Abstract
Pollen limitation (PL) is thought to be an important factor driving the evolution of floral traits in alpine plants. However, results of pollen supplementation experiments in alpine plants do not always show high levels of PL, and a general review of the importance of PL on alpine-plant reproduction is needed. We assessed to what extent alpine plants are pollen limited, and whether the intensity of PL differs between alpine and lowland species. Moreover, we assessed whether or not PL of alpine species depends on their reproductive system and, finally, whether the outcome of PL studies depends on whether supplemental-pollination was done at the whole-plant level or within a subset of available flowers. We performed both classical and phylogenetic meta-analysis. Our results show that alpine plants are pollen limited. However, PL did not differ significantly between alpine and lowland species. In the alpine, self-incompatible and self-compatible species show similar levels of PL. We did not find differences in PL between different manipulation levels. These results will help understand the real importance of PL of seed production in the alpine. We identify gaps in our knowledge of PL in the alpine that could serve to guide future directions of research in this field.
Introduction
The attraction of animal pollinators carrying an adequate quantity and quality of pollen is an essential part in the reproductive cycle of most angiosperm species. When plant reproductive success is reduced because of a shortage in the supply of pollen, it suffers from pollen limitation (PL), a widespread phenomenon among angiosperms (CitationBurd, 1994; CitationLarson and Barrett, 2000; CitationAshman et al., 2004). Several recent reviews have studied different aspects of PL. Among-species variation in PL depends on species' reproductive systems (PL self-incompatible species > PL self-compatible species), their habitats (PL in forested habitats > PL in open habitats), and distribution (PL in tropical areas > PL in temperate areas) (CitationLarson and Barrett, 2000). Added to this, the extent of PL in a community is positively correlated with species richness (CitationVamosi et al., 2006), whereas the magnitude of PL in plant populations depends on both historical constraints and ecological factors (CitationKnight et al., 2005). More recently, the theoretical models explaining the relationship between PL and abiotic resource availability have been improved, and some aspects of the ecological and evolutionary causes and consequences of PL have been clarified (CitationAshman et al., 2004). Moreover, reallocation processes have been revealed to be important in the results of PL experiments (CitationKnight et al., 2006). Finally, the standard pollen-supplementation technique used to estimate PL has been recently criticized because it may not accurately estimate PL, confounding the effects of pollen quantity and quality limitation on reproduction (CitationAizen and Harder, 2007).
Intuitively, PL should be particularly strong in plant populations occurring in habitats where the availability of pollinators is low. One such habitat may be the arctic and alpine tundra, and indeed several earlier reviews of the ecology of arctic and alpine plants emphasize that their reproduction depends on pollination by insects (CitationKevan, 1972, Citation1973), thus it is strongly challenged by a shortage of them (CitationMüller, 1881; CitationMani, 1962; CitationBillings and Mooney, 1968; CitationBliss, 1971; CitationKevan and Baker, 1983; CitationBillings, 1987). As stated by CitationKörner (1999) in his book on alpine plant ecology, “If one browses the more general alpine literature, one gets the impression that abundance and activity of pollinators decreases with elevation, so that alpine plants have a problem.” Basically, the supposed strong PL in alpine and arctic species is attributed to severe weather conditions, such as low temperature and strong winds that restrict the flight activity of individual flower visitors, resulting in decreased flower visitation rates with altitude (CitationArroyo et al., 1985; CitationHeinrich, 1993; CitationMcCall and Primack, 1992; CitationTotland, 1994). The supposed strong PL in alpine and arctic species is believed to have wide-reaching evolutionary consequences. Low availability of pollinators has caused the evolution of relatively large flowers with particularly bright coloration (CitationBillings and Mooney, 1968; CitationBliss, 1971) that supposedly enhance the ability of plants to attract the few pollinators available. Moreover, there is a higher frequency of plant species that use several different groups of pollinators (i.e. that are pollination generalists) in the alpine/arctic compared to elsewhere (CitationTotland, 1993; CitationLarson et al., 2001), and it could drive the evolution of pollinator-generalization in alpine species (CitationTotland, 1993). With regard to sexual reproduction, very important in the alpine in terms of species numbers (CitationKörner, 1999), and present even in the High Arctic flora (CitationKevan, 1972), the high level of inbreeding in alpine/arctic taxa has evolved because strong PL causes selection to favor selfing as a reproductive assurance mechanism (CitationCrawford, 1989). The same causal pathway has been proposed to explain the high level of asexual reproduction in alpine species, including clonal reproduction and apomixis (CitationMüller, 1881; CitationRichards, 1997). Finally, with regard to pollination strategy, the high proportion of wind-pollinated or ambophilous (species using a mixture of wind- and animal-pollination) species in alpine/arctic habitats could be due to low pollinator service that selected for the evolution of wind-pollination traits from animal-pollinated ancestors (CitationCulley et al., 2002). Thus, the belief that pollinators are in limited supply in tundra habitats has had a prominent position in explaining the evolution of several floral traits that are believed to be typical of alpine and tundra plant species.
Nevertheless, during the last decade it has been proposed that the link between the frequency or quality of pollinator visitation and the resulting reproductive output may not be so obvious. Other factors than pollen availability, such as severe weather, low nutrient availability, and short growing season, also impact on the reproductive success of alpine plants and are suggested to constrain seed production to a level that prevents plants from taking advantage of a high deposition of pollen on their stigmas (CitationHaig and Westoby, 1988; CitationZimmerman and Pyke, 1988; CitationAshman et al., 2004). The “Haig and Westoby equilibrium” (CitationAshman et al.; 2004) between the resources allocated to pollinator attraction and seed provisioning predicts that there would not be an augmentation of seed set as a response to higher pollen loads. However, PL is common among flowering plants (CitationBurd, 1994; CitationAshman et al., 2004; CitationKnight et al., 2006), suggesting that departures from that equilibrium are common in nature. CitationBurd (1995) explained it as a bet hedging strategy where flowers should be oversupplied with ovules relative to the average pollen load received, as an adaptation to stochasticity in pollen receipt. The alpine pollination environment is, as introduced above, highly unpredictable, so that plant populations in the alpine would depart from the Haig and Westoby equilibrium, and higher levels of PL could be expected in the alpine compared to lowland plant populations.
Added to this, to truly reveal PL in plants, it is necessary to supplement pollination of the majority of flowers on experimental individuals (hereafter plant-level experiments) (CitationZimmerman and Pyke, 1988). If the experiment is made only on a subset of the flowers (hereafter partial-level experiments), reallocation of resources from naturally pollinated to supplemented flowers on the same plant may lead to an overestimation of PL at the whole plant level (CitationKnight et al., 2006), which is the relevant unit for evolutionary responses to pollen limitation. This issue may be particularly important in alpine species because their seed production may be strongly constrained by abiotic conditions that also could hamper resource reallocation processes.
The proposed causal pathway from severe weather conditions that result in a low pollinator visitation frequency, coupled by a low quality of visits (due to relatively inefficient pollinators), further causing a strong PL is apparently so obvious and convincing that few, if any, have critically opposed this idea. In this paper we use both classical and phylogenetically corrected meta-analysis to synthesize quantitatively experimental studies on PL in alpine plant species. In particular we ask: (1) Are alpine plants in general pollen limited? (2) Is the intensity of PL different in alpine compared to lowland species? (3) Does PL of alpine species depend on their reproductive system (self-compatible vs. self-incompatible)? (4) Does the outcome of PL studies depend on the manipulation level of the supplemental pollination experiment (plant-level vs. partial-level)?
Methods
Scope of The Meta-Analysis and Data Base Building
We conducted a search of papers on the effect of cross-pollen addition on the reproductive output of arctic and alpine plants by means of hand-pollination experiments by using the ISI Web of Science (1945–2005). We used “poll* limitation,” “supp* poll*,” “hand poll*,” “breeding system,” and “reproductive success” in combination with the terms “alpine” or “arctic” as search terms. We also used the lists of references within the papers on PL in alpine/arctic plants obtained through the ISI search and the paper collection of the authors to identify as many studies as possible that quantify PL in alpine/arctic species. We also included unpublished results if they fulfilled the requirements listed below.
We only included in the meta-analysis the articles fulfilling the following a priori requirements: (1) Hand-pollination experiments were accomplished using outcross pollen, and both control and treatment plants were open pollinated. We included one paper where the treated plants were bagged because the author explicitly noted that the treatment provided an excess of outcross pollen (CitationShykoff, 1988). We did not consider studies including emasculations of control or treatment plants. (2) The study was conducted under natural conditions. We did not include garden or glasshouse experiments because under such experimental conditions the pollinator assemblages would be modified. (3) The study included an estimate of female reproductive success to measure the plant response to the pollen-addition treatment. Among the different estimates, we included relative (seed-set, fruit-set, and seed/flowers ratio), and absolute (seeds per plant, seeds per flower, seeds per fruit) variables. When a single study reported different estimates of reproductive success for the same independent experiment we only included one of them in the analysis. We selected the variable most related to the success of seed production (mainly seed-set and analogous variables), in order to avoid bias attributable to the response variable used (CitationKnight et al., 2006). (4) In studies where only indexes of reproductive success or PL (compounded from some of the above-mentioned variables) were reported as response variables of the hand-pollination experiments, we asked the authors for the original data used to calculate the indexes (CitationKasagi and Kudo, 2003).
As independent data points for the meta-analysis, we recorded data from experiments carried out at separate populations or at different years in the same population, as has been done in other meta-analyses on PL (CitationAshman et al., 2004; CitationKnight et al., 2005) (see [available free of charge at MetaPress: http://instaar.metapress.com/content/120707 or at BioOne: http://www.bioone.org/perlserv/?request=get-archive&issn=1523-0430]).
From each study we recorded the means, the standard deviations (SD) and the sample sizes from both the pollen-addition treatment and the control. When only standard errors were available, we transformed them into SD. When the data were only available in figures, we used the ImageJ free software (http://rsb.info.nih.gov/ij/) to obtain the mean and SD directly from PDF files or after digitalizing the papers. When necessary, we asked the authors to clarify uncertainties or to provide missing data.
Among the experiments on PL obtained from the bibliographic search described above, we differentiated those in which authors noted that hand-pollinations had been done in more than or equal to 80% of the flowers in individual plants (plant-level) from those where only a small subset of flowers were manipulated (partial-level). With this data set, we tested the hypothesis that PL at the plant-level is lower than PL at the partial-level, presumably reflecting a resource limitation of reproduction via reallocation processes (CitationKnight et al., 2006). Moreover, we obtained information on the breeding system (self-compatible vs. self-incompatible) of the species included in the meta-analysis according to the information given in the papers, to test the hypothesis that PL depended on the ability of plants to produce seeds by self-pollen and also potentially in the absence of pollinators. Finally, we tested the hypothesis that PL should be higher in alpine compared to lowland species. To do this, we compared CitationAshman et al.'s (2004) data set (lowland sample) to our data set from the alpine (alpine sample). To enable this comparison, we modified CitationAshman et al.'s (2004) data set by removing the alpine species included in it and calculating Hedges' d (CitationGurevitch and Hedges, 2001) as the measure of effect sizes. In this comparison we used only our data from plant-level pollination experiments carried out in the alpine, because the CitationAshman et al. (2004) data set only included plant-level experiments.
Statistical Analysis
We used Hedges' d (CitationGurevitch and Hedges, 2001) as the measure of effect size in our meta-analysis. In this study it calculates a standardized difference between the estimates of female reproductive success of the control and the pollen supplementation treatment. It has been used in a number of meta-analyses (e.g. CitationAshman et al., 2004; CitationMaestre et al., 2005) and its properties are well known.
We used two different statistical approaches to test our hypotheses. First, we analyzed the data using classical meta-analysis, treating each data record in the data set as an independent sample. This approach does not account for the common ancestry of the species included in the analysis, resulting in a pseudoreplication bias if there is a phylogenetic signal in the data set (CitationGurevitch et al., 2001; CitationGarland et al., 2005). To account for this, as a second approach, we also used a phylogenetically controlled meta-analysis (CitationVerdú and Traveset, 2004, Citation2005).
We conducted the meta-analyses with MetaWin version 2.1.4 (CitationRosenberg et al., 2000). The Q statistic (CitationHedges and Olkin, 1985) was used to test the differences in the intensity of PL between experiments at the plant-level and those at the partial-level in alpine plants, and also to test whether there were differences in the levels of PL depending on the breeding system of the species. The Q statistic was also used to test whether there were differences in the intensity of PL between alpine and lowland plants. We used a randomization procedure (5000 iterations) for all significance tests.
We used random-effects models in all the meta-analyses. Such models assume that studies differ not only by sampling error, but also by a random component in effect sizes between studies, termed “pooled study variance” (CitationRosenberg et al., 2000). These models are more suitable than fixed-effects ones when analyzing ecological data. Fixed-effect models' assumption that all the observed variation is attributable to the sampling error is difficult to meet when using data from a broad range of studies (CitationMaestre et al., 2005).
We used a resampling procedure (5000 iterations each) to generate bootstrap confidence intervals (95%) that are more conservative (wider) than parametric ones. Confidence intervals obtained by this method are recommended when the sample sizes are low and the assumptions of normality are not met (CitationAdams et al., 1997; CitationGurevitch and Hedges, 1997).
We calculated Rosenthal's fail-safe numbers to test the presence of publication bias in the data sets. These numbers represent the number of non-significant, unpublished, or missing studies that would need to be added to a meta-analysis in order to change the results from significance to non-significance (CitationRosenberg et al., 2000). If the fail-safe number is larger than five times the sample size plus 10, it is safe to conclude that the results are robust with regard to publication bias (CitationVerdú and Traveset, 2005).
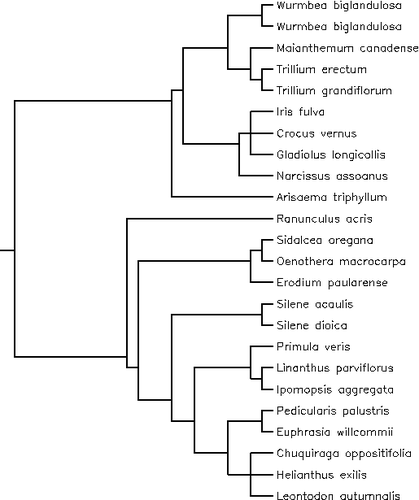
We carried out phylogenetic meta-analysis as first outlined by CitationVerdú and Traveset (2005). For this analysis the sample sizes are the number of species because the weighted average effect sizes are used. These weighted average effect sizes are single (cumulative) effect sizes per species, the weight being the inverse of the variance (CitationRosenberg et al., 2000). By means of the PDAP software (T. Garland, Jr., P. E. Midford, J. A. Jones, A. W. Dickerman, R. Díaz-Uriarte, unpublished manuscript “PDAP manual”) we examined whether a phylogenetic signal was present in the calculated effects sizes (see CitationVerdú and Traveset, 2005; and the “PDAP manual” for further explanations). We did this by using phylogenetically independent contrasts and comparing the variance of the observed weighted average effect sizes with the variance of a set of permuted data. If the variance of the real data is lower than 95% of the variances of the permuted data, a phylogenetic signal exists. After this, we used the lm.phylog function in the PHYLOGR package for R written by R. Díaz-Uriarte and T. Garland for the Comprehensive R Archive Network (http://cran.r-project.org) to implement the phylogenetic information in the meta-analysis models. Using this function we carried out weighted generalized least squares in which the phylogenetic information is included by means of the phylogenetic trees. The significance tests are obtained by contrasting the observed values (weighted average effect sizes) against simulated distributions obtained by running 1000 permutations in the PDSIMUL module of the PDAP software (CitationGarland et al., 1993). We included the inverse of the effect size variance as the vector of weights, as meta-analyses do. We used the on-line software utility Phylomatic (CitationWebb and Donoghue, 2004) to obtain the phylogenetic trees for the phylogenetic meta-analysis. The phylogenetic trees (Appendices 2 and 3), one for the alpine plants data set and one for the alpine-lowland plants data set, were constructed with CitationDavies et al. (2004) phylogeny for angiosperms. We set the branch lengths to unity.
Results
The Alpine Plants Data Set
The data set comprised 18 studies including 24 species and 71 independent data records (). It contained 7 studies (studying 5 species and including 11 independent data records) where the hand-pollinations were carried out at the plant-level, and 11 studies (studying 19 species and including 60 independent data records) where the hand-pollinations were accomplished at the partial-level. In the data set, 13 species were self-incompatible (n = 40) and 11 self-compatible (n = 31). Forty-eight percent of the cases (and 50% of the species) were significantly pollen limited (). All the studies included in this data set were carried out in alpine habitats. We only found one paper studying PL in the Arctic (CitationPhilipp et al., 1996) and fulfilling all the a priori requirements of the bibliographic search. In this paper Pedicularis lanata is pollen limited (d = 0.67, var(d) = 0.02). We did not include it in further analysis, so that our analysis and discussion are focused on alpine plants.
Figure 1 Distribution of standardized effect sizes (Hedges' d, vertical bars) and sampling variances (horizontal bars) for 70 cases where pollen supplementation was conducted in alpine plants. Overall mean effect size of PL in alpine plants (arrow) was 0.74 with a bootstrap confidence interval of 0.59–0.89. Individual effect sizes with a sampling variance that do not overlap 0 are significant (p < 0.05).
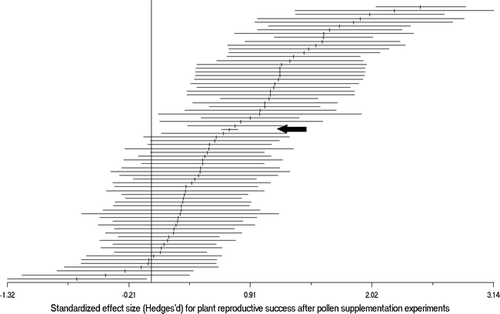
The overall mean effect size of PL in alpine plants was 0.72 throughout with a bootstrap confidence interval that did not include zero (0.59–0.89), showing that PL in alpine plants is significantly higher than zero. Overall heterogeneity tests for this data set were non-significant (see below). The observed variance in the effect sizes (0.32) was lower than the 95% of the variances of the permuted data (p = 0.009), showing the presence of a phylogenetic signal in the effect sizes.
Plant- Vs. Partial-Level Pollen Limitation
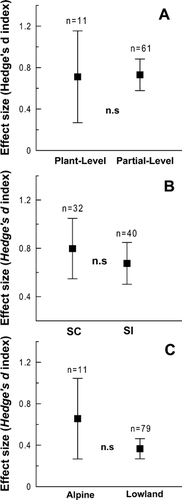
When comparing the hand-pollination manipulation levels, the total heterogeneity test was non-significant (Qtotal = 67.33, df = 69, p = 0.60), suggesting that the model explained the variance found in the data. Neither the Rosenthal's fail-safe number test (2135.5 > 360, fail-safe number > safe threshold), nor the weighted histogram () denoted the presence of a publication bias. We did not find significant differences in PL between plant- (n = 11) and partial-level pollen supplementation experiments (n = 60) in PL (Q = 0.07, df = 1, p = 0.9) (). This result is consistent when controlling for common ancestry (Coefficient = 0.11, F = −19.93, p = 0.75).
Figure 2 Weighted histograms of the effect sizes from pollen supplementation experiments in alpine plant species. (A) Plant-level (black), and partial-level experiments (white); (B) self-compatible (black), and self-incompatible alpine plant species (white); (C) alpine (black), and lowland plant species (white).
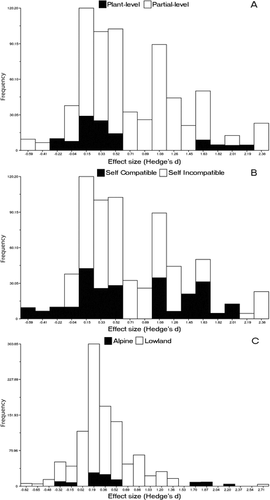
Figure 3 Mean effect sizes of PL and 95% bootstrap confidence intervals. (A) Plant-level (n = 11, 5 species) and partial-level (n = 60, 19 species); (B) SC (self-compatible) (n = 31, 11 species) and SI (self-incompatible) alpine species (n = 39, 13 species); (C) alpine (n = 11) and lowland plants (n = 79). Squares represent mean effect sizes of PL and vertical lines 95% bootstrap confidence intervals. n: the sample size, corresponding to the number of independent data records; n.s.: no significant differences between groups.
Pollen Limitation In Self-Incompatible VS. Self-Compatible Alpine Species
The overall heterogeneity test was not significant (Qtotal = 63.48, df = 69, p = 0.66) when testing the effects of breeding system on PL, suggesting that the model explained the variance found in the data. Neither the Rosenthal's fail-safe number (2106.3 > 360), nor the weighted histogram () denote a publication bias. We did not find significant differences in PL between self-incompatible (n = 40) and self-compatible species (n = 31) (Q = 0.59, df = 1, p = 0.44) (). The phylogenetically controlled meta-analysis did not show significant differences in PL between species with different breeding systems (Coefficient = 0.18, F = −19.91, p = 0.73).
Pollen Limitation in Alpine and Lowland Species
This data set was formed by data from our subset of alpine plants (plant-level pollen supplementation, 7 studies, 5 species, and 11 independent data records) and the modified CitationAshman et al. (2004) data set (23 studies, 19 species, 79 independent data records). The result was a data set with 90 independent data points from 24 species ( [available free of charge at MetaPress: http://instaar.metapress.com/content/120707 or at BioOne: http://www.bioone.org/perlserv/?request=get-archive&issn=1523-0430]).
The total heterogeneity test on this data set was non-significant (Qtotal = 108.28, df = 89, p = 0.08), so that our model explained the variability of the data. According to the Rosenthal's fail-safe number (1955.2 > 460), and the weighted histogram (), there is no indication of a publication bias. The observed variance (0.45) was not lower than 95% of the variances of the permuted data (p = 0.73), so that there was no indication of a phylogenetical signal in the effect sizes of this data set.
We did not find significant differences in PL between alpine (n = 11) and lowland (n = 79) plants (Q = 3.35, df = 1, p = 0.07) (), a result consistent with the phylogenetically “informed” meta-analysis (Coefficient = −0.87, F = 6.1, p = 0.34).
Discussion
In general, 62–73% of plant species or populations have their female reproduction constrained by pollen availability (CitationBurd, 1994; CitationAshman et al., 2004; CitationKnight et al., 2006), showing that pollen limitation is a widespread phenomenon among the angiosperms. Our results showed lower percentages of prevalence of PL among alpine plants than in the data sets mentioned above: 48.05% of the cases (and 50% of the species) were significantly pollen limited. This percentage is still appreciable. We did not find significant differences in the levels of PL between alpine and lowland plants. The validity of this result is supported by the randomization method used to obtain it, the fail-safe numbers and the histogram. However, because of the low number of species examined for plant-level PL (5 alpine species for plant-level experiments, 11 independent data records) we have to be cautious about the conclusions drawn from this limited sample. These results do not agree with the presumption that PL should be particularly strong in alpine species primarily because severe weather conditions may constrain pollinator flower visitation activity.
We did not find differences in PL between plant- and partial-level pollen-supplementation experiments in alpine plants. It has been widely accepted that female reproductive success in flowering plants is limited both by pollen and resource availability (CitationHaig and Westoby, 1988; CitationZimmerman and Pyke, 1988; CitationAshman et al., 2004). There are studies in the alpine reporting either PL (CitationGalen, 1985; CitationMiller et al., 1994) or resource limitation (CitationArft et al., 1999; CitationTotland and Eide, 1999; CitationGalen, 2000; CitationGaudeul and Till-Bottraud, 2004; CitationMuñoz et al., 2005) as the main factors limiting female reproductive success. We expected PL to be higher in partial-level pollen-addition experiments than in plant-level ones, because in the partial-level reallocation of resources from other parts of the plant could increase female reproductive success of the hand-pollinated flowers at the expense of seed production in naturally pollinated flowers (CitationZimmerman and Pyke, 1988; CitationAshman et al., 2004; CitationKnight et al., 2006). This difference between partial-level and plant-level pollen addition experiments, interpreted as driven by resource reallocation, has already been shown for an extensive data set of angiosperms (CitationKnight et al., 2006). Future experimental studies in the alpine should focus on the relationships between PL and resource limitation, to test to what extent the seed production of alpine plants is limited by abiotic conditions, plants being unable to allocate extra resources to the supplementarily pollinated flowers. Apart from our evidence of the existence of PL in the alpine, there is other evidence supporting the idea that both PL and resources limit seed production in the alpine (CitationTotland and Eide, 1999; CitationTotland, 2001), the levels of PL in the alpine being lower than expected because resource limitation might constrain the use of extra-pollen loads by experimental plants.
We did not find differences in PL between studies using self-compatible and self-incompatible alpine species. This result contrasts with that of CitationLarson and Barrett (2000) in an extensive data set across many habitats. They found lower levels of PL in self-compatible species than in self-incompatible ones. Our result suggests that self-compatibility in alpine species does not necessarily contribute to reduced PL. Some alpine species may have acquired self-compatibility before colonizing the alpine habitat, so that many of their traits (including their levels of PL) would not be a response to the alpine selective environment but rather an effect of phylogenetic inertia, as proposed by CitationArroyo et al. (2006). Furthermore, constraints from abiotic conditions could be invoked here as an additional explanation for the lack of difference in PL between self-compatible and self-incompatible species. Self-compatible species may potentially receive more compatible pollen on their stigmas than self-incompatible ones, but certain levels of resource limitation might not let them take advantage of it.
Although plant species in the alpine are visited by insects even under the adverse conditions of nights (CitationKendall et al., 1981; CitationKevan and Kendall, 1997), pollinator visitation rates to plants in alpine habitats appear to be relatively low compared to plants at lower altitudes (CitationArroyo et al., 1982, Citation1985; CitationInouye and Pyke, 1988; CitationTotland, 1993), possibly because of environmental constraints on insect activity in alpine ecosystems (CitationMcCall and Primack, 1992; CitationStenström and Bergman, 1998; CitationTotland, 1994). However, even if flowers of alpine plants are less visited by pollinators, they may benefit from a higher efficiency of the pollinators compared to those at lower altitudes, primarily through the higher abundance of efficient bumblebees (CitationArroyo et al., 1985; CitationGalen and Stanton, 1989; Bingham and Orthner, 1999; but see CitationTotland, 1993). Moreover, alpine plants may have an extended flower longevity compared to lowland plants, (CitationArroyo et al., 1985; Bingham and Orthner, 1999), which may compensate for a lower visitation frequency in terms of their reproductive success. These factors, related to the pollination environment, combined with abiotic constraints on reproduction, may serve to explain the levels of PL in alpine species.
There is clearly a need for more studies on PL in alpine plants before we can assess with certainty the general importance of pollinators for seed production in alpine species. Moreover, PL studies conducted on alpine species on the plant-level are scarce, as well as data on pollinator visitation rates. We suggest that a fruitful approach to the study of PL in the alpine would be to conduct replicated supplementary pollination experiments along altitude gradients, supplemented by measurements of pollinator visitation rates and pollen deposition along the same gradient. Those future experiments should take into account CitationAizen and Harder's (2007) suggestions to avoid under and overestimation of PL, and also distinguish between pollen quantity and quality limitation of seed production. Furthermore, there is a need to reduce the “geographical bias” in studies of PL, because as far as we know, there is a lack of studies in the Arctic and on tropical mountains. Nevertheless, despite both methodological and geographical limitation of PL studies on alpine plants, the results of these studies, as revealed by our meta-analysis, should serve to stimulate a new critical look at the evolution of pollination and reproductive traits in alpine plants.
uaar_a_11957380_sm0001.pdf
Download PDF (100.6 KB)uaar_a_11957380_sm0004.pdf
Download PDF (100.6 KB)uaar_a_11957380_sm0003.pdf
Download PDF (101.9 KB)Acknowledgments
We thank Dr. Miguel Verdú and Rubén Torices for help with the phylogenetically corrected meta-analysis, and Felix Gugerli and Gaku Kudo for providing their original data. Comments by Dr. Adrián Escudero, Dr. Mary Price, Rubén Torices, and anonymous referees improved the manuscript. This work was funded by the Research Council of Norway and the Madrid Government's program of short stays for predoctoral fellowships (F.P.I Orden 3334/04).
References Cited
- Adams, D. C. , J. Gurevitch , and M. S. Rosenberg . 1997. Resampling tests for meta-analysis of ecological data. Ecology 78:1277–1283.
- Aizen, M. A. and L. D. Harder . 2007. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88:271–281.
- Arft, A. M. , M. D. Walker , J. Gurevitch , J. M. Alatalo , M. S. Bret-Harte , M. Dale , M. Diemer , F. Gugerli , G. H R. Henry , M. H. Jones , R. D. Hollister , I. S. Jónsdóttir , K. Laine , E. Lévesque , G. M. Marion , U. Molau , P. Mølgaard , U. Nordenhäll , V. Raszhivin , C. H. Robinson , G. Starr , A. Stenström , M. Stenström , Ø Totland , P. L. Turner , L. J. Walker , P. J. Webber , J. M. Welker , and P. A. Wookey . 1999. Responses of tundra plants to experimental warming: meta-analysis of the International Tundra Experiment. Ecological Monographs 69:491–511.
- Arroyo, M. T K. , R. B. Primack , and J. J. Armesto . 1982. Community studies in population ecology in the high temperate Andes of central Chile I. Pollination mechanisms and altitudinal variation. American Journal of Botany 69:82–97.
- Arroyo, M. T K. , J. J. Armesto , and R. B. Primack . 1985. Community studies in population ecology in the high temperate Andes of central Chile II. Effect of temperature on visitation rates and pollination possibilities. Plant Systematics and Evolution 149:187–203.
- Arroyo, M. T K. , M. S. Muñoz , C. Henríquez , I. Till-Bottraud , and F. Pérez . 2006. Erratic pollination, high selfing levels and their correlates and consequences in an altitudinally widespread above-tree-line species in the high Andes of Chile. Acta Oecologica 30:248–257.
- Ashman, T-L. , T. M. Knight , J. A. Steets , P. Amarasekare , M. Burd , D. R. Campbell , M. R. Dudash , M. O. Johnson , S. J. Mazer , R. J. Mitchell , M. T. Morgan , and W. Wilson . 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421.
- Billings, W. D. 1987. Constraints to plant growth, reproduction, and establishment in arctic environments. Arctic and Alpine Research 19:357–365.
- Billings, W. D. and H. A. Mooney . 1968. The ecology of arctic and alpine plants. Biological Review 43:481–529.
- Bingham, R. A. and A. R. Orthner . 1998. Efficient pollination of alpine plants. Nature 391:238–239.
- Bliss, L. C. 1971. Arctic and alpine plant life cycles. Annual Review of Ecology and Systematics 2:405–438.
- Burd, M. 1994. Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review 60:83–139.
- Burd, M. 1995. Ovule packaging in stochastic pollination and fertilization environments. Evolution 49:100–109.
- Crawford, R. M M. 1989. Studies in Plant Survival: Ecological Case Histories of Plant Adaptation to Adversity. Oxford Blackwell Scientific Publications. 296.
- Culley, T. M. , S. G. Weller , and A. N. Sakai . 2002. The evolution of wind pollination in angiosperms. Trends in Ecology and Evolution 17:361–369.
- Davies, T. J. , T. G. Barraclough , M. W. Chase , P. S. Soltis , D. E. Soltis , and V. Savolainen . 2004. Darwin's abominable mystery: insights from a supertree of the angiosperms. Proceedings of the National Academy of Sciences of the USA 101:1904–1909.
- Galen, C. 1985. Regulation of seed-set in Polemonium viscosum : floral scents, pollination, and resources. Ecology 66:792–797.
- Galen, C. 2000. High and dry: drought stress, sex-allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae). American Naturalist 156:72–83.
- Galen, C. and M. L. Stanton . 1989. Bumble bee pollination and floral morphology: factors influencing pollen dispersal in the alpine sky pilot, Polemonium viscosum (Polemoniaceae). American Journal of Botany 76:419–426.
- Garland Jr, T. , A. W. Dickerman , C. M. Janis , and J. A. Jones . 1993. Phylogenetic analysis of covariance by computer simulation. Systematic Biology 42:265–292.
- Garland Jr, T. , A. F. Bennet , and E. L. Rezende . 2005. Phylogenetic approaches in comparative physiology. Journal of Experimental Biology 208:3015–3035.
- Gaudeul, M. and I. Till-Bottraud . 2004. Reproductive ecology of the endangered alpine species Eryngium alpinum L. (Apiaceae): phenology, gene dispersal and reproductive success. Annals of Botany 93:711–721.
- Gómez, J. M. 2002. Self-pollination in Euphrasia willcommii Freyn (Scrophulariaceae), an endemic species from the alpine of the Sierra Nevada (Spain). Plant Systematics and Evolution 232:63–71.
- Gurevitch, J. and L. V. Hedges . 1997. Statistical issues in ecological meta-analyses. Ecology 80:1142–1149.
- Gurevitch, J. and L. V. Hedges . 2001. In Sheiner, S. M. and J. Gurevitch . Meta-analysis:combining the results of independent experiments. Design and Analysis of Ecological Experiments. New York Oxford University Press. 347–369.
- Gurevitch, J. , P. S. Curtis , and M. H. Jones . 2001. Meta-analysis in ecology. Advances in Ecological Research 32:200–247.
- Haig, D. and M. Westoby . 1988. On limits of seed production. The American Naturalist 131:757–759.
- Hedges, L. V. and I. Olkin . 1985. Statistical Methods for Meta-Analysis. New York Academic Press. 369.
- Heinrich, B. 1993. The Hot-Blooded Insects: Strategies and Mechanisms of Thermoregulation. Berlin Springer. 602.
- Inouye, D. W. and G. H. Pyke . 1988. Pollination biology in the Snowy Mountains of Australia: comparisons with montane Colorado, USA. Australian Journal of Ecology 13:191–210.
- Kasagi, T. and G. Kudo . 2003. Variations in bumble bee preference and pollen limitation among neighbouring populations: comparisons between Phyllodoce caerulea and Phyllodoce aleutica (Ericaceae) along snowmelt gradients. American Journal of Botany 90:1321–1327.
- Kendall, D. M. , P. G. Kevan , and J. D. Lafontaine . 1981. Nocturnal flight activity of moths (Lepidoptera) in alpine tundra. Canadian Entomologist 113:607–614.
- Kevan, P. G. 1972. Insect pollination of High Arctic flowers. The Journal of Ecology 60:831–847.
- Kevan, P. G. 1973. Flowers, insects, and pollination ecology in the Canadian High Arctic. Polar Record 16:667–674.
- Kevan, P. G. and H. G. Baker . 1983. Insects as flower visitors and pollinators. Annual Review of Entomology 28:407–453.
- Kevan, P. G. and D. M. Kendall . 1997. Liquid assets for fat bankers: summer nectarivory by migratory moths in the Rocky Mountains, Colorado, U.S.A. Arctic and Alpine Research 29:478–482.
- Knight, T. M. , J. A. Steets , J. C. Vamosi , S. J. Mazer , M. Burd , D. R. Campbell , M. R. Dudash , M. O. Johnson , R. J. Mitchell , and T-L. Ashman . 2005. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology and Systematics 36:467–497.
- Knight, T. M. , J. A. Steets , and T-L. Ashman . 2006. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. American Journal of Botany 93:271–277.
- Körner, C. 1999. Alpine Plant Life. 2nd ed . Berlin Springer-Verlag. 338.
- Larson, B. M H. and S. C H. Barrett . 2000. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society 69:503–520.
- Larson, B. M H. , P. G. Kevan , and D. W. Inouye . 2001. Flies and flowers: taxonomic diversity of anthophiles and pollinators. Canadian Entomologist 133:439–465.
- Maestre, F. T. , F. Valladares , and J. F. Reynolds . 2005. Is the change of plant-plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. Journal of Ecology 93:748–757.
- Mani, M. S. 1962. Introduction to High Altitude Entomology. Insect Life above the Timber-Line in the North-West Himalaya. London Methuen & Co. 302.
- McCall, C. and R. B. Primack . 1992. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. American Journal of Botany 79:434–442.
- Miller, J. , M. Litvak , S. Kelso , and A. Vargo . 1994. Comparative reproductive biology of two alpine primrose species. Arctic and Alpine Research 26:297–303.
- Müller, H. 1881. Alpenblummen, ihre Befruchtung durch Insekten und ihre Anpassungen an dieselben. Leizpig Engelmann. 611.
- Muñoz, A. A. , C. Celedon-Neghme , L. A. Cavieres , and M. T K. Arroyo . 2005. Bottom-up effects of nutrient availability on flower production, pollinator visitation, and seed output in a high-Andean shrub. Oecologia 143:126–135.
- Philipp, M. , S. R J. Woodell , J. Böcher , and O. Mattsson . 1996. Reproductive biology of four species of Pedicularis (Scrophulariaceae) in West Greenland. Arctic and Alpine Research 28:403–413.
- Richards, A. J. 1997. Plant Breeding Systems. London Chapman & Hall. 529.
- Rosenberg, M. S. , D. C. Adams , and J. Gurevitch . 2000. Meta Win: Statistical Software for Meta-Analysis with Resampling Tests. Version 2.0 . Sunderland Sinauer Associates. 128.
- Shykoff, J. A. 1988. Maintenance of gynodioecy in Silene acaulis (Caryophyllaceae): stage-specific fecundity and viability selection. American Journal of Botany 75:844–850.
- Stenström, M. and P. Bergman . 1998. Bumblebees at an alpine site in northern Sweden: temporal development, population size, and plant utilization. Ecography 21:306–316.
- Totland, Ø . 1993. Pollination in alpine Norway: flowering phenology, insect visitors, and visitation rates in two plant communities. Canadian Journal of Botany 71:1072–1079.
- Totland, Ø . 1994. Influence of climate, time of day and season, and flower density on insect flower visitation in alpine Norway. Arctic and Alpine Research 26:66–71.
- Totland, Ø . 2001. Environmental-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82:2233–2244.
- Totland, Ø and W. Eide . 1999. Environmentally-dependent pollen limitation on seed production in alpine Ranunculus acris . Ecoscience 6:173–179.
- Totland, Ø and M. Sottocornola . 2001. Pollen limitation of reproductive success in two sympatric alpine willows (Salicaceae) with contrasting pollination strategies. American Journal of Botany 88:1011–1015.
- Vamosi, J. C. , T. M. Knight , J. A. Steets , S. J. Mazer , M. Burd , and T-L. Ashman . 2006. Pollination decays in biodiversity hotspots. Proceedings of the National Academy of Sciences of the USA 103:956–951.
- Verdú, M. and A. Traveset . 2004. Bridging meta-analysis and the comparative method: a test of seed size effect on germination after frugivore's gut passage. Oecologia 138:414–418.
- Verdú, M. and A. Traveset . 2005. Early emergence enhances plant fitness: a phylogenetically controlled meta-analysis. Ecology 86:1385–1394.
- Webb, C. O. and M. J. Donoghue . 2004. Phylomatic: tree assembly for applied phylogenetics. Molecular Ecology Notes 5:181–183.
- Zimmerman, M. and G. H. Pyke . 1988. Reproduction in Polemonium : assessing the factors limiting seed set. The American Naturalist 131:723–738.
Appendix 1
List Of References Used In The Meta-Analysis But Not Cited In The Text
-
Gómez, J. M.
and
R. Zamora
. 1996. Wind pollination in high mountain populations of
Hormatophylla spinosa
(Cruciferae). American Journal of Botany 83:580–585. <!--${if: isGetFTREnabled}-->
-
Gugerli, F.
1998. Effect of elevation on sexual reproduction in alpine populations of
Saxifraga oppositifolia
(Saxifragaceae). Oecologia 114:60–66. <!--${if: isGetFTREnabled}-->
-
Kudo, G.
and
S. Suzuki
. 2002. Relationships between flowering phenology and fruit-set of dwarf shrubs in alpine fellfields in northern Japan: a comparison with a subarctic heathland in northern Sweden. Arctic, Antarctic, and Alpine Research 34:185–189. <!--${if: isGetFTREnabled}-->
-
Lundemo, S.
and
Ø Totland
. 2007. Within population spatial variation in pollinator visitation rates, pollen limitation on seed set and flower longevity in an alpine species. Acta Oecologica 32:262–268. <!--${if: isGetFTREnabled}-->
-
Muñoz, A. A.
and
M. T K. Arroyo
. 2006. Pollen limitation and spatial variation of reproductive success in the insect-pollinated shrub
Chuquiraga oppositifolia
(Asteraceae) in the Chilean Andes. Arctic, Antarctic, and Alpine Research 38:608–613. <!--${if: isGetFTREnabled}-->
-
Pickering, C. M.
1995. Breeding systems of Australian
Ranunculus
in the alpine region. Nordic Journal of Botany 17:613–620. <!--${if: isGetFTREnabled}-->
-
Spira, T. P.
and
O. D. Pollak
. 1986. Comparative reproductive biology of alpine biennial and perennial gentians (
Gentiana
: Gentianaceae) in California. American Journal of Botany 73:39–47. <!--${if: isGetFTREnabled}-->
-
Stenström, M.
and
U. Molau
. 1992. Reproductive ecology of
Saxifraga oppositifolia
: phenology, mating system, and reproductive success. Arctic and Alpine Research 24:337–343. <!--${if: isGetFTREnabled}-->
-
Totland, Ø
. 1997a. Limitations on reproduction in alpine
Ranunculus acris
. Canadian Journal of Botany 75:136–144. <!--${if: isGetFTREnabled}-->
-
Totland, Ø
. 1997b. Effects of flowering time and temperate on growth and reproduction in
Leontodon autumnalis
var.
taraxaci
, a late-flowering alpine plant. Arctic and Alpine Research 29:285–290. <!--${if: isGetFTREnabled}-->
-
Totland, Ø
and
B. Schulte-Herbrüggen
. 2003. Breeding system, insect flower visitation, and floral traits of two alpine
Cerastium
species in Norway. Arctic, Antarctic, and Alpine Research 35:242–247. <!--${if: isGetFTREnabled}-->
Appendix 2
Appendix 2 Phylogenetic tree including the species of the alpine plants data set obtained using the online software utility Phylomatic ( Citation Webb and Donoghue, 2004 ) and constructed with the Citation Davies et al. (2004) phylogeny for angiosperms. We set the branch lengths to unity.
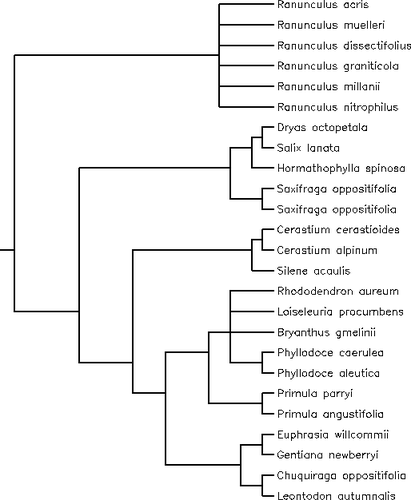
Appendix 3
Appendix 3 Phylogenetic tree including the species of the alpine-lowland plants data set obtained using the online software utility Phylomatic ( Citation Webb and Donoghue, 2004 ) and constructed with the Citation Davies et al. (2004) phylogeny for angiosperms. We set the branch lengths to unity.