Abstract
The colonization of the underside of rocks normally requires that the material is sufficiently translucent to allow the penetration of photosynthetically active radiation. We examined the underside of 950 opaque rocks in sixteen locations in the Arctic for hypolithic colonization by photosynthetic microorganisms. Greater than 90% of rocks were colonized. The mean width of the bands of colonization was 3.1 ± 1.9 cm on Devon Island, and 3.0 ± 1.6 cm on Cornwallis Island. The width of the bands of colonization was less in the interior of frost-sorted polygons compared to their edges (in the arctic location, 0.7 ± 0.8 and 3.6 ± 1.4 cm in the interior and at the edges, respectively), suggesting the importance of frost sorting in enhancing the penetration of light around the edges of rocks to their undersides, and thus allowing colonization by photosynthetic organisms. We observed a similar pattern of colonization in antarctic polygons. The hypolithic habitat provides protection from environmental extremes. We show that within the hypolithic habitat organisms are protected from UV radiation. From radiolabeled carbon uptake measurements we estimate the productivity of the arctic communities to be approximately 0.8 g m−2 a−1, potentially as high as above-ground productivity. We discuss the potential implications of climate change for these communities.
Introduction
In extreme hot and cold deserts, the underside of rocks can provide a refugium for microorganisms, both photosynthetic and non-photosynthetic (CitationCameron and Blank, 1965; CitationFriedmann and Galun, 1974; CitationSchlesinger et al., 2003). The organisms are referred to as “hypoliths” and the community as “hypolithon” (CitationGolubic et al., 1981). In the antarctic polar desert the stones warm the hypolithic biota and, during the summer, mitigate freeze-thaw (CitationBroady, 1981).
For photosynthetic organisms, the limiting factor for the depth of colonization around the underside of the rock is set by the depth at which light levels are below those required for photosynthesis, resulting in well defined “bands” of growth around rocks or in the case of thin rocks, complete colonization of their underside. As photosynthetic microorganisms provide a source of carbon for heterotrophic microorganisms, the hypolithic colonization of translucent rocks by photosynthetic microorganisms can benefit non-photosynthetic microorganisms (CitationSmith et al., 2000).
All studies to date have focused on the colonization of rocks that are translucent to visible light, particularly quartz. Hypolithic colonization of quartz stones has now been documented in many locations, for example: the Vestfold Hills, Antarctica (CitationSmith et al., 2000), the Mojave Desert, U.S.A. (CitationSchlesinger et al., 2003), the Negev desert, Israel (CitationBerner and Evenari, 1978), and the Namib Desert, Africa (CitationBudel and Wessels, 1991).
As wind transport of fine material tends to fill openings around the edges of rocks, it is generally the case that hypolithic colonization requires that the rock itself permits visible light to reach the communities on their underside. Investigations on the colonization of the underside of translucent flint in the Negev desert showed that the more translucent flint types were colonized in a greater number of instances compared to less translucent flints (CitationBerner and Evenari, 1978).
However, translucent or transparent rocks such as quartz are a special geologic case. Most rocks, including gneisses, igneous rocks, and sedimentary rocks such as dolomites are generally opaque in the wavelengths required for photosynthesis (400 to 700 nm). As a result, they are not usually regarded as suitable sites for hypolithic colonization (we use the term “opaque” here to mean any rock where the flux of light that penetrates through a rock of given size is lower than the minimum light level required for photosynthesis; this level is taken to be 10 nmoles m−2 s−1, based on field measurements [CitationLittler et al., 1986] and 0.1 μmoles m−2 s−1 based on theoretical calculations (CitationRaven et al., 2000)—in either case rocks in our field locations of greater thickness than 1 mm are opaque).
Our investigations of hypolithic colonization focused on the polar desert. Polar deserts constitute approximately 5 × 106 km2 of Earth's surface, and these regions cover a substantial part of the Arctic and coastal non–ice-covered regions of Antarctica. They are regions where annual precipitation is less than 250 mm and the mean temperature during the warmest month is less than 10°C. A common feature of polar deserts is areas of frost-sorted rocks or “patterned ground” (CitationKessler and Werner, 2003; CitationSletten et al., 2003).
In this paper, we describe a large photosynthetic ecosystem in the polar desert under opaque rocks and we estimate the productivity of these communities. Our colonization and productivity measurements focus on the Arctic, but we also discuss some colonization measurements from the Antarctic.
Materials and Methods
STUDY LOCATIONS
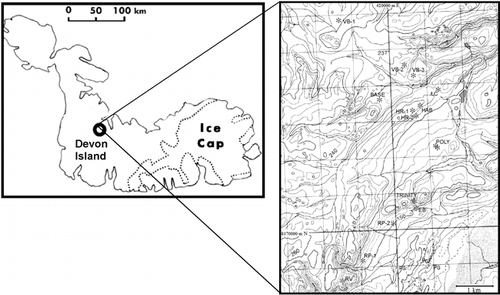
Our study sites were split between Arctic and Antarctic locations. In the Canadian Arctic, our measurements were made on locations on Devon Island in a region bounded by the coordinates 75°23.13′–75°26.70′N and 89°54.21′–89°48.14′W and around the town of Resolute, Cornwallis Island (74°41′N, 94°54′W), (, ). Measurements were made in these locations during July and August 2001 and 2002. The rocks sampled in the Antarctic were located in a deglaciated region of Alexander Island, (71.9°S, 68.2°W) (). Measurements were made at this location on 18 and 19 November 2001.
MEASUREMENTS OF HYPOLITHIC COLONIZATION
Rocks were randomly selected for measurement. This was accomplished by throwing a flagged marker stone blindly over the shoulder. This methodology was important to prevent bias in the rock selection. The rock nearest to the flagged marker rock was removed from the ground and the following parameters were measured (see ): (a) the length of rock and width to the nearest mm; (b) the height above ground of the exposed portion of the rock; (c) the depth of penetration of rock below ground; and (d) colonization by hypolithic organisms or not (determined by eye) (in the case of colonized rocks the depth of the band of colonization was measured in three places for each rock to the nearest mm).
In the Antarctic, rocks were recorded as being either inside (n = 100) or on the edges of sorted polygons (n = 100). In the Arctic, the colonization patterns (inside and outside polygons as shown in and shown in as “polygons”) were recorded for a total of 100 samples at 75°25.19′N, 89°48.14′W. For all other Arctic samples (n = 750 on Devon and n = 100 at Resolute) there was no discrimination of location within the patterned ground. Sampling sites were within polygonal terrain and large areas of randomly frost-sorted rocks.
Using the data, two parameters were calculated: (1) the mean width of the zone of colonization (band width); and (2) the estimated surface area of colonization normalized to the estimated total surface area of the rock to provide a measure of the fraction of the rock surface that was colonized.
MICROSCOPY
Photosynthetic microorganisms were carefully scraped from the arctic rocks using a sterile blade and examined under bright-field microscopy with an Olympus BX-51 universal microscope (Olympus America Inc, Melville, New York, U.S.A.) under 1000× magnification.
TEMPERATURE MEASUREMENTS
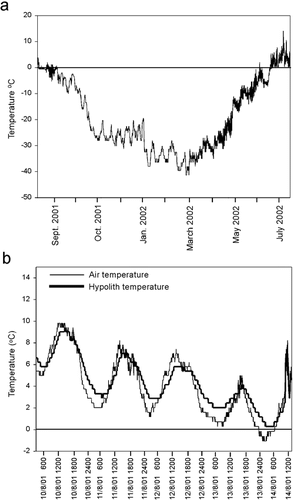
Measurements of temperatures in the hypolithic environment were collected in the Arctic over a one-year period using a Hobo™ data logger (Onset Computers, Bourne, Massachusetts, U.S.A.) attached to an external thermistor (Hobo™ TMC6-HB temperature sensor). A rock of dimensions 5.5 cm length × 4.2 cm width, 4.8 cm below-ground depth and 3.4 cm above-ground height was used at location 75°25′52.39″N, 89°49′28.05″W (Sample 1). The thermistor was implanted at the soil-rock interface in the region showing hypolithic growth. A second logging device was deployed in an identical way in a rock 1 m away with similar dimensions (Sample 2). The readings were within 10% of Sample 1 (data not shown). The loggers were set to record at 2 h intervals from 18 August 2001 to 11 July 2002. The temperature 5 cm above the rock (here referred to as “air temperature”) was also recorded. In the case of Sample 1, recordings of hypolithic and air temperatures were also taken at 3 min intervals from 9 to 14 August 2001.
MEASUREMENT OF ULTRAVIOLET RADIATION AND PAR PENETRATION
Penetration of UV radiation to the underside of the arctic rocks (using the same rocks as described for temperature measurements) was estimated using the Deutsches Zentrum für Luft- und Raumfahrt: Biofilm or DLR-Biofilm (CitationQuintern et al., 1992; CitationHorneck et al., 1996; CitationRettberg et al., 1999; CitationCockell et al., 2001). This dosimeter uses the inactivation of a monolayer (∼5 × 105 spores cm−2) of Bacillus subtilis spores to measure the biological effect of UV radiation. After exposure to UV radiation, the biofilms were incubated in nutrient broth, and the protein that was produced by the growing microorganisms (those that were not inactivated) was stained using Coomassie blue dye (CitationQuintern et al., 1992). The absorbance at 590 nm, determined by image analysis, provides a measure of the inactivation of the spores. The inactivation of the spores was expressed as an effective irradiance at 254 nm, i.e., the dose from a laboratory 254 nm UV radiation source required to produce the same inactivation as that seen in the field on a calibration biofilm. The sheets of Bacillus subtilis were protected by a thin layer of UV transparent Whirlpak® (Nasco, Ft. Atkinson, Wisconsin, U.S.A.) plastic and had dimensions of 1 × 2 cm. The biofilms conform to the principal of reciprocity, whereby response is dependent upon total dose, not irradiance (CitationQuintern et al., 1992). The method provides data on the integrated UV exposure over hours or days.
The dosimeters were carefully slotted into the hypolithic habitat at the edge of rocks with the dosimeter field pointing outward. Control dosimeters were placed on a horizontal surface near to the rock. Six dosimeters were placed around the edges of two different rocks (three dosimeters per rock). Control dosimeters were retrieved after 24 h, dosimeters in the hypolithic habitat were retrieved after 4 days. The dosimeters were deployed at 20:00 h on 11 August 2001 and retrieved at the same time on 15 August 2001. During this period of the year at the arctic location there is a 24-h photoperiod.
To determine whether the rocks might be transmitting PAR (Photosynthetically Active Radiation) through to the organisms beneath, in analogy to quartz rocks, we measured penetration through rock slices. Three 1 mm slices of dolomite from Devon Island were prepared. The irradiance between 400 and 700 nm at 1 nm intervals was measured through a 4 mm diameter cosine corrected collector at the end of a 1 m fiber optic cable attached to an S2000 Avantes spectroradiometer (Anglia Instruments, Ely, U.K.). The collector was fixed in an upright position in a clamp. Around the edge of the collector a small amount of dried opaque sealant was fixed so that slides could be pressed down onto the sealant to prevent stray light from the sides entering the collector. Thus, only light passing through the slide was measured. On a clear day when incident light levels were constant over an interval of several minutes, three control scans were obtained of the solar spectrum under a glass slide. Immediately afterward the three rock sections were placed over the collector with the rock section facing down on the collector, and spectral scans were obtained. A control scan was taken again to verify the constancy of the solar spectrum during the measurements. The mean of the scans at 1 nm intervals was calculated.
MEASUREMENT OF PRODUCTIVITY
We estimated the productivity of hypoliths in the arctic location. Productivity was measured using sodium bicarbonate. We added 18.5 MBq of bicarbonate (ICN Biomedicals, Canada) (2.1 GBq mmol−1) to 10 mL of water obtained from the edge of a sorted polygon. Rocks at location 75°26.36′N, 89°50.22′W were carefully removed and 100μL of 14C-bicarbonate was brushed onto an area of 1 cm2 on each of six rocks for each incubation and the area was marked using a pencil. Incubations were run for 5 min, 20 min, 1 h, and 2 h from 13:30 to 15:30 on 16 July 2002, with all incubations finishing at 15:30 and in triplicate. Dark controls were run by covering rocks in foil and were run in triplicate. The rocks were carefully replaced into their holes. The temperature of the ground during the incubations was 6°C. After the incubation period, the biomass was carefully removed from the rock using a scalpel and placed into a 5 mL scintillation vial (ICN Biomedicals, California, U.S.A.). To each vial, 200 μL of acetic acid (Sigma-Aldrich Corp., St Louis, Missouri, U.S.A.) was added to drive off excess bicarbonate. The biomass was dried and the samples were returned to the laboratory. Five mL of Ecolume scintillation fluid (ICN Biomedicals, California, U.S.A.) were added, and the disintegrations per minute in each vial were determined in a scintillation counter (Packard 1600CA, Packard Instruments, California, U.S.A.) using an instrument efficiency of 88%. Uptake of bicarbonate was linear up to 2 h. Dark controls gave values less than one-tenth of the light-exposed samples. The mean (± s.d.) background Dissolved Inorganic Carbon (DIC) concentration for these regions of the Arctic is measured as 16.7 ± 3.5 mg L−1 (CitationLim and Douglas, 2003) and was used to calculate 14C carbon uptake in the samples. The isotope discrimination factor was taken as 1.022. To estimate annual productivity of the hypolithon, we assumed that productivity of the value that we measured for the 20 min incubation occurs for 5 h in the day around midday. (Our 20 min incubation was from 15:10 to 15:30 and solar noon is at approximately 13:00. Thus, if we assume that productivity at the level we measured stops at 15:30, but occurs at this level from solar noon until this time, this would be a conservative estimate of the period of productivity.) We assume there are 45 days of the year when productivity can occur (from mid-July to the end of August), based on the number of days when temperatures at noon were above freezing. We assume that the carbon uptake equates to the increase in biomass, which will make our estimates of the productivity of the hypolithon an underestimate. However, comparison with the work of CitationBliss et al. (1984) requires carbon losses in respiration to be considered, which might yield a net primary productivity lower than gross primary productivity. To calculate the productivity per m2 of ground, we estimated the surface area of hypolithic colonization per m2 of above-ground area by taking a mean rock size and the mean band width. The hypolithon was estimated to cover one-tenth of the above-ground area in the regions studied.
Results
HYPOLITHIC COLONIZATION
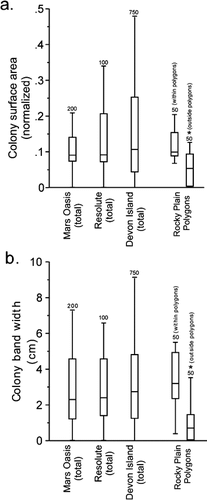
The percentage of rocks colonized in the arctic sites (no discrimination of patterned ground type) was 95% on Devon Island and it was 94% on Cornwallis Island. The mean normalized surface area of colonization was 0.16 for Devon Island (n = 750) and 0.15 for Cornwallis Island (n = 100). The mean (± s.d.) band width of the hypolithic communities was 3.1 ± 1.9 cm on Devon Island and 3.0 ± 1.6 cm on Cornwallis Island ().
For the Arctic polygons, the percentage of rocks colonized at the edges of polygons was 100%. The value within the polygons was 68%. The mean normalized surface areas inside and outside were 0.06 and 0.12, respectively (). The mean (± s.d.) band widths within and outside the polygons were 0.7 ± 0.8 and 3.6 ± 1.4 cm, respectively.
For the Antarctic location, the percentage colonization at the edges of polygons was 100%, the value within the polygons was 5%. The mean normalized surface areas within and outside were 0.05 and 0.12, respectively. The mean (± s.d.) band widths inside and outside the polygons were 0.7 ± 0.1 and 2.1 ± 0.3 cm, respectively.
The width of the bands of hypolithic colonization was not found to be statistically different between the Arctic and Antarctic locations (unpaired t tests, t = 0.53, P < 0.05).
MICROSCOPY
Cyanobacteria observed in the arctic hypoliths included Gloeocapsa cf. atrata (Kützing), Gloeocapsa cf. punctata (Nägeli), Gloeocapsa cf. kuetzingiana (Nägeli), Aphanothece- and Aphanocapsa-like cells, and Chroococcidiopsis-like cells. Various filamentous forms were observed, primarily Oscillatoriales, similar to Leptolyngbya and Scytonema. Unicellular algal chlorophytes were observed. These organisms were collected from the underside of rocks with no discrimination of depth.
TEMPERATURE MEASUREMENTS
The lowest air temperature recorded was −45.3°C on 25 February 2002 (). From 18 June to 11 July 2002, when light was available for photosynthesis and mean daytime temperatures were above freezing, the mean air temperature was 4.1°C and the mean temperature in the hypolithic habitat was 3.5°C. Diurnal differences in the air and hypolithic temperature regimen are observed (). Temperatures in the hypolithic habitat lag behind the air temperatures because of the thermal inertia of the rock. The heat retained in the rock is also sufficient to maintain higher temperatures in the hypolithic habitat during the times of lowest air temperatures. On 14 August this was sufficient to prevent freeze-thaw in the hypolithic habitat when the air temperature was −1.1°C.
ULTRAVIOLET RADIATION EXPOSURE AND PAR PENETRATION
After 24 h, the spores in the control dosimeters were all killed. The effective equivalent dose at 254 nm was greater than 200 J. However, spores in the hypolithic habitat showed no measurable inactivation after 4 days.
There was no measurable penetration of PAR through the 1 mm sections of dolomite.
PRIMARY PRODUCTIVITY
Linear uptake for 14C-bicarbonate was observed until 2 h when saturation had occurred. We took 20 min incubation as the basis for our productivity calculations. The mean of six incubations was 28773 ± 15521 dpm. The assumptions we made on productivity (described in Methods) led to an estimated mean carbon uptake of 7.7 g m−2 hypolithic colonization a−1. The hypolithic cover per m2 of ground area is estimated to be 0.1 m2, leading to an estimated hypolithic productivity of 0.8 ± 0.3 g m−2 a−1.
Discussion
Frost-sorted ground is a ubiquitous feature of polar deserts. The movement of rocks by periglacial processes creates openings around the edges of sorted rock features (CitationHallet and Prestrud, 1986; CitationMarchant et al., 2002; CitationSletten et al., 2003; CitationKessler and Werner, 2003). This process has important biological implications because, as we show here, by creating openings around the edges of rocks, periglacial processes allow opaque rocks to be colonized on their underside by photosynthetic microorganisms.
The productivity of this below-ground ecosystem is potentially very high as our preliminary investigations suggested (CitationCockell and Stokes, 2004). The productivity values we measure are subject to some necessary estimates, such as the period of time when net productivity occurs. However, our values suggest that the below-ground primary productivity of the hypolithon could be substantial during the summer. CitationBliss et al. (1984) estimated the above-ground productivity of arctic polar deserts accounted for by plants, lichens, and bryophytes. Their values range from 0.07 to 1.70 g m−2 a−1. The values they measured for Devon Island had a mean of 1.0 ± 0.38 g m−2 a−1 (CitationBliss et al., 1984). The values we obtain are the same order of magnitude to these values and suggest that the hypolithon is an unappreciated source of primary productivity in the polar desert, potentially contributing at least as much to carbon sequestration as above-ground biomass in the regions in which we recorded its presence.
The hypolithon associated with polar desert terrain is a widespread habitat because, in contrast to the colonization of quartz rocks, the habitat does not presume any geologic attributes of the rock, other than it is not toxic. Thus, potentially any substrate can support hypolithic colonization. The more extensive colonization of the edges of well-defined sorted polygons and their larger band widths compared to the interiors of polygons, where finer soil is sorted around the edges of rocks, suggests the importance of sorting in facilitating light penetration by creating openings around rocks at the edges of polygons. The edges of stone borders not only have larger spaces between the rocks in contrast to their centers, but they are also subject to greater cryoturbation compared to the relatively quiescent centers, thus potentially exposing surfaces for colonization at a faster rate than the centers. These findings show that an otherwise highly biologically detrimental stressor in the polar regions—freeze-thaw—is beneficial for photosynthetic microorganisms by greatly expanding their available habitat.
The photosynthetic organisms we found in the arctic habitat were mainly coccoid cyanobacteria. Similar coccoid, Chroococcidiopsis-like cells and filamentous species grouping with Lyngbya/Phormidium/Plectonema were reported by CitationSmith et al. (2000) and CitationBroady (1981) in Antarctic hypolithic communities. Broady reported more than 17 taxa of algae in antarctic hypoliths. We also found Gloeocapsa species to be abundant within this habitat.
Our environmental data show that the habitat provides significant advantages to these organisms compared to living on the surface of the rocks. UV radiation damage is greatly reduced and in some instances freeze-thaw is mitigated. Concomitantly, however, the photosynthetically active radiation must be reduced compared to living on the surface.
The hypolithic habitat can provide improvements in water availability compared to the surface. We noted that often the underside of the rocks was wet. Meltwater tends to drain around the edges of polygons, whereas the surface of the rocks was often wind-dried. The undersides of quartz stones in Antarctica were found to be moister than the surrounding soils (CitationSmith et al., 2000). The higher water availability in the Antarctic was found to result in a higher count of heterotrophic (organic-utilizing) bacteria under the rocks compared to the surrounding soils (CitationSmith et al., 2000). It is also likely that the photosynthetic productivity of the cyanobacteria contributed to the increased biomass of the heterotrophs by supplying organics.
The air temperatures that we measured in the summer were on average higher than the temperatures in the hypolithic environment. These data are in contrast with those reported by other workers. Quartz stones in the Vestfold Hills, Antarctica, could provide temperatures 10°C in excess of ambient air temperatures (CitationSmith et al., 2000). Similarly, the underside of quartz rocks in the Negev desert was elevated above air temperature (CitationBerner and Evenari 1978), and in these hot deserts, temperatures under stones was sometimes greater than 50°C, potentially increasing the stress experienced by the hypolithon.
We explain our observations by the fact that the rocks are embedded into ground close to polar ground ice. In this region of the Arctic the active layer is approximately 40 cm deep. When air temperatures transiently rise at midday, the underside of the rocks is maintained at the lower temperature by the cooled ground and, unlike quartz, infrared radiation is not readily transmitted through the opaque rock. Conversely, however, when air temperatures transiently drop below freezing, the rocks tend to be buffered by the ground temperatures and can, in rare instances, protect against freeze-thaw. One similarity between the antarctic and arctic communities is that during the dark polar winter the hypoliths are subjected to long periods of deep freeze, when lack of light and freezing temperatures will render them metabolically inactive.
The depth of colonization of the rocks is likely to be set by the minimum light levels required for photosynthesis. This is analogous to the way in which the lower depth of colonization of quartz rocks is set by their translucence. CitationBerner and Evenari (1978) showed that colonization under flint stones in the Negev desert was correlated to their transparency. More opaque flints had two times less colonization in terms of number of rocks colonized than the more transparent flints.
Vogel found that 0.06% of incident light penetrated into quartz rocks at a depth of 25 mm (CitationVogel, 1955). Less than 0.01% of incident light penetrated to below 40 mm in quartz rocks in the Negev (CitationBerner and Evenari, 1978), and light was reduced to 0.08% of incident under 25 mm of quartz rock from the Mojave desert (CitationSchlesinger et al., 2003). At these depths colonization became limited. Broady found similarly large attenuations in Antarctic quartz rock (CitationBroady, 1981). Raven et al. estimated the minimum light level required for photosynthesis to be 0.1 μmoles m−2 s−1 (CitationRaven et al., 2000), such that the reduction of visible light to approximately 0.06% of ambient (assuming it to be ∼2000 μmoles m−2 s−1 at midday) would be sufficient to extinguish photosynthesis, consistent with the observations reported for colonization of quartz rocks. In our studies, the hypothesis that light limitation is also responsible for determining the area of colonization is supported by the fact that where colonization does occur in the interior of the polygons, where the rocks are surrounded by fines, the depths of the bands of colonization and the surface areas are about half of that at the edges of the polygons. In some rocks at the edges of the polygons, where the openings around the rocks are large, the entire underside of the rock can be colonized. This accounts for the large range values in the band widths that we measured.
In regions of polygonal terrain, existing colonized polygons must act as a source of propagules for newly formed polygons. We speculate that colonization of the underside of the rocks occurs when organisms are carried by meltwater during the spring from one rock to another. Rocks that become exposed by wind scouring as a result of sorting might also cause the wind dispersal of the organisms that then land on the ground and get carried by meltwater to the underside of rocks.
The implications of climate change for these communities are uncertain. The warming of the Arctic, for instance, might reduce periglacial processes and reduce habitat availability for hypolithic communities. Although this could reduce their productivity and thus their contribution to carbon sequestration, a concomitant increase in above-ground vascular plant cover might cancel, or even exceed, this reduction. As these relative changes are likely to be complex, it is clear that hypolithic communities deserve greater attention in estimating the flux of carbon in arctic ecosystems.
Conclusions
Periglacial processes can cause movements in rocks, which in turn create openings around the edges of rocks that allow the penetration of photosynthetically active radiation to the underside of rocks. These processes allow for the colonization of the underside of opaque rocks by photosynthetic organisms. Our preliminary measurements suggest that the productivity of these communities may be as high as the above-ground productivity accounted for by plants. More widespread measurements would allow for more accurate comparisons that take into account differences in hydrology and nutrient flow between above-ground locations and hypolithic habitats. These data reveal the importance of freeze-thaw in generating new habitats for polar microbial life. The data suggest that climate warming could reduce the availability of these habitats by affecting the extent of periglacial processes.
FIGURE 3. Hypolithic colonization of frost-sorted polygons. (a) Schematic showing localization of hypolithic growth. (b) Photograph showing an excavated rock colonized by cyanobacteria. (c) Patterns of frost-sorted ground. White lines separate “inside” and “outside” of polygons, as used to study colonization patterns in this paper. Image is 2 m across. (d) Diagram showing measurements taken to quantify colonization (described in Methods)
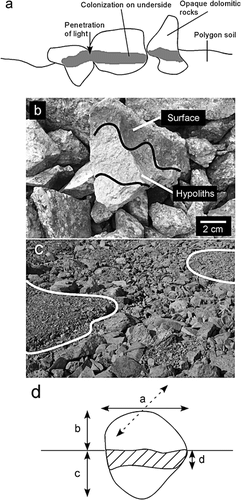
FIGURE 4. Nonparametric plot showing the surface area and band widths of hypolithic cyanobacteria and algae. Samples were measured on Alexander Island in the Antarctic and on Devon Island and Resolute in the Canadian High Arctic (n = 200 in the Antarctic, n = 850 on Devon Island, n = 100 at Resolute). The line in the middle of each box is the mean, the outer ends of the box show one standard deviation, the whiskers denote the range
Acknowledgments
We thank Paul Broady, University of Canterbury, Christchurch, New Zealand, for his identification of hypolithic cyanobacteria. This work was made possible by a Leverhulme grant to study UV penetration into microhabitats (no. F/00 802/A) and British Council Academic Research Collaboration (ARC) Project no. 1210. We thank the NASA Haughton-Mars project for logistical access to the arctic field sites and the British Antarctic Survey for access to the antarctic field site. We thank the DLR (Deutsches Zentrum für Luft- und Raumfahrt), Cologne, for provision and interpretation of the biofilm data results. We also thank two anonymous reviewers for thoughtful review comments and Suzanne Anderson for thoughtful and helpful editorial oversight of our paper.
References Cited
- Berner, T. and M. Evenari . 1978. The influence of temperature and light penetration on the abundance of the hypolithic algae in the Negev Desert of Israel. Oecologia 33:255–260.
- Bliss, L. C. , J. Svoboda , and D. I. Bliss . 1984. Polar deserts, their plant cover and plant production in the Canadian High Arctic. Holarctic Ecology 7:305–324.
- Broady, P. A. 1981. The ecology of sublithic terrestrial algae at the Vestfold Hills, Antarctica. British Phycology Journal 16:231–240.
- Budel, B. and D. C J. Wessels . 1991. Rock inhabiting blue-green algae from hot arid regions. Archiv für Hydrobiologie 92:385–398.
- Cameron, R. E. and G. B. Blank . 1965. Soil studies—microflora of desert regions. VIII. Distribution and abundance of desert microflora. Space Programs Summary 4:193–202.
- Cockell, C. S. and M. D. Stokes . 2004. Widespread colonisation by polar hypoliths. Nature 431:414.
- Cockell, C. S. , D. D. Wynn-Williams , G. Horneck , P. Scherer, Rettberg , and A. Gugg-Helminger . 2001. Influence of ice and snow covers on the UV exposure of microbial communities—dosimetric studies. Journal of Photochemistry and Photobiology 68:23–32.
- Friedmann, E. I. and M. Galun . 1974. Desert algae, lichens, and fungi. In Brown, G. W. (ed.), Desert biology. Volume II. New York: Academic Press, 165–212.
- Golubic, S. , I. Friedmann , and J. Schneider . 1981. The lithobiontic ecological niche, with special reference to microorganisms. Journal of Sedimentary Petrology 51:475–478.
- Hellet, B. and S. Prestrud . 1986. Dynamics of periglacial sorted circles in western Spitzbergen. Quaternary Research 26:81–99.
- Horneck, G. , P. Rettberg , E. Rabbow , W. Strauch , G. Seckmeyer , R. Facius , G. Reitz , K. Strauch , and J. U. Schott . 1996. Biological UV dosimetry of solar radiation for different simulated ozone column thicknesses. Journal of Photochemistry and Photobiology B: Biology 32:189–196.
- Kessler, M. A. and B. T. Werner . 2003. Self-organization of sorted patterned ground. Science 299:380–383.
- Lim, D. S S. and M. S V. Douglas . 2003. Limnological characteristics of 22 lakes and ponds in the Haughton Crater region of Devon Island, Nunavut, Canadian High Arctic. Arctic, Antarctic, and Alpine Research 35:509–519.
- Littler, M. M. , D. S. Littler , S. M. Blair , and J. N. Norris . 1986. Deep-water plant communities from an uncharted seamount off San Salvador Island, Bahamas: distribution, abundance and primary production. Deep-Sea Research 33:881–892.
- Marchant, D. R. , A. R. Lewis , W. M. Phillips , E. J. Moore , R. A. Souchez , G. H. Denton , D. E. Sugden , N. Potter , and G. P. Landis . 2002. Formation of patterned ground and sublimation till over Miocene glacier ice in Beacon Valley, Southern Victoria Land, Antarctica. Geological Society of America Bulletin 114:718–730.
- Quintern, L. E. , G. Horneck , U. Eschweiler , and H. Bücker . 1992. A biofilm used as ultraviolet-dosimeter. Journal of Photochemistry and Photobiology 55:389–395.
- Raven, J. A. , J. E. Kübler , and J. Beardall . 2000. Put out the light, and then put out the light. Journal of the Marine Biological Association of the UK 80:1–25.
- Rettberg, P. , R. Sief , and G. Horneck . 1999. The DLR-Biofilm as personal UV dosimeter. In Baumstark-Khan, C., Kozubek, S., and Horneck, G. (eds.), Fundamentals for the assessment of risks from environmental radiation. NATO Science Series, vol. 55. Dordrecht: Kluwer Academic Publishers, 367–370.
- Schlesinger, W. H. , J. S. Pippen , M. D. Wallenstein , K. S. Hofmockel , D. M. Klepeis , and B. E. Mahall . 2003. Community composition and photosynthesis by photoautotrophs under quartz pebbles, Southern Mohave Desert. Ecology 84:3222–3231.
- Sletten, R. S. , B. Hallet , and R. C. Fletcher . 2003. Resurfacing time of terrestrial surfaces by the formation and maturation of polygonal patterned ground. Journal of Geophysical Research–Planets, 108: article no. 8044, doi: 10.1029/2002JE001914.
- Smith, M. C. , J. P. Bowman , F. J. Scott , and M. A. Line . 2000. Sublithic bacteria associated with antarctic quartz stones. Antarctic Science 12:177–184.
- Vogel, S. 1955. Niedere “Fensterpflanzen” in der südafrikanischen Wüste, ein ökolosische Schilderung. Beitrage zur Biologie der Pflanzen 31:45–135.