ABSTRACT
In 2003 a partial bison skull was recovered by Ashley National Forest archaeologists from an elevation of 3840 m (12,600 ft) above mean sea level on Gilbert Peak in the Uinta Mountains of Utah. The skull consists of a portion of the frontal, occipital region, and horn cores including horn sheaths. Through the analysis of the individual cones of the horn sheath, a record of the animal’s dietary and migration patterns were obtained. While high-altitude bison remains have been discussed in the scientific literature periodically, they have not gone beyond the descriptive. In addition to descriptive and metric analysis of the skull, radiometric assay and stable isotope analyses were applied. The radiocarbon age of the specimen is 150 ± 40 yr BP (cal. a.d. 1725–1778). Metric analysis of the skull indicates it was an older adult male, which compares well with Bison bison athabascae (wood bison) in size and is larger than either Bison bison bison (plains bison) specimens or other high-altitude bison. However, it is probable this individual represents a member of the species Bison bison bison, but phenotypic characteristics (e.g., large horn size) may be the result of gene flow. More definitive taxonomic placement of the Gilbert Peak bison may not be resolved without genetic analysis.
Introduction
I doubt the bison had much respect for altitude, and I believe they went as high as they choose (CitationWarren, 1927: p. 61).
Documenting the range of mammalian species was an important focus for biologists working in the late 19th and early 20th centuries, especially with respect to shrinking habitat from expanding settlement in the Intermountain West of the United States. This was particularly true of the North American bison (Bison bison Linnaeus 1758); it had been reduced to a few hundred individuals at the beginning of the 20th century, and its long-term preservation was uncertain (CitationShell, 2002: p. viii).
Altitudinal range, as well as latitudinal and longitudinal, were often reported in scientific journals. CitationFryxell (1926) reported on bison specimens in the Medicine Bow Mountains of Wyoming at altitudes ranging from 2895 to 3658 m (9500 to 12,000 ft), followed by a report on high-altitude bison records from Colorado, Wyoming, and Montana (CitationFryxell, 1928). High-altitude bison sightings in southern and northern Colorado are also reported by CitationCook (1930), CitationWarren (1927), and CitationBeidleman (1955). More recently, CitationWilson (1974a) reported on bison remains from above timberline in the Bighorn Mountains of northern Wyoming. With climate-driven ice/snowfield melting in high-altitude and high-latitude regions, a new source of bison remains has been uncovered (CitationLee et al., 2006).
Unfortunately, few high-altitude records of bison have been reported in Utah. CitationMadsen (2000) reports that 30 genera of late Quaternary fauna have been found in alpine settings. However, only two high-altitude sites, Mastodon Sinkhole (3232 m [10,604 ft]) in Sanpete County (CitationGillette and Madsen, 1993, citing W. Miller, personal communication) and Huntington Canyon (2981 m [9780 ft]) near the crest of the Wasatch Plateau (CitationGillette and Madsen, 1993; CitationMadsen, 2000), produced bison. The paucity of high-altitude bison remains from Utah is therefore noteworthy. While the Gilbert Peak bison represents a single individual, it does contribute significantly to our knowledge of high-elevation fauna.
This study takes its cue from CitationFryxell (1928: p. 139), a statement which may be more imperative today:
It is now out of the question to turn bison loose on the plains. The only portions, if any, of the former range of the bison that could now possibly be restocked are certain uninhabited tracts in or adjacent to the mountains—within the national forests and national parks. Hence, obviously, the importance of preserving such scraps of information as can be obtained at this late period concerning the former range and life habits of the bison in our western mountains.
Therefore, it is the purpose of this study to provide some of these scraps of information using contemporary research techniques (e.g., CitationCannon, 1997; CitationCannon, 2001). These include radiometric dating, metric analysis of the specimen and its relationship to other intermountain and high-altitude specimens, and stable isotope analyses. Stable carbon and nitrogen isotope analyses have the potential to yield information on the diet and ecology of an individual. As CitationWalker (1992) explained, there are still temporal and spatial gaps in the Holocene record of bison, and isolated skulls can help fill them. Isolated bison skulls can provide specific information for understanding the paleoecology and evolution of the species. Information derived from detailed study of isolated bison skulls can have implications for a number of social and ecological issues (CitationVan Vuren, 1987: p. 65), such as the management and restoration of ecosystems (CitationLyman, 1996; CitationLyman and Cannon, 2004), zoogeography (CitationLyman and Livingstone, 1983), and ethnography (CitationBamforth, 1988; CitationReeves and Peacock, 2001).
While this study focuses on the results of various analyses conducted on the Gilbert Peak bison, the context of the study is regional in scope. This study, along with an earlier one (CitationCannon, 1997), provide the initiation of a larger research goal to understand the biogeography of bison in the Intermountain West within the context of long-term climate change (CitationCannon, 2001; CitationLyman, 2004).
Environmental Context
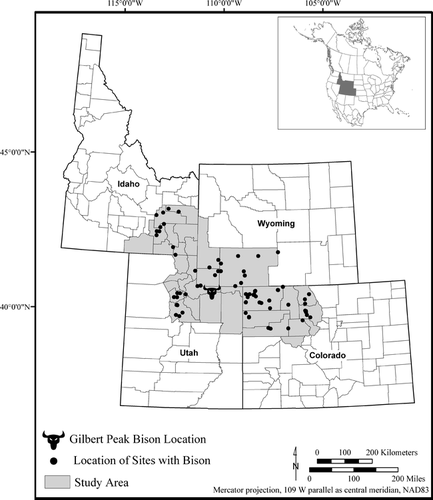
U.S. Forest Service archaeologists recovered the Gilbert Peak bison skull from a steep gravel- and boulder-covered ridge southwest of Gilbert Peak at an elevation of 3840 m (12,600 ft) above mean sea level (AMSL) in Summit County along the boundary between the Ashley and Wasatch National Forests (). The area consists of alpine vegetation with surface cobbles common. Soils are shallow and well-drained. Tundra vegetation is present in the immediate area. As defined by CitationEdwards et al. (1995), common species include perennial herbs (Geum rossi, Silen acaulis, Paronychia pulvinata, Arenaria obtusiloba, Trifolium nanum, Kobresia myouroides, Polygonum bistoroides, Eriophorum chamissonis), perennial grasses (Deschampsia caespotosa, Festuca ovina, Koeleria cristata, Trisetum spicatum), sedges (Carex sp.), and willow (Salix sp.).
Figure 1 Regional map illustrating location of precontact sites with bison remains in relation to Gilbert Peak bison find location.
To the east, the landscape slopes dramatically into a boggy area with an unnamed lake that is drained by Gilbert Creek, near the headwaters of the Uinta River. Engelmann spruce (Picea engelmannii) are present along the edge of the boggy area. The wet meadow vegetation consists mostly of grasses, forbs, sedges, and rushes.
Regional Evidence of Bison
The record of bison remains from late Quaternary sites in the region is extensive, and almost exclusively the result of human predation (CitationCannon, 2004; ). The distribution in northern Utah (CitationLupo and Schmitt, 1997), southeastern Idaho (CitationButler, 1978; CitationCannon, 1997; CitationPlew and Sundell, 2000), and southwestern Wyoming (CitationLubinski, 2000) indicates bison were present throughout the Holocene with population density possibly fluctuating in relation to climate patterns. However, several authors have argued that the sagebrush-steppe never supported large herds of bison (CitationMack and Thompson, 1982; CitationDaubenmire, 1985), therefore making for an inconsistent resource (CitationHenrikson, 2003, Citation2004).
CitationLubinski's (2000) research in southwestern Wyoming focused on the dietary selection of antelope and bison within the context of shifting climatic regimes (CitationEckerle and Hobey, 1995). His results indicate that archeological bison were more prevalent during moist periods of the Holocene (10,000–9000 yr BP; 3500–1800 yr BP; 500–150 yr BP).
In the southern Rocky Mountains, preservation of faunal remains may influence interpretations. While bison in Colorado were probably most common in the eastern grasslands, high-elevation parks represented areas that appear to have been attractive to bison (CitationKornfeld and Frison, 2000; CitationStiger, 2001; CitationPitblado, 2003).
High-altitude bison remains in Utah are rare, as are high-altitude local faunas in general (CitationGillette and Madsen, 1993). CitationMadsen (2000) has suggested a seasonal use of high-altitude valleys in the Wasatch Plateau during post-glacial times by mammoth (Mammuthus sp.), mastodon (Mammut sp.), horse (Equus sp.), camel (Camelops sp.), and bison (Bison sp.). These patterns are similar to modern migrations of ungulates through multiple floristic zones, for example the seasonal migration of bison in the Henry Mountains of southeastern Utah (e.g., CitationVan Vuren, 1982) and Yellowstone National Park (CitationMeagher, 1973).
Regional evidence indicates bison have been a consistent member of the Holocene mammalian community, although climate may have influenced herd numbers and the availability of bison as a human prey item.
Description and Examination of the Skull
Description
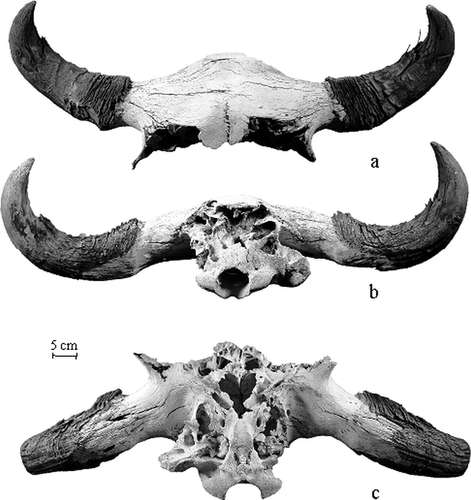
The Gilbert Peak bison skull is represented by the proximal portion of the cranium including the horn cores with preserved horn sheaths. A portion of the frontal bone is also preserved, while the occipital region is eroded with only the occipital condyles and the area around the foramen magnum present (). Colonies of the lichen Xanthoria elegens are present on the horn sheaths and in the interior of the skull. This species of lichen is common on calcium-rich rocks, old bones, and old wood in alpine regions of the Rocky Mountains (CitationKershaw et al., 1998: p. 344).
Sexing of the Gilbert Peak bison was initially based upon relative robustness of the skull and horn core morphology. Female skulls are typically smaller in size, and some features, such as horn cores and eye orbits, are less massive. Male horn cores have a distinct burr or rim at the dorsal base, while females typically do not have a distinct burr, and the horn core often blends with the neck and frontals (CitationMcDonald, 1981a: p. 44). The Gilbert Peak skull has distinct burrs at the base of the horn cores, suggesting it is a bull.
A less subjective means of assessing gender is the metric comparison of the Gilbert Peak skull with other skulls of known gender. All available skull measurements follow those defined by CitationMcDonald (1981a: pp. 43–47) as modified from CitationSkinner and Kaisen (1947) and are presented in . Utilizing CitationMcDonald's (1981b) data for other western United States bison, the dorso-ventral diameter of the horn core was plotted against spread of the horn cores. The plot indicates that the Gilbert Peak bison skull is a large male (; ).
Table 1 Skull measurements following CitationMcDonald (1981a). All measurements are presented in millimeters except for those marked in degrees. Data on Bison antiquus occidentalis are from CitationMcDonald (1981a:Table 25), Bison bison athabascae from CitationMcDonald (1981a: Table 34), Bison bison bison from CitationMcDonald (1981a: Table 29), and high altitude bison from CitationMcDonald (1981b: 550–553) and Citation CitationWilson (1974: Table III). All specimens used in analysis are males.
Figure 3 Plot of Gilbert Peak bison skull in relation to male bison skulls from western United States specimens. Data presented in . WY = Wyoming, MT = Montana, CO = Colorado, and OR = Oregon.
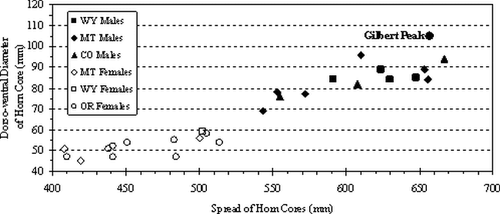
Table 2 Craniometric data for high altitude and western bison used in comparative study. Measurement abbreviations are presented in . All measurements are presented in millimeters except for those marked in degrees. AMNH = American Museum of Natural History, CoMNH = Colorado Museum of Natural History, UCy = University of Calgary, UCM = University of Colorado Museum, UM = University of Montana, USGS = U.S. Geological Survey, USNM = University of Nebraska State Museum, UWy = University of Wyoming, BH = Bighorn Mountains, Wyoming, specimen, FC = Fawn Creek, Idaho, specimen.
Assessing age at death of archaeological bison is typically conducted through wear patterns of mandible teeth (e.g., CitationFrison and Reher, 1970). Unfortunately, the teeth are not preserved in this specimen. CitationMcHugh (1958) and CitationFuller (1959) suggested that the size and shape of the horn can be used to determine age. While this can be used as a relative means for assessing age in populations, it is not of particular value for individuals. Counting the number of growth rings on the horn sheath was also suggested by CitationMcHugh (1958) as a means of estimating age. However, CitationReynolds et al. (1982) disregarded the counting of rings as a reliable estimate of age.
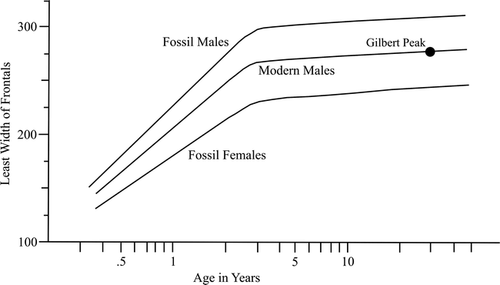
Suture closure is another means of assessing relative age (CitationMcDonald, 1981a). For the Gilbert Peak skull, the sagittal frontal suture is almost completely fused (), suggesting an adult. CitationWilson (1975: Figs. 7 and 8) presented a preliminary study of age based upon allometric growth. Plotting two skull measurements against known ages, he illustrated logarithmic curves for estimating age. CitationWalker (1992) applied this technique in his study of an isolated bison skull from the Seminoe Reservoir, Carbon County, Wyoming. For the Gilbert Peak bison skull, only one skull measurement was available for study: least width of frontals. Superimposing the Gilbert Peak measurement onto CitationWalker's graph (1992: Fig. 7 lower), an estimated age at death for the Gilbert Peak bison is 12 years ().
Figure 4 Semi-log plot of least width of frontals to individual age in late Pleistocene and modern bison skulls (adapted from CitationWalker, 1992: Fig. 7).
Preparation
The bison skull was recovered from the surface on the southwest slope of Gilbert Peak. The skull had been known for several years in the Ashely National Forest prior to it being recovered. A cursory survey of the immediate area did not produce any post-cranial elements. While the context of the substrate would normally not preserve bone, and transport by humans is possible and has been noted ethnohistorically (CitationReeves and Peacock, 2001), I am assuming the bison died close to its find spot. No formal stabilization of the specimen was conducted, except for the removal of adhering sediments with a soft brush.
Radiocarbon Dating Results
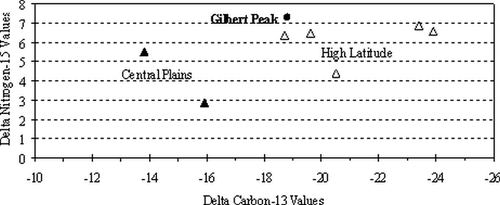
An 11.79 g sample from the occipital bone of the skull was removed and submitted to Beta Analytic (Miami, Florida) for radiocarbon assay using the accelerator mass spectrometry (AMS) technique. The radiocarbon dating procedure proceeded normally, and the sample contained adequate carbon for dating. The 13C/12C ratio was measured at −18.8‰, which is within the expected range for ungulates. This measurement indicates that sufficient collagen was present for an accurate radiocarbon age. The 15N/14N ratio was also measured for this specimen, which produced a value of 7.3‰ (CitationHood, 2004a). Published δ15N values for bison range from 2.9‰ to 6.9‰ (CitationCannon, 1997; ; ).
Table 3 Results of stable isotope analysis of modern North American bison specimens. Alaska data from CitationBocherens et al. (1994: Table 4); Konza, Kansas; Niobrara, Nebraska; Wood Buffalo, Northwest Territories; and Yellowstone, Wyoming, data from CitationTieszen et al. (1996); Wind Cave, South Dakota, data from CitationTieszen (1994: Table 5); and Fawn Creek, Idaho, data from CitationCannon (1997: Table 6). δ13C values presented for the pre-1800 specimens, Gilbert Peak and Fawn Creek are those measured and uncorrected. The values in parentheses are adjusted by 1.5‰ as suggested by CitationTieszen et al. (1996) when comparing Holocene samples prior to a.d. 1800 with modern samples.
Figure 5 Plot of δ15N against δ13C values for modern North American bison specimens from high altitude, high latitude, and central Great Plains. Data from CitationBocherens et al. (1994), CitationTieszen (1994), CitationTieszen et al. (1996), and CitationCannon (1997).
The radiocarbon age obtained is 150 ± 40 yr BP (Beta-192144). At the 2δ level (95%), the calibrated results range from cal. a.d. 1660 to 1950 with intercepts at cal a.d. 1680, 1740, 1810, 1930, and 1950 (CitationTalma and Vogel, 1993; CitationStuiver et al., 1998; ). Statistically, this bison likely died during the early to mid-18th century.
Table 4 Results of calibration using CALIB 4.4.
Bison Taxonomy
Since the 19th century, there has been controversy surrounding the species of bison occupying the Rocky Mountains. The earliest accounts are rife with descriptions of the exploits of the “Woodland or Mountain Bison” (e.g., CitationChristman, 1971). Various historical accounts (e.g., CitationNorris, 1880) of the “mountain” bison indicate they “were more hardy, fleet, and wary, and had darker finer, curlier hair” than the plains bison (CitationMeagher, 1973: pp. 14–15).
In fact, considering the geographic range of bison in North America, some authors have suggested there may have been several distinct geographic forms. However, with the near extinction of the bison in North America, a comprehensive study of its geographic variation has been precluded (Citationvan Zyll de Jong, 1986: p. 1). In the latter part of the 19th century, biologists recognized a distinct form of bison in northern Canada, formally described as the subspecies B. b. athabascae by CitationRhoads (1897), based on a single specimen he did not directly observe (Citationvan Zyll de Jong, 1986: p. 1). While most biologists agreed with Rhoads's designation of B. b. athabascae being at least subspecifically distinct (e.g., CitationSkinner and Kaisen, 1947; CitationMcDonald, 1981a), some felt that the differences in the two subspecies, B. b. athabascae and B. b. bison, were of little consequence (Citationvan Zyll de Jong, 1986: p. 1).
The decimation of the bison herds prior to firsthand study and the small number of specimens available for study contributed to the diversity of opinions (Citationvan Zyll de Jong, 1986). In one of the first quantitative studies of museum specimens—primarily crania—CitationSkinner and Kaisen (1947) argued for an overlap in distribution of the two subspecies—B. b. athabascae and B. b. bison—along the eastern slopes of the Rocky Mountains. However, their argument was unconvincing due to the lack of cranial and postcranial specimens for comparison.
In response to Skinner and Kaisen, CitationMcDonald (1981a) presented metric data from a limited sample that shows evidence that the B. b. athabascae range was limited to the northern Rocky Mountains and the boreal forests of Canada. He suggested a phylogenesis of modern North American bison from an indigenous Nearctic line (B. b. antiquus), with B. b. athabascae evolving directly from the ancestral B. b. antiquus, or a more recent adaptive differentiation from B. b. bison, as suggested by the larger body size of B. b. athabascae. However, Citationvan Zyll de Jong (1986) suggested that body size is just one of a number of presumably genetic characteristics that differentiates the two modern species. According to his analyses, B. b. athabascae is more probably “a direct and little differentiated descendant of [Beringian] B. b. occidentalis” (Citationvan Zyll de Jong, 1986: p. 54). His analysis found that B. b. bison shows a marked difference in horn core measurements, reflecting a general reduction in horn core size in comparison to B. b. occidentalis, whereas with B. b. athabascae there is only a reduction in horn core length (Citationvan Zyll de Jong, 1986: p. 18; ).
Figure 6 Ratio diagram comparing 10 skull dimensions of the Gilbert Peak specimen in comparison with Bison bison bison, Bison bison athabascae, and Bison antiquus occidentalis, with the latter serving as a standard. All measurements included in analysis are from male skulls. (B. antiquus occidentalis [N = 31] data from Citationvan Zyll de Jong, 1986: Table 1; B. b. athabascae and B. b. bison from CitationMcDonald, 1981a: Tables 29 and 34, respectively). McDonald's data used in analysis is only from males. Only those measurements that were available from the Gilbert Peak bison skull were used in this analysis. See for measurement abbreviations.
![Figure 6 Ratio diagram comparing 10 skull dimensions of the Gilbert Peak specimen in comparison with Bison bison bison, Bison bison athabascae, and Bison antiquus occidentalis, with the latter serving as a standard. All measurements included in analysis are from male skulls. (B. antiquus occidentalis [N = 31] data from Citationvan Zyll de Jong, 1986: Table 1; B. b. athabascae and B. b. bison from CitationMcDonald, 1981a: Tables 29 and 34, respectively). McDonald's data used in analysis is only from males. Only those measurements that were available from the Gilbert Peak bison skull were used in this analysis. See Table 1 for measurement abbreviations.](/cms/asset/e28a4d49-4c95-46b6-9774-64ef1935c1b0/uaar_a_11957201_f0006.gif)
The interaction of ecological and behavioral factors, gene flow, and natural selection can account for the maintenance of the distinctiveness of the two modern species according to Citationvan Zyll de Jong (1986: pp. 54–55). Specifically, the boreal forest ecotone acted as a natural barrier to contact with B. b. bison in the grasslands to the south minimizing interbreeding and potentially promoting “differential directional selection” of a specific allele frequency or phenotype that provided them with a greater degree of fitness for surviving in their respective environments.
In a genetic study, CitationWilson and Stroebeck (1999) surveyed 11 microsatellite loci of 11 bison populations in order to calculate the genetic variation and genetic distances of wood and plains bison; their expectation was that large genetic distance should exist between wood and plains bison. CitationStroebeck's (1992) results from an earlier study led him to conclude that the genetic isolation of bison populations produced the morphological variability apparent in these populations.
One result of their study was that all the sampled bison populations are genetically distinct from one another. According to the authors, this was not unexpected and was probably a result of the founder effect and genetic drift which resulted “from the small number of transfers between herds that have occurred, [which] are probably responsible for the uniqueness of these populations” (CitationWilson and Stroebeck, 1999: p. 493). Of particular interest to this study is that the genetic distance between the Yellowstone bison is not as large as expected if these bison were a distinct population (i.e., mountain bison). The authors indicated indigenous Yellowstone bison were “driven to the area by hunters” (CitationWilson and Stroebeck, 1999: p. 493) and, therefore, represent mountain-dwelling plains bison (emphasis mine).
Understanding the taxonomy of the Gilbert peak bison may not be adequately addressed without genetic analysis, but morphological differences in adult skulls has been commonly used to determine how individual animals, or populations, compare. In differentiating between B. b. athabascae and B. b. bison, CitationMcDonald (1981a) used horn core morphology as key characteristics both in size and shape.
Using a ratio diagram (CitationSimpson, 1941) we can explore how the Gilbert Peak bison compares to other populations. The ratio diagram was applied by Citationvan Zyll de Jong (1986) in trying to establish an evolutionary history between modern bison species and the extinct Bison antiquus occidentalis. The ratio diagram is a univariate technique in which measurements can be compared to a standard. To replicate Citationvan Zyll de Jong's study (1986: Fig. 16), as well as that of CitationWalker (1992: Fig. 6), B. antiquus occidentalis is used as the standard. The technique is a plot of the difference between the log of a measurement from a comparative specimen or the mean of a population (e.g., high-altitude bison) and the log of the measurement of a conspecific specimen. The comparative specimen is set to zero with dimensions with negative values indicating the characteristic is smaller with a positive value indicating a larger attribute (CitationLyman, 2004).
The results of this comparison are interesting and suggest the Gilbert Peak bison is larger in some dimensions and smaller in others than the standard (). Specifically, a comparison of select horn core measurements indicates that the Gilbert Peak bison skull is within the range of each of these groups ().
Figure 7 Comparison of select horn core measurements of Gilbert Peak bison skull with other taxonomic and regional groups to illustrate amount of overlap in range of measurements. See for measurement abbreviations.
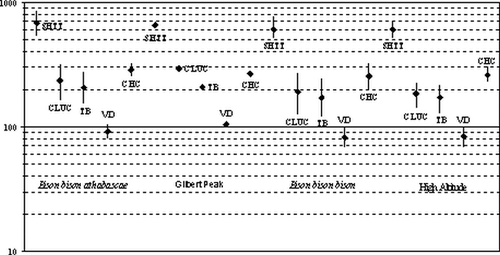
The Gilbert Peak skull horn core morphology is more similar to B. bison athabascae, being larger than either the high-altitude or the B. bison bison populations. The large size of the Gilbert Peak skull and its incompleteness may influence the results of the comparison. Another possibility is that genetic isolation of populations may be expressed in the phenotype (see CitationWilson and Stroebeck, 1999). CitationWilson (1974b) illustrated a similar situation from the 10,000-year-old Casper site in which phenotypic characteristics of two populations may have contributed to the single herd. However, he suggested gene flow between the two populations. Genetic isolation of intermountain populations in the West may be expressed phenotypically, as discussed by Citationvan Zyll de Jong (1986). More interestingly, the Gilbert Peak bison is larger than all of the sample populations, including the standard (B. antiquus occidentalis) in several horn core dimensions (). A more definitive understanding of the genetic relatedness of intermountain populations will have to wait for genetic analysis, such as mtDNA (e.g., CitationShapiro et al., 2004).
Results of Stable Isotope Analyses
One aspect of this research is addressing the ecology and seasonal movement of mammals that no longer occupy their original range. This can provide us with information that may be useful in reestablishment of habitats and other conservation biology issues (e.g., CitationLyman and Cannon, 2004), but may also contribute to understanding past behavior of bison as a prey species for past human groups (e.g., CitationWidga, 2004).
One way of approaching this predicament is by applying analyses of diet to the study of foraging patterns. If bison were moving through various ecosystems during seasonal migrations, and if these environments had different food resources, we should expect this to be evident in the bison's diet (CitationChisholm et al., 1986: p. 193; ). Stable isotope analysis has been applied to modern ungulates (e.g., CitationTieszen et al., 1979; CitationVogel et al., 1978) as well as to fossil vertebrates (e.g., CitationBocherens et al., 1994; CitationHeaton, 1995) in order to understand their dietary selection.
Stable Carbon Isotope Analysis
The application of carbon isotope analysis to ecological studies became apparent with the publication of an article by CitationBender (1968), which described a systematic relationship between the photosynthetic pathways (C3 and C4) and the stable isotopic ratios of carbon in grasses (CitationTieszen, 1994). The dietary application of carbon isotope studies involves the quantification of ratios of 13C/12C isotopic abundance in bone collagen, which is linked through the food web to the primary producers—photosynthetic plants (CitationBocherens et al., 1994). In terrestrial environments, two main categories of plants are recognized based on their carbon-fixation pathways, which are clearly distinguished by their stable carbon isotope ratios. The C3 plants represent about 90% of all plants and include all trees and herbaceous plants from cold and temperate climates. Their δ13C values range between −23‰ and −32‰ with an average of about −26‰.
Warm weather and tropical herbaceous plants, such as blue grama (Bouteloua gracilis), big bluestem (Andropogon gerardi), and buffalo grass (Buchloe dactylides), are classified as C4 and have a δ13C value between −9 and −16‰ with an average around −13‰ (CitationSmith and Brown, 1973; CitationBocherens et al., 1994). Patterns of C4 diversity in North America indicate a strong positive relationship with growing season temperature. On the Great Plains, both relative and absolute C4 grass abundances correlate with mean annual temperature (MAT) and mean annual precipitation (MAP). In contrast, C3 grass abundance decreases with MAT and summer precipitation. Since C3 plants do most of their growing in the spring and early summer, ideal conditions for productivity are cool temperatures with adequate winter precipitation. Warm summers accompanied by summer precipitation favor C4 grasses. With this understanding of the δ13C values, the amount of C3 and C4 plants consumed by herbivores can be quantified and applied to various biogeographic questions.
A third group of plants uses the CAM (crassulacian acid metabolism) photosynthetic pathway and includes succulents, such as cactus. These plants are probably not relevant to bison or other herbivores as forage and are not included in this discussion.
The presence and distribution of C3 and C4 plants in the environment is not random but related to environmental factors, specifically temperature. C3 and C4 plant abundance can be predicted from surface energy and moisture balance characteristics of the soil. With increasing latitude and altitude, a corresponding increase in C3 species is expected (CitationBoutton et al., 1980; CitationTieszen, 1994).
On the Great Plains, an increase in C3 grasses is correlated with increasing latitude. In south and southwest Texas, C4 grasses are represented at 68 and 82%, respectively, decreasing to 35% in South Dakota. Browse species, such as the sedges, do not show as clear a temperature dependent distribution as grasses. Carex, a common genus of sedge in the mountains, is C3 (CitationTieszen, 1994).
It is therefore expected that generalist consumers of grass biomass should have a modern isotopic signal that reflects the mixture of C3 and C4 species in the utilized environment. However, since climatic changes have been demonstrated for various periods during the course of the Holocene, vegetation values should be expected to reflect these climatic shifts (e.g., CitationThompson et al., 1993). This temporal variable is another complicating factor involved in the interpretation of isotopic signals from paleosamples (CitationTieszen, 1994). A number of recent studies have demonstrated the value of herbivore isotopic signatures for discerning changes in vegetation communities (e.g., CitationConnin et al., 1998) within herd variability (e.g., CitationGadbury et al., 2000) and behavior (e.g., CitationLarson et al., 2001).
Stable Nitrogen Isotope Analysis
Another stable isotope that is linked to trophic level and potentially important for investigating diet and herbivore migration is δ15N. Nitrogen (N2) enters the soil through the atmosphere, by precipitation, or from parent rock decomposition. Once in the system, nitrogen is taken up by plants and moves up through the food chain, where it progressively becomes enriched by 2–5‰ through each trophic level (CitationAmbrose, 1991; CitationBocherens et al., 1994). Nitrogen isotopes have been most widely applied to discerning the contribution of marine and terrestrial foods in human diets (e.g., CitationAmbrose, 1991).
The potential of dietary stress can also be assessed by examining δ15N values. In examining horn sheath annuli of bison from the Central Plains, CitationTieszen (1994) identified a change in δ13C values accompanied by changes in δ15N that he interpreted as a large degree of stress undergone by these individuals with shifts in diet. The researcher did not identify the specific cause of the dietary stress but suggested it may have been related to illness or water stress (i.e., drought).
CitationAmbrose and DeNiro (1986) noted a strong correlation between annual rainfall and herbivore δ15N ratios. They suggested enrichment may be caused by physiological adaptations to water stress and low-protein diets in arid habitats. For example, when drought-tolerant mammals are water stressed they will concentrate 15N in their tissues and eliminate 14N in urea, producing greater isotopic variability and more tolerant values than obligate drinkers. In arid or saline environments (<400 mm yr−1 of precipitation), these herbivores are more depleted than browsers living in the same environment (CitationAmbrose and DeNiro, 1986; CitationHeaton et al., 1986).
CitationHughes (2003), in her analysis of Holocene bighorn sheep (Ovis canadensis) from Mummy Cave in northwestern Wyoming, argued that changes in δ15N values were related to migration of sheep between summer and winter range. She argued that changes in these values reflect shifts in migratory behavior resulting from climate change. Bison from the high altitudes moving into lower elevation grasslands of the surrounding basins should illustrate similar patterns: low δ15N values for high-elevation grasslands and high δ15N values for low-elevation grasslands.
Results
The horn is a solid, bony core which is part of the animal's skull and is covered by a sheath of hard fibrous horn. The shape that the horn sheath takes is formed by the inner bony core, both of which continue to grow and are correlated with age (CitationMiura, 1985).
Horn sheath samples were removed from the dorsal portion of the right horn using a Dremel variable-speed rotary tool. A 10- × 4-cm section was removed from the sheath and placed in distilled water for approximately 8 h in order to rehydrate the sheath to allow for the separation of the individual cones. Although the growth rings or cones do not necessarily represent an annual record (CitationReynolds et al., 1982), they do provide a periodic record of the individual bison that can provide us with information on its ecology, diet, health, and possible migration.
Ten samples of the horn sheath, each representing a single horn cone, were submitted to Beta Analytic for stable carbon and nitrogen isotopic analysis. The samples were numbered consecutively from the oldest (HS-1) to most recent (HS-10). The pretreatment protocol used by Beta Analytic involves collagen extraction with alkali. Each sample supplied sufficient carbon and nitrogen for accurate measurements, and the analyses proceeded normally ().
Table 5 Results of stable isotopic analyses of horn sheath samples (CitationHood, 2004b). Adj δ13C values illustrate enrichment of 3.1‰ between keratin and diet (CitationCerling and Harris, 1999). Percent C4 vegetation is following CitationBrooks (1995).
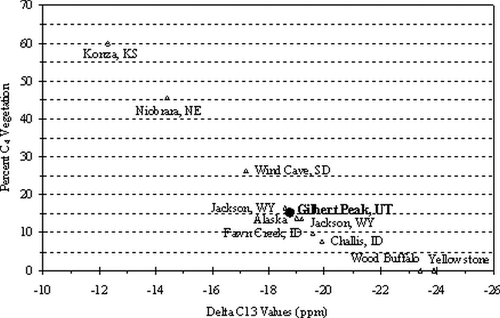
The analyses indicate that the horn sheaths provide a record of stable carbon and nitrogen isotope signatures of assimilated foods as was demonstrated in an earlier study by CitationTieszen (1994). The values, adjusted by 3.1‰ for enrichment between keratin and diet (CitationCerling and Harris, 1999), indicate variability in δ13C values that range between −17.2‰ and −19.0‰, with a mean of −22.51‰, which indicates a diet that is oriented toward C3 vegetation (). This is not unexpected and represents a diet that is similar to modern bison in the Henry Mountains (CitationVan Vuren, 1984). However, the large percentage of C4 vegetation in the diet is unusual for a bison living in high-altitude environments. The C4 vegetation may result from seasonal migrations to low elevations, but may also represent a climatic signal of drought conditions during the 18th century.
Figure 8 Percent C4 vegetation is individual bison diet based upon δ13C values as computed by CitationBrooks (1995: p. 77). Samples are arranged from south (left) to north (right). KS = Kansas, NE = Nebraska, SD = South Dakota, WY = Wyoming, ID = Idaho, and UT = Utah.
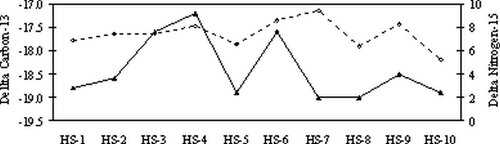
The pattern of changes in δ15N values of the Gilbert Peak bison tend to follow that of δ13C values (). In general, as δ15N values increase, the δ13C values become less negative. The Gilbert Peak values may reflect a similar migratory pattern of this bison using various habitats over the course of its life.
Figure 9 Results of stable carbon and nitrogen analyses preformed on horn sheath cones of the Gilbert Peak bison. Results are oriented from oldest (HS-1) on the left to youngest (HS-10) on the right. Upper dotted line is δ15N values. δ13C values (solid line) have been adjusted for keratin enrichment of 3.1‰.
Another possible explanation involves nitrogen waste through urine. CitationSteele and Daniel (1978) have found that in herbivorous mammals, urea is the main form of nitrogen waste, and the 15N isotopic composition in urine is lower than the 15N isotopic composition of the vegetation they consume. With the elimination of urea collagen, δ15N values are elevated. As CitationFizet et al. (1995) related, protein quality also has an effect of lower δ15N values in herbivores due to less urea in the urine. CitationAmbrose and DeNiro (1986) demonstrated that modern East African herbivorous mammals living in arid areas have increasingly higher δ15N values. These higher levels are related to the excretion of urine highly concentrated in urea, a strategy for water conservation.
Based upon this information, CitationFizet et al. (1995) argued that higher δ15N values of mammal collagen from the French Pleistocene Marillac Cave layer #7 was related to an episode of aridity between 45,000 and 40,000 yr BP. Other paleoclimatic proxy data support this interpretation. The elevated levels of δ15N values for the Gilbert Peak bison may reflect periods of increased aridity at periods during its life and may provide evidence of short-term climate shifts.
The period from approximately a.d. 1450 to 1800 has been labeled the Little Ice Age (CitationFagan, 2000) and was characterized by reduced temperatures and increased effective moisture, as indicated by a final high stand of the Great Salt Lake (CitationCurrey, 1990). An increase in sagebrush pollen and higher water levels at Ruby Marsh during the last 500 years indicates “the coolest and/or moistest conditions since the early Holocene,” presumably a Little Ice Age signal (CitationThompson, 1992). Glacial advances have also been suggested for this period in the Wind River Range (CitationRichmond, 1986), the Colorado Front Range (CitationBenedict, 1973), and the Teton Range (CitationRichmond, 1986). Mesic indicator small mammal species reappear in deposits from Lamar Cave in Yellowstone National Park (CitationHadly, 1996), and bison are reported as being at their highest Holocene levels (CitationButler, 1978; CitationFrison and Reher, 1980). Using historic and modern photographic evidence from the northern Uinta Mountains, CitationMunroe (2003) provided evidence of growing season depression prior to a.d. 1870. While uniform cooler temperatures and increased effective moisture have traditionally been suggested for the Little Ice Age, recent research is suggesting more variability (e.g., CitationJacoby and D'Arrigo, 1989; CitationKaplan et al., 2002).
Local tree-ring records from the Uinta Basin Watershed indicate “the most intense dry regime in the proxy record [a.d. 1226–2000] occurred during the late 18th century from 1772 through 1786.” While this was not an exceptionally long drought, it did encompass four of the driest years (1773, 1774, 1780, and 1786) in the entire record (CitationGray et al., 2004: p. 953). Earlier in the 18th century, decade-long droughts are also present. These periods of drought overlap with the radiocarbon date obtained from the Gilbert Peak bison and may provide an explanation for elevated δ15N values and the small C4 vegetation component derived from the δ13C value obtained from the skull (−18.8‰ or 15.17%). Another possibility is that the bison lived a majority of its life at lower alpine elevations, and the skull was transported to the high country by humans.
Conclusions
In 2003 a partial bison skull was recovered by Ashley National Forest archaeologists from an elevation of 3840 m (12,600 ft) AMSL in the Uinta Mountains. The skull consists of a portion of the frontal, occipital region, and horn cores including horn sheaths. While high-altitude bison remains have been discussed in the scientific literature periodically, they have not gone beyond the descriptive. The radiocarbon age of the specimen is 150 ± 40 yr BP (cal a.d. 1725–1778). Metric analysis of the skull indicates it was an older adult male, which compares well with Bison bison athabascae (wood bison) in size and is larger than either Bison bison bison (plains bison) specimens or other high-altitude bison. However, it is probable this individual represents a member of the species Bison bison bison, but phenotypic characteristics (e.g., large horn size) may be the result of gene flow. More definitive taxonomic placement of the Gilbert Peak bison may not be resolved without genetic analysis. Stable carbon and nitrogen isotopic signatures were obtained from 10 individual cones from the preserved horn sheath. The results suggest a diet oriented toward cool season C3 vegetation but with a significant contribution from warm season C4 vegetation. The more positive δ13C values and higher δ15N values may be a climate signal of warmer or more arid conditions, a record preserved in the local Uinta Basin tree-ring record. As this study has demonstrated, and previously articulated by CitationWalker (1992), isolated bison skulls can provide significant information that can be used to fill gaps in our understanding of bison paleoecology.
Acknowledgments
The results of this study were originally facilitated through an interagency agreement with the U.S. National Park Service's Midwest Archaeological Center (MWAC) and the U.S. Forest Service's Ashley National Forest, and is available online at http://www.cr.nps.gov/mwac/Publications/misc_pdf/cannon.htm. To begin, I express my gratitude to Byron Loosle, Heritage Program Leader for the Ashley National Forest (ANF), for supporting this research endeavor. I also thank Clay Johnson (ANF) and Brian Storm (ANF) for their support. Also, thanks to Ralph Hartley of the MWAC for allowing me the opportunity to do the work.
A number of individuals have assisted me over the years in understanding the application of stable isotope analysis to mammal ecology. These individuals include Sheri Fritz, Susan Hughes, Alan Osborn, and Larry Tieszen. I also thank Mary Anne Davis, Kristen Jensen, Joel Janetski, and Mary Sullivan for providing information on bison site locales. Danny Walker, Jerry McDonald, Michael Wilson, and C. R. van Zyll de Jong also graciously provided me with information on bison and access to their databases.
Production of this manuscript is attributed to a number of individuals: Molly Boeka Cannon (MWAC) produced , and Alan Smith photographed the skull. I also acknowledge John Andresen, Dawn Bringelson, and Molly Boeka Cannon (MWAC), and the two reviewers, Suzann Henrikson and Mathew Hill, for their editorial comments in helping to make this a more concise document. Even though a number of people worked on this project, the interpretations presented here are mine and any errors in the data reporting or interpretation are my burden.
References Cited
- Ambrose, S. H. 1991. Effects of diet, climate, and physiology on nitrogen isotope abundances in terrestrial foodwebs. Journal of Archaeological Science 18:293–317.
- Ambrose, S. H. and M. J. DeNiro . 1986. The isotopic ecology of East African mammals. Oecologia 69:395–406.
- Bamforth, D. 1988. Ecology and Human Organization on the Great Plains New York Plenum Press.
- Beidleman, J. W. 1955. An altitudinal record for the bison in northern Colorado. Journal of Mammalogy 36:470–471.
- Bender, M. M. 1968. Mass spectrometric studies of carbon 13 variations in corn and other grasses. Radiocarbon 10:468–472.
- Benedict, J. B. 1973. Chronology of cirque glaciation, Colorado Front Range. Quaternary Research 3:4 585–599.
- Bocherens, H. , M. Fizet , and A. Mariotti . 1994. Diet, physiology, and ecology of fossil mammals as inferred from stable and nitrogen isotope biogeochemistry: implications for Pleistocene bears. Palaeogeography, Palaeoclimatology, Palaeoecology 107:213–225.
- Boutton, T. W. , A. T. Harrison , and B. N. Smith . 1980. Distribution of biomass of species differing in photosynthetic pathway along and altitudinal transect in southeastern Wyoming grassland. Oecologia 45:287–298.
- Brooks, M. R. 1995. Paleoclimate of the Late Pleistocene and Holocene: Stable Carbon Isotope Evidence of a Moist-Hot Altithermal Period from Bone Collagen of Prehistoric Bison M.A. Thesis. Laramie Department of Anthropology, University of Wyoming.
- Butler, R. B. 1978. A Guide to Understanding Idaho Archaeology: the Upper Snake River and Salmon River Country Third edition. Boise Idaho State Historic Preservation Office.
- Cannon, K. P. 1997. The Analysis of a Late Holocene Bison Skull from Fawn Creek, Lemhi County, Idaho, and Its Implications for Understanding the History and Ecology of Bison in the Intermountain West Lincoln, Nebraska National Park Service, Midwest Archaeological Center.
- Cannon, K. P. 2001. What can the past provide: contribution of prehistoric bison studies to modern management. Great Plains Research 11:145–174.
- Cannon, K. P. 2004. The Analysis of a Late Holocene Bison Skull from the Ashley National Forest, Utah Lincoln, Nebraska National Park Service, Midwest Archaeological Center.
- Cerling, T. E. and J. M. Harris . 1999. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia 120:347–363.
- Chisholm, B. S. , J. Driver , S. Dube , and H. P. Schwarc . 1986. Assessment of prehistoric bison foraging and movement via stable-carbon isotopic analysis. Plains Anthropologist 31:193–205.
- Christman, G. M. 1971. The Mountain Bison. American West 8:44–47.
- Connin, S. L. , J. Betancourt , and J. Quade . 1998. Late Pleistocene C4 plant dominance and summer rainfall in the southwestern United States from isotopic study of herbivore teeth. Quaternary Research 50:179–193.
- Cook, H. J. 1930. Occurrence of mammoth and giant bison in glacial moraines in the high mountains of Colorado. Science 72:1855 68.
- Currey, D. R. 1990. Quaternary paleolakes in the evolution of semidesert basins, with special emphasis on Lake Bonneville and the Great Basin, U.S.A. Palaeogeography, Palaeoclimatology, Palaeoecology 76:189–214.
- Daubenmire, R. 1985. The western limits of the range of the American bison. Ecology 66:622–624.
- Eckerle, W. E. and J. Hobey . 1995. Environmental background. In Francis, J. E. , editor. ed. Root Procurement in the Upper Green River Basin, Wyoming: Data Recovery at 48SU1002 Laramie Office of the Wyoming State Archaeologist, 4–6.
- Edwards, T. C. , C. G. Homer , S. D. Bassett , A. Falconer , R. D. Ramsey , and D. W. Wright . 1995. Utah Gap Analysis: an Environmental Information System Utah Cooperative Fish and Wildlife Research Unit, Technical Report 95-1. Logan Utah State University.
- Fagan, B. 2000. The Little Ice Age: How Climate Made History 1300–1850 New York Basic Books.
- Fizet, M. , A. Mariotti , H. Bocherens , B. Lange-Badré , B. Vandermeersch , J. P. Borel , and G. Bellon . 1995. Effect of diet, physiology, and climate on carbon and nitrogen stable isotopes of collagen in a Late Pleistocene anthropic palaeoecosystem: Marillac, Charente, France. Journal of Archaeological Science 22:67–79.
- Frison, G. C. and C. A. Reher . 1970. Appendix I: age determination of buffalo by teeth eruption and wear. In Frison, G. C. , editor. ed. The Glenrock Buffalo Jump, 48CO304: Late Prehistoric Period Buffalo Procurement and Butchering on the Northwestern Plains Plains Anthropologist, Memoir 7. 46–47.
- Frison, G. C. and C. A. Reher . 1980. The Vore Site, 48CK302, a Stratified Buffalo Jump in the Wyoming Black Hills Plains Anthropologist, Memoir 16.
- Fryxell, F. M. 1926. A new high altitude record for American bison. Journal of Mammalogy 7:102–109.
- Fryxell, F. M. 1928. The former range of the bison in the Rocky Mountains. Journal of Mammalogy 1928:129–139.
- Fuller, W. A. 1959. The horns and teeth as indicators of age in bison. Journal of Wildlife Management 23:342–344.
- Gadbury, C. , L. Todd , A. H. Jaren , and R. Admundson . 2000. Spatial and temporal variations in the isotopic composition from the Early Holocene Hudson-Meng Bone Bed, Nebraska. Palaeogeography, Palaeoclimatology, Palaeoecology 157:79–93.
- Gillette, D. D. and D. B. Madsen . 1993. The Columbian Mammoth, Mammuthus columbi , from the Wasatch Mountains of central Utah. Journal of Paleontology 67:4 669–680.
- Gray, S. T. , S. T. Jackson , and J. L. Betancourt . 2004. Tree-ring based reconstructions of interannual to decadal scale precipitation variability for northeastern Utah since 1226 a.d.. Journal of the American Water Resources Association 49:4 947–960.
- Hadly, E. A. 1996. Influence of Late-Holocene climate on northern Rocky Mountain mammals. Quaternary Research 46:298–310.
- Heaton, T. H. E. 1995. Interpretation of 13C values from vertebrate remains of the Alexander Archipelago, S.E. Alaska. Current Research in the Pleistocene 12:95–97.
- Heaton, T. H. E. , J. C. Vogel , G. von la Chevallerie , and G. Collett . 1986. Climatic influence on the isotopic composition of bone nitrogen. Nature 322:822–823.
- Henrikson, L. S. 2003. Bison freezers and hunter-gatherer mobility: archaeological analysis of cold lava tube caves on Idaho's Snake River Plain. Plains Anthropologist 48:187 263–285.
- Henrikson, L. S. 2004. Frozen bison and fur trapper's journals: building a prey choice model for Idaho's Snake River Plain. Journal of Archaeological Science 31:903–916.
- Hood, D. 2004a. Radiocarbon dating result for sample MWAC1039/1. Letter report dated 4 June 2004. Miami, Florida Beta Analytic, Inc.
- Hood, D. 2004b. Isotopic analysis results for samples HS-1…HS-10. Letter report dated 8 October 2004. Miami, Florida Beta Analytic, Inc.
- Hughes, S. S. 2003. Beyond the Altithermal: the Role of Climate Change in the Prehistoric Adaptations of Northwestern Wyoming Ph.D. dissertation. Seattle Department of Anthropology, University of Washington.
- Jacoby, G. C. and R. D. D'Arrigo . 1989. Reconstructed Northern Hemisphere annual temperature since 1671 based on high-latitude tree-ring data from North America. Climate Change 14:39–49.
- Kaplan, M. R. , A. P. Wolfe , and G. H. Miller . 2002. Holocene environmental variability in southern Greenland inferred from lake sediments. Quaternary Research 58:149–159.
- Kershaw, L. , A. MacKinnon , and J. Pojar . 1998. Plants of the Rocky Mountains Renton, Washington Lone Pine Publishing.
- Kornfeld, M. and G. C. Frison . 2000. Paleoindian occupation of the high country: the case of Middle Park, Colorado. Plains Anthropologist 45:172 129–154.
- Larson, R. M. , L. C. Todd , E. F. Kelly , and J. M. Welker . 2001. Carbon isotopic analysis of bison dentition. Great Plains Research 11:1Proceedings of the Fifth Circumpolar Ecosystems Conference and Workshop, Churchill, Manitoba, Canada, February 2004. Special section: pp. 1-24 25–64.
- Lee, C. M. , J. B. Benedict , and J. B. Lee . 2006. Ice patches and remnant glaciers: paleontological discoveries and archaeological possibilities in the Colorado high country. Southwestern Lore 72:1Proceedings of the Fifth Circumpolar Ecosystems Conference and Workshop, Churchill, Manitoba, Canada, February 2004. Special section: pp. 1-24 26–43.
- Lubinski, P. M. 2000. Of bison and lesser mammals: prehistoric hunting patterns in the Wyoming Basin. In Madsen, D. B. and M. D. Metcalf , editors. eds. Intermountain Archaeology Salt Lake City University of Utah Press Anthropological Papers no. 122. 176–188.
- Lupo, K. D. and D. N. Schmitt . 1997. On Late Holocene variability in bison populations in the northeastern Great Basin. Journal of California and Great Basin Anthropology 19:1Proceedings of the Fifth Circumpolar Ecosystems Conference and Workshop, Churchill, Manitoba, Canada, February 2004. Special section: pp. 1-24 50–69.
- Lyman, R. L. 1996. Applied zooarchaeology: the relevance of faunal analysis to wildlife management. World Archaeology 28:110–125.
- Lyman, R. L. 2004. Late-Quaternary diminution and abundance of prehistoric bison ( Bison sp.) in eastern Washington State, USA. Quaternary Research 62:76–85.g.
- Lyman, R. L. and K. P. Cannon , editors. 2004. Zooarcheology and Conservation Biology Salt Lake City University of Utah Press.
- Lyman, R. L. and S. D. Livingstone . 1983. Late Quaternary mammalian zoogeography of eastern Washington. Quaternary Research 20:360–373.
- Mack, R. N. and J. N. Thompson . 1982. Evolution in steppe with few large, hooved mammals. American Naturalist 119:757–773.
- Madsen, D. B. 2000. A high-elevation Allerød–Younger Dryas megafauna from the west-central Rock Mountains. In Madsen, D. B. and M. D. Metcalf , editors. eds. Intermountain Archaeology Salt Lake City University of Utah Press Anthropological Papers, no. 122. 100–113.
- McDonald, J. N. 1981a. North American Bison: Their Classification and Evolution Berkeley University of California Press.
- McDonald, J. N. 1981b. Bison biometric data. Available from author.
- McHugh, T. 1958. Social behavior of the American buffalo ( Bison bison bison ). Zoologica 43:1–40.
- Meagher, M. 1973. The Bison of Yellowstone National Park Washington, D.C National Park Service, Scientific Monograph 1.
- Miura, S. 1985. Horn and cementum annulation as age criteria in Japanese serow. Journal of Wildlife Management 49:1Proceedings of the Fifth Circumpolar Ecosystems Conference and Workshop, Churchill, Manitoba, Canada, February 2004. Special section: pp. 1-24 152–156.
- Munroe, J. S. 2003. Estimates of Little Ice Age climate inferred through historical rephotography, northern Uinta Mountains, U.S.A. Arctic, Antarctic, and Alpine Research 35:4 489–498.
- Norris, P. W. 1880. Annual Report of the Superintendent of Yellowstone National Park to the Secretary of Interior Washington, D.C U.S. Government Printing Office.
- Pitblado, B. L. 2003. Late Paleoindian Occupation of the Southern Rocky Mountains: Early Holocene Projectile Points and Land Use in the High Country Boulder University Press of Colorado.
- Plew, M. G. and T. Sundell . 2000. The archeological occurrence of bison on the Snake River Plain. North American Archaeologist 21:2 119–137.
- Reeves, B. and S. Peacock . 2001. “Our Mountains are Our Pillows” an Ethnographic Overview of Glacier National Park Glacier, Montana Glacier National Park.
- Reynolds, H. W. , R. D. Glaholt , and A. W. L. Hawley . 1982. Bison: Bison bison . In Chapman, J. A. and G. A. Feldhamer , editors. eds. Wild Mammals of North America: Biology, Management, and Economics Baltimore Johns Hopkins University Press. 972–1007.
- Rhoads, S. N. 1897. Notes on living and extinct species of North American Bovidae. Proceedings of the Academy of Natural Sciences in Philadelphia 49:483–502.
- Richmond, G. M. 1986. Stratigraphy and correlation of glacial deposits of the Rocky Mountains, the Colorado Plateau, and the ranges of the Great Basin. In Sibrava, V. , D. Q. Bowen , and G. M. Richmond , editors. eds. Quaternary Glaciations in the Northern Hemisphere Quaternary Science Reviews No. 5. New York Pergamon Press. 129–144.
- Shapiro, B. , A. J. Drummond , A. Rambaut , M. C. Wilson , P. E. Matheus , A. V. Sher , O. G. Pybus , M. T. P. Gilbert , I. Barnes , J. Binladen , E. Willerslev , A. J. Hansen , G. F. Baryshnikov , J. A. Burns , S. Davydov , J. C. Driver , D. G. Froese , C. R. Harington , G. Keddie , P. Kosintsev , M. L. Kunz , L. D. Martin , R. O. Stephenson , J. Storer , R. Tedford , S. Zimov , and A. Cooper . 2004. Rise and fall of the Beringian Steppe bison. Science 306:1561–1565.
- Shell, H. R. 2002. Introduction: finding the soul in the skin. In Hornaday, W. T. , editor. ed. The Extermination of the American Bison. Revised edition Washington D.C Smithsonian Institution Press. viii–xxiii.
- Simpson, G. G. 1941. Large Pleistocene felines of North America. American Museum Novitates 1136:1–27.
- Skinner, M. F. and O. C. Kaisen . 1947. The fossil Bison of Alaska and preliminary revision of the genus. Bulletin of the American Museum of Natural History 89:127–256.
- Smith, B. N. and W. V. Brown . 1973. The Kranz Syndrome in the Gramineae as indicated by carbon isotopic ratios. American Journal of Botany 60:6 505–513.
- Steele, K. W. and R. M. Daniel . 1978. Fractionation of nitrogen isotopes by animals: a further complication to the use of variations in the natural abundance of 15N for tracer studies. Journal of Agricultural Science 90:7–9.
- Stiger, M. 2001. Hunter-gatherer archaeology of the Colorado high country Boulder University Press of Colorado.
- Stroebeck, C. 1992. Molecular Genetic Research and DNA Repository; Final Report 1992 Edmonton, Canada Manuscript in possession of Stroebeck, Department of Biology, University of Alberta.
- Stuiver, M. , et al 1998. INTCAL98 radiocarbon age calibration. Radiocarbon 40:3 1041–1083.
- Talma, A. S. and J. C. Vogel . 1993. A simplified approach to calibrating C14 dates. Radiocarbon 35:2 317–322.
- Thompson, R. S. 1992. Late Quaternary environments in Ruby Valley, Nevada. Quaternary Research 37:1–15.
- Thompson, R. S. , C. Whitlock , P. J. Bartlein , S. P. Harrison , and W. G. Spaulding . 1993. Climatic changes in the western United States since 18,000 yr B.P. In Wright Jr, H. E. , J. E. Kutzbach , T. Webb III , W. F. Ruddiman , F. A. Street-Perrott , and P. J. Bartlein , editors. eds. Global Climates Since the Last Glacial Maximum Minneapolis University of Minnesota Press. 468–513.
- Tieszen, L. L. 1994. Stable isotopes on the plains: vegetation analyses and diet determinations. In Owsley, D. W. and R. L. Jantz , editors. eds. Skeletal Biology in the Great Plains Washington D.C Smithsonian Institution Press. 261–282.
- Tieszen, L. L. , M. M. Senyimba , S. K. Imbamba , and J. H. Troughton . 1979. The distribution of C3 and C4 grasses and carbon isotope discrimination along an altitudinal and moisture gradient in Kenya. Oecologia 37:337–350.
- Tieszen, L. , J. V. Kool , and C. McMurty . 1996. Interpretation of seasonal diet patterns in bison from stable isotopic analysis of horn sheaths Unpublished manuscript. Sioux Falls, South Dakota Department of Biology, Augustana College.
- Van Vuren, D. 1982. Comparative ecology of bison and cattle in the Henry Mountains, Utah. In Peek, J. M. and P. D. Dalke , editors. eds. Wildlife-Livestock Relationships Symposium: Proceedings 10 Moscow, Idaho University of Idaho, Forest, Wildlife, and Range Experiment Station. 449–457.
- Van Vuren, D. 1984. Summer diets of bison and cattle in southern Utah. Journal of Range Management 37:3 260–261.
- Van Vuren, D. 1987. Bison west of the Rocky Mountains: an alternative explanation. Northwest Science 61:2 65–69.
- van Zyll de Jong, C. G. 1986. A systematic study of recent bison, with consideration of the wood bison ( Bison bison athabascae Rhoads 1898). Publications in Natural Sciences no. 6. Ottawa, Canada National Museum of Natural Sciences.
- Vogel, J. C. , A. Fuls , and R. P. Ellis . 1978. The geographic distribution of kranz grasses in southern Africa. South African Journal of Science 75:209–215.
- Walker, D. N. 1992. A Bison bison bison skull from 48CR4897, Seminoe Reservoir, Carbon County, Wyoming. The Wyoming Archaeologist 36:3–4 71–84.
- Warren, E. R. 1927. Altitude limit of bison. Journal of Mammalogy 8:60–61.
- Widga, C. 2004. Early archaic subsistence in the Central Plains: the Spring Creek (25FT31) fauna. Plains Anthropologist 49:25–58.
- Wilson, G. A. and C. Stroebeck . 1999. Genetic variation within and relatedness among wood and plains bison populations. Genome 42:483–496.
- Wilson, M. 1974a. History of the bison in Wyoming, with particular reference to Early Holocene forms. In Wilson, M. , editor. ed. Applied Geology and Archaeology: the Holocene History of Wyoming Reports of Investigations 10. Laramie Wyoming Geological Survey. 91–99.
- Wilson, M. 1974b. The Casper Local Fauna and its fossil bison. In Frison, G. C. , editor. ed. The Casper Site: a Hell Gap Bison Kill on the High Plains New York Academic Press. 125–172.
- Wilson, M. 1975. Holocene Fossil Bison from Wyoming and Adjacent Areas M.A. thesis. Laramie Department of Anthropology, University of Wyoming.