclimate under isolated Rhododendron stands and under the closed Rhododendron populations; these modifications were favorable to biological soil activity. The canopy, the stems, and branches of shrubs which had a sheltering effect, reduced desiccation during the summer. Better moisture conditions were characterized by establishment in closed heathland of moisture plants (Adenostyles alliariae at Belledonne and Taillefer) and mosses; these conditions were favorable to Rhododendron, which had high water needs (CitationLandolt, 1977; CitationRichard and Pautou, 1982; CitationPornon, 1994). Under Rhododendron closed heathlands, during vegetative period (May to September), the humus temperatures were 2.5°C to 4°C below temperatures of meadows humus (CitationPornon and Doche, 1995; CitationPierre, 1996). The effect of plant cover on humus temperatures was variable and the latter in turn had variable effects on phenology, plant growth, and more precisely root growth (Bannan and Bode in CitationTranquillini, 1979), nutrient absorption (CitationBlackman, 1936; CitationDadykin, 1958), pedological processes as mineralization (CitationBonneau, 1980; CitationRoze, 1986; CitationTavant, 1986), and on microbiological activity.
Lower mineral nitrogen amounts were observed in closed heathland with lower soil temperatures (CitationPornon and Doche, 1995). The variations of different mineral nitrogen forms could be important in the long term because the ability of plants to absorb NH4 + or NO3 − and the stability of these ions were different. Woody plants, as the Ericaceae, are likely to absorb NH4 + (CitationBoxam and Roelofs, 1988; CitationScheromm and Plassard, 1988; CitationHoffmann, 1966; CitationHoffmann and Fiedler, 1966), while herbaceous are not (CitationHull and Muller, 1977; CitationJenny et al., 1950; CitationSalsac et al., 1987) except Deschampsia flexuosa and Nardus stricta (CitationLe Tacon et al., 1982; CitationTroelstra et al., 1995). With ericoïd mycorrhizal roots, ericaceous shrubs used organic nitrogen forms in the humus (CitationRead, 1983, Citation1991, Citation1996) and consequently the heathland shrubs were more competitive for nitrogen nutrition than meadow plants. At Taillefer, the growth of current-year twigs was also high in closed populations (CitationPornon et al., 1997) where senescence patches were not observed. CitationPornon et al. (2000) mentioned that the range of genetic diversity diminished from the open to the closed populations; there was a selection of the most competitive genotypes (high growth) in old closed heathlands.
All the changes in the environment under Rhododendron canopy are induced by its own development and lead to an improvement in growth conditions (CitationPornon et al., 1996). This process in which a population (or a community) modifies the environment, making it more suitable for this population (or community) is connected with the concept of “positive-feedback switches”. CitationWilson and Agnew (1992) defined four types of switches in accordance with the community's number and with the changes of an environmental factor “in” and “out” of the patches generated by each community (“one-sided” and “external reaction” switches with one community; “symmetric” and “two-factors” switches with two communities). The Rhododendron population induces changes in the environment, in patches where it is present and the boundaries cannot be stable because populations can invade the unmodified environment (one-sided switch). The Rhododendron shrub maintains (pH) or improves (porosity principally) some parameters of humus but it is also the structure of closed heathland (news microclimatic conditions), which explains the “positive-feedback switch”. The consequences of the ability of plants to influence soil processes remain unexplored and therefore the plant-soil feedbacks are still unrecognized (CitationBever, 1994; CitationVan Der Putten, 1997). The ability of Rhododendron to absorb NH4 +, the only nitrogen form available below a certain threshold of low temperatures, its ability to modify its reproduction strategy (CitationPornon et al., 1997), the growth of current-year twigs highest in closed population and the absence of senescence patches are indicators of a positive-feedback mechanism. The humus conditions resulting from Rhododendron dynamics allow species that have changed the humus to remain, to monopolize solely by layering the space from 60 to 80 cm in depth. Normally, the effects of plants on soil results in a beneficial future for generations of the same species (CitationVan Der Putten, 1997) and conversely to Rhododendron populations which do not have offspring under the canopies of closed heathlands. CitationBever et al. (1997) emphasize that asexual reproduction may be advantageous when genotypes within a population experience a strong positive environmental feedback on fitness.
With all the environmental modifications, the shrub populations compete more efficiently with other species (grasses, dwarf shrubs, trees) and contribute to stop the forest colonization. In the long term, on the north-facing slopes of wet subalpine level (external and intermediate Alps), the closed heathlands are successful in monopolizing large areas; on a human scale, these plant community remain physiognomically stable but their internal evolution must be studied.
TABLE 1 Ecological features of the three sites
TABLE 2 Structural and biological characteristics of each physiognomic stage in the three sites. M = meadow, O.H. = open heathland, C.H. = closed heathland
TABLE 3 The chemical parameters of humus and meadow soils in each physiognomic stage in the three sites. Mean values (± standard deviation) of 3 samples in humus (0–10 cm). M = meadow, O.H. = open heathland, C.H. = closed heathland
TABLE 4 The main soil types in each site (Baize and Girard, 1992; italics, C.P.C.S. classification, 1967)
TABLE 5 “Site” and “vegetation type” effects for 14 physical and chemical variables (ANOVA, Duncan grouping). Site (B = Belledonne, T = Taillefer, Br = Briançonnais) and type (M = meadow, O.H. = open heathland, C.H. = closed heathland). Shaded boxes have a significant effect
TABLE 6 Correlation number between “vegetation type” and “site” for 14 physical and chemical variables (Duncan grouping). M = meadow, O.H. = open heathland, C.H. = closed heathland
TABLE 7 The humus types under every physiognomic stage in the three sites (Duchaufour, 1997; italics, Baize and Girard, 1992)
TABLE 8 Micromorphologic characterization of 27 thin sections of humus using standardized methods (9 per site). In each column, the results (aggregate size, structure, aggregation degree, porosity, etc.) represent the analysis of 3 thin sections by humus (about 120–140 cm2) and by physiognomic stage. M = meadow, O.H. = open heathland, C.H. = closed heathland
TABLE 9 Evolution and comparison of microfauna in humus under each physiognomic stage in the three sites (3 × 300 cm3 are analyzed per stage). M = meadow, O.H. = open heathland, C.H. = closed heathland
Acknowledgments
This work was supported by the French ministry of environment (E.G.P.N. 95 085 : Dynamique des écosystèmes subalpins : interférences forêts, pelouses et landes intra- et supra-sylvatiques : dynamique extrasylvatique des populations d'Ericacées subalpines et délimitation des clones), the Centre National de la Recherche Scientifique (UMR-CNRS 55 53) and the Université Joseph Fourier (Grenoble).
Notes
Revised ms submitted July 2005
References Cited
- AFNOR, 1985. Qualité des sols, détermination de la capacité d'échange cationique et des cations échangeables. Paris: Afnor, 12 pp.
- André, J. 1994. Régénération de la pessière à myrtille: allélopathie, humus et mycorhizes. Acta Botanica Gallica 141:4 551–558.
- Aubert, G. 1976. Les Ericacées en Provence. Répartition, édaphologie, phytosociologie, croissance et floraison. Thèse d'Etat, Université d'Aix Marseille III, France, 286 pp.
- Bachelier, G. 1978. La faune des sols; son écologie et son action. Paris: Editions de l'ORSTOM, 391 pp.
- Barclay-Estrup, P. and C. H. Gimingham . 1969. The description and interpretation of cyclical processes in a heath community. I) Vegetational change in relation to the Calluna cycle. Journal of Ecology 57:737–758.
- Berendse, F. 1994. Litter decomposability—a neglected component of plant fitness. Journal of Ecology 82:187–190.
- Bernier, N. and J. F. Ponge . 1994. Humus form dynamics during the sylvogenetic cycle in a mountain spruce forest. Soil Biology and Biochemistry 26:2 183–220.
- Bernier, N. , J. F. Ponge , and J. André . 1994. Comparative study of soil organic layers in two bilberry-spruce forest stands (Vaccinio-Piceetea). Relation to forest dynamics. Geoderma 59:89–108.
- Bever, J. D. 1994. Feedback between plants and their soil communities in an old field community. Ecology 75:7 1965–1977.
- Bever, J. D. , K. M. Westover , and J. Antonovics . 1997. Incorporating the soil community into plant dynamics: the utility of the feedback approach. Journal of Ecology 85:561–573.
- Blackman, G. E. 1936. The influence of temperature and available nitrogen supply on the growth of pasture in the spring. Journal Agricultural Science 26:620–647.
- Bonneau, M. 1980. Production d'azote minéral dans divers types de landes du Massif Central. Annales des Sciences Forestières 37:3 173–188.
- Boullard, B. 1964. Mycorhizes et reboisement des landes à Calluna vulgaris . (commentaire sur une étude récente de Handley). Revue Forestière Française 2:140–143.
- Boxam, A. W. and J. G M. Roelofs . 1988. Some effects of nitrate versus amonium nutrition on the nutrient fluxes in Pinus sylvestris seedlings. Effects of mycorrhizal infection. Canadian Journal of Botany 66:1091–1097.
- Clement, B. and J. Touffet . 1976. Biomasse végétale aérienne et productivité des landes des Monts d'Arrée (Bretagne). Oecologia Plantarum 1:4 345–360.
- Coquillard, P. and J. Gueugnot . 1991. Regressive dynamic and denudation processes of vegetation on volcanoes in the “Chaine des Puys” (French Massif Central): assay of interpretation. Vegetatio 93:131–141.
- Dadykin, V. P. 1958. Plant physiological research problems of the far North. Probl. North (National Research Council of Canada) 1:205–216.
- Doche, B. 1986. Déterminisme et expression cartographique des successions végétales. Exemple de l'Aubrac montagnard (Massif Central Français). Thèse Doct. ès Sciences, Univ. Joseph Fourier, Grenoble I, France, 251 pp.
- Doche, B. 1990. Les successions articulées autour des landes à Calluna vulgaris dans le Massif Central français. Comparaisons avec quelques autres systèmes à Ericacées. Bulletin d'Écologie 21:1 43–50.
- Doche, B. , A. Pornon , and N. Escaravage . 1997. Analyse comparative de quelques aspects de la dynamique et du fonctionnement des landes à Ericacées en fonction de l'altitude (France). Ecologie 28:4 293–306.
- Duchaufour, Ph 1977. Pédologie. I—Pédogénèse et classification. Paris: Editions Masson, 469 pp.
- Duchaufour, Ph 1989. Pédologie et groupes écologiques I—Rôle du type d'humus et du pH. Bulletin d'Écologie 20:1 1–6.
- Escaravage, N. , S. Questiau , A. Pornon , B. Doche , and P. Taberlet . 1998. Clonal diversity in a Rhododendron ferrugineum L. (Ericaceae) population inferred from AFLP markers. Molecular Ecology 7:975–982.
- Fedoroff, N. , P. Bullock , A. Jongerius , G. Stopps , T. Tursinat , and U. Babel . 1985. Handbook for soil thin section description. Waine Research Publications, 152 pp.
- Gallet, C. and P. Lebreton . 1994. Les composés phénoliques d'une pessière d'altitude: origine et transferts. Revue Valdotaine d'Histoire Naturelle 48:395–398.
- Gandoy, C. 2000. Approche comparative des relations végétation/environnement sur substrats calcaire et siliceux à l'étage subalpin dans le massif des Grandes Rousses (Alpes du Nord françaises). Thèse de l'Université Joseph Fourier, Grenoble, France, 244 pp.
- Gilot, J. Cl 1967. Note écologique sur divers groupements à Rhododendron ferrugineum L. se développant sur substrat calcaire: xemple des Préalpes occidentales françaises. Oecologia Plantarum II:139–162.
- Gimingham, C. H. 1972. Ecology of heathlands. London: Chapman and Hall, 266 pp.
- Gimingham, C. H. 1978. Calluna and its associated species: some aspects of co-existence in communities. Vegetatio 36:179–186.
- Gloaguen, J. C. , J. Touffet , and F. Forgeard . 1980. Vitesse de décomposition et évolution minérale des litières sous climat atlantique. II. Les principales espèces des landes de Bretagne (France). Oecologia Plantarum 1:15 257–273.
- Gross, K. L. , K. S. Pregitzer , and A. J. Burton . 1995. Spatial variation in nitrogen availability in three successional plant communities. Journal of Ecology 83:357–367.
- Grossi, J. L. and J. J. Brun . 1997. Effect of climate and plant succession on lumbricid populations in french Alps. Soil Biology and Biochemistry 29:3/4 329–333.
- Groten, K. and H. Bruelheide . 1997. Differences in soil conditions between heathlands and grasslands on Zechstein gypsum soils. Flora 12:347–359.
- Haynes, R. J. and K. M. Goh . 1978. Ammonium and nitrate nutrition of plants. Biological Review 53:465–510.
- Hobbie, S. E. 1995. Direct and indirect effects of plant species on biogeochemical processes in arctic ecosystems. Arctic and alpine biodiversity; patterns, causes and ecosystem consequences. Berlin: F. S. Chapin III and C. Körner.
- Hoffmann, F. 1966. Untersuchungen zur Stickstoffernährung junger Koniferen. II—Die Aufnahme von Ammonium und Nitratstickstoff durch Fichtensamlinge unter verschiedenen Bedingungen. Archiv Fortwesen 15:10 1093–1103.
- Hoffmann, F. and H. Fiedler . 1966. Die Stickstoffernährung junger Koniferen. Biologische Rundschau 4:3 138–155.
- Hull, J. C. and C. H. Muller . 1977. The potential for dominance by Stipa pulchra in a California grassland. American Midland Naturalist 97:147–175.
- Jenny, H. , J. Ulmars , and W. E. Martin . 1950. Creehouse essay of fertility of California soils. Hilgardia 20:1–18.
- Kubiena, W. L. 1953. Bestimmungsbuch und Systematik der Böden Europas. Stuttgart: Enke.
- Landolt, E. 1977. Okologische Zeigerwerte zur Schweizer flora. Veröffent-lichungen des Geobotanischen Institutes der Eidg. techn. Hochschule, Stiftung Rübel, in Zurich, 64. 208 pp.
- Le Tacon, F. , J. Timbal , and J. M. Waldenaire . 1982. Influence de la forme d'azote minéral sur la croissance d'espèces herbacées forestières. Oecologia Plantarum 3:17 307–318.
- Lechowicz, M. J. and G. Bell . 1991. The ecology and genetics of fitness in forest plants. II. Microspatial heterogeneity of the edaphic environment. Journal of Ecology 79:687–696.
- Lepart, J. and J. Escarre . 1983. Les successions végétales, mécanismes et modèles, analyse bibliographique. Bulletin d'Écologie 14:3 133–178.
- Lovett Doust, L. 1981. Interclonal variation and competition in. Ranunculus repens. New Phytologist 89:495–502.
- Miles, J. 1979. Outline Studies in Ecology. Vegetation dynamics. London and New York: Chapman and Hall, 79 pp.
- Miles, J. 1981. Problems in heathland and grassland dynamics. Vegetatio 46:61–74.
- Miles, J. 1985. The pedogenic effects of different species and vegetation types and the implications of succession. Journal of Soil Science 36:571–584.
- Northup, R. R. , Z. Yu , R. A. Dahlgren , and K. A. Vogt . 1995. Polyphenol control of nitrogen release from pine litter. Trends in Ecology and Evolution 377:227–229.
- Ozenda, P. 1981. Végétation des Alpes sud-occidentales. Notice détaillée des feuilles 60 Gap—61 Larche—67 Digne—68 Nice—75 Antibes. Carte de la Végétation de la France à 1/200000. Paris: Editions C.N.R.S., 258 pp.
- Ozenda, P. 1985. La végétation de la chaîne alpine dans l'espace montagnard européen. Paris: Masson, Editeur, 344 pp.
- Ozinga, W. A. , J. Van Andel , and M. P. McDonnell-Alexander . 1997. Nutritional soil heterogeneity and mycorrhiza as determinants of plant species diversity. Acta Botanica Neerl 46:3 237–254.
- Pellissier, F. 1994. Effect of phenolic compounds in humus on the natural regeneration of spruce. Phytochemistry 36:4 865–867.
- Pesson, P. 1971. La vie dans les sols. Paris: Edition Gauthier-Villard. 115pp.
- Pierre, L. 1996. Hétérogénéité du milieu et dynamique des populations subalpines d'Ericaceés: analyse à l'échelle du versant (adret/ubac) et de la communauté végétale (Massif du Taillefer, Alpes du Nord occidentales). DEA, Université Joseph Fourier, Grenoble, France, 72 pp.
- Ponge, J-F. and L. Delhaye . 1995. The heterogeneity of humus profiles and earthworm communities in a virgin beech forest. Biology and Fertility of Soils 20:24–32.
- Ponge, J-F. , J. Andre , N. Bernier , and C. Gallet . 1994. La régénération naturelle: connaissances actuelles. Le cas de l'Epicéa en forêt de Macot (Savoie). Revue Forestière Francaise XLVI:1 25–45.
- Pornon, A. 1994. Dynamique et fonctionnement des populations de Rhododendron ferrugineum L.(Ericacée)—(étage subalpin; Alpes Nord-Occidentales). Thèse d'Université, Lab. de Biologie des populations d'altitude, Université J. Fourier, Grenoble, France, 163 pp.
- Pornon, A. and B. Doche . 1995. Minéralisation et nitrification de l'azote dans différents stades de colonisation des landes subalpines à Rhododendron ferrugineum L. (Alpes du Nord; France). Comptes Rendus de l'Academie des Sciences 318:887–895.
- Pornon, A. , R. Bligny , E. Gout , and B. Doche . 1996. Growth rates and nutrition status of an open and a closed population of Rhododendron ferrugineum L. in the northwestern Alps (France). Trees 11:91–98.
- Pornon, A. , N. Escaravage , I. Till-Bottraud , and B. Doche . 1997. Variation of reproductive traits in Rhododendron ferrugineum L. (Ericaceae) populations along a successional gradient. Plant Ecology 130:1–11.
- Pornon, A. , N. Escaravage , P. Thomas , and P. Taberlet . 2000. Dynamics of genotypic structure in clonal Rhododendron ferrugineum (Ericaceae) populations. Molecular Ecology 9:1099–1111.
- Prentice, J. C. , O. Van Tongeren , and J. T. De Smidt . 1987. Simulation of heathland vegetation dynamics. Journal of Ecology 75:203–219.
- Read, D. J. 1983. The biology of mycorrhiza in the Ericales. Canadian Journal of Botany 61:985–1004.
- Read, D. J. 1991. Mycorrhizas in ecosystems. Experientia 47:376–391.
- Read, D. J. 1996. The structure and function of the ericoïd mycorrhizal root. Annals of Botany 77:365–374.
- Richard, L. and G. Pautou . 1982. Alpes du Nord et Jura méridional. Notice détaillée des feuilles 48 Annecy, 54 Grenoble. Carte de la Végétation de la France à 1/200000. Paris: Editions C.N.R.S., 316 pp.
- Richter, D. D. , D. Markewitz , C. G. Wells , H. L. Allen , R. April , P. R. Heine , and B. Urrego . 1994. Soil chemical change during three decades in an old-field loblolly pine ( Pinus taeda L.) ecosystem. Ecology 75:5 1463–1473.
- Roze, F. 1986. Le cycle de l'azote dans les landes bretonnes. Thèse de Doct. ès Sciences Nat., Université de Rennes, France, 292 pp.
- Saetre, P. 1998. Decomposition, microbial community structure, and earthworm effects along a birch-spruce soil gradient. Ecology 79:3 834–846.
- Salsac, L. , S. Chaillou , J. F. Morot-Gaudry , C. Lesaint , and E. Jolivet . 1987. Nitrate and Amonium nutrition in plants. Plant Physiology and Biochemistry 25:6 805–812.
- Scheromm, P. and C. Plassard . 1988. Nitrogen nutrition of no mycorrhized pine ( Pinus pinaster ) grown on nitrate or amonium. Plant Physiology and Biochemistry 26:261–269.
- Schvartz, Ch 1975. Evolution des hydrosolubles de litières de Ca1lune et le Hétre au cours du processus d'humification. Thèse Docteur-Ingénieur, Université Nancy I, France, 81 pp.
- Tavant, Y. 1986. Dynamique saisonnière des matières organiques et de l'azote des sols forestiers brunifiés et calcimagnésiques humifères des séquences bioclimatiques du Jura. Thèse d'Université Sciences de la Vie, Besançon, France, 109 pp.
- Tian, X-J. , H. Takeda , and T. Ando . 1997. Application of a rapid thin section method for observations on decomposing litter in mor humus form in a subalpine coniferous forest. Ecological Research 12:289–300.
- Toutain, F. 1974. Étude écologique de l'humification dans les hêtraies acidiphiles. Thèse de Doctorat d'État, Université de Nancy, France, 114 pp.
- Tranquillini, W. 1979. Physiologic Ecology of the Alpine Timberline. Ecological studies. Berlin, Heidelberg, New-York: Springer Verlag, 137 pp.
- Troelstra, S. R. , R. Wagenaar , and W. Smant . 1995. Nitrogen utilization by plant species from acid heatland soils. I—Comparison between nitrate and ammonium nutrition at constant low pH. Journal of Experimental Botany 46:290 1103–1112.
- Van Der Putten, W. H. 1997. Plant-soil feedback as a selective force. Tree 12:5 169–170.
- Vannier, G. 1970. Réactions des microarthropodes aux variations de l'état hydrique du sol. Techniques relatives à l'extraction des arthropodes du sol. Paris: Editions du C.N.R.S., 315 pp.
- Vedy, J. C. 1973. Relations entre le cycle biochimique des cations et l'humification en milieu acide. Thése de Doctorat d'État, Université de Nancy I, France, 116 pp.
- Vinton, M. A. and I. C. Burke . 1995. Interactions between individual plant species and soil nutrient status in shortgrass steppe. Ecology 76:4 1116–1133.
- Watkinson, A. R. 1998. The role of the soil community in the plant population dynamics. Trends in Ecology and Evolution 13:5 171–172.
- Weiner, J. 1990. Asymmetric competition in plant populations. Trends in Ecology and Evolution 5:11 360–364.
- Wilson, J. B. and A. D Q. Agnew . 1992. Positive-feedback switches in plant communities. Advances in Ecological Reasearch 23:263–336.