Abstract
The Khangchendzonga Biosphere Reserve in the Sikkim State, India, with an area of 2620 km2, forms an important reserve to protect biodiversity, habitats, and landscapes of the eastern Himalayan region. More than 18% of the area supports alpine vegetation, which is extensively used as summer grazing grounds by transhumance and nomadic graziers. Despite the global recognition of the region, unfortunately no report has so far been made available on the vegetation composition of the reserve. The present paper reports on floral diversity, economic use, and nutrient contents of selected alpine pastures at elevations between 3800 and 4800 m above sea level in the Khangchendzonga Biosphere Reserve. The growing season lasts from April to October, and during the rest of the months, the area remains under snow. The pastureland showed high species richness: as many as 202 plant species were present. These belong to 38 families, of which 90% of the species are dicotyledons and 9% are monocotyledons. Plant diversity and density increased from April until August and decreased thereafter. Monocotyledon species, such as Poa angustifolia, show high dominance during the early and late parts of the growing season, whereas dicot species proliferate mainly during the middle part of the growing season. Asteraceae, Ranunculaceae, Ericaceae, Primulaceae, and Rosaceae are dominant plant families in the area. Life-form distribution patterns showed that >50% of the species were chamaephytes, showing high adaptation by the plants. Besides using the area for grazing, the local people also collect various species for medicine, incense, tea-substitute, and aesthetic purposes. The belowground plant parts contributed nearly 90% of total plant biomass, whereas the aboveground biomass contributed just 10%. Such partitioning of biomass is considered beneficial in pastureland, as the belowground biomass helps the immediate recovery of vegetation after grazing as well as at the start of the growing season. Most of the species are highly nutritive and have high mineral contents, and animals showed a preference for species with low lignin content. In order for the area to continue to provide an important pasturage for animals in the future, the grazing pressure must not exceed an optimum level so that the high species diversity can be conserved.
Introduction
The Himalaya is recognized as a major biodiversity center in the world (CitationKhoshoo, 1992). The eastern Himalaya is very rich in biodiversity and harbors the greatest number of endemic species in India (CitationMacKinnon and MacKinnon, 1986; CitationMyers, 1988). It is located at the juncture of the Indo-Malayan and Indo-Chinese biogeographical realms and includes both Himalayan and Peninsular Indian elements (CitationKhoshoo, 1991). It is considered that this region, along with contiguous regions in China and Southeast Asia, constituted the evolutionary cradle for flowering plants (CitationTakhtajan, 1969). The Khangchendzonga Biosphere Reserve in Sikkim Himalaya falls among the most important protected areas in the eastern Himalaya (CitationSingh et al., 2002). It has extensive alpine areas that provide important pasturage for local and transhumance herders who graze their livestock, such as yaks, dzos, cattle, horses, and sheep on common pastures along with the pack animals for trekking groups and expeditions (CitationRai and Sundriyal, 1997). The influx of tourists and trekkers to the alpine pastures has begun to have a negative environmental impact because of excessive grazing near the camping grounds at select places, removal of rhododendron bushes for fuelwood, collection of medicinal plants and nontimber forest products, and uncontrolled disposal of trash (CitationSingh et al., 2002).
Alpine vegetation in the western Himalaya have been the subject of previous ecological investigations (CitationMani, 1978; CitationRam et al., 1988; CitationRawat and Pangtey, 1987; CitationSundriyal, 1992). There are a few studies related to floral diversity of the alpine vegetation of selected areas (CitationSemwal and Gaur, 1981; CitationDhar and Kachroo, 1993). Reports on the floral diversity of a few sites in the Sikkim Himalaya started in the nineteenth century (CitationHooker, 1875) and have continued ever since (CitationSmith and Cave, 1911; CitationSmith, 1913; CitationHara, 1963; CitationPradhan and Lachungpa, 1990). These studies, however, lack information on structure, economic use, and nutrient contents of the plant species. The baseline data on floristic and structural patterns and economic utility of plant resources are critical for ensuring the sustainable management of any area. The areas that the transhumance and nomadic graziers visit must be able to support their animals in large herds and also provide plants for other diverse purposes. Therefore the present study was undertaken in selected parts of the Khangchendzonga Biosphere Reserve in the Sikkim Himalaya to acquire data on the floristic composition, structure, flowering pattern, nutrient contents, and economic uses of the alpine vegetation. The goal was to provide some valuable information that may further strengthen the management of such an important reserve in near future.
Study Area
Sikkim is a tiny state of India (area 7096 km2) that falls in the eastern Himalaya. The Khangchendzonga Biosphere Reserve (27°25′N to 27°55′N and 88°30′E to 88°38′E) is located in the North and West Districts of Sikkim (). The biosphere reserve provides a picturesque view of snow-clad mountains, lakes, alpine vegetation, thick forests and ground vegetation in temperate and subalpine zones, and rich wildlife. Thus the reserve has high implications for conservation that relate to the entire eastern Himalayan region. The total area of the biosphere reserve is 2620 km2; the alpine region (scrubs + pastures + barrens) accounts for 18.4% of the area. The present study area covers three major alpine pastures, namely, Dzongri, Goechala-Thangsing, and Base Camp; these cover an area of ∼225 km2 and have an altitudinal range of 3800–4800 m a.s.l. The alpine region is the popular trekking corridor for more than 2000 national and international tourists every year. A large number of dzos and horses are used as pack animals during tourist season (March to May and September to November). The local communities practice the agro-pastoral system of livelihood, and livestock animals form the backbone of this system (CitationSingh et al., 2003).
The plant-growing season at alpine sites starts in April with the snow melt, and during June–September the area shows a lush green appearance. The vegetation starts drying from late September through the end of October, and thereafter the area is covered with snow until March of the next year. Seasonal animal relocation from lower hills to alpine meadows during the plant-growth season is a common strategy for securing sufficient forage for grazing animals. A large number of livestock—namely, yaks, dzos, horses, cattle, and sheep—graze in the alpine pastures during the snow-free period (May to October) and are brought down to the lower-altitude forested areas during colder months (November to April). Besides, the reserve is also visited by local communities for collection of fuel, fodder, and medicinal plants.
Geologically the Khangchendzonga Biosphere Reserve is composed of rocks of the Darjeeling Group; these are mainly high-grade gneisses, consisting of quartz and feldspar with streaks of biotite (CitationGSI, 1984). The soils are loose with 50–60% sand and acidic in nature (pH varies from 4.93 to 5.41) (). The organic carbon, nitrogen, and phosphorus contents of soils are fairly good. The area has a monsoon-controlled climate; more than 80% of the average annual precipitation of 2300 mm comes in June through September (). The scant precipitation during November to March is mainly in the form of snow. The mean monthly maximum temperature is 13°C (September), and the mean minimum is −8°C (January).
Methods
The field survey was carried out during 1996–1999 on monthly basis from April to November (during the rest of the months, the ground is covered with snow). Random quadrats of 5 × 5 m size were laid at six different locations, namely, Dzongri, Base Camp, Goechala, Thangsing, Chowrigang, and Deorali (n = 25 at each location). All the plant species encountered were collected and identified with the help of experts. A checklist of all plant species encountered in the sampling along with their respective life-forms and flowering period are arranged according to Bentham and Hooker's sequence (). Biological spectra were prepared that separated different plant growth and life-forms, following CitationRaunkiaer (1934) and CitationRawat and Pangtey (1987). The detailed structural analysis of vegetation was conducted in 1998 and 1999, and subsequently the data were combined, because climate, seasonality, and periodicity of growth and phytomass did not vary significantly during the two-year period. The plant density, frequency (F), and abundance (A) (CitationMisra, 1968) and the A/F ratio (CitationWhitford, 1949) were analyzed for all the species from the randomly sampled 1 × 1 m plots (n = 25) at three main alpine pastures (namely, Dzongri, Base Camp, and Thangsing-Goechala) during April, June, August, and October. The density of any given species at a site was calculated by dividing the total number of individuals of that species in all quadrats with the total number of quadrats studied at that site. The relative dominance was computed by summing the total basal cover of a species in all quadrats at a given site times 100 and then dividing that value by the total basal area of all species in all quadrats at that site (CitationMisra, 1968). The importance value index (IVI) was determined as the sum of relative dominance, relative frequency, and relative density (CitationPhillips, 1959). Species richness (CitationMargalef, 1957), diversity (CitationShannon, 1948), equitability (CitationBuzas and Gibson, 1969), and dominance (CitationSimpson, 1949) were also estimated for each site. To assess the plant biomass at each of the three major alpine pastures, 15 random quadrats of 1 × 1 m size were harvested during April, June, August, and October. The aboveground plant material was clipped close to the ground and separated according to species. Belowground phytomass was separated from the monoliths. Litter material was hand-picked after harvesting of aboveground material. The belowground plant material was collected from 15 randomly collected soil monoliths of 25 × 25 cm down to a depth of 30 cm. Further details of the methodology are presented in CitationSingh (2000).
Nutrient contents were analyzed for the plant samples collected during August, which is peak growth period, following standard methods (CitationAllen, 1989; CitationAnderson and Ingram, 1993). Crude fiber was determined by the acid and alkali digestion method using Tecator AB Fibretec apparatus System M2 (1017 hot extract unit and 1018 cold extract unit, Hoganas, Sweden). Acid detergent–soluble lignin was determined by defatting a known mass of plant sample initially with acetone (cold extraction) and thereafter with acid detergent solution (hot extraction). Cellulose was determined by difference of acid detergent–soluble fiber minus acid detergent–soluble lignin. Hemicellulose was determined as the difference between detergent nutrient fiber and acid detergent–soluble fiber by using a Fibretec apparatus. Nitrogen was estimated following a modified Kjeldahl method (CitationAllen, 1989). Crude protein was obtained by multiplying the nitrogen percent by a factor of 6.25, which is based on the assumption that plant protein consists of 16% nitrogen. Phosphorus was estimated by colorimetric determination using molybdate reagent and ascorbic acid, and absorbance was measured at 880 nm in a UV Spectrascan unit (CitationAnderson and Ingram, 1993). Organic carbon in the soil samples was estimated after partial oxidation with an acidified dichromate solution by using a modified Walkey-Black method (CitationAnderson and Ingram, 1993).
Results
The Khangchendzonga Biosphere Reserve covers nearly 36% of the total land area of the Sikkim State (). The environment of the area shows great seasonality; major rainfall and moderate temperatures from May to October make that period the best growing season (). For rest of the year, the snow cover does not allow any plant to grow. The soils of the area under investigation have a sandy-loam structure with more nitrogen content in the upper part of the soil cross section and high phosphorus and organic carbon at lower soil depths ().
FLORAL COMPOSITION, STRUCTURE, LIFE-FORMS, AND BIOMASS
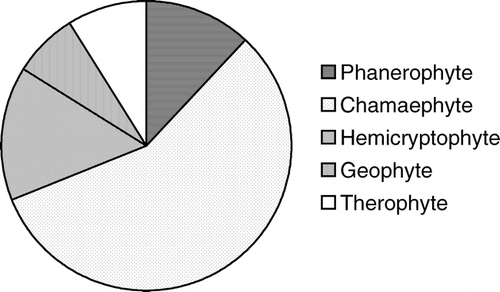
Plants germinated soon after the end-of-April snow melt that commenced the growing season. Maximum species growth was registered during June–August, which was the period of higher rainfall and warmer temperature that provided the best growing conditions. A total of 202 plants species including 102 genera and 38 families (29 dicots, 8 monocots, and 1 gymnosperm) were collected from the study area. These species varied in their life-forms, vegetative growth habits, and flowering period (). Nearly 90% are dicots and 9% are monocots (). The most dominant families in the study area are Asteraceae (22 species), Ranunculaceae (19 species), Ericaceae (16 species), and Primulaceae (14 species) (). Among different life-forms, chamaephytes showed a clear dominance as more than 50% of the species were in this category, followed by therophytes (15.3%), hemicryptophytes (14.9%), phanerophytes (12.4%), and geophytes (7.4%) ().
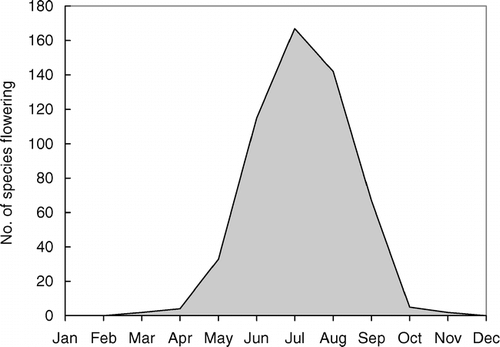
Selected species flowered just after the start of the growing season, e.g., Bergenia ciliata started flowering by end of April. Hemiphragma heterophyllum also started early flowering that lasted until August (). June to August formed the peak flowering period, and as many as 115, 167, and 142 species recorded blooming in these months, respectively (). The plant-species density increased from April onward; it peaked in August and decreased thereafter. The plant density ranged between 920 and 2075 plants per square meter in different months. Poa angustifolia was the species growing at the highest density during April (447 plants per square meter), August (604 plant/m2), and October (534 plants per square meter), and Bistorta affinis showed the highest density for June (387 plants per square meter). The highest A/F ratios were also shown by these species in the same respective months. The species having the highest importance value index was Poa angustifolia during April and October, Potentilla coriandrifolia during June, and Potentilla peduncularis during August ().
Plant-species richness and diversity indices were also highest in August. On the contrary, the equitability and dominance indices were higher during the initial growth period, showing that only a few species dominated at that period (). The aboveground biomass registered a continuous increase from April onward and peaked during August, and it ranged between 134 and 310 g m−2 for different months (). On the contrary, the proportion of belowground biomass reached a minimum in August. The belowground biomass was fairly high at the start of growing season, decreased in subsequent months, and again peaked in October before close of the growing season. It ranged from 927 to 1104 g m−2 for different months. The contribution of belowground biomass to total plant biomass was always higher than the aboveground biomass, which shows the importance of the underground parts of plants in such a harsh environment. High litter mass was registered at the start of growing season, which was from the previous year's phytomass. It ranged between 9 and 40 g m−2 during different sampling intervals (). The root:shoot ratio was higher at the start and end of growing season owing to lower aboveground biomass at that period ().
ECONOMIC UTILITIES OF PLANTS
The alpine plant species are used for diverse purposes by the local peoples. Uses of plant species include medicinal (11 species), incense (6 species), tea-substitute and edible (a single species each), fuelwood (6 species), garden pot plants, and field implements purposes (). The plants are collected to cure fever, jaundice, diabetes, diarrhea, gastric problems, influenza, hair loss, epilepsy, cuts and wounds, bruise and injuries, bone fractures, body aches, and chest pain. The root of Rheum australe is used in both fresh and dry forms as a tea-leaf substitute by the nomads and also cures body aches. Branches of selected species such as Rhododendron, Juniperus, and Cremanthodium were collected in large quantities and used as incense; the branches are commonly sold in the local markets. The branches are also used for incense in the Buddhist monasteries.
NUTRIENTS
Nutrient concentrations of the 10 most dominant alpine plants were analyzed (). Crude fiber content of the plant species ranged between 11.41 and 28.45%, the highest being in Potentilla peduncularis and the least in Poa pratensis (). Cellulose content ranged between 4.59 and 33.19%; the highest values were recorded for Aletris pauciflora, and the least were for Bistorta affinis. Hemicellulose content ranged between 19.71 and 35.84%; the highest were for Hemiphragma heterophyllum, and the lowest were for Potentilla coriandrifolia. Lignin content ranged between 9.32 and 23.11% among various species. Total nitrogen content was estimated at 1.06–1.87%, whereas the phosphorus content varied between 0.185 and 0.377% among the studied species. The crude protein ranged between 6.63 and 11.69%; the highest content was recorded for Poa pseudamoena, and the lowest was for Aletris pauciflora.
Discussion
The present investigation was limited to a few select pastures that showed high species diversity. By extrapolation, the results indicate that the region has a very rich vegetation composition. Dicots were the most dominant plant types, a result that is well comparable with the data from the central Himalaya where 92% of the species were dicots and 8.3% were monocots (CitationRawat and Pangtey, 1987). The percentage of chamaephytes (50%) was higher at the present study site than has been reported for the alpine vegetation of the central Himalaya (46.7%) and western Himalaya (46.4%) (CitationDhar and Kachroo, 1993). The chamaephyte percentage was much higher than was recorded for the Rudranath flora (31%) (CitationRam et al., 1988). The present study site had more woody plants than alpine sites of the central and western Himalaya. The geophytes (7.4%) are comparatively rarer at the present site than at the Rudranath flora site (28.9%), indicating that rhizomatous plants are rarer in the present study area. Asteraceae, Ranunculaceae, Ericaceae, Rosaceae, and Saxifragaceae were the most dominant families (i.e., they had higher numbers of plant species), thus showing a wider adaptability to cold alpine environment. Such species composition may be attributed to relatively better environmental conditions in the eastern Himalayan region than the central and western ones. Most of the plants bloomed during June to August when the temperature and rainfall were relatively higher than in other months. This finding was in accordance with the reports presented on the phenology of alpine species from central Himalaya (CitationSundriyal et al., 1987; CitationRam et al., 1988, CitationRikhari et al., 1992). The aboveground plant biomass showed an increasing trend from start of the growing season, peaked during August, and decreased thereafter again. On the contrary, the belowground biomass was high at the start and end of the growing season. Similar reports are available from the western and central Himalaya (CitationKaul and Sapru, 1973; CitationRam et al., 1988; CitationRikhari et al., 1992; CitationSundriyal and Joshi, 1990).
The rainy season (June–August) provided better growth conditions; therefore plants achieved higher aboveground biomass in this period, which helped to store and accumulate higher belowground biomass before closure of the season. The greater volume of belowground biomass is considered beneficial for growing new tissue at the start of next growing season (CitationSundriyal, 1992). It is an important adaptation in plants for survival under snow because after the snow melts, underground plant parts regenerate the new aboveground parts. The nutrient levels, particularly nitrogen and phosphorus contents, of various species are in accordance with those reported for the species from central Himalayan alpine vegetation (CitationSundriyal and Joshi, 1992). The cellulose and lignin contents are related to palatability of the species. Poa pseudamoena and Juncus thomsonii were highly palatable, and the protein content of these species was also estimated high. On the contrary, Potentilla peduncularis and Bistorta affinis were least palatable, probably because of their high lignin content. A higher concentration of unpalatable species at a particular pasture location was perhaps an indication that that area suffers high grazing pressure, as most of the palatable species have been removed by grazing (CitationSundriyal and Joshi, 1992; CitationSundriyal, 1995).
The influx of trekkers and mountaineers to the alpine areas has an obvious environmental impact in the form of excessive grazing near the camping grounds. It also led to the removal of rhododendrons for fuelwood and the disposal of trash at many sites (CitationSingh et al., 2002). Furthermore, trampling of vegetation by trekkers and pack animals has resulted in an increase of patches of bare ground and conspicuous erosion near the trails. Heavy grazing occurred during the growing season.
Two years' protection of vegetation from livestock grazing (1997–1998) increased the pastures' aboveground biomass by 50% and plant density by 40% (CitationSingh et al., 2003). This result indicates that there is a need to adopt a grazing regime to avoid the adverse impact of overgrazing.
Because a large number of species are also collected for diverse needs, the populations of some of these species have been reduced. Aconitum hookeri, Picrorhiza kurooa, Orchis latifolia, Rheum australe, and Nardostachys jatamansi have been particularly affected, as all these species are collected for medicinal purposes (CitationRai et al., 2000). Furthermore, the trekkers and local porters commonly collect attractive plant species without knowing their importance. Therefore, awareness is needed to educate these trekkers and local porters.
In the economy of high-altitude dwellers in Sikkim, animals play an important role (CitationSingh, 2000). They provide valuable animal proteins in the form of milk, meat, and other by-products. Large numbers of them are also used as pack animals for tourists and trekkers, which allows their owners to earn direct money. Grazing is adopted as the easiest mean to support animals. Therefore, banning of livestock grazing in the alpine zone is not advisable; instead, a management option such as rotational grazing can be suggested based on the grazing carrying capacity of different pastures. This approach will ensure better pasture status as well as animal health, and the pastureland vegetation could be conserved for years to come.
FIGURE 1. Location map of Sikkim Himalaya showing the Khangchendzonga Biosphere Reserve (KBR) (dashed shading)
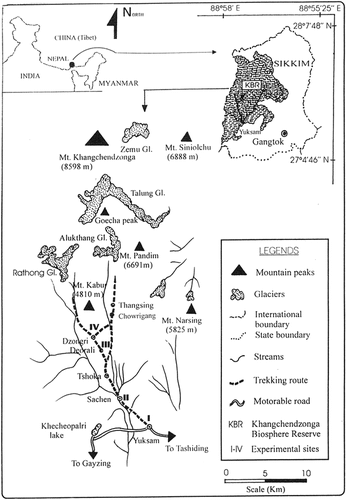
FIGURE 2. Annual rainfall and temperature of Dzongri alpine meadow in the Khangchendzonga Biosphere Reserve, Sikkim Himalaya
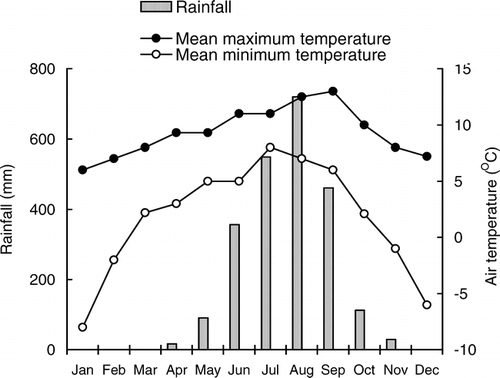
FIGURE 3. Major families and number of plant species encountered in the alpine pasture study area in the Khangchendzonga Biosphere Reserve, Sikkim Himalaya
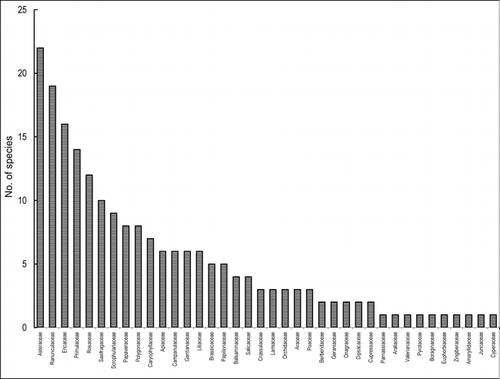
TABLE 1 Physical and chemical properties of soils of the alpine zone in the Khangchendzonga Biosphere Reserve of the Sikkim Himalaya (±1 SE)
TABLE 2 Statistical analysis of the alpine plants of the Khangchendzonga Biosphere Reserve
TABLE 3 Structure of alpine vegetation in the Khangchendzonga Biosphere Reserve (±1 SE). Where the species was either absent or not encountered in the sampling quadrats, the cell is marked with a dash (—); A/F is abundance/frequency ratio
TABLE 4 Species richness, diversity, equitability, and concentration of dominance of alpine vegetation (± 1 SE)
TABLE 5 Economic plant wealth of alpine region in the Khangchendzonga Biosphere Reserve
TABLE 5 (Cont.)
TABLE 6 Nutrient concentration of dominant alpine plant species in the Khangchendzonga Biosphere Reserve (±1 SE)
Acknowledgments
We thank the Director of the G. B. Pant Institute of Himalayan Environment and Development (GBPIHED) for facilities. E. Sharma is thanked for providing valuable input during the study. This work is an outcome of the study supported by Biodiversity Conservation Network, U.S. Agency for International Development, and was jointly managed by the GBPIHED and the Mountain Institute, USA, as the Sikkim Biodiversity and Ecotourism Project. One of the authors (Singh) acknowledges the partial support from International Development Research Centre (IDRC), Canada. We also acknowledge the help of the Botanical Survey of India, Sikkim Circle for plant identification.
Notes
Revised ms submitted March 2004
References Cited
- Allen, S. E. 1989. Chemical analysis of ecological materials. Oxford: Blackwell Scientific Publications, 368 pp.
- Anderson, J. M. and J. S I. Ingram . 1993. Tropical soil biology and fertility: A handbook of methods. Wallingford, U.K.: CAB International, 171 pp.
- Buzas, M. A. and T. G. Gibson . 1969. Species diversity: Benthonic foraminifera in Western North Atlantic: Science 163, 72–75.
- Dhar, U. and P. Kachroo . 1993. Alpine flora of the Kashmir Himalaya (review). Jodhpur, India: Scientific Publishers, 280 pp.
- GSI, 1984. Geology and mineral resources of West District of Sikkim State. Calcutta: Geological Survey of India, Government of India, 14 p.
- Hara, H. 1963. Spring flora of Sikkim Himalaya. Osaka, Japan, 118 pp.
- Hooker, J. D. 1875. The flora of British India. London: L. Reeve & Company, volumes I–VII.
- Kaul, U. and B. L. Sapru . 1973. The phytosociology and biomass production relations of seven meadowlands in Srinagar. Vegetatio 28:1 19–39.
- Khoshoo, T. N. 1991. Conservation of biodiversity in Biosphere. In Khoshoo, T. N., and Sharma, M. (eds.), Indian Geosphere-Biosphere. New Delhi: Vikas Publications, p. 178–233.
- Khoshoo, T. N. 1992. Plant diversity in the Himalaya: Conservation and utilization, II. Pt. G. B. Pant Memorial Lecture. Almora, India: G. B. Pant Institute of Himalayan Environment and Development, 129 p.
- MacKinnon, J. and K. MacKinnon . 1986. Review of the Protected areas system in the Indo-Malayan realm. Gland and Cambridge, IUCN, 284 pp.
- Mani, M. S. 1978. Ecology and phytogeography of high altitude plants of the northwest Himalaya. Introduction to high altitude botany. New Delhi: Oxford and IBH Publishing Company, 205 p.
- Margalef, D. R. 1957. Information theory in ecology. Memorias de la Real Academia de Ciencias y Artes de Barcelona 23:378–449.
- Misra, R. 1968. Ecology Work Book. New Delhi: Oxford and IBH Publishing Company, 244 p.
- Myers, N. 1988. Threatened biotas: “Hot spots” in tropical forestry. The Environmentalist 8:1–20.
- Phillips, E. A. 1959. Methods of vegetation study. New York: Holt, Rinehart and Winston, 107 pp.
- Pradhan, U. and S. Lachungpa . 1990. Sikkim Himalayan Rhododendrons. Kalimpong, West Bengal, India: Primulaceae Books, 130 pp.
- Rai, S. C. and R. C. Sundriyal . 1997. Tourism and biodiversity conservation: The Sikkim Himalaya. Ambio 26:4 235–242.
- Rai, L. K. , P. Prasad , and E. Sharma . 2000. Conservation threats to some important medicinal plants of the Sikkim Himalaya. Biological Conservation 93:1 27–33.
- Ram, J. , S. P. Singh , and J. S. Singh . 1988. Community level phenology of grassland above treeline in the Central Himalaya. Arctic and Alpine Research 20:3 325–332.
- Raunkiaer, C. 1934. The life forms of plants and statistical plant geography, being the collected papers of C. Raunkiaer. Oxford: Clarendon Press, 639 p.
- Rawat, G. S. and Y. P S. Pangtey . 1987. Floristic structure of snowline vegetation in central Himalaya, India. Arctic and Alpine Research 19:2 195–201.
- Rikhari, H. C. , G. C S. Negi , G. B. Pant , B. S. Rana , and S. P. Singh . 1992. Phytomass and primary productivity in several communities of a central Himalayan alpine meadow, India. Arctic and Alpine Research 24:4 344–351.
- Semwal, J. K. and R. D. Gaur . 1981. Alpine flora of Tungnath in the Garhwal Himalaya. Journal of Bombay Natural History Society 78:498–512.
- Shannon, C. E. 1948. A mathematical theory of communication. Bell System Technical Journal 27:1 and 2 379–423.
- Simpson, E. H. 1949. Measurement of diversity. Nature 163:688.
- Singh, H. B. 2000. Grazing impact on plant diversity and productivity along a tourist trekking corridor in the Khangchendzonga Biosphere Reserve of Sikkim. Ph.D. thesis, University of North Bengal, India, 252 pp.
- Singh, H. B. , R. C. Sundriyal , and E. Sharma . 2002. Khangchendzonga Biosphere Reserve, Sikkim: Vegetation dynamics and livestock-rangeland linkages along a tourist trekking corridor. Himalayan Biosphere Reserves 4:1 and 2 1–27.
- Singh, H. B. , R. C. Sundriyal , and E. Sharma . 2003. Livestock grazing in the Khangchendzonga Biosphere Reserve of Sikkim Himalaya, India: Implications for management. Indian Forester 129:5 611–623.
- Smith, W. W. 1913. The alpine and sub-alpine vegetation of South-east Sikkim. Botanical Survey of India Records, IV (7): 431 pp.
- Smith, W. W. and G. H. Cave . 1911. The vegetation of the Zemu and Llonakh valley of Sikkim. Botanical Survey of India Records, IV (5): 285 pp.
- Sundriyal, R. C. 1992. Plant structure, productivity and energy flow in an alpine grassland community. Journal of Vegetation Science 3:15–20.
- Sundriyal, R. C. 1995. Grassland forage production and management in the Himalaya: A review. Journal of Hill Research 8:2 135–150.
- Sundriyal, R. C. and A. P. Joshi . 1990. Effect of grazing on standing crop, productivity and efficiency of energy capture in an alpine grassland ecosystem at Tungnath (Garhwal Himalaya), India. Tropical Ecology 31:2 84–97.
- Sundriyal, R. C. and A. P. Joshi . 1992. Annual nutrient budget for an alpine grassland in the Garhwal Himalaya. Journal of Vegetation Science 3:21–26.
- Sundriyal, R. C. , A. P. Joshi , and R. Dhasmana . 1987. Phenology of high altitude plants at Tungnath in the Garhwal Himalaya. Tropical Ecology 28:289–299.
- Takhtajan, G. 1969. Flowering plants: Origin and dispersal. Edinburgh: Oliver and Boyd, 207 pp.
- Whitford, P. B. 1949. Distribution of woodland plants in relation to succession and clonal growth. Ecology 30:199–208.
APPENDIX 1
APPENDIX 1 List of plant species encountered from the study area. Life-form abbreviations are as follows: Ph = Phanerophytes; Ch = Chamaephytes; He = Hemicryptophytes; Ge = Geophytes, Th = Therophytes
APPENDIX 1 (Cont.)
APPENDIX 1 (Cont.)
APPENDIX 1 (Cont.)
APPENDIX 1 (Cont.)
APPENDIX 1 (Cont.)