ABSTRACT
Low temperatures, short growing seasons, and strong winds, which constrain the abundance and activity of insect pollinators, characterize alpine ecosystems. In northern hemisphere alpine environments, the reproductive output of several insect-pollinated plants has been reported to be pollen-limited. Using a supplemental hand-pollination experiment, we assessed the magnitude of pollen limitation (PL) in the obligate outcrossing insect-pollinated shrub Chuquiraga oppositifolia in the lower alpine scrub vegetation belt in the central Chilean Andes. We also assessed spatial variation in its reproductive success by comparing seed production among three additional sites. Hand-pollination resulted in a two- to three-fold increase in seed output above natural levels, thus demonstrating PL in this species. Nevertheless, percentage seed output remained low, increasing from 2.0 to 5.7%. Seed weight was reduced by 15% in hand-pollinated plants. Seed output was also low in the three additional sites, but did not differ among them, suggesting that low seed output is a spatially widespread phenomenon in this species. Our results, together with previous research, suggest that both pollinator visitation and abiotic resources (soil nutrients) constrain the reproductive output of this shrub. Longer-term research should unravel potential future vegetative and reproductive costs of current-year enhanced reproductive output and determine if PL is a recurrent phenomenon in the Chilean Andes.
Introduction
In flowering plants, much research has shown seed and fruit output to be constrained by insufficient pollen delivery to stigmas, a phenomenon known as pollen limitation (hereafter PL) (e.g., CitationGalen, 1985; CitationWhelan and Goldingay, 1989; CitationBurd, 1994; CitationLarson and Barrett, 2000; CitationAshman et al., 2004). At the whole plant level, PL is demonstrated empirically when supplemental hand pollination of flowers increases seed output of individuals compared to open pollinated controls. In a literature review, CitationBurd (1994) reported significant PL at some times or in some sites in 62% of 258 species examined. More recently, CitationLarson and Barrett (2000) documented PL to be ca. twice as intense in obligate outcrossing species than in self-compatible and autogamous species, which can be explained by the dependence of outcrossers on variable and unpredictable insect pollinator visitation.
Due to the low ambient temperatures, short snow-free growing seasons, and strong winds that high-mountain or alpine areas experience, various studies have reported low diversity, abundance, and activity levels of insect pollinators in these cold ecosystems around the world (e.g., CitationArroyo et al., 1982, Citation1985; CitationKearns, 1992; CitationMcCall and Primack, 1992; CitationKudo, 1993; CitationTotland, 1994a, Citation1994b, CitationBingham and Orthner, 1998; CitationMedan et al., 2002; CitationKasagi and Kudo, 2003; CitationMuñoz and Arroyo, 2004). Reduced pollinator visitation rates can be expected to be particularly critical for plant reproductive success in the alpine where entomophily is common (e.g., CitationArroyo et al., 1982; CitationKörner, 1999; CitationMedan et al., 2002), and outbreeding is often the dominant breeding system, especially among long-lived species (e.g., CitationArroyo and Squeo, 1990; CitationGugerli, 1998). Thus, PL of seed and fruit output could be a widespread and recurrent phenomenon here. However, current evidence for this prediction is equivocal, with some studies having reported moderate to strong PL in some species and populations (e.g., CitationGalen, 1985; CitationCampbell and Halama, 1993; CitationGugerli, 1998; CitationTotland, 2001; CitationKudo and Suzuki, 2002; CitationKasagi and Kudo, 2003) while others found none (e.g., CitationYoung, 1982; CitationSandvik et al., 1999; CitationTotland, 2004).
Despite the growing amount of literature on PL of plant reproductive success and its evolutionary and ecological causes and consequences in alpine ecosystems in various parts of the world, scarce research on this topic has been published for high-mountain environments in the Southern Hemisphere (but see CitationArroyo and Uslar [1993] in a montane sclerophyllous forest). Further, the paucity of studies on PL in this part of the world becomes particularly striking for the Andes Mountains of central Chile as reduced species richness, abundance, and activity levels of insect pollinators in this high-mountain ecosystem have been known for over 20yr, with a high percentage of species being insect-pollinated (CitationArroyo et al., 1982, Citation1985).
In the present study we focused our attention on the fully self-incompatible, insect-pollinated, dwarf shrub species Chuquiraga oppositifolia (Asteraceae), a dominant component of the lower alpine belt (subandean scrub vegetation belt) ranging between ca. 2000 and 2700m a.s.l. in the Andean Cordillera in central Chile (CitationArroyo et al., 1982; CitationRozzi, 1990; CitationCavieres et al., 2000). Using a supplemental hand-pollination experiment, we evaluated whether seed production in C. oppositifolia is pollen-limited. Given the frequently low pollinator visitation rates, and enhanced seed output following increased pollinator visitation reported for this shrub species (CitationMuñoz and Arroyo, 2004; CitationMuñoz et al., 2005), we hypothesized that hand-pollination of C. oppositifolia would result in a strong increase in its female reproductive success, thus providing evidence for PL. In addition, we assessed whether there is spatial variation in its reproductive success. To address this question, we compared seed production of C. oppositifolia among three different sites within the subandean scrub vegetation belt in the central Chilean Andes. Given the frequently low abundance and activity levels of insect pollinators reported for this alpine ecosystem (CitationArroyo et al., 1982, Citation1985; CitationRozzi, 1990; CitationMuñoz and Arroyo, 2004??), we predicted that seed output would be low at all three sites. Such a result would be suggestive of pollen limitation being a spatially widespread phenomenon in the central Chilean Andes. Thus, we asked the following questions: (1) Is seed production in this alpine shrub limited by pollen availability and (2) Does its female reproductive output vary spatially within the lower alpine scrub vegetation belt
Methods
The Plant Species
The dwarf <45-cm-high spiny shrub Chuquiraga oppositifolia (Asteraceae: Mutisieae) is the dominant shrub species at many sites within the subandean scrub vegetation belt (2000–2700m elevation) in the central Chilean Andes (CitationCavieres et al., 2000; A.A.M., personal observations). It flowers late in the growing season (January to April) (CitationArroyo et al., 1981) and becomes replete with large 10- to 12-mm-diameter, golden-yellow capitula containing 9 to 12 morphologically identical disc florets on average (CitationRozzi, 1990; CitationMuñoz, 2003), which are insect-pollinated (CitationArroyo et al., 1982; CitationRozzi, 1990). In this species, all florets are fertile and each is capable of developing a single seed. Percentage seed set within each capitulum ranges widely from 0 to 100% (CitationMuñoz, 2003) although mean number of achenes produced per capitulum is <1 (CitationMuñoz and Arroyo, 2004). A breeding system analysis using bagging starting at the bud stage and controlled self- and cross-pollinations showed that C. oppositifolia requires visitation to produce seeds (CitationRozzi, 1990; Rozzi, unpublished data). Bagged capitula, which insects could not access, on 15 individual shrubs, did not produce any seeds, thus showing that C. oppositifolia is not autogamous (or capable of self-fertilization). Open pollination by insects resulted in seed set, whereas bagging only did so following cross-pollination, thus demonstrating that it is fully self-incompatible and hence outcrossing (CitationRozzi, 1990; Rozzi, unpublished data). The most important pollinators of C. oppositifolia are the satyrid butterfly Cosmosatyrus chilensis, the syrphid fly Scaeva melanostoma, and the andrenid bee Heterosarus sp. although it is also visited by many other taxa including bombyliid and tachinid flies, other small andrenid bees, and the bumblebee Bombus dahlbomii (CitationMuñoz, 2003; CitationMuñoz and Arroyo, 2004; see also CitationArroyo et al., 1982). Detailed pollen-load analyses demonstrated that the main pollinators of C. oppositifolia carry abundant pollen (CitationRozzi, 1990).
Study Sites
Research was conducted at four sites within the subandean scrub vegetation belt in the central Chilean Andes (32–33°S). The supplemental hand-pollination experiment was performed at an 18-ha site at 2600m elevation near the Valle Nevado (VN) Ski Resort (33°21′S, 70°16′W) between January and May 2002. Here, climate is alpine with Mediterranean influence, with a 5- to 8-mo snow-free growing season, commonly extending from mid-October to mid-May (CitationArroyo et al., 1981). This study site is south-facing with gentle (<15°) slopes. Vegetation is dominated by low (< 45cm) spiny shrubs of Chuquiraga oppositifolia (Asteraceae), Anarthrophyllum cumingii (Papilionaceae), and Berberis empetrifolia (Berberidaceae). Herbaceous species, such as Acaena pinnatifida (Rosaceae), Phacelia secunda (Hydrophyllaceae), Stachys philippiana (Labiatae), and various species of Adesmia (Papilionaceae) and Senecio (Asteraceae), are also abundant.
To evaluate whether low seed production in Chuquiraga oppositifolia is a spatially widespread phenomenon in the central Chilean Andes, three additional study sites were selected in December 2002. These sites were: Lagunillas (LA) at 2000m a.s.l. (33°33′S, 70°16′W) and ca. 30km south of VN, Farellones (FA) at 2300m a.s.l. (33°19′S, 70°17′W) and ca. 5km west of VN, and Portillo (PO) at 2550m a.s.l. (32° 51′S, 70°10′W) and ca. 60km north of VN. LA contains a mixture of subandean scrub vegetation dominated by C. oppositifolia, Acaena pinnatifida, Mutisia sinuata (Asteraceae), and various species of Senecio and Adesmia, together with vegetation characteristic of the montane sclerophyllous matorral immediately below it, dominated by the trees Kageneckia angustifolia (Rosaceae) and Schinus montanus (Anacardiaceae), and the shrub Colliguaja integerrima (Euphorbiaceae). This study site is west-facing with gentle (<20°) slopes. Vegetation at FA is very similar to that described for the VN site, with gentle (<15°) slopes but has a west-facing aspect. Vegetation at PO is dominated again by C. oppositifolia and B. empetrifolia. The other dominant species here are Tropaeolum polyphyllum (Tropaeolaceae) and Lathyrus sp. (Papilionaceae). This site is south-facing with steep (20–50°) slopes.
Pollen Limitation Experiment
We employed supplemental hand-pollination to assess whether the amounts of pollen reaching stigmas constrain seed production in Chuquiraga oppositifolia . In January 2002, in a fairly flat 200 × 75m (1.5ha) sector within the 18-ha site at VN, we randomly selected a total of 30 similarly sized (ca. 0.2–0.3m3 ) shrubs. Selected experimental shrubs were located at least 5m away from each other so as to study clearly distinct individual shrubs, thus ensuring independence of replicates. All shrubs studied possessed 350 to 500 apical floral buds at this stage, being representative of individuals growing in the general area in terms of size and floral display. Each shrub was randomly assigned to either a supplemental pollination (n = 15 shrubs) or control (n = 15 shrubs) treatment. On each individual we tagged 50 randomly selected twiglets, each with its apical floral bud as in CitationMuñoz et al. (2005) . Each twiglet can produce only one capitulum. Given that this species is self-incompatible (see above), we collected the pollen used to hand pollinate the 15 plants under the supplemental pollen treatment from shrubs that were growing at least 50m away from the 1.5-ha experimental sector of the study site. A 50-m distance allowed us to avoid potentially disturbing nontarget control plants within the experimental sector. At the same time, this distance was well within the flying range of the main insect pollinators of this species including the butterfly Cosmosatyrus chilensis, the fly Scaeva melanostoma, and the bumblebee Bombus dahlbomii (personal observations). Thus, natural cross-pollination could also be expected between donor and target plants. To accomplish pollen addition, we collected a large quantity of florets with dark orange pollen-laden anthers from the above plants and carefully brushed these across the receptive stigmas of florets of most (>80%) of the total number of capitula on the supplemental pollination plants (including all 50 capitula that were tagged). Thus, our experimental procedure allowed us to test for PL at the whole-plant level (CitationZimmerman and Pyke, 1988). We hand-pollinated shrubs on three occasions during peak flowering of each individual between late January and late February 2002.
We covered all monitored capitula following withering in late February–early March with 8×6cm yellow mesh bags so as to prevent the potential loss of the developing wind-dispersed achenes (one-seeded fruit) as in CitationMuñoz and Arroyo (2004). We retrieved all bags in April–May (thus allowing sufficient time for seed development), and analyzed each capitulum for seed output (number of achenes) and seed quality (weight of achenes). Seed output was expressed as (1) percentage of capitula per shrub that set one or more achenes (%CA), (2) mean number of achenes per capitulum per shrub (NAC), and (3) percentage seed set (%SS), which was measured as the percentage of ovaries of open florets per shrub that set seeds (i.e., that could potentially have been fertilized). Percentage seed set (%SS) is equivalent to the seed∶ovule (s∶o) ratio reported in other studies (e.g., CitationGugerli, 1998; CitationTotland, 2004). When calculating NAC, we included all monitored capitula regardless of whether the capitulum produced any achene or not (i.e. including the zero-achene capitula). Additionally, we determined the frequency distribution of seed output per capitulum (i.e., the percentage of capitula with 0, 1, 2, 3, >3 achenes) for control and supplemental hand pollination shrubs. Then, we individually weighed dry achenes (including the pappus) to the nearest milligram using a digital balance. We analyzed differences in seed output and quality in control vs. supplemental pollination shrubs using Mann-Whitney U tests because the assumption of normality of data for parametric tests was not met, even when appropriately transformed (CitationZar, 1996).
Spatial Patterns of Seed Production in the Subandean Scrub Belt
We assessed whether the reproductive success in C. oppositifolia , in terms of the quantity and quality of achenes produced, varies spatially within the subandean scrub vegetational belt by comparing seed production in shrubs at the three sites (LA 2000m, FA 2300m, and PO 2550m) described above. In December 2002, within a 1- to 1.5-ha area at each of these three sites, we randomly selected 10 similarly-sized (ca. 0.2–0.3m3) shrubs of C. oppositifolia, located at least 5m away from each other, and monitored them for seed production between January and April 2003. These shrubs were representative of individuals growing in their respective general study areas in terms of size and floral display. Floral display of these shrubs did not differ among sites (mean ±1 SE = 730 ±214 capitula/shrub at LA, 741 ±147 capitula/shrub at FA, and 608 ±81 capitula/shrub at PO, one-way ANOVA: F = 0.220, P = 0.804). At each site, during late January to mid-February 2003, and once most of the capitula on each shrub had florets showing signs of withering, we tagged 30 randomly selected capitula on each shrub and covered these with 8×6-cm yellow mesh bags so as to later assess mature seed production as above. We retrieved all bags in March–April (thus allowing sufficient time for seed development), and analyzed each capitulum for seed output (number of achenes). As above, we individually weighed achenes (including the pappus) to the nearest mg. Differences in the number (%CA, NAC, and %SS) and quality (weight) of seeds produced among shrubs growing at LA, FA, and PO were analyzed by one-way ANOVA, with site as the factor (CitationZar 1996), after testing for normality and homogeneity of variances using the Shapiro-Wilks and Bartlett tests, respectively. Data were transformed appropriately when the normality condition was not met. A posteriori multiple comparisons were conducted using the Tukey HSD Test.
Results
Pollen Limitation Experiment
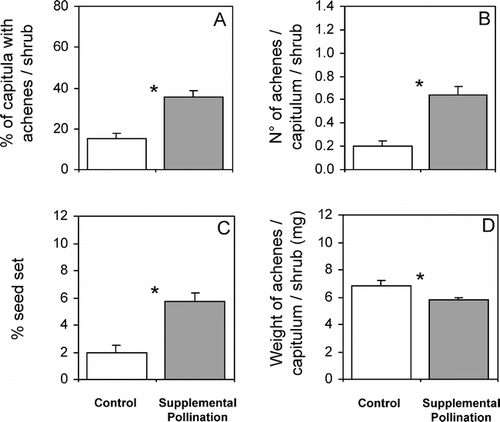
Supplemental hand-pollination resulted in a significant enhancement in seed output of C. oppositifolia (). Mean percentage of capitula per shrub that set one or more achenes (%CA) was more that two-fold higher in hand-pollinated shrubs compared to control shrubs (Mann-Whitney U test, U = 24.0, z = − 3.65, P< 0.001, ). Likewise, hand-pollinated shrubs produced a > three-fold greater number of achenes per capitulum per shrub (NAC) than control shrubs (U = 23.5, z = − 3.65, P< 0.001, ). Percentage seed set (%SS) was also significantly lower in control compared to hand-pollinated shrubs (U = 24.5, z = − 3.65, P< 0.001, ). Additionally, almost three times as many capitula from supplemental hand pollination shrubs (34.05%) produced 1 to 3 achenes compared to capitula on control shrubs (12.27%). However, seed quality, expressed as the mean achene mass per shrub, was slightly reduced in hand-pollinated vs. control shrubs (U = 64.0, z = 2.012, P = 0.044, ).
Figure 1 Mean seed output expressed as (A) the percentage of capitula per shrub that set one or more achenes (%CA), (B) the number of achenes per capitulum per shrub (NAC), and (C) percentage seed set (% of ovaries of open florets per shrub that set seed) (%SS), and seed quality expressed as (D) weight of achenes per capitulum per shrub (mg) in control (white bars) and hand supplementary pollinated (gray bars) shrubs of the insect-pollinated dwarf shrub Chuquiraga oppositifolia (Asteraceae) at a 2600m elevation site (Valle Nevado–33°21′S, 70°16′W) in the lower alpine belt (subandean scrub vegetation belt), Andes of central Chile. Bars are means +1 SE. *significant differences (P< 0.05). Each treatment had 15 replicates.
Spatial Patterns of Seed Production in the Subandean Scrub Belt
Seed output in shrubs of C. oppositifolia did not differ among the three sites studied when expressed as %CA (F 1,27 = 1.019, P = 0.374), NAC (F 1,27 = 1.368, P = 0.272), and %SS (F 1,27 = 1.542, P = 0.232) (. However, mean weight of achenes produced by shrubs at the FA and PO sites was ca. 20 and 30% lower, respectively, than that of achenes produced at the LA, site (, F 1,27 = 16.056, P< 0.01, a posteriori≠ = Tukey HSD, LA FA PO, P< 0.05).
Figure 2 Mean seed output expressed as (A) %CA, (B) NAC, and (C) (%SS), and seed quality expressed as (D) weight of achenes per capitulum per shrub (mg) in shrubs of Chuquiraga oppositifolia at three different sites in the lower alpine belt (subandean scrub vegetation belt) in the central Chilean Andes. Lagunillas (LA—33°33′S, 70°16′W) at 2000m (white bars), Farellones (FA —33°19′S, 70°17′W) at 2300m (light gray bars), and Portillo (PO—32°51′S, 70°10′W) at 2550m (dark gray bars). Bars are means +1 SE. Treatments (sites) sharing the same letter for any given response variable do not differ significantly (P> 0.05). Ten replicate shrubs were monitored at each site.
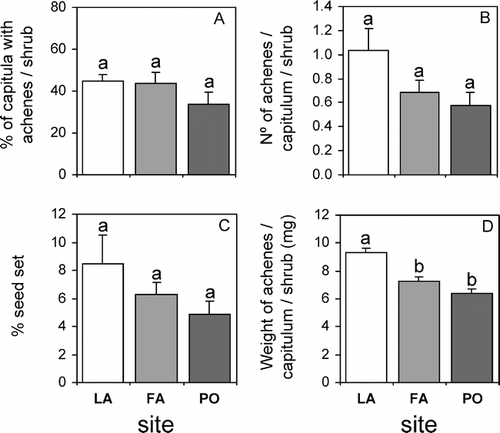
On the other hand, control (natural) seed output at the experimental site at Valle Nevado, was significantly lower compared to all three other sites, expressed as %CA (F 1,41 = 9.388, P< 0.01, a posteriori Tukey HSD, P< 0.05), NAC (F 1,41 = 7.871, P< 0.05, a posteriori Tukey HSD, P< 0.05), and %SS (F 1,41 = 8.918, P< 0.01, a posteriori Tukey HSD, P< 0.05) (, ).
Discussion
Female reproductive success of the fully self-incompatible insect-pollinated dwarf shrub Chuquiraga oppositifolia was strongly limited by pollen availability at the Valle Nevado site, with seed production of naturally open pollinated shrubs being very low there. Supplemental hand pollination at the individual shrub level resulted in a two- to three-fold increase in seed output above natural levels at the Valle Nevado site. This result concurs with reviews documenting that long-lived, woody, and self-incompatible species are often pollen limited (CitationBurd, 1994; CitationLarson and Barrett, 2000). As far as we are aware, our study is the first to directly test for, and document, PL in an alpine species in a southern hemisphere high-altitude ecosystem in general, and in the central Chilean Andes in particular. Additionally, seed output was also low and did not differ among the three additional sites examined (Lagunillas, Farellones, and Portillo) within the subandean scrub vegetation belt, suggesting that low seed output is a spatially widespread phenomenon in this shrub species within this ecosystem. Nevertheless, it is worth pointing out that seed output at these three sites was higher than at Valle Nevado, with supplemental hand pollination there having brought seed production into the range of natural reproductive output at Lagunillas, Farellones, and Portillo.
In other studies in alpine ecosystems, a lack of PL of the reproductive success of some species or populations has sometimes been reported (e.g., CitationYoung, 1982; CitationSandvik et al., 1999; CitationTotland, 2004). This has often been attributed to the possession of mixed (e.g., insect and wind) pollination strategies (CitationTotland and Sottocornola, 2001), or the capacity for self-pollination (autogamy) (CitationYoung, 1982; CitationKasagi and Kudo, 2003) and self-fertilization (self-compatibility) (CitationSandvik et al., 1999; CitationTotland and Schulte-Herbrüggen, 2003). Such species are not fully dependent on cross-pollination by insects to produce seeds as in C. oppositifolia.
Strong PL was expected in C. oppositifolia in view of its reliance on cross-pollination for seed production (CitationRozzi, 1990) and the already reported low pollinator visitation rates to this shrub species in previous field experiments (CitationMuñoz and Arroyo, 2004; CitationMuñoz et al., 2005). Indeed, the occurrence of PL at a number of northern hemisphere high-mountain ecosystems, including the Rocky Mountains of North America (CitationGalen, 1985; CitationCampbell and Halama, 1993), alpine southwest Norway (e.g., CitationTotland, 2001; CitationTotland and Sottocornola, 2001) and the Taisetsu Mountains of northern Japan (CitationKudo and Suzuki, 2002; CitationKasagi and Kudo, 2003) could be explained by the low diversity, abundance, and activity levels of insect pollinators in cold-weather alpine ecosystems (e.g., CitationArroyo et al., 1982, Citation1985; CitationGalen, 1985; CitationKearns, 1992; CitationMcCall and Primack, 1992; CitationKudo, 1993; CitationTotland, 1994a, Citation1994b; CitationBingham and Orthner, 1998; CitationMedan et al., 2002; CitationMuñoz and Arroyo, 2004). However, CitationTotland (2001) cautioned that low pollinator availability or visitation at high altitudes does not always lead to strong PL of seed output, even in self-incompatible species. Seed set of the perennial herb Ranunculus acris in a high-altitude population in alpine southwest Norway was not enhanced via supplemental hand-pollination despite naturally low pollinator visitation rates, presumably because seed production there was constrained by resource availability, particularly by low temperatures (CitationTotland, 2001, Citation2004). In contrast, seed output in a lower-altitude population, where pollinator visitation rates were three times higher compared to the high-altitude population, was pollen limited (CitationTotland, 2001).
In our study, despite a three-fold enhancement in seed set of C. oppositifolia following supplemental hand-pollination, seed set still remained very low, reaching only 5.7% (despite that capitula are capable of maturing all seeds produced). This result, along with the recent demonstration that seed output in C. oppositifolia is also strongly limited by soil nitrogen resource availability (CitationMuñoz et al., 2005), reinforces the notion that plant reproductive success in alpine ecosystems can be constrained by both resources and pollinators (CitationHaig and Westoby, 1988; CitationCampbell and Halama, 1993; CitationTotland, 2004). In our nitrogen addition study (CitationMuñoz et al., 2005), we argued that the ca. three- to four-fold enhancement in seed output in shrubs that had received nitrogen fertilizer was probably a result, not only of the greater amount of resources available for seed maturation, but also of the ca. two-fold enhancement in floral display, ending in a ca. two-fold increase in pollinator visitation, and ultimately in the liberation of these shrubs from PL.
Alternatively, the observed low seed output in this self-incompatible alpine shrub (both naturally at the four sites and when hand pollinated) may be caused by natural selection for large floral displays in this system, (where pollinator abundance and activity levels are low), resulting in the production of many more capitula and florets than would be needed to achieve full seed set. Large floral displays would enhance male fitness (siring success) through pollen removal (e.g., CitationMutikainen and Delph, 1996; CitationStrauss, 1997; CitationKrupnick and Weis, 1999). Here, we assessed the reproductive success of C. oppositifolia through the female function only (i.e., seed output).
Additionally, a perspective that may be relevant in the C. oppositifolia–insect pollinator system is that this abundant, annually flowering, and long-lived woody plant, through its often large floral displays, may be maintaining the populations of pollinators via its food offerings. This type of plant-pollinator mutualism, suggested for in other systems (e.g., CitationScott et al., 1993), may be important in the central Chilean Andes since it is known that, on the one hand, a high percentage of species are insect-pollinated (CitationArroyo et al., 1982; Muñoz, unpublished data) and, on the other, visitation rates are very low (CitationArroyo et al., 1985; CitationMuñoz and Arroyo, 2004).
Nevertheless, because our study was conducted during a single growing season only, we cannot assume that the reproductive output of C. oppositifolia necessarily would be pollen limited during subsequent seasons. Relatively few studies have examined PL over multiple growing seasons (see CitationBurd, 1994; and CitationLarson and Barrett, 2000, for reviews), and, to our knowledge, only two recent studies (CitationKasagi and Kudo, 2003; CitationTotland, 2004) have done so for high-altitude plant species. In other environments, PL has been shown to vary seasonally (e.g., CitationRamsey, 1995; CitationBaker et al., 2000; CitationGoldingay, 2000), with interyear shifts in pollinator abundance and activity being invoked to explain such changes in PL (e.g., CitationRamsey, 1995). In the Andes Mountains of central Chile, the recently documented extremely low insect pollinator visitation rates to C. oppositifolia during two growing seasons (2001–2002 and 2003–2004) at the VN study site (CitationMuñoz and Arroyo, 2004; CitationMuñoz et al., 2005), together with the low visitation rates at nearby sites during the 1984–1985 growing season (CitationRozzi, 1990), are suggestive that seed output in C. oppositifolia is often pollen limited. Interannual variation in seed output can also be due to costs of reproduction. Some experimentally hand-pollinated species showed reduced vegetative growth or reproduction in subsequent years (e.g., CitationAckerman and Montalvo, 1990; CitationPrimack and Hall, 1990; CitationMattila and Kuitunen, 2000), although such costs have not been seen in others (e.g., CitationRamsey, 1995; CitationGoldingay, 2000). Thus, through elevated seed output in one season, some plants may experience a reduction in lifetime fitness. Although we currently lack data on future potential costs of enhanced seed output in hand-pollinated shrubs, our results showing an immediate (current season) reduction in the mass of achenes produced by supplemental pollinated shrubs suggest that such costs occur in C. oppositifolia.
To attempt to further understand the relative importance of pollen vs. resource limitation towards the longer-term reproductive success of C. oppositifolia and the alpine flora of central Chile in general, future empirical research should manipulate both factors during a number of seasons (CitationMuñoz et al., 2005). This way, we can begin to unravel, not only if pollen and resource limitation are recurrent phenomena in the central Chilean Andes, but also the interplay between them including whether high reproductive output in one season incurs in future reproductive costs.
Acknowledgments
P. Chacn, M. Correa L. Daz, S. Henrquez, B. Muñoz, A. Rivera, and L. Sotomayor provided helpful field assistance. . Totland and L. A. Cavieres, as well as P. E. Scott and an anonymous reviewer are thanked for their valuable comments on earlier versions of the manuscript that improved its clarity and overall quality. Our research benefited from discussions with I. Till-Bottraud. R. Leatherbee is acknowledged for permission to conduct work on the El Colorado Ski Complex (ANDACOR) property. Our study was funded by FONDECYT 2010032 doctoral research grant (20012003) to A.A.M. Aid from the Millennium Center for Advanced Studies in Ecology and Research in Biodiversity (CMEB) funded through grants P99-103-F-ICM and P02-051-F-ICM is also acknowledged.
References Cited
- Ackerman, J. D. and A. M. Montalvo . 1990. Short- and long-term limitations to fruit production in a tropical orchid. Ecology 71:263–272.
- Arroyo, M. T. K. and F. A. Squeo . 1990. Relationship between plant breeding systems and pollination. In Kawano, S. , editor. ed. Biological Approaches and Evolutionary Trends in Plants New York Academic Press. 205–227.
- Arroyo, M. T. K. and P. Uslar . 1993. Breeding systems in a temperate Mediterranean-type climate montane sclerophyllous forest in central Chile. Botanical Journal of the Linnean Society 111:83–102.
- Arroyo, M. T. K. , J. J. Armesto , and C. Villagrán . 1981. Plant phenological patterns in the high Andean Cordillera of central Chile. Journal of Ecology 69:205–223.
- Arroyo, M. T. K. , R. Primack , and J. Armesto . 1982. Community studies in pollination ecology in the high temperate Andes of central Chile. I. Pollination mechanisms and altitudinal variation. American Journal of Botany 69:82–97.
- Arroyo, M. T. K. , J. J. Armesto , and R. B. Primack . 1985. Community studies in pollination ecology in the high temperate Andes of central Chile II. Effect of temperature on visitation rates and pollination possibilities. Plant Systematics and Evolution 149:187–203.
- Ashman, T. L. , T. M. Knight , J. A. Steets , P. Amarasekare , M. Burd , D. R. Campbell , M. R. Dudash , M. O. Johnston , S. J. Mazer , R. J. Mitchell , M. T. Morton , and W. G. Wilson . 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421.
- Baker, A. M. , S. C. H. Barrett , and J. D. Thompson . 2000. Variation of pollen limitation in the early flowering Mediterranean geophyte Narcissus assoanus (Amaryllidaceae). Oecologia 124:529–535.
- Bingham, R. A. and A. R. Orthner . 1998. Efficient pollination of alpine plants. Nature 391:238–239.
- Burd, M. 1994. Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. The Botanical Review 60:83–139.
- Campbell, D. R. and K. J. Halama . 1993. Resource and pollen limitations to lifetime seed production in a natural plant population. Ecology 74:1043–1051.
- Cavieres, L. A. , A. Peñaloza , and M. K. Arroyo . 2000. Altitudinal vegetation belts in the high-Andes of central Chile (33°). Revista Chilena de Historia Natural 73:331–344.
- Galen, C. 1985. Regulation of seed set in Polemonium viscosum : Floral scents, pollination, and resources. Ecology 66:792–797.
- Goldingay, R. L. 2000. Further assessment of pollen limitation in the waratah ( Telopea speciosissima ). Australian Journal of Botany 48:209–214.
- Gugerli, F. 1998. Effect of elevation on sexual reproduction in alpine populations of Saxifraga oppositifolia (Saxifragaceae). Oecologia 114:60–66.
- Haig, D. and M. Westoby . 1988. On limits to seed production. American Naturalist 131:757–759.
- Kasagi, T. and G. Kudo . 2003. Variations in bumble bee preference and pollen limitation among neighboring populations: comparisons between Phyllodoce caerulea and Phyllodoce aleutica (Ericaceae) along snowmelt gradients. American Journal of Botany 90:1321–1327.
- Kearns, C. A. 1992. Anthophilous fly distribution across an elevation gradient. American Midland Naturalist 127:172–182.
- Körner, C. 1999. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems Berlin Springer-Verlag. 338.
- Krupnick, G. A. and A. E. Weis . 1999. The effect of floral herbivory on male and female reproductive success in Isomeris arborea . Ecology 80:135–149.
- Kudo, G. 1993. Relationship between flowering time and fruit set of the entomophilous alpine shrub, Rhododendron aureum (Ericaceae), inhabiting snow patches. American Journal of Botany 80:1300–1304.
- Kudo, G. and S. Suzuki . 2002. Relationships between flowering phenology and fruit-set of dwarf shrubs in alpine fellfields in northern Japan: a comparison with a subarctic heathland in northern Sweden. Arctic, Antarctic, and Alpine Research 34:185–190.
- Larson, B. M. H. and S. C. H. Barrett . 2000. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society 69:503–520.
- Mattila, E. and M. T. Kuitunen . 2000. Nutrient versus pollination limitation in Platanthera bifolia and Dactylorhiza incarnata (Orchidaceae). Oikos 89:360–366.
- McCall, C. and R. B. Primack . 1992. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. American Journal of Botany 79:434–442.
- Medan, D. , N. H. Montaldo , M. Devoto , A. Mantese , V. Vasellati , G. G. Roitman , and N. H. Bartoloni . 2002. Plant-pollinator relationships at two altitudes in the Andes of Mendoza, Argentina. Arctic, Antarctic, and Alpine Research 34:233–241.
- Muñoz, A. A. 2003. Evaluación experimental de la importancia de efectos indirectos descendentes y ascendentes sobre el éxito reproductivo de Chuquiraga oppositifolia (Asteraceae) en la Cordillera de Los Andes en Chile central. PhD dissertation,. Santiago Facultad de Ciencias, Universidad de Chile.
- Muñoz, A. A. and M. T. K. Arroyo . 2004. Negative impacts of a vertebrate predator on insect pollinator visitation and seed output in Chuquiraga oppositifolia , a high Andean shrub. Oecologia 138:66–73.
- Muñoz, A. A. , C. Celedon-Neghme , L. A. Cavieres , and M. T. K. Arroyo . 2005. Bottom-up effects of nutrient availability on flower production, pollinator visitation, and seed output in a high-Andean shrub. Oecologia 143:126–135.
- Mutikainen, P. and L. F. Delph . 1996. Effects of herbivory on male reproductive success in plants. Oikos 75:353–358.
- Primack, R. B. and P. Hall . 1990. Costs of reproduction in the pink lady's slipper orchid: a four-year experimental study. American Naturalist 136:638–656.
- Ramsey, M. 1995. Causes and consequences of seasonal variation in pollen limitation of seed production in Blandfordia grandiflora (Liliaceae). Oikos 73:49–58.
- Rozzi, R. 1990. Períodos de floración y especies de polinizadores en poblaciones de Anarthrophyllum cumingii y Chuquiraga oppositifolia que crecen sobre laderas de exposició n norte y sur. Masters thesis,. Santiago Facultad de Ciencias, Universidad de Chile.
- Sandvik, S. M. , Ø Totland , and J. Nyléhn . 1999. Breeding system and effects of plant size and flowering time on reproductive success in the alpine herb Saxifraga stellaris L. Arctic, Antarctic, and Alpine Research 31:196–201.
- Scott, P. E. , S. L. Buchmann , and M. K. O'Rourke . 1993. Evidence for mutualism between a flower-piercing carpenter bee and ocotillo: use of pollen and nectar by nesting bees. Ecological Entomology 18:234–240.
- Strauss, S. Y. 1997. Floral characters link herbivores, pollinators, and plant fitness. Ecology 78:1640–1645.
- Totland, Ø 1994a. Influence of climate, time of day and season, and flower density on insect flower visitation in alpine Norway. Arctic and Alpine Research 26:66–71.
- Totland, Ø 1994b. Intraseasonal variation in pollination intensity and seed set in an alpine population of Ranunculus acris in southwestern Norway. Ecography 17:159–165.
- Totland, Ø 2001. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82:2233–2244.
- Totland, Ø 2004. No evidence for a role of pollinator discrimination in causing selection on flower size through female reproduction. Oikos 106:558–564.
- Totland, Ø and B. Schulte-Herbrüggen . 2003. Breeding system, insect flower visitation, and floral traits of two alpine Cerastium species in Norway. Arctic, Antarctic, and Alpine Research 35:242–247.
- Totland, Ø and M. Sottocornola . 2001. Pollen limitation of the reproductive success in two sympatric alpine willows (Salicaceae) with contrasting pollination strategies. American Journal of Botany 88:1011–1015.
- Whelan, R. J. and R. L. Goldingay . 1989. Factors affecting fruit-set in Telopea speciosissima (Proteaceae): The importance of pollen limitation. Journal of Ecology 77:1123–1134.
- Young, T. P. 1982. Bird visitation, seed-set, and germination rates in two species of Lobelia on Mount Kenya. Ecology 63:1983–1986.
- Zar, J. H. 1996. Biostatistical Analysis. 3rd ed Upper Saddle River, N.J Prentice Hall. 662.
- Zimmerman, M. and G. H. Pyke . 1988. Reproduction in Polemonium : assessing the factors limiting seed set. American Naturalist 131:723–738.