ABSTRACT
Breeding penguins are the major source of nutrients for terrestrial ecosystems in the Maritime Antarctic. The impact of penguin rookeries on vegetation patterns and diversity was examined by descriptive and quantitative methods along a transect from penguin rookeries to areas distant from penguin impact. Several vegetation zones related to varying degrees of rookery impact have been recognized: (1) areas under the immediate influence of fresh penguin guano and trampling support little or no vegetation; (2) the adjacent zone is covered with nitrophilous green algae, locally also cyanobacteria; (3) the next zone is dominated by the Antarctic hair-grass; (4) further on, a zone dominated by mosses is formed; (5) finally, the zone least affected by penguin impact is dominated by lichens. With increasing distance from penguin rookeries, vegetation zones become less distinct and more complex; along consecutive zones vegetation richness and diversity increase and dominance decreases. Change in species composition is gradual with a broad overlap of species distributions, and the distinct zones are the result of differences in life-forms and growth-forms of the dominant species. This ecological gradient, comprising a few species and being relatively simple, offers unique opportunities to study hypotheses concerning ecological processes and patterns.
Introduction
Understanding patterns in species distribution, abundance, and diversity along environmental gradients is of primary interest to community ecology (CitationDale, 1999). In vegetation science most studies concerning this issue are done in diverse and complex ecosystems of the northern temperate zones. Even though multivariate methods now enable quantitative analyses of large and complex data sets, interpretation of the results and establishing casual relationships between species assemblages and environmental gradients is often a difficult task (CitationJongman et al., 1995). Antarctic terrestrial assemblages comprise of a few species, and their component interactions are few compared with more diverse and complex assemblages elsewhere (see CitationLewis Smith, 1984; CitationOchyra, 1998; CitationØvstedal and Lewis Smith, 2001). Therefore they provide an ideal opportunity for studying patterns of species assemblages and testing explicit hypotheses on their functional relationships (CitationBlock, 1994).
Antarctic terrestrial ecosystems are generally poor in nutrients, i.e. polar deserts. However, locally there is high nutrient input from seals and sea birds. These marine animals feed in the surrounding ocean but reproduce, molt, and rest on land. Consequently, they deposit large quantities of excrement and fertilize the terrestrial ecosystems. Breeding penguins play the most important role in this process, being the dominant contributor of nutrients to the terrestrial ecosystems (CitationAllen et al., 1967; CitationLewis Smith, 1985; CitationMyrcha et al., 1985; CitationMyrcha and Tatur, 1991; CitationTatur, 2002). For instance, on King George Island the entire population of Pygoscelis penguins nesting on the west shore of Admiralty Bay (ca. 30,000–50,000 pairs) during the breeding season deposits about 6.35 tonnes of dry guano on the land daily, whereas all flying birds deposit only about 0.14 tonnes per day. Within a penguin rookery area, the intensity of manuring may reach 10 kg of dry excreta per square meter during the breeding season (CitationTatur, 2002). However, high nutrient input is not strictly confined to the area of penguin rookeries. Water runoff washes out and redistributes guano and nutrient-laden solutions over the surrounding areas. Additionally, strong winds transport and widely redistribute fine particles of guano and volatilized ammonia over a much greater area. Thus penguin influence covers considerable areas of the Antarctic terrestrial ecosystems (CitationWodehouse and Parker, 1981; CitationGreenfield, 1992; CitationErskine et al., 1998; CitationTatur, 2002).
It has long been recognized that penguin rookeries and their attendant nutrient supply are of great importance in determining the distribution and abundance of the terrestrial vegetation (CitationSmith, 1978; CitationLewis Smith, 1984; CitationMyrcha and Tatur, 1991; CitationTatur, 2002). Some previous papers have also commented on the zonation of vegetation in relation to penguin nesting sites (CitationLewis Smith, 1984; CitationZarzycki, 1993; CitationSmykla, 2001; CitationOlech, 2002; CitationSmykla et al., 2006). However, apart from the work of CitationSmykla (2001), no quantitative assessment of the effects of penguin rookeries on vegetation patterns has been presented.
The primary objective of this project was to determine how the presence of penguin nesting sites affects vegetation patterns and diversity. To address this question, a transect from a penguin rookery to a location distant from the rookery was established, and the vegetation along the transect surveyed. This paper describes and interprets the zonation patterns of the vegetation found around the penguin rookeries.
Study Area
The research was conducted on King George Island, near the Polish Research Station “Henryk Arctowski” (62°10′S, 58°28′W) within the Antarctic Specially Protected Area (ASPA) No. 128 (formerly Site of Special Scientific Interest SSSI No. 8), Western Shore of Admiralty Bay ().
Figure 1 Map of King George Island (after CitationBraun et al., 2001, modified) showing the location of the Polish Antarctic Station “Arctowski” (flag) and the ASPA (Antarctic Specially Protected Area) no. 128 (formerly the Site of Special Scientific Interest SSSI no. 8). Insert shows position of the island in the Antarctic.
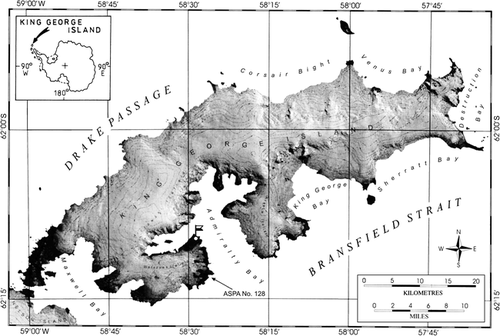
King George Island, the largest in the South Shetland archipelago, is located between 61°50′–62°15′S and 57°30′–59°01′W. The island lies approximately 770 km southeast of Cape Horn, from which it is separated by Drake Passage, and about 160 km north of Trinity Peninsula, the northernmost part of the Antarctic Peninsula. The cold climate with mean annual temperature of −1.7°C (2.4° in January and −6.8° in July), high relative humidity (84%) with a strong oceanic influence, and high precipitation (530 mm a−1) is typical of the northern part of the Maritime Antarctic. A major climatic feature of this area is strong katabatic winds, which often reach hurricane force. Despite the harsh environmental conditions, ice-free areas which constitute only about 10% of the island provide conditions favorable for supporting a relatively diverse terrestrial biota.
The vegetation of the island is almost exclusively cryptogamic, comprising mostly mosses, liverworts, lichens, algae, and cyanobacteria. The vascular flora is represented by only two native species, the Antarctic hair-grass Deschampsia antarctica Desv. (Poaceae) and the Antarctic pearlwort Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae). King George Island, particularly the west shore of Admiralty Bay, is one of the richest botanical areas in the entire Antarctic (CitationOchyra, 1998; CitationKrzewicka and Smykla, 2004). Detailed descriptions of the terrestrial ecosystems of the Admiralty Bay area have already been presented in several publications (e.g. CitationRakusa-Suszczewski, 1993; CitationOchyra, 1998; CitationBeyer and Bölter, 2002).
Materials and Methods
Data Collection
The fieldwork was carried out as part of a project conducted by the first author during the XXth and XXVIth Antarctic Expeditions of the Polish Academy of Sciences, from December 1995 to December 1996 and from December 2001 to March 2002, respectively.
The vegetation survey was made along a transect running across two rookeries of gentoo penguins (Pygoscelis papua Forster) to a site distant from the rookeries. The transect was established parallel to the shore at more or less similar elevation along all its length, so that any potential influence of distance from the sea coast and elevation on vegetation patterns was excluded. The length of the transect was approximately 500 m and ended where the topography did not allow us to go further.
Stands of relatively homogeneous vegetation ( = plant assemblages), each of ca. 20–50 m2, were identified along the whole transect by subjective visual interpretation. Sharp and clear boundaries between all identified plant assemblages provided easy delimitation of particular stands. These stands represented different plant assemblages, the floristic composition of which varied according to the degree of rookery impact. Within each of 22 such stands all living taxa (vascular plants, mosses, lichens, algae, and cyanobacteria) were recorded and their abundance estimated. However, in the case of algae and cyanobacteria, only species forming macroscopically detectable “mats” were recorded. At the penguin rookeries and in adjacent areas, the vegetation survey was done late in the breeding season after the nesting penguins had departed, so that no disturbance to occupied nests occurred.
Species abundance was estimated visually as the percentage cover of the vertically projected area of the above-ground plant parts. Within each stand, 50 randomly distributed measurements were taken. From these data the mean percentage cover of every taxon was calculated for each plant assemblage. To estimate the cover values, a metal circle of area 400 cm2 (1/25 m2) was used. It has been shown in previous studies (see CitationGimingham and Lewis Smith, 1970; CitationLewis Smith, 1972, Citation1988, Citation1999) that for Antarctic cryptogamic assemblages, a sampler of such a size is adequate for such analyses.
To evaluate the degree of penguin colony influence on particular vegetation stands, the following set of variables, which are related to penguin colony influence, were considered: (1) distance from the penguin colony, (2) intensity of trampling, and (3) intensity of guano manuring. The values of (1) were measured, whereas (2) and (3) were subjectively estimated using a four-grade scale (3 = strong, 2 = moderate, 1 = low, 0 = none).
After obtaining the cover data, specimens from all taxa were collected to check identity. The collected specimens were identified and nomenclature assigned according to CitationGreene and Greene (1963) for vascular plants, CitationOchyra (1998) for mosses, CitationØvstedal and Lewis Smith (2001) for lichens, and CitationKomárek and Komárek (1999) for algae and cyanobacteria. Specimens of all the collected taxa have been deposited in the Herbarium of the W. Szafer Institute of Botany of the Polish Academy of Sciences in Cracow (KRAM). For the purpose of this paper, lichens and cyanobacteria are included in the terms “flora,” “plants,” and “vegetation.”
Data Analysis
Initially the vegetation stands were arbitrarily arranged into groups representing a series of plant assemblages with different degrees of penguin rookery influence. In order to derive an objective assessment of the vegetation zones based on the similarity of their floristic composition, the data were subjected to numerical classification and ordination. Multivariate analyses were performed on the matrix species × stands with vegetation cover data using software MVSP 3.1 (CitationKovach, 1998). The data matrix consisted of the entire set of recorded taxa and 20 stands. Two stands (nos. 3 and 11) located within penguin rookeries were excluded from the numerical analysis because they were almost completely devoid of visible vegetation.
Cluster analysis was conducted using Ward's method with Squared Euclidean Distance as a dissimilarity measure. This hierarchical agglomerative classification successively amalgamates samples into groups according to their degree of similarity, based on minimum variance strategy. Detrended Correspondence Analysis (DCA) was applied to obtain ordination of the vegetation stands and clarify the vegetation patterns. This indirect gradient analysis extracts the ordination axes from the species data alone. It arranges samples so that the final output matrix places similar samples adjacent to each other, with differences in value between the sample scores reflecting their relative differences in species composition. The obtained DCA taxa ordinations (the estimated taxa distributions along the ordination axes) were used to generate the hypotheses about the environmental basis of the computed vegetation gradients. Pearson's correlation coefficients (r) were calculated to detect pair-wise relationships between DCA axis and environmental variables related to penguin colony influence (distance from the penguin colony, intensity of trampling, and intensity of guano manuring). The analyses were done with a significance level of α = 0.05.
The indirect ordination technique was preferred over a direct ordination because of difficulty in obtaining reliable environmental data, which in a direct approach implies a risk of modeling the response of species to subordinate gradients (CitationEjrnæs, 2000). Moreover, measurements of the environmental variables determining observed zonation patterns, i.e. penguin disturbance (trampling) and nutrient availability (particularly in the case of air-born ammonia), are difficult, time-consuming to obtain, and unreliable. Further, it must also be emphasized that since growth of the Antarctic vegetation is slow, the present distribution patterns may reflect past environmental gradients rather than the present situation. Species composition may therefore be a more informative indicator of environment than any given set of measured environmental variables.
The numerical classification and ordination techniques used in the data analysis have been fully described by CitationJongman et al. (1995).
To assess the patterns of species richness and diversity, the data from vegetation stands located within the same zone were pooled by calculating mean species cover over all stands included in the zone. Then the total number of species ( = species richness), Shannon's Diversity Index (H′), and Simpson's Dominance Index (λ) were calculated for each zone. The indices were calculated as:
1 and
2 where pi
is the proportional abundance (i.e. proportion of the cover) of ith
species (CitationLudwig and Reynolds, 1988).
Results
Species Composition
During the fieldwork, 35 taxa were encountered in all 22 study plots comprising 2 vascular plants, 11 mosses, 19 lichens, 1 green alga, and 2 cyanobacteria. The complete list of taxa and their abundance are presented in .
Table 1 Occurrence and abundance of particular taxa in a series of vegetation zones around penguin rookeries on King George Island, Maritime Antarctic. Thickness of the bars reflects the relative abundance (mean % cover); + indicates presence without estimated abundance.
Floristic Analysis
The first four DCA ordination axes had eigenvalues of λI = 0.842, λII = 0.180, λIII = 0.101, and λIV = 0.013, and gradient lengths of 4.57, 1.96, 1.49, and 0.91 standard deviation (SD) units, respectively. The data thus reflect a single strong environmental gradient; therefore subsequent interpretations focused only on the first DCA axis (). The first DCA axis was significantly related to distance from penguin colony (r = 0.724; P = 0.0003), trampling (r = −0.863; P < 0.0001), and manuring intensity (r = −0.901; P < 0.0001). These correlations clearly indicate that this major environmental gradient reflected by the first DCA axis is related to intensity of the penguin colony influence.
Figure 2 Numeric Detrended Correspondence Analysis (DCA) ordination of 20 vegetation stands, illustrating the vegetation gradient related to penguin rookery impact. The encircled clusters correspond to the vegetation zones from the adjacent (no. 1) to the most distant zone (no. 5) from penguin rookeries. The lines identify the vegetation zones distinguished on the basis of a subjective approach (broken lines) and the groups of study plots identified by numerical classification (solid lines). Axes are scaled in standard deviation (SD) units.
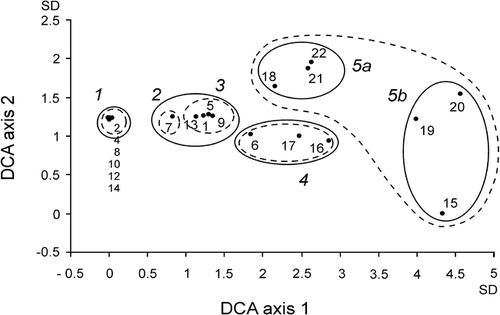
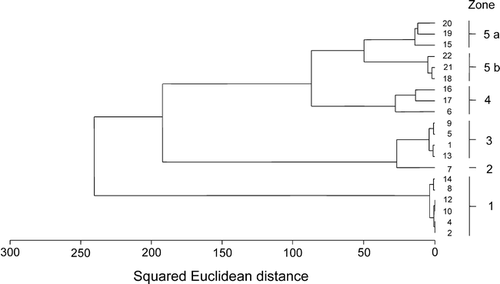
Cluster analysis produced five well-defined groups of vegetation stands that further highlighted the observed relations (). Stands nos. 3 and 11, not included in the multivariate analyses, were grouped in a separate cluster (zone 0). The clusters and their ordination obtained by numerical methods coincided with the subjectively derived zonation pattern ( and ). From a penguin rookery (zone 0) to stands least affected by penguin impact (zone 5), six different vegetation zones were distinguished ().
Figure 3 Dendrogram displaying classification of the vegetation stands. The numbers and lines on the right margin indicate the vegetation zones from the adjacent (no. 1) to the most distant zone (no. 5) from penguin rookeries. The classification was achieved using Ward's method with Squared Euclidean Distance as a dissimilarity measure.
General Characteristics of the Vegetation Zones
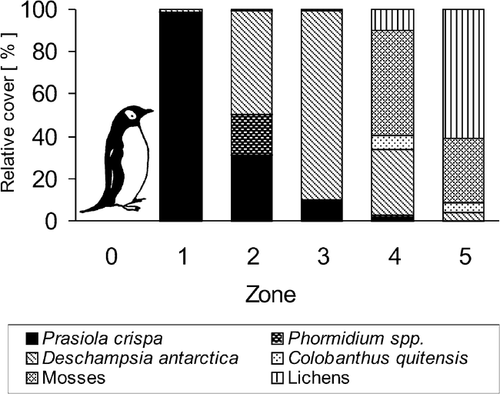
The ground within the active penguin rookeries (zone 0) was covered by fresh guano constantly trampled by penguins and therefore almost entirely devoid of vegetation. Only small thalli of the green alga Prasiola crispa were found there. However, shortly after the breeding season, the deserted rookeries were colonized by P. crispa, which can rapidly develop extensive mats covering the whole area of the rookeries. Other alga species and cyanobacteria also occurred in the fresh guano, but they could not be detected macroscopically, and their description is beyond the scope of this paper. Within the active rookeries only large rocks and boulders projecting from the nesting area, and therefore not disturbed by penguins, were covered by relatively diverse assemblages of ornithocoprophilous lichens. Since the rock and boulder surfaces clearly differ from the ground of the active rookeries, both in penguin disturbance and in nutrient availability, these lichen assemblages are not considered in this treatment.
The topographical features of the penguin colonies enabled downflow of guano deposited near the nesting area. Trampling of the ground was not so intensive here as in the rookeries, thereby allowing the development of vegetation. This vegetation zone (no. 1) was almost completely covered with Prasiola crispa thalli. In moist depressions and areas with continuous water supply, “mats” of the nitrophilous cyanobacteria Phormidium attenuatum and Ph. scottii also occurred. Small stones were covered by ornithocoprophilous lichens such as Acarospora macrocyclos, Buellia falklandica, Caloplaca sublobulata, Rhizoplaca aspidophora, and Turgidosculum complicatulum. Occasionally, single small tufts of Deschampsia antarctica were also found there. However, the grass, as well as lichens, occurred within this zone only in very small amounts, and their total contribution to the plant cover was less than 1%.
Beyond the belt of Prasiola crispa (zone 1), dense, lawn-like swards of Deschampsia antarctica usually formed the next vegetation zone (no. 3). The transition between these zones was usually very distinct and occurred within a range of only a few centimeters. However, in some localities, a transition zone (no. 2) was found, where the green alga Prasiola crispa and cyanobacteria Phormidium spp. intermingled with Deschampsia antarctica patches. The abundance of these nitrophilous alga and cyanobacteria exceeded 50%. The grass was also very abundant, covering over 50% of the area in this zone. The ornithocoprophilous lichens listed above and small patches of mosses also occurred here, but their coverage did not exceed 1%.
In the Deschampsia antarctica zone (no. 3) the grass formed lush, almost uniform, lawn-like swards which covered more than 90% of the ground. The nitrophilous Prasiola crispa was still frequent, but its cover did not exceed 10%. Occasionally, among the grass tufts and in wet depressions irrigated by nutrient-rich water, “mats” of the cyanobacteria Phormidium spp. were also found. In the Deschampsia antarctica sward zone, Colobanthus quitensis and several moss and lichen species also occurred, but their total contribution to the plant cover did not exceed 1%. Among lichens, mainly crustose saxicolous species were found, but the muscicolous Ochrolechia frigida also occurred here in very small amounts.
The succeeding zone (no. 4) was characterized by a high incidence of mosses (50%). The vascular plants were also significant in this zone (Deschampsia antarctica—31%, Colobanthus quitensis—7%), together with lichens (10%), while the nitrophilous alga Prasiola crispa and cyanobacteria Phormidium spp. covered only 3%. Several moss and lichen species were found here. In moist places, the most abundant moss was Sanionia georgico-uncinata, with Brachythecium austrosalebrosum also locally abundant. In drier localities, the most abundant taxon was Polytrichastrum alpinum, with Polytrichum piliferum, Bryum pseudotriquetrum, Ceratodon purpureus, Syntrichia princeps, and S. saxicola also abundant locally. Among the lichens epibryophytic species were the most numerous. Ochrolechia frigida provided the largest coverage in general, together with Megaspora verrucosa, Psoroma hypnorum, and Rinodina olivaceobrunnea locally, whereas only small, single thalli of the ornithocoprophilous lichens occurred within this zone.
The zone most distant from penguin rookeries (zone 5) and least affected by penguin impact was dominated by lichens and mosses (60% and 30%, respectively). Among the mosses the dominant species were Polytrichastrum alpinum, Syntrichia saxicola, S. princeps, and Polytrichum piliferum. The most numerous lichens were the crustose Ochrolechia frigida and fruticose Usnea antarctica, which formed a canopy over a diverse understory of mosses and crustose lichens. Locally, the nitrophilous Caloplaca sublobulata was also relatively abundant, but its distribution was probably restricted to stones fertilized by flying birds. The nitrophobous lichen Leptogium puberulum, which did not occur in the zones under greater influence from penguin colonies, was also locally frequent here. The cover of vascular plants was relatively low and did not exceed 10%. The nitrophilous Prasiola crispa occurred here only exceptionally, its thalli usually not exceeding 1 cm and its total contribution to the plant cover amounting to only a fraction of one percent.
Patterns of Richness and Diversity
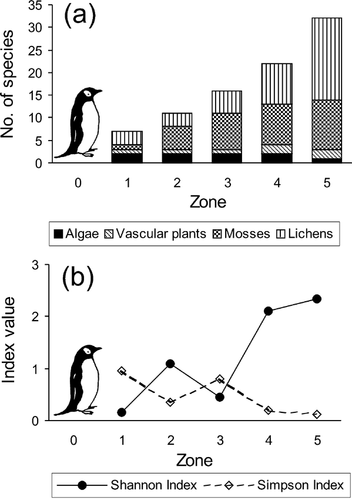
The data show a clear trend in the richness and diversity of vegetation related to the degree of penguin rookery impact. The lowest number of species is found within the penguin rookeries (zone 0). In the consecutive zones, the number of species increases () so that the highest number is found in the zone least affected by the rookeries (zone 5). Similarly, the diversity measure (H′) shows an identical tendency, and its value increases from zone 1 to zone 5. The opposite pattern is observed only for dominance (λ), the lowest dominance being found in zone 5, while zone 1 has the highest (). These results indicate that with decreasing penguin rookery impact there is a consistent increase in plant species richness and diversity with a simultaneous decrease in species dominance.
Discussion
Floristic Gradient Related to Penguin Rookeries
The results of this survey provide quantitative and qualitative evidence of the impact of penguin rookeries on the floristic composition of plant assemblages and the spatial pattern of the terrestrial vegetation at Admiralty Bay, King George Island, in the Maritime Antarctic. The described zonation pattern of vegetation is in broad agreement with earlier studies conducted in this area by CitationZarzycki (1993). Although not presenting quantitative evidence, he pointed out the pronounced zonation pattern of the terrestrial vegetation related to the penguin rookeries. He also suggested that plant assemblages, which form different vegetation zones, floristically “are not sharply separated.” The data reported in the present study are consistent with his observations. clearly demonstrates that the distinct zones occurring around penguin rookeries are floristically similar. Species composition and their abundance change gradually along the zonation gradient, with a broad overlap of species distribution. The pronounced vegetation zones are identified only by different life-forms and growth-forms of the dominant taxa.
“Mats” of the nitrophilous foliose alga Prasiola crispa and, in wet areas, also the cyanobacteria Phormidium spp. occupy sites with extremely high nutrient concentrations and high disturbance level (i.e. active rookeries and adjacent areas). It is well-known that occurrence of these taxa depends on the high trophic level of a site and is usually associated with bird nesting sites (CitationLewis Smith, 1984; CitationKomárek and Komárek, 1999). Although in this study Prasiola crispa was found throughout the transect, its abundance decreased markedly in consecutive vegetation zones away from the penguin influence. The presence of this nitrophilous alga as well as that of ornithocoprophilous lichens (e.g. Caloplaca citrina, C. sublobulata) in localities distant from the rookeries is probably attributable to their microsites being fertilized by droppings of flying birds, and thus having higher nutrient concentrations. Transportation by wind, which can widely redistribute small particles of guano and volatilized ammonia (CitationAllen et al., 1967; CitationGreenfield, 1992; CitationErskine et al., 1998; CitationTatur, 2002), may perhaps also be responsible for the occurrence of these nitrophilous taxa in localities distant from bird nesting sites. However, their presence may also reflect past nutrient status of these sites.
In the zone adjacent to that of the nitrophilous alga and cyanobacteria, in moderately manured areas around penguin rookeries, Deschampsia antarctica became the dominant species. The data demonstrate that the grass has a wide ecological range with relatively high abundance over most of the vegetation zones except for the extremely disturbed and excessively nutrient-enriched active rookeries and the adjacent zone. Its abundance also decreased in sites distant from the rookeries with low nutrient availability. Data from other studies suggest that the grass has a wide ecological amplitude (CitationGreene and Holtom, 1971; CitationEdwards, 1972; CitationLewis Smith, 1996, Citation2003; CitationTatur et al., 1997; CitationBarcikowski et al., 2001, Citation2003). Although the grass can occur on soils with 0.29–3.16% total nitrogen concentration (CitationLewis Smith, 1996), the data from the present study, as well as in the studies mentioned above, indicate that the most favorable conditions for the grass occur in moderately manured areas near penguin rookeries where it forms the most luxuriant growth. According to CitationTatur et al. (1997), nutrient-rich relict ornithogenic soils at abandoned rookeries also offer optimum habitats for the growth of this grass.
Colobanthus quitensis was also widespread, but it was considerably less abundant and seemed to have a narrower ecological range than Deschampsia antarctica. This finding is consistent with earlier studies (CitationGreene and Holtom, 1971; CitationEdwards, 1972; CitationFowbert and Lewis Smith, 1994; CitationLewis Smith, 2003). Although both species are usually found together, the grass is generally more abundant and occupies a wider range of habitats. The grass can also tolerate a higher degree of animal-derived contamination. On the other hand, Colobanthus quitensis seems to be less tolerant of high nutrient concentrations than Deschampsia antarctica and its importance consistently increases in zones with a lower penguin impact where conditions for its growth are, presumably, more favorable. The data cited above, as well as those presented in this paper, suggest that, despite their co-occurrence, the two species have significantly different ecological ranges and optima.
In the zones with low penguin impact, mosses and lichens predominated with only a relatively small incidence of vascular plants. Compared with other taxonomic groups, lichens have the lowest nutrient requirements and therefore are able to colonize and persist in habitats with very low and unpredictable nutrient availability (CitationKappen, 1993, Citation2000).
Alongside the shift in life-forms related to the change in dominance of different taxonomic groups (i.e. algae, vascular plants, mosses, and lichens), a gradual change in the dominant moss and lichen growth-forms was also clearly visible between different zones. Among mosses, carpet-, tall cushion-, and tall turf-forming species were the most abundant close to the rookeries, whereas short turf- and small cushion-forming species predominated farther away. It is clear that availability of water is an important factor in determining the presence of moss species with a particular growth-form within a habitat (CitationGimingham and Lewis Smith, 1971; CitationLewis Smith, 1972, Citation1984; CitationOchyra, 1998; CitationDavey, 1999). According to some authors, nutrient availability does not appear to be an important determinant of the moss distribution pattern (CitationDavey, 1999; CitationLewis Smith, 1999); however, it has been reported that elevated nutrient levels considerably increases growth in sub-Antarctic moss species (CitationSmith, 1993). There is also some evidence that habitats in which hydric mosses predominate have higher nitrogen and phosphorus levels than habitats dominated by mesic or xeric mosses (CitationHoldgate et al., 1967; CitationDavis, 1981). It is therefore likely that the shift in moss species composition and related change in their dominant growth-forms, reported in the present study, may also result from changes in the concentration and availability of penguin-derived nutrients. Whether such a shift in moss growth-forms is to be expected as a result of change in the zone nutrient status, and to what extent nutrients are essential for the existence and distribution pattern of bryophytes in Antarctica, could be tested by a controlled fertilizer application experiment.
Among lichens close to the rookeries, only epilithic crustose and foliose taxa occurred, with colorful ornithocoprophilous species predominating. With increased abundance of mosses, epibryophytic lichens became the most numerous, with a lesser incidence of the nitrophilous species. Finally, fruticose macrolichens dominated the zone most distant from the rookeries. As in other parts of Antarctica (see CitationLewis Smith, 1972; CitationKappen, 1993, Citation2000), such a pattern of lichen distribution reflects the distinction in occurrence between the eutrophic species that can cope with the fertilizer effects of bird excrement, and the oligotrophic (nitrophobous) species that avoid such enriched sites.
As shown above, the general trend in the change of the vegetation structure between consecutive zones is marked by the decreasing abundance of nitrophilous species with the simultaneous appearance and increasing abundance of nitrophobous species. Therefore, it may be inferred that the penguin-derived fertility gradient is a major factor in determining the zonation pattern of vegetation presented in this paper. The distribution of each species, and consequently of plant assemblages forming particular vegetation zones, is most probably related mainly to the degree of fertilization by penguin-derived nutrients.
Patterns of Richness and Diversity
The data demonstrate a trend in species richness and diversity related to the penguin-enrichment gradient. With increasing distance from the penguin rookeries, the vegetation zones become less distinct and more complex (; ). Plant species richness and diversity increase along consecutive zones, from the lowest values at the nutrient-rich rookeries to the highest in the zone with the lowest nutrient availability. The opposite pattern was observed only for species dominance (). Lower values of diversity measures at the penguin-affected sites have also been reported for soil algae (CitationOhtani et al., 2000; CitationMataloni and Tell, 2002). CitationMataloni and Tell (2002) suggested the existence of a decreasing trend in richness and diversity of the soil algae in association with an increase in penguin-derived enrichment. According to these authors this may be caused by toxic substances, which act as a constraint on the growth of many species.
These seem to be conflicting results since it has been shown previously that species richness and diversity of lichen-dominated vegetation on the Antarctic Continent were higher under the high nutrient input from breeding seabirds compared with sites away from the nests (CitationRyan and Watkins, 1989; CitationLeishman and Wilde, 2001). However, it should be noted that their studies included sites affected by the breeding activity of flying birds (i.e. snow petrels and south polar skuas). Although they demonstrated considerable nutrient input in the vicinity of the nests of these birds, penguin-derived fertilization is of a much greater magnitude and thus it may have a different effect on the vegetation. The soil nutrient concentrations in sites adjacent to the nests of flying birds were found to be 8 to 11 times greater than in those remote from them (CitationRyan and Watkins, 1989; CitationLeishman and Wilde, 2001). In contrast, soils enriched by penguin guano may have nutrient concentrations hundreds of times higher than sites unaffected by penguin activity. For example, the total soil nitrogen at the nests of flying birds was 0.22% N compared with 0.05% N in sites not affected by birds, whereas soils influenced by penguin guano may have 9–16% of the total N value (see CitationLeishman and Wilde, 2001).
The sites surveyed during the present study farthest from the penguin rookeries were located only about 500 m from the colonies. During warm days, with winds blowing from the direction of the rookeries, the smell of ammonia was very noticeable at these localities. CitationErskine et al. (1998) demonstrated that, at a distance of 600 m from rookeries, the level of penguin-derived ammonia deposited in the ecosystem may still be significantly high. Additionally, the presence of flying birds, with nests dispersed nearby, was a regular feature of the surveyed sites most distant from the rookeries. Therefore it seems likely that the input of bird-derived nutrients at these sites could still be relatively high. This conclusion is also supported by the presence of nitrophilous taxa (i.e. Prasiola crispa, Caloplaca citrina, C. sublobulata) which were found within these sites (see ).
In fact, the results reported in the present paper, and those by CitationMataloni and Tell (2002), may be complementary to those found by CitationRyan and Watkins (1989) and CitationLeishman and Wilde (2001). Observed differences are associated with various magnitudes of bird-derived fertilization. The toxic levels of manuring and trampling by dense penguin populations cause vegetation damage. The few taxa which are able to tolerate these conditions may benefit and increase their dominance. A decrease in penguin-derived fertilizer input allows the concentration of nutrients to fall to levels favorable for the growth of many different taxa, enabling more diverse plant assemblages to develop. By contrast, vegetation around the individual nests of flying birds is usually not devastated, as the moderate input of nutrients around these nests acts as a fertilizer stimulating the growth of various taxa. However, a further decrease of nutrient availability inhibits the growth of many taxa, thereby causing a decrease in species richness and diversity. Thus, on a local scale, the species richness and diversity of the Antarctic vegetation is probably greatest when intermediate nutrient levels occur. This is consistent with general models (e.g. CitationGrime, 2001) which predict a unimodal curve of species richness and diversity, with low values at nutrient-rich and nutrient-deficient habitats and high values in habitats with intermediate nutrient levels, while the opposite pattern is predicted for species dominance.
Concluding Remarks
This study provides quantitative and qualitative evidence that there are clear vegetation patterns and differences in the structure of the Antarctic plant assemblages related to the intensity of penguin rookery impact. Although the present survey was carried out within a relatively small area on King George Island in the Maritime Antarctic, previously published data suggest that the conclusions concerning vegetation patterns can probably be generalized to include all the Southern Polar Zones. However, in the Subantarctic, the Deschampsia antarctica sward zone is replaced by other grass species and, at low-nutrient sites, plant assemblages with higher vascular plant diversity develop (e.g. CitationSmith, 1978; CitationLewis Smith, 1984; CitationVidal et al., 2003). On the other hand, in Continental Antarctic where vascular plants are absent, the nitrophilous algae and cyanobacteria zone abuts directly with various moss and lichen assemblage zones (e.g. CitationLewis Smith, 1984, Citation1988). Studies of vegetation succession on abandoned penguin rookeries (CitationHuntley, 1971; CitationTatur et al., 1997), despite lacking quantitative analysis, suggest also similar temporal vegetation patterns.
This paper provides not only a description of the zonation of vegetation related to penguin rookeries, but also a basis for establishing an ecological gradient model. Such a simple biological model may, because of the poverty in species composition and simple gradient structure, provide excellent facilities for empirical studies and the testing of ecological hypotheses. Detailed analyses of the penguin-vegetation relationship with respect to the problem of plant community classification, plant life strategies, and plant eco-physiological adaptations to the penguin-enrichment gradient will be published in separate papers.
Acknowledgments
The authors owe special thanks to Prof. Kazimierz Zarzycki, whose ideas initiated this survey, for his generous encouragement and support throughout, without which its accomplishment could not have been possible. The authors also thank Ryszard Ochyra, Lucyna Śliwa, and Dag Olav Øvstedal for taxonomic consultations; Jakub Cieślak, who constructed ; and Krystyna Grodzińska and Arthur Copping (Diss, UK) for their constructive comments on the manuscript. Thanks are also due to all the XXth and XXVIth “Arctowski” expeditionary teams for logistical aid and many fruitful discussions. The first author is especially indebted to his wife Renata Malejki for her encouragement when embarking upon this work and her assistance throughout. The field survey was made possible by the logistical and financial support of the Department of Antarctic Biology, Polish Academy of Sciences. This work was supported by the State Committee for Scientific Research grant No. 2 P04F 001 27.
References Cited
- Allen, S. E. , H. M. Grimshaw , and M. W. Holdgate . 1967. Factors affecting the availability of plant nutrients on an Antarctic island. Journal of Ecology 55:381–396.
- Barcikowski, A. , J. Czaplewska , I. Giełwanowska , P. Loro , J. Smykla , and K. Zarzycki . 2001. Deschampsia antarctica (Poaceae)—The only native grass from Antarctica. In Frey, L. , editor. ed. Studies on grasses in Poland Kraków W. Szafer Institute of Botany, Polish Academy of Sciences. 367–377.
- Barcikowski, A. , J. Czaplewska , P. Loro , A. Łyszkiewicz , J. Smykla , and A. Wojciechowska . 2003. Ecological variability of Deschampsia antarctica in the area of Admiralty Bay (King George Island, Maritime Antarctic). In Frey, L. , editor. ed. Problems of grass biology Kraków W. Szafer Institute of Botany, Polish Academy of Sciences. 393–407.
- Beyer, L. and M. Bölter , editors. 2002. Geoecology of Antarctic Ice-free Coastal Landscapes Ecological Studies 156. Berlin Springer. 427.
- Block, W. 1994. Terrestrial ecosystems. Antarctica. Polar Biology 14:293–300.
- Braun, M. , J. C. Simões , S. Vogt , U. F. Bremer , N. Blindow , M. Pfender , H. Saurer , F. E. Aquino , and F. A. Ferron . 2001. An improved topographic database for King George Island: compilation, application and outlook. Antarctic Science 13:41–52.
- Dale, M. R. T. 1999. Spatial pattern analysis in plant ecology Cambridge Cambridge University Press. x +. 336 pp.
- Davey, M. C. 1999. The elemental and biochemical composition of bryophytes from the maritime Antarctic. Antarctic Science 11:157–159.
- Davis, R. C. 1981. Structure and function of two Antarctic terrestrial moss communities. Ecological Monographs 51:125–143.
- Edwards, J. A. 1972. Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv.: V.Distribution, ecology and vegetative performance on Signy Island. British Antarctic Survey Bulletin 28:11–28.
- Ejrnæs, R. 2000. Can we trust gradients extracted by Detrended Correspondence Analysis?. Journal of Vegetation Science 11:565–572.
- Erskine, P. D. , D. M. Bergstorm , S. Schmidt , G. R. Stewart , C. E. Tweedie , and J. D. Shaw . 1998. Subantarctic Macquarie Island—A model ecosystem for studying animal-derived nitrogen sources using 15N natural abundance. Oecologia 117:187–193.
- Fowbert, J. A. and R. I. Lewis Smith . 1994. Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arctic and Alpine Research 26:290–296.
- Gimingham, C. H. and R. I. Lewis Smith . 1970. Bryophyte and lichen communities in the Maritime Antarctic. In Holdgate, M. W. , editor. ed. Antarctic ecology. Vol. 2 London Academic Press. 752–785.
- Gimingham, C. H. and R. I. Lewis Smith . 1971. Growth form and water relations of mosses in the Maritime Antarctic. British Antarctic Survey Bulletin 25:1–21.
- Greene, D. M. and A. Holtom . 1971. Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv.: III. Distribution, habitats and performance in the Antarctic botanical zone. British Antarctic Survey Bulletin 26:1–29.
- Greene, S. W. and D. M. Greene . 1963. Check list of the sub-Antarctic and Antarctic vascular flora. Polar Record 11:411–418.
- Greenfield, L. G. 1992. Precipitation nitrogen at maritime Signy Island and continental Cape Bird, Antarctica. Polar Biology 11:649–653.
- Grime, J. P. 2001. Plant strategies, vegetation processes and ecosystem properties Chichester, UK John Wiley & Sons. xxxvii +. 417 pp.
- Holdgate, M. W. , S. E. Allen , and J. G. Chambers . 1967. A preliminary investigation of the soils of Signy Island, South Orkney Islands. British Antarctic Survey Bulletin 12:53–71.
- Huntley, B. J. 1971. Vegetation. In van Zinderen Bakker Sr, E. M. , J. M. Winterbottom , and R. A. Dyer , editors. eds. Marion and Prince Edward Islands Cape Town Balkema. 98–160.
- Jongman, R. H. G. , C. J. F. ter Braak , and O. F. R. van Tongeren . 1995. Data analysis in community and landscape ecology Cambridge Cambridge University Press. xxi +. 299 pp.
- Kappen, L. 1993. Lichens in the Antarctic region. In Friedmann, E. I. , editor. ed. Antarctic microbiology New York Wiley-Liss. 433–490.
- Kappen, L. 2000. Some aspects of the great success of lichens in Antarctica. Antarctic Science 12:314–324.
- Komárek, O. and J. Komárek . 1999. Diversity of freshwater and terrestrial habitats and their oxyphototroph microflora in the Arctowski Station region, South Shetland Islands. Polish Polar Research 20:259–282.
- Kovach, W. L. 1998. MVSP—A MultiVariate Statistical Package for Windows. ver.3.0 Pentraeth, Wales, U.K Kovach Computing Services. iv +. 127 pp.
- Krzewicka, B. and J. Smykla . 2004. The lichen genus Umbilicaria from the neighbourhood of Admiralty Bay (King George Island, Maritime Antarctic), with a proposed new key to all Antarctic taxa. Polar Biology 28:15–25.
- Leishman, M. R. and C. Wilde . 2001. Vegetation abundance and diversity in relation to soil nutrients and soil water content in Vestfold Hills, East Antarctica. Antarctic Science 13:126–134.
- Lewis Smith, R. I. 1972. The vegetation of the South Orkney Islands, with particular reference to Signy Island. British Antarctic Survey Scientific Reports 68:1–124.
- Lewis Smith, R. I. 1984. Terrestrial plant biology of the sub-Antarctic and Antarctic. In Laws, R. M. , editor. ed. Antarctic ecology. Vol. 1 London Academic Press. 61–162.
- Lewis Smith, R. I. 1985. Nutrient cycling in relation to biological productivity in Antarctic and sub-Antarctic terrestrial and freshwater ecosystems. In Siegfried, W. R. , P. R. Condy , and R. M. Laws , editors. eds. Antarctic nutrient cycles and food webs Berlin, Heidelberg, New York Springer-Verlag. 138–155.
- Lewis Smith, R. I. 1988. Classification and ordination of cryptogamic communities in Wilkes Land, Continental Antarctica. Vegetatio 76:155–166.
- Lewis Smith, R. I. 1996. Terrestrial and freshwater biotic components of the western Antarctic Peninsula. In Ross, R. M. , E. E. Hofmann , and L. B. Quetin , editors. eds. Foundation for ecological research west of the Antarctic Peninsula American Geophysical Union, Antarctic Research Series. 70:15–59.
- Lewis Smith, R. I. 1999. Biological and environmental characteristics of three cosmopolitan mosses dominant in continental Antarctica. Journal of Vegetation Science 10:231–242.
- Lewis Smith, R. I. 2003. The enigma of Colobanthus quitensis and Deschampsia antarctica in Antarctica. In Huiskes, A. H. L. , W. W. C. Gieskes , J. Rozema , R. M. L. Schorno , S. M. van der Vies , and W. J. Wolff , editors. eds. Antarctic Biology in a Global Context Leiden Backhuys Publishers. 234–239.
- Ludwig, J. A. and J. F. Reynolds . 1988. Statistical ecology. A primer on methods and computing New York John Wiley & Sons. xviii +. 337 pp.
- Mataloni, G. and G. Tell . 2002. Microalgal communities from ornithogenic soils at Cierva Point, Antarctic Peninsula. Polar Biology 25:488–491.
- Myrcha, A. and A. Tatur . 1991. Ecological role of current and abandoned penguin rookeries in the land environment of the Maritime Antarctic. Polish Polar Research 12:3–24.
- Myrcha, A. , S. J. Pietr , and A. Tatur . 1985. The role of pygoscelid penguin rookeries in nutrient cycles at Admiralty Bay, King George Island. In Siegfried, W. R. , P. R. Condy , and R. M. Laws , editors. eds. Antarctic nutrient cycle and food webs Berlin, Heidelberg, New York Springer-Verlag. 156–162.
- Ochyra, R. 1998. The moss flora of King George Island, Antarctica Cracow W. Szafer Institute of Botany, Polish Academy of Sciences. xxiv +. 279 pp.
- Ohtani, S. , K. Suyama , H. Yamamoto , Y. Aridomi , R. Itoh , and Y. Fukuoka . 2000. Distribution of soil algae at the monitoring sites in the vicinity of Syowa Station between austral summers of 1992/1993 and 1997/1998. Polar Bioscience 13:113–132.
- Olech, M. 2002. Plant communities on King George Island. In Beyer, L. and M. Bölter , editors. eds. Geoecology of Antarctic Ice-free Coastal Landscapes Ecological Studies 156. Berlin Springer. 215–231.
- Øvstedal, D. O. and R. I. Lewis Smith . 2001. Lichens of Antarctica and South Georgia. A Guide to their Identification and Ecology Cambridge Cambridge University Press. xii +. 411 pp + 104 pls pp.
- Rakusa-Suszczewski, S. , editor. 1993. The Maritime Antarctic Coastal Ecosystem of Admiralty Bay Warsaw Department of Antarctic Biology, Polish Academy of Sciences. 216.
- Ryan, P. and B. Watkins . 1989. The influence of physical factors and ornithogenic products on plant and arthropod abundance at an inland nunatak group in Antarctica. Polar Biology 10:151–160.
- Smith, V. R. 1978. Animal-plant-soil nutrient relationships on Marion Island (Subantarctic). Oecologia (Berlin) 32:239–253.
- Smith, V. R. 1993. Effect of nutrients on CO2 assimilation by mosses on a sub-Antarctic island. New Phytologist 123:693–697.
- Smykla, J. 2001. Zmiany składu gatunkowego i strategii życiowych roślin w gradiencie oddziaływania kolonii pingwinów (Wyspa Króla Jerzego, Antarktyka Morska). [Changes in the species composition of plant communities and plant life strategies related to the penguin rookery impact gradient (King George Island, Maritime Antarctic)]. Ph.D. thesis. Kraków, Poland W. Szafer Institute of Botany, Polish Academy of Sciences. 90 pp + IX tables +12 figures [unpublished].
- Smykla, J. , J. Wołek , A. Barcikowski , and P. Loro . 2006. Vegetation patterns around penguin rookeries at Admiralty Bay, King George Island, Maritime Antarctic: preliminary results. Polish Botanical Studies 22. in press.
- Tatur, A. 2002. Ornithogenic ecosystems in the Maritime Antarctic—Formation, development and disintegration. In Beyer, L. and M. Bölter , editors. Ecological Studies 156. eds. Geoecology of Antarctic Ice-free Coastal Landscapes Berlin Springer. 161–184.
- Tatur, A. , A. Myrcha , and J. Niegodzisz . 1997. Formation of abandoned penguin rookery ecosystems in the Maritime Antarctic. Polar Biology 17:405–417.
- Vidal, E. , P. Jouventin , and Y. Frenot . 2003. Contribution of alien and indigenous species to plant-community assemblages near penguin rookeries at Crozet archipelago. Polar Biology 26:432–437.
- Wodehouse, E. B. and B. C. Parker . 1981. Atmospheric ammonia nitrogen, a potential source of nitrogen eutrophication of freshwater Antarctic ecosystems. Antarctic Research Series 30:155–167.
- Zarzycki, K. 1993. Vascular plants and terrestrial biotopes. In Rakusa-Suszczewski, S. , editor. ed. The Maritime Antarctic Coastal Ecosystems of Admiralty Bay Warsaw Department of Antarctic Biology, Polish Academy of Sciences. 181–187.