Abstract
Effects of abiotic factors on daily and annual photosynthetic carbon gain were evaluated in Abies lasiocarpa and Picea engelmannii at three sites across an alpine treeline ecotone in the Medicine Bow Mountains of southeastern Wyoming (U.S.A.). In addition, the year 2001–2002 was characterized as an episodic drought (seventh driest year since 1895), including a winter that generated only 45% of normal snowpack. Both species had approximately 50% lower xylem water potentials and photosynthesis compared to previous studies for the same species and locale. In A. lasiocarpa, estimated total photosynthesis for the measurement period (A tot) was greatest (28.7 mol m−2) at the mid-ecotone site (∼3198 m), followed by the alpine site (24.6 mol m−2) at ∼3286 m, and then the forest site (19.4 mol m−2) below timberline (∼2965 m). Similar results occurred in P. engelmannii (17.6, 23.4, and 25.3 mol m−2, respectively). These differences appeared to be most influenced by stomatal rather than non-stomatal effects based on comparisons of photosynthesis, leaf conductance, and internal CO2 concentrations through summer. Although photosynthesis over the summer appeared limited primarily by annual water limitation, the two higher elevation sites had significantly greater values that were associated with microclimatic differences in sunlight incidence and, possibly, temperature.
Introduction
Physiochemical factors that control the altitudinal limit of forest trees have been studied for over a century (CitationTranquillini, 1979; CitationWardle, 1968). The majority of these investigations have concluded that, above certain altitudes, trees are unable to assimilate enough photosynthetic carbon for growth and survival. In contrast, CitationKörner (1998, Citation1999) proposed that the altitudes of treelines may be restricted by low soil temperatures that inhibit the processing of assimilated carbon, not its acquisition. In support of this argument, studies have shown constant, or even increasing, values of non-soluble carbohydrate with altitude, indicating little limitation in carbon gain compared to processing (e.g., see CitationKörner, 2003, for review). However, CitationSmith et al. (2003) proposed that the altitudinal extent of both timberlines and treelines is strongly dependent on the ecological facilitation of establishing seedlings, which leads to an improved photosynthetic carbon gain, and, thus, is crucial for the upward movement of timberline boundaries. Regardless, there have been no studies comparing annual carbon gain of any species across the full extent of the alpine treeline ecotone, or differences in carbon gain capabilities according to specific abiotic factors that could be limiting photosynthesis at different times during a day or season.
Several abiotic factors (solar radiation, water, and temperature) appeared to limit photosynthetic carbon gain differently on a daily as well as seasonal basis in these timberline tree species (CitationSmith and Knapp, 1990; CitationSmith et al., 2003). Particularly important for the 2002 measurement period was the unusually small amount of winter snowpack (45% of normal), an early warm period in spring, and the subsequent low water availability during the following summer. This dry winter may have been only slightly attenuated by modest rainfall throughout the spring and summer. This extremely dry year provided a unique opportunity to observe the physiological response of P. engelmannii and A. lasiocarpa to low water availability under relatively normal sunlight and temperature conditions at three locations spanning the altitudinal breadth of the treeline ecotone. Moreover, under current scenarios of future climate change, these types of episodic stress events may become more frequent and intense, and show early impacts on plant boundary systems, in particular (CitationBrown and McLachlan, 2002).
Materials and Methodology
To test for the influence of altitude and site microclimate, photosynthetic carbon gain and water status of mature, cone-bearing trees of Picea engelmannii Parry ex. Englem. and Abies lasiocarpa (Hook.) Nutt. were monitored at three sites throughout the summer of 2002 (22 June–20 September). Abiotic factors (sunlight, temperatures, and plant water status) were also measured at each site and associated with differences in the shoot-level photosynthesis and water relations parameters measured.
STUDY SITES
In the south-central Rocky Mountains, U.S.A., subalpine forest occurs at near 2300 m, emerging from mixed short-grass prairie and shrub steppe that is interspersed with riparian forest along stream bottoms. This subalpine forest consists primarily of a mixture of P. engelmannii, A. lasiocarpa, Pinus contorta, P. flexilis, and Populus tremuloides. With increasing elevation, tree species composition changes to the two co-dominants, A. lasiocarpa and P. engelmannii. At the high elevation limit of the subalpine forest, ribbon-like forest glades are formed by strong prevailing wind effects on snow deposition (CitationSmith and Knapp, 1990). Within the adjacent treeline (alpine) ecotone, spruce and fir become tightly grouped into tree “islands” separated by tens of meters of surrounding alpine meadow. With further procession toward the treeline limit, mature trees group together in dense clusters and exhibit a windswept appearance, while inter-island distances increase. The extreme upper portion of the treeline ecotone is composed of smaller, shrub-like krummholz mats that are widely spaced. This change in distribution and growth form is highly variable at a given altitude, and depends primarily on wind exposure, topography, and snow deposition (CitationDaubenmire, 1954; CitationWardle, 1968; CitationSmith et al., 2003). Other associated species include P. flexilis, P. contorta, shrubs (Salix spp. and Ribes montigenum), and many herbaceous species such as Caltha leptosepala, Helianthella quinquenervis, and Erythronium grandiflorum (CitationBillings, 1969; CitationPeet, 1988).
Three study sites (approximately 40 × 40 m each) were selected for comparison in the treeline ecotone of the Snowy Range of the Medicine Bow Mountains in southeastern Wyoming, U.S.A. (41°20′N, 106°13′W). The selected sites and trees involved in this study were considered to be representative of the three general regions associated with the treeline ecotone. One site was located within in a mixed A. lasiocarpa, P. engelmannii subalpine forest (FS) at the lower timberline edge (2965 m elevation). The second site was composed of a mosaic of tree islands (both A. lasiocarpa and P. engelmannii) located within alpine meadow near the center of the ecotone (TS) at 3198 m. The third site was located at the farthest extent of where trees occurred (AS) at 3256 m, where krummholz mats formed stunted tree islands spaced tens of meters apart (CitationSmith et al., 2003). All three sites were located on east-southeast (approximately 106° for FS, 114° for TS, and 118° for AS) slopes at <15° incline for all sites.
PHOTOSYNTHESIS AND WATER STATUS
Net photosynthetic CO2 exchange (μmol CO2 m−2 s−1) and accompanying transpiration (mmol H2O m−2 s−1) were monitored in mature trees (5–10 m in height at FS and TS, and <2 m in height at AS, and 25–150 years in age) using gas-exchange measurements throughout the summer of 2002 (22, 23, and 26 Jun; 12, 13, and 17 Jul; 30, 31 Jul and 2 Aug; 17, 18, and 19 Aug; 20, 21, and 22 Sep). Measurements were taken five times per day at approximately 0900, 1100, 1400, 1600, and 1800 hours solar time (CitationList, 1971). Each sampling included south-facing sun shoots at mid-canopy (five for each species) from five individual trees of each species, healthy in appearance and selected as representative of the site (tree size and spacing).
Mean net photosynthesis (A) and transpiration (E) were calculated by averaging all photosynthesis measurements in each species and sample period (900, 1100, 1400, 1600, and 1800 hours). Integrated daily photosynthetic carbon gain (A tot) was then computed from mean values for each time period, multiplying by the amount of time in each interval, and integrating for the entire day. This procedure was repeated 100 times, while randomly resampling from the five mean photosynthesis measurements for each of the 5 diurnal sample periods for both species. Resampling and other calculations were analyzed using Microsoft Excel and Visual Basic according to techniques described in CitationCrowly (1992). Corresponding water use efficiencies (WUE) were calculated as A/E.
Photosynthesis of the attached conifer shoots and previous-year (1 yr old) needle cohorts was measured using a LICOR-6200 portable photosynthesis system (LICOR Inc., Lincoln, Nebraska) for shoots orientated naturally to the sun. Photosynthesis was computed on a total leaf area basis by measuring the average number of needles per cm of stem length within the sample cuvette, the area of individual needles, and then multiplying the two by stem length. Based on 12 shoot samples (south-facing) for each species, 34.0 ± 3.1 cm2 and 30.4 ± 2.2 cm2 of total needle area occurred per 10 cm of stem length in A. lasiocarpa and P. engelmannii, respectively (similar to results of CitationThompson and Leyton, 1971; CitationHadley and Smith, 1987; CitationSmith et al., 1991). Photosynthesis expressed on a silhouette leaf area basis was between 1.5 and 2 times greater for both species, as previously reported in CitationSmith et al. (1991).
Xylem water potential (ψ) was measured using a Scholander-type pressure chamber (model 1000-XT, PMS Instrument Co., Corvallis, Oregon) at approximately two-week intervals between 1400 and 1500 solar time from to 10 Jun–22 Sep 2002. Stems (∼10 cm lengths) with 2- to 3-yr-old needles were excised (cut surface immediately covered with petroleum jelly) and placed into air-tight freezer bags on ice until measurements were completed, approximately 1 h later. Field measurements of ψ were taken within 2 min of excision and compared to those of shoots transferred to the lab. No significant changes in water status were detected due to transfer and storage time during the length of the measurement period (N = 20, α = 0.05).
T leaf , T air , PPFD, AND PRECIPITATION
Photosynthetic photon flux density (PPFD, 400–700 nm wavelength), air temperature (T air), and soil temperature (T soil) were measured at central locations at each site within a few meters of all study trees. Measurements were recorded every 10 min for a total of 67 d (26 Jun–1 Jul, 5–24 Jul, 28 Aug, 15–25 Aug, and 31 Aug–22 Sep). PPFD was measured using LiCOR LI-190 PPFD sensors oriented horizontally at 1 m height, T air using two fine-wire (0.02 mm diameter) copper/constantan thermocouples (Omega Engineering, Stamford, Connecticut) at 1 m height (shielded from direct solar radiation), and T soil using three thermistors buried at 15 cm depths (Omega Engineering, Stamford, Connecticut). All sensors were monitored using dataloggers (model 21x, Campbell Scientific, Logan, Utah). PPFD levels were also measured during photosynthetic measurements using the LI-190 PPFD sensor attached to the leaf cuvette and oriented in the same plane as the primary axis of the measurement shoot. Needle temperatures (T leaf) were monitored in the field and during photosynthesis measurements with attached, fine-wire thermocouples (CitationHadley and Smith, 1987).
Weekly precipitation data were obtained from the National Atmospheric Deposition Program, WY00 site (41°23′N, 106°16′W, 3284 m elevation), located less than 1 km from the research sites. Rainfall was measured daily using an Alter-shielded Belfort rain gauge and summed weekly.
STATISTICS
Statistical comparisons between means of replicated data for a given time period are shown as standard errors, while interspecific and between-site comparisons of mean values were conducted using single factor analysis of variance (ANOVA) (CitationZar, 1999).
Results
PRECIPITATION
The year 2001–2002 was one of the driest on record for the southern Rocky Mountain region, leading to widespread drought and fire. The period from April to September 2002 was the seventh driest on record for this region since the National Oceanic and Atmospheric Administration (NOAA) began recording in 1895 (NOAA Drought Information Center). Sporadic rainfall occurred from approximately 22 June through September, rising above 5 mm per week on nine occasions, and above 15 mm on three occasions (e.g., during the two weeks before the 22 Sep gas exchange measurements) (from CitationJohnson et al., 2004). Total recorded rainfall was 148 mm for most of the summer growth period (1 Jun–28 Sep 2002).
SUNLIGHT, TEMPERATURE AND WATER RELATIONS
Photosynthetic photon flux density (PPFD) tended to be higher at highest alpine site (AS), followed by the middle treeline (TS) and forest (FS) sites, except for the first measurement period (22, 23, and 26 Jun) where TS measurements were greatest (). Corresponding integrated PPFD for the summer measurement period was 3.9, 5.1, and 5.2 kmol m−2 at FS, TS, and AS, respectively ().
Mean daily air temperatures (T air) and leaf temperatures (T leaf) at FS were substantially higher (up to 4.4°C) than at TS and AS throughout much of summer, while Tleaf was greater than corresponding T air values at all sites for nearly the entire summer measurement period (). Soil temperature (T soil) at all sites remained relatively constant, between about 12 and 16°C, throughout summer, but with a marked decline to near 5°C for the 20 Sep measurements ().
The mean leaf-to-air vapor pressure deficit (LAVD) varied from 1.5 to 2.7 kPa with no consistent pattern for either species, except the significant decrease to near 1.0 kPa (22 Sep) following a cooling period (and significant rainfall) in early September (). However, A. lasiocarpa trees at FS had the lowest as well as the greatest LAVD (2.7 kPa) measured during early summer. LAVD remained somewhat variable through mid-summer, with FS trees typically having higher LAVD than TS and AS trees (with the exception of the 31 Jul measurement date; ). Measured xylem water potentials (Ψ) were at a minimum early in summer with the lowest value (−3.4 MPa) measured for A. lasiocarpa at TS during the first sample date (). Ψ remained almost constant in both species at all sites through mid-summer, followed by a slight increase in A. lasiocarpa at FS.
GAS EXCHANGE PHYSIOLOGY
Mean photosynthesis (A) was greatest for both species at the TS and AS sites, with little difference between the two species at any site (). Among the two species, maximum summer values for A (slightly above 3 μmol m−2 s−1) occurred at FS and TS, but not AS, on the 20 Sep measurement date. A at FS and AS showed little change throughout the entire summer period of measurement, except for the 20 Sep date at FS ().
In both A. lasiocarpa and P. engelmannii, leaf conductance (g) and transpiration (E) at FS and AS decreased, in general, throughout the summer, but with slight increases at FS and TS on 20 Sep, but not AS (). Changes in g and E tracked changes in A at all sites, but were more variable at TS than FS or AS. Similar patterns occurred for C i, although calculated WUE (A/E) increased steadily during most of the measurement period in both species, with a substantial increase in both species at AS. Also, C i did not increase abruptly on 20 Sep as measured at FS and TS ().
At the highest elevation alpine site (AS), the greatest A values occurred on the earliest sampling date (26 Jun) and corresponded to the highest g and C i values measured for the entire summer sampling period (). Similar results occurred for the FS and TS sites (data not plotted). In addition, much stronger positive linear correlations occurred for the A vs. g data (r 2 > 0.57) than for the A vs. C i data (r 2 < 0.22) on all sample dates, with the exception of the 22 Sep measurements. In addition, the slopes of the regression lines for A vs. g were not significantly different between measurement dates at P = 0.05 (ANOVA) ().
At all three sites for both species, a strong positive linearity occurred between the ratio of A to C i (A:C i) and date as the summer progressed, but especially at AS (). When comparing A:C i data for all sites (FS, TS, and AS), AS measurements had the steepest slope (greatest sensitivity between A:C i and date), followed by the TS and FS sites. Linear regressions for the FS, TS, and AS data were calculated as follows, (FS) y = 4(10−5)x − 3.75, r 2 = 0.72; (TS) y = 2(10−4)x − 7.12, r 2 = 0.64; (AS) y = 3(10−4)x − 10.46, r 2 = 0.94) ().
INTEGRATED SUNLIGHT AND CARBON GAIN
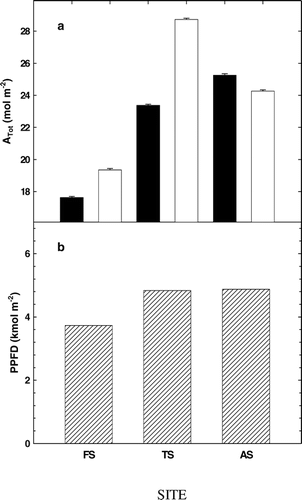
In P. engelmannii, total photosynthetic carbon gain for the summer measurement period (A tot) was greatest at the higher elevation TS and AS sites followed by the lowest elevation FS site (28.7, 24.6, and 19.4 mol m−2, respectively) (). Maximum integrated carbon gain occurred for P. engelmannii at TS. A tot in A. lasiocarpa was also similar at TS and AS (p < 0.05; ANOVA) and yet, greater compared to FS (17.6, 25.3, and 23.4 mol m−2, respectively). Integrated sunlight was also similar at TS and AS, and greater than measured at FS (P < 0.05; ).
Discussion
In the same approximate locale as the present study, CitationCarter et al. (1988) reported mean daily photosynthesis in A. lasiocarpa and P. engelmannii of about 50% greater than measured here, and a leaf conductance (g) during early summer of almost three times greater. The effects of this drought were also reflected in the low xylem water potentials (ψ) measured throughout summer, particularly during early summer (see CitationSmith, 1985; CitationSmith and Knapp, 1990, for reviews). In contrast to previous studies of seasonal photosynthesis in subalpine conifer trees, low values of A and ψ occurred during June in both species at all sites (except the forest site, FS). As a result, this enabled comparison of the gas exchange responses in A. lasiocarpa and P. engelmannii during an unusually dry year with an otherwise typical pattern of sunlight and temperature regimes.
PHOTOSYNTHESIS (A)
Despite the general depression in A throughout summer, differences in photosynthetic values showed considerable variation among the three sites. The lowest A values occurred at the lowest elevation, subalpine forest site (FS), which also corresponded to the lowest PPFD values of the three sites. Also, A values remained fairly stable throughout summer, except for an abrupt increase on the final sample date in September following a significant precipitation event. Both TS and AS (alpine site) trees received similar amounts of incident PPFD throughout the measurement period, approximately 1.3 times more than FS. Thus, PPFD may have limited photosynthesis at FS compared to TS and AS, despite the strong overall limitation induced by drought at all three sites. However, trees at TS were influenced by adjacent forest that blocked a significant portion of early morning PPFD, as well as nighttime sky, both of which can be important causes of low-temperature photoinhibition of photosynthesis (CitationJordan and Smith, 1994; CitationGermino and Smith, 1999, Citation2000; CitationSmith et al., 2003).
Increased sky exposure is known to negatively affect photosynthesis in a variety of alpine plants, especially with respect to low-temperature photoinhibition of photosynthesis (see CitationLoreto et al., 2004, for review). Thus, trees at FS experienced lower incident PPFD, as well as warmer air and needle temperatures during early morning, while the tree mats at AS and the TS trees within islands experienced colder nighttime temperatures (greater sky exposure), as well as greater sunlight exposure early the following morning. The high levels of incident sunlight at these two sites, plus no relationship between maximum A values on a daily basis and daily maximums in PPFD (data not shown) suggest that some factor other than water and sunlight may have been responsible for the measured differences in A between TS and AS. The possibility exists that greater low temperature photoinhibition may have been a factor at both of the more exposed, higher sites (TS and AS).
As noted above, morning A measurements typically showed some of the greatest differences between sites. Soil temperature differences were also greatest in the mornings, particularly between TS and AS. Early in the season, TS and AS trees had similar A values, while T soil at AS was ∼2.5°C warmer than measure red at TS. In addition, on 20 Sep when TS trees had much higher A than at AS, T soil at AS was the lowest of all three sites through most of the day. Conversely, carbon gain at AS increased slightly through the summer, while T soil decreased nearly 10°C over the same period. TS trees also had the greatest photosynthetic carbon gain at the end of summer (early fall) following significant rainfall, and when soil temperatures were coldest for the summer measurement period. Thus, little correlation between cold soil temperatures and photosynthetic performance was apparent between the three sites (see CitationKörner, 1998, for a contrasting hypothesis).
STOMATAL (g) VERSUS NON-STOMATAL EFFECTS ON A
For both species at all three sites, E showed a general decline throughout summer while A remained relatively constant, although a rebound in E occurred on 20 Sep at FS and TS following rainfall (). A corresponding decline followed by a late-season increase also occurred in g, while WUE increased throughout summer at all three sites, especially the highest elevation site (AS). September (20–22 Sep) measurements at all sites were markedly different compared to those taken throughout the rest of summer, most likely due to the significant precipitation (2.2 cm of rainfall) on 19 Sep. The previous rainfall event was 0.95 cm and occurred on 2 Sep 2002 (NOAA site, WY00). Computed increases in WUE were due to the relatively constant A, and the accompanying decreases in g and E. Moreover, needle warming to well above air temperature occurred in both A. lasiocarpa and P. engelmannii due to a greater needle aggregation in sun-type shoots, with only minor declines in photosynthesis due to mutual shading among adjacent needles (CitationSmith and Carter, 1988; CitationSmith and Brewer, 1994; Niinemets et al., 2001). The decreasing E pattern throughout the summer for all sites might also be attributed to the seasonal trend of decreasing T air and T leaf, although decreases in the leaf-to-air vapor pressure deficit (LAVD) at all sites was not apparent until the last measurement period (20 Sep) when WUE also increased dramatically at AS. The lack of a rebound in A and g at AS following rainfall at the end of summer might have been associated with the colder T air, T leaf, and T soil measured at this site.
C i in A. lasiocarpa and P. engelmannii also decreased during mid-summer, with a return to higher values on 20 Sep at FS and TS. Past research has shown that C3 plants grown in low light conditions (e.g., FS trees) may require, in general, a higher internal CO2 concentration (C i) to offset a lower internal mesophyll cell conductance, while those experiencing warmer temperatures may also require a higher C i in support of greater CO2 demand by mesophyll cells (CitationBauer et al., 1983). Overall, lower C i is often interpreted as a decrease in CO2 availability for carboxylation in the chloroplasts of mesophyll cells due to a decline in the diffusion gradient from the mesophyll air space to the chloroplasts Citation(Cowan and Farquhar, 1997).
In general, seasonal changes in internal CO2 concentration (C i) also appeared to track changes in leaf conductance (g), while WUE showed opposite trends, in both species. Thus, A appeared to be most strongly influenced by stomatal rather than non-stomatal limitations. Thus, an apparently greater mesophyll demand (sink) for CO2 led to the higher A recorded for trees at TS. Trees at AS showed the strongest decreases in C i throughout the growth season while maintaining a relatively constant A, most likely due to the corresponding declines in g. A second possibility for the higher C i measured in AS trees, even though TS and AS had similar g, would be a lower mesophyll cell demand for CO2, at the same degree of stomatal opening, implicating greater sink limitations due to increased stress in mesophyll cells of AS trees.
INTERSPECIFIC AND SITE DIFFERENCES
At both FS and TS, A. lasiocarpa had significantly greater photosynthetic carbon gain than P. engelmannii based on a single factor ANOVA (P <0.05). Total integrated carbon gain was also significantly different between the three sites (P < 0.05). In addition, the larger trees characteristic of FS, and to a certain extent at TS, generated substantially longer periods of shade throughout these sites during the early morning and late evening hours. Moreover, greater shade tolerance in A lasiocarpa may have contributed to the higher carbon gain measured for this species in the lower light environment at FS (CitationCarter and Smith, 1988). Abies lasiocarpa at TS also had higher A, g, and E throughout the season, while maintaining WUE similar to P. engelmannii, suggesting a greater capability to respond to the TS environment. However, these physiological trends reversed at AS, where P. engelmannii had slightly higher carbon gain than A. lasiocarpa. However, leaf area index at the whole-crown level could also have an important influence on carbon uptake differences between individuals of both species, as well as sites, in addition to the site- and shoot-level (interspecific) differences measured here.
SUMMARY
Greater A and total photosynthetic carbon gain in A lasiocarpa and P. engelmannii occurred at the highest elevation sites (TS and AS) compared to the subalpine forest site (FS), indicating no apparent, detrimental effect of increased altitude across the full breadth of the treeline ecotone, at least for this unusually dry year. However, microsite differences in sunlight and, possibly, temperatures at the three sites may have significantly influenced photosynthetic carbon gain. In addition, stomatal adjustments that generated similar A values, but at higher values of WUE, appeared to be operative in response to gradual declines in plant water potential through summer. Moreover, the linear increase in the ratio of A:C i through summer, despite steadily declining levels of g and C i, implicates a strong, feed-forward response mechanism to ensure water conservation (higher WUE). Thus, an understanding of the interaction of stomatal behavior with microclimatic effects (possibly involving ecological facilitation) appeared to override any effects of this rather narrow span in altitude (CitationSmith et al., 2003). Similar results have been found for establishing conifer seedlings in this same treeline ecotone (CitationJohnson et al., 2004). These results also suggest that global-change effects leading to warmer temperatures, altered sunlight regimes due to cloud cover changes, and/or summer precipitation patterns could result in significant impacts on photosynthesis and growth in these treeline conifers, even during relatively severe drought years (CitationBrown and McLachlan, 2002).
FIGURE 1. (A) Mean photosynthetic photon flux density (PPFD) received throughout 2002 growing season at FS (closed circles, subalpine forest, 2965 m), TS (closed squares, upper treeline ecotone, 3198 m), and AS (open triangles, alpine treeline, 3256 m). Vertical bars represent standard errors. (B) Mean daily T air (black symbols), T leaf (open symbols), and T soil (lower curves) for the three study sites: FS (circles, subalpine forest, 2965 m), TS (squares, upper treeline ecotone, 3198 m), and AS (triangles, alpine treeline, 3256 m). Vertical bars are standard errors
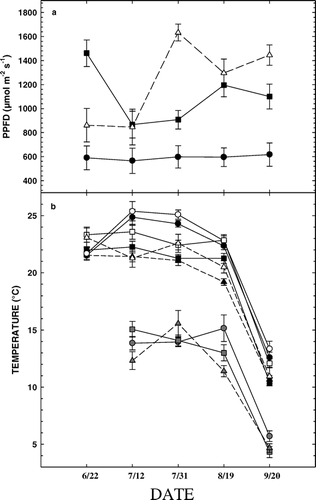
FIGURE 2. (A) Mean leaf to air vapor pressure deficit (LAVD) for A. lasiocarpa and P. engelmannii at FS (closed circles, subalpine forest, 2965 m), TS (open squares, upper treeline ecotone, 3198 m), and AS (solid triangles, alpine treeline, 3256 m). (B) Mean daily xylem water potentials (ψ) for A. lasiocarpa (open symbols) and P. engelmannii (closed symbols) for the three study sites FS (circles, subalpine forest, 2965 m), TS (squares, upper treeline ecotone, 3198 m), and AS (triangle, alpine treeline, 3256 m) through the 2002 growing season. Vertical bars are standard errors
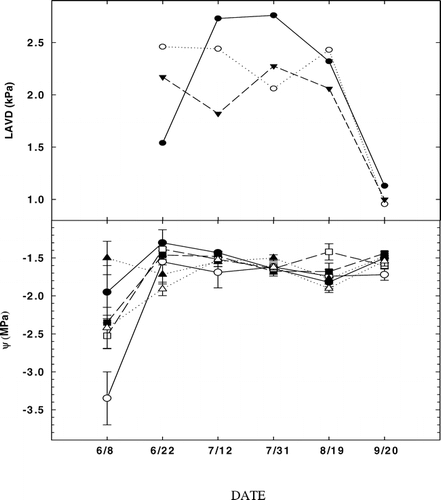
FIGURE 3. Mean photosynthesis (A), leaf conductance (g), transpiration (E), water use efficiency (WUE), and intracellular concentration of CO2 (C i). in A. lasiocarpa (open symbols) and P. engelmannii (closed symbols) for the three study sites FS (subalpine forest, 2965 m), TS (upper treeline ecotone, 3198 m), and AS (alpine treeline, 3256 m) through the 2002 growing season. Values for WUE should be multiplied by 10−4, and vertical bars are standard errors
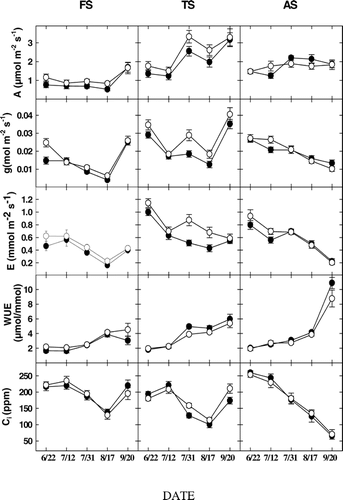
FIGURE 4. Mean daily photosynthesis (A) versus leaf conductance (g) and intracellular CO2 concentration (C i) for A. lasiocarpa (open symbols) and P. engelmannii (solid symbols) over five measurement days (26 June, 17 July, 30 July, 18 August, and 23 September 2002) at AS
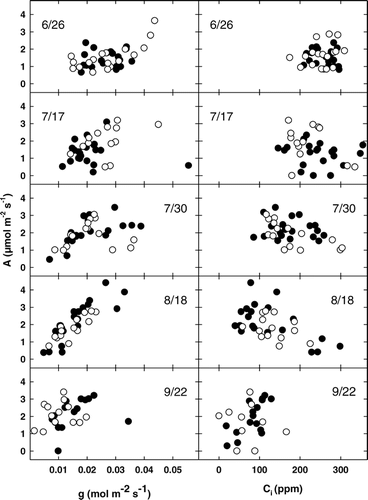
FIGURE 5. Seasonal change in the ratio of mean daily photosynthesis (A) to CO2 concentration of the mesophyll air space inside the leaf (C i), or A:C i, measured at the FS (solid circles), TS (solid squares), and AS (open triangles) study sites. FS (subalpine forest, 2965 m), TS ( treeline ecotone, 3198 m), and AS (alpine treeline, 3256 m). Solid lines represent best-fit linear regressions (FS: r 2 = 0.57; TS: r 2 = 0.70; AS: r 2 = 0.94)
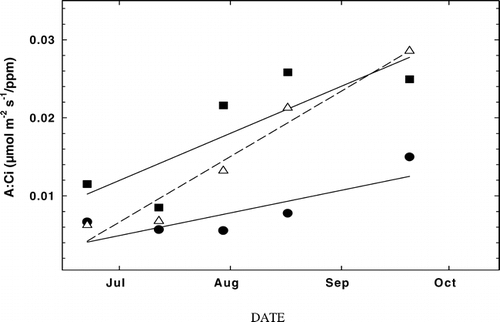
FIGURE 6. (A) Total carbon gain (A tot) and (B) integrated photosynthetically active radiation (PPFD) for the 2002 growing season in A. lasiocarpa (black bars) and P. engelmannii (gray bars) at the three sites, FS (subalpine forest, 2965 m), TS (treeline ecotone, 3198 m), and AS (alpine treeline, 3256 m)
Acknowledgments
We gratefully acknowledge the support of the National Science Foundation (Ecological and Evolutionary Physiology Program); the U.S. Forest Service, Fort Collins, Colorado, U.S.A.; the Vecellio and Sullivan Scholarship Fund, the Department of Biology and the Graduate School Research Fund, Wake Forest University.
References Cited
- Bauer, H. , P. Martha , B. Kirchner-Heiss , and I. Mairhofer . 1983. The CO2 compensation point of C3 plants—a re-examination II. Intraspecific variability. Zeigen der Pfanzenphysiolgia 109:143–154.
- Billings, W. D. 1969. Vegetational pattern near alpine timberline as affected by fire-snowdrift interactions. Vegetatio 19:192–207.
- Brown, A. C. and A. McLachlan . 2002. Sandy shore ecosystems and the threats facing them: some predictions for the year 2025. Environmental Conservation 29:62–77.
- Carter, G. A. and W. K. Smith . 1988. Microhabitat comparisons of transpiration and photosynthesis in three subalpine conifers. Canadian Journal of Botany 66:963–969.
- Carter, G. A. , W. K. Smith , and J. L. Hadley . 1988. Leaf conductance in three conifer species at different elevations during summer in Wyoming. Canadian Journal of Forest Research 18:242–246.
- Cowan, I. R. and G. D. Farquhar . 1977. Stomatal function in relation to leaf metabolism and environment. Symposium for the Society of Experimental Biology 21:471–505.
- Crowly, P. H. 1992. Resampling methods for computation-intensive data analysis in ecology and evolution. Annual Review of Ecology and Systematics 23:405–447.
- Daubenmire, R. 1954. Alpine timberlines in the Americas and their interpretation. Butler University Botanical Studies 2:119–136.
- Germino, M. J. and W. K. Smith . 1999. Sky exposure, crown architecture, and low-temperature photoinhibition in conifer seedlings at alpine treeline. Plant, Cell and Environment 22:407–415.
- Germino, M. J. and W. K. Smith . 2000. Differences in microsite, plant form, and low-temperature photoinhibition in alpine plants. Arctic and Alpine Research 32:388–396.
- Hadley, J. L. and W. K. Smith . 1987. Influence of krummholz mat microclimate on needle physiology and survival. Oecologia 73:82–90.
- Johnson, D. M. , M. J. Germino , and W. K. Smith . 2004. Abiotic factors limiting photosynthesis in Abies lasiocarpa seedlings below and above alpine timberline. Tree Physiology 24:377–386.
- Jordan, D. N. and W. K. Smith . 1994. Energy-balance analysis of nighttime leaf temperatures and frost formation in a sub-alpine environment. Agricultural and Forest Meteorology 71:359–372.
- Körner, C. 1998. A re-assessment of high elevation treeline positions and their explanation. Oecologia 115:445–459.
- Körner, C. 1999. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Berlin: Springer, 372 pp.
- Körner, C. 2003. Carbon limitation in trees. Journal of Ecology 91:4–17.
- List, R. J. 1971. Smithsonian Meteorological Tables. Washington, D.C.: Smithsonian Institution Press, 527 pp.
- Loreto, F. , N. R. Baker , and D. R. Ort . 2004. Environmental constraints: chloroplast to leaf. In Smith, W. K., Vogelmann, T. C., and Cristchley, C. (eds.), Photosynthetic Adaptation: Chloroplast to Landscape. New York: Springer, 231–261.
- Niinemets, Ü and O. Kull . 2001. Sensitivity of photosynthetic electron transport to photoinhibition in a temperate deciduous forest canopy: photosystem II center openness, non-radiative energy dissipation, and excess irradiance under field conditions. Tree Physiology 21:899–914.
- Peet, R. K. 1988. Forests of the Rocky Mountains. In Barbour, M. G., and Billings, W. D. (eds.), North American Terrestrial Vegetation. Cambridge: Cambridge University Press, 63–102.
- Smith, W. K. 1985. Western montane forests. In Chabot, B. F., and Mooney, H. A. (eds.), Physiological Ecology of North American Plant Communities. New York: Chapman and Hall, 95–126.
- Smith, W. K. and C. A. Brewer . 1994. The adaptive importance of shoot and crown architecture in conifer trees. American Naturalist 143:528–532.
- Smith, W. K. and G. A. Carter . 1988. Shoot structural effects on needle temperatures and photosynthesis in conifers. American Journal of Botany 75:496–500.
- Smith, W. K. and A. K. Knapp . 1990. Ecophysiology of high elevation forests. In Osmond, C. B., Pitelka, L. F., and Hidy, G. M. (eds.), Plant Biology of the Basin and Range. Berlin: Springer-Verlag, 87–142.
- Smith, W. K. , A. W. Schoettle , and M. Cui . 1991. Importance of leaf area measurement to the interpretation of gas exchange parameters of complex shoots (e.g. conifers). Tree Physiology 8:121–127.
- Smith, W. K. , M. J. Germino , T. E. Hancock , and D. M. Johnson . 2003. Another perspective on altitudinal limits of alpine timberlines. Tree Physiology 23:1101–1112.
- Thompson, F. B. and L. Leyton . 1971. Method for measuring the surface area of complex shoots. Nature 229:572.
- Tranquillini, W. 1979. Physiological Ecology of the Alpine Timberline: Tree Existence at High Altitudes with Special Reference to the European Alps. New York: Springer-Verlag, 137 pp.
- Wardle, P. 1968. Engelmann spruce at its upper limits on the Front Range, Colorado. Ecology 49:483–495.
- Zar, J. H. 1999. Biostatistical Analysis. 4th edition. New Jersey: Prentice Hall, 663 pp.