Abstract
Tundra soils contain large amounts of organic carbon (C) that might become available to microbial decomposition as soils warm. To elucidate the C sources currently sustaining CO2 emissions from striped tundra soils (soil respiration) in Northwest Greenland, we studied the seasonal pattern and radiocarbon (14C) signature of soil respiration and of CO2 within the pore space, respired from roots and non-root–associated microbes, and of bulk soil organic matter.
Old C pools are present in the topsoil of both barren ridges (1000–5000 yrs) and vegetated troughs (modern to 600 yrs). Before leaf-out, soil respiration was depleted in 14C relative to atmospheric CO2, root and microbial respiration within the topsoil, demonstrating a substantial contribution of C fixed before 1950. As the growing season progressed, the contribution of older C pools decreased, but remained apparent in the soil respiration from ridges and in pore space CO2. Soil respiration from troughs became dominated by recently fixed C.
As the active layer deepens with permafrost thaw, buried C may become an increasingly larger component of soil respiration. Detecting microbial decomposition of older C pools requires continuous monitoring of soil and microbial respiration and better constraints on soil C pools.
Introduction
Soils of the arctic permafrost region contain an estimated 496 Pg organic carbon (C) within the top meter and 1024 Pg C within the top three meters (CitationTarnocai et al., 2009). Recent climate change (CitationBox, 2002; CitationHinzman et al., 2005; CitationLawrence et al., 2008b) may intensify microbial activity (CitationSchimel et al., 2006) and amplify losses of C to the atmosphere as carbon dioxide (CO2) (CitationJones et al., 2000; CitationNordstroem et al., 2001; CitationIlleris et al., 2003; CitationWelker et al., 2004) or methane (CitationMastepanov et al., 2008). Increased C losses could turn the region from a C sink to a source providing a positive climate feedback until increases in plant productivity and vegetative cover offset respiratory losses (CitationOechel et al., 1995; CitationSullivan et al., 2008a; CitationSchuur et al., 2009).
The High Arctic's (N of ~70° latitude) C biogeochemistry is poorly constrained by measurements, especially for prostrate-shrub tundras (539 × 106 km2; CitationWalker et al., 2005) where patterned ground (CitationKessler and Werner, 2003) complicates spatially accurate estimates of soil C pools and fluxes (CitationMichaelson et al., 1996). A recent study (CitationHorwath, 2007; CitationHorwath et al., 2008) concluded that high arctic soils (1.984 × 106 km2) contain 12.06 Pg C—an order of magnitude more C than previously estimated (CitationBliss and Matveyeva, 1992). Given that permafrost thawing will expose deeper-lying pools of soil C, the lack of data on C depth distributions and its exchange with the modern atmosphere constrains our ability to model accurately the pan-arctic C cycle and its climate feedback potential (CitationSitch et al., 2007).
The main pathway returning C from well-drained arctic soils to the atmosphere is decomposition of organic matter by microorganisms to CO2 (soil respiration). There are multiple sources of soil C, including above- and belowground plant litter, root exudates, and microbial byproducts that may vary in age from days to millennia (CitationHorwath et al., 2008). Quantifying the relative contribution of these multiple sources to the total respiration flux is especially important today as the deepening of the active layer with climate warming can result in older, previously inactive soil C becoming a larger component of the modern ecosystem respiration flux (CitationSchuur et al., 2009).
One technique to assess contributions of different sources of C to the overall CO2 fluxes from ecosystems involves radiocarbon (14C) measurements of soil respiration and its main sources (CitationTrumbore, 2006). This approach quantifies the 14C content of CO2 respired from plant roots and microorganisms whose C sources are plant exudates or short-lived roots (root and rhizosphere respiration) and from microorganisms decomposing other organic matter (microbial respiration). The 14C content of respired CO2 is a measure of the time interval since this C was fixed by plants from the atmosphere. The technique can be used to estimate the mean age of C pools in soils on several time scales. Radiocarbon dating measures the mean age of C in pools that are hundreds up to about 50,000 yrs old—based on radioactive decay after an organism dies. In addition, we can use ‘bomb’-C, produced by aboveground nuclear weapons testing in the 1950s and 1960s as a tracer to estimate the mean age of soil C pools that cycle on decadal to centennial time scales. Here, the 14C content of a C pool or flux is compared to the 14C content of atmospheric CO2 between 1950 and the year of sampling. The 14C content of CO2, and also that of recent photosynthetic assimilates, peaked in the northern hemisphere in 1963–1964 and is declining as this ‘bomb’ 14CO2 exchanges with C reservoirs in the oceans and biosphere, and becomes diluted by fossil-fuel derived (>50,000 yrs) CO2 (CitationLevin et al., 2008).
Our study focused on two questions: (1) How much do root and microbial respiration contribute to the overall soil respiration flux in high arctic polar semi-deserts? (2) Are soils currently losing old C that had been unavailable to microorganisms under the climatic conditions of the last few centuries?
Material and Methods
SITE DESCRIPTION
We studied two high arctic prostrate dwarf shrub tundra (or “polar semi-desert”) systems (“South Mountain” and “Polar Desert”) in Northwest Greenland near Thule Air Base (76°32′N, 68°50′W; 200–350 m a.s.l.) () as part of the U.S. National Science Foundation Biocomplexity Program (CitationWalker et al., 2008). Mean annual air temperature averages −11.6 °C, with a mean annual precipitation of 112 mm (1971–2004) (Thule Air Base unpublished; from CitationSullivan et al., 2008b). During the growing season (June, July, August) air temperature averages 3.5 °C. Annual air temperature has increased by 0.5 °C per decade from 1971 to 2000 (CitationSullivan et al., 2008b). The soils are Cryosols with an active layer depth of about 0.6 to 1.2 m (). The underlying geology is composed of Proterozoic dolostone, siltstone, and sandstone covered by glacial drift of mixed lithology (in CitationHorwath et al., 2008). Due to a gentle slope (<10%), the soil surface is dominated by non-sorted stripes (): alternating 0.5-m-wide, vegetated troughs and 1- to 3-m-wide, barren ridges. Polar stripes form from frost cracking, eolian in-filling, colonization by higher plants, and freeze/thaw dynamics. This landscape type represents ~1/3 of the entire high arctic terrestrial land cover (CitationBliss and Matveyeva, 1992).
Figure 1 Picture of non-sorted, polar stripes in NW Greenland. Stripes consist of alternating 0.5-m-wide, vegetated (mountain-avens [Dryas integrifolia Vahl] and arctic willow [Salix arctica Pall.]) troughs and 1- to 3-m-wide, barren ridges.
![Figure 1 Picture of non-sorted, polar stripes in NW Greenland. Stripes consist of alternating 0.5-m-wide, vegetated (mountain-avens [Dryas integrifolia Vahl] and arctic willow [Salix arctica Pall.]) troughs and 1- to 3-m-wide, barren ridges.](/cms/asset/119348ce-00e6-449c-bfb8-08f1dd92a6c3/uaar_a_11957482_f0001.gif)
Table 1 Overview of sampling sites (from CitationHorwath, 2007).
SOIL RESPIRATION RATES AND PORE SPACE CO2 CONCENTRATIONS
We measured soil respiration and the CO2 concentrations in the soil pore space as well as in ambient air approximately weekly throughout the growing season of 2007. Soil respiration was measured using paired, dynamic chambers (30 cm i.d., ~8 L air V) in troughs (n = 3) and ridges (n = 3) at both study sites. To restrict diffusion of ambient air into the chambers, chamber bases were inserted about 5 cm into the mineral soil in late May 2007, sealed with soil material on the outside, and left in place until the end of the growing season. Vegetation was not clipped. Respiration rates were measured by closing a chamber with an opaque lid and circulating the air in the chamber's headspace between the chamber and an infrared gas analyzer (LI-6200, LI-COR Biosciences, Lincoln, Nebraska, U.S.A.). The respiration rate was calculated from the slope of time vs. CO2 concentration curves (CitationGaudinski et al., 2000). With each chamber measurement we recorded air and soil temperature at 5 cm depth.
At South Mountain only, we measured the concentration of CO2 in the soil pore space. Stainless steel probes (0.35 cm i.d., 0.6 cm o.d.) were inserted vertically into the mineral soil to 20, 30, or 60 cm depths in ridges and to 20 cm in troughs, and all were closed off with a rubber septum (Blue septa, Grace, Deerfield, Illinois, U.S.A.). Probes were inserted as soon as the thaw of the active layer allowed and remained in the same location until the end of the growing season. Soil gas was obtained with 60 mL syringes (BD, Franklin Lakes, New Jersey, U.S.A.). After discarding the air present within the probe, 60 mL of soil air was injected into an infrared gas analyzer (LI-800, LI-COR) and the concentration monitored with a data logger (LI-1400, LI-COR).
CO2 SAMPLING FOR ISOTOPE ANALYSIS
Isotope samples were taken once a month. To sample CO2 from soil respiration, CO2 from ambient air was removed from the flux chambers by circulating the air in the chambers' headspace through a CO2 trap (soda lime, Fisher Scientific, Pittsburgh, Pennsylvania, U.S.A.) for 25 min at a flow rate of ~1 L min−1 (>3 × chamber V). Afterwards the chamber was left closed for approximately 24 hrs to accumulate sufficient CO2 for 14C analysis (~0.5–1 mg C). The CO2 was sampled by circulating the head space air through drierite (W.A. Hammond Drierite Co. Ltd., Xenia, Ohio, U.S.A.) followed by an activated molecular sieve trap for 15 min (CitationGaudinski et al., 2000). The molecular sieve (powder-free 13× 8/12 beads, Grace) was pre-conditioned prior to the construction of a trap by baking at ≤750 °C under vacuum for ≥12 hrs. Traps were activated by baking at 650 °C under vacuum for ≥45 min (see below).
We obtained air from within the soil pore space by connecting 2 L evacuated stainless steel canisters to the soil probes. To minimize the disturbance of the soil CO2 concentration gradient and the risk for sampling soil air from other than the sampling depth, the cans were filled via stainless steel capillaries (0.010 × 0.063 × 30 cm, Fisher Scientific), which restrict the flow rate, over an approximately 4 hour period (CitationGaudinski et al., 2000). CO2 from ambient air was sampled by flushing air through drierite followed by a molecular sieve trap for 15 min.
To sample CO2 respired from roots we manually extracted all roots from a block of 0–5 cm mineral soil the same day the soil was sampled. Roots were rinsed with water and placed into a 1 L mason jar. The air in the jar was circulated through soda lime for 10 min at a flow rate of ~1 L min−1 to remove CO2 from ambient air. After 12 hrs, the evolved CO2 was collected on a molecular sieve trap for 10 min (~1 L min−1).
To sample CO2 respired by microorganisms we collected soil cores (0–10 cm mineral soil) from troughs (n = 6) and ridges (n = 6) in early June 2007 using a steel tube (approximately 5 cm i.d.). Sampling the surface soil does not capture the respiration of all microorganisms in the active layer, but it should provide an estimate of the dominant fraction of microbial respiration, since microbial activity in soils declines with depth, often in parallel with the distribution of roots. To ensure structural integrity, samples were wrapped in aluminum foil and packed into plastic bags in the field. Samples were frozen and transported to the University of California (UC) Irvine on ice. The frozen cores were placed into 1 L mason jars. To conserve their structural integrity, the cores remained in the aluminum foil, but the foil was removed from the top and pierced along the core to facilitate gas and water flow. Soils were thawed at 6.4 °C for four days. Before and after thawing, jars were flushed with CO2-free air (ambient air flushed through soda lime). Two out of the six cores from each site and landscape position (ridge or trough) remained at 6.4 °C, two were moved to about −4.7 °C, and two to 15.0 °C. After two weeks, the headspace air was transferred to evacuated 0.5 L canisters. Throughout the incubation period CO2 concentrations were monitored every 1–5 days (data not shown); CO2 concentrations in the jars were kept ≤2%).
CARBON ISOTOPE ANALYSES OF CO2
Carbon dioxide was released from molecular sieve traps by baking at 650 °C for ≥45 min or extracted from canisters using a vacuum line, purified cryogenically and reduced to graphite using Zn reduction (CitationXu et al., 2007). A split of the CO2 was analyzed for its stable C isotope ratio (DeltaPlus, GasBench II, Thermo Scientific, Waltham, Massachusetts, U.S.A.). The 14C content of the graphite was measured with accelerator mass spectrometry (NEC 0.5MV 1.5SDH-2 AMS) at the KCCAMS laboratory of UC Irvine (CitationSouthon and Santos, 2007). Radiocarbon data are reported relative to NIST OX-I (SRM 4990a) and OX-II (SRM 4990c) following CitationStuiver and Polach (1977).
Atmospheric CO2 was removed from the chambers prior to sampling (see above), however, leakage of atmospheric CO2 into the chambers during the accumulation period cannot be excluded in these rocky soils. Leakage can affect the 14C content of the sampled CO2, in particular when respiration fluxes are low. We calculated the amount of ambient CO2 in each sample (fair
) based on the δ13C ratios of CO2 in the sample (δ
13
Cobs
.), in ambient air (δ
13
Cair
), and respired from either roots or microbes (δ
13
Cresp
.) ():
The calculation of fair is sensitive to the choice of δ 13 Cresp .. We estimated fair for each soil respiration sample using the δ13C of root respiration (−28‰ in June, −26‰ in July and August). In addition, we also calculated fair using the minimum and maximum of observed δ13C of microbial respiration (−19 or −29‰).
Using these three estimates for fair
, we then calculated three air-corrected 14C signatures (Δ14
Ccor
.) from each observed soil respiration 14C signature (Δ14
Cobs
.):
We report air-corrected Δ14C signatures of soil respiration as average ± SD (n = 3). The measurement uncertainty for Δ14C was <2‰.
SOIL ORGANIC MATTER AND CARBONATE ANALYSIS
After incubation, soils were dried at 60 °C and sieved to <2 mm. A subsample was ground and carbonates were removed by shaking overnight with 0.5 M HCl. Rinsed, freeze-dried samples were analyzed for C content and stable isotopic composition in duplicate (Carlo Erba, Milano, Italy; DeltaPlus, Thermo Scientific). For each site and landscape position, the samples with the highest and lowest C content were analyzed for their 14C content. Samples were combusted to CO2 in pre-combusted, evacuated quartz tubes (6 mm i.d.) with CuO and Ag for 2 hrs at 900 °C, and the CO2 was processed as described above.
For each site, we analyzed other subsamples (ridges, n = 2; troughs, n = 1) for their concentration and isotopic composition of inorganic C (carbonates). CO2 was released from carbonates by acidifying soil samples (0.1 g trough, 1 g ridge soils) with 0.8 mL phosphoric acid in pre-evacuated glass tubes and purified on a vacuum line and graphitized as described above.
Results and Discussion
SOIL CARBON POOLS
The concentration of organic C in the mineral soil (0–10 cm) was significantly higher in troughs than in ridges, averaging 2.8 ± 0.3 vs. 0.8 ± 0.2% C (mean ± SE, n = 12; t-test p < 0.01) (). Concentrations in troughs or ridges were similar at both sites and in the same range as previously reported (CitationHorwath, 2007; CitationHorwath et al., 2008).
Table 2 Concentration and isotopic properties of organic matter and carbonates in the top 10 cm of mineral soil (<2 mm). Organic matter data are averages (SD), carbonate data are point samples.
As expected, the δ13C signature of the soil organic C indicated that C3 plants were the dominant source (−25.7 ± 0.2‰; ). The 14C signature of organic C in troughs was younger than in ridges (). Radiocarbon ages in troughs or ridges were similar at both sites, although spatial variability was very high. The mean conventional 14C ages of C varied from 630 yrs to modern in troughs, and from 5040 to 1230 yrs in ridges.
The temporal and spatial dynamics of cryoturbation in striped soils are poorly understood (CitationWalker et al., 2008). Nevertheless, the distribution of organic C and its 14C ages within the surface mineral soil of these landscapes seems to reflect the current distribution of vegetative cover. The surface soil of vegetated troughs receives annual inputs of plant leaf and root litter. In the barren ridges, organic matter is either a remnant of past vegetative cover and/or has and is being transported from troughs via cryoturbation and as dissolved organic matter. The first detailed mapping of age vs. depth profiles by CitationHorwath et al. (2008) showed that at South Mountain troughs are associated with more stable, sand-rich A horizon wedges and ridges with more mobile, silty loam and carbonate-cemented C horizons. In troughs, 14C ages of bulk soil organic matter increased with depth (0–60 cm) from 65 to 2695 yrs, while ridges contained pockets of buried organic matter between 50 and 70 cm depth with 14C ages of about 30,000 yrs.
The carbonate content of the soils was low (<0.2%-mass) and not all samples yielded enough CO2 for isotope analysis (). Carbonates had 14C ages older than 17,000 yrs and a δ13C ratio of +2‰. Thus, carbonate Δ14C signatures were significantly depleted and carbonate δ13C ratios were significantly enriched relative to the isotopic signatures of soil organic C.
SOIL PORE SPACE CO2
Concentrations of CO2 in the soil pore space (measured only at South Mountain) were consistently higher in troughs than ridges for a given soil depth, averaging 1713 ± 76 vs. 752 ± 76 ppm CO2 at 20 cm depth (mean ± SE, n = 5–6, t-test p < 0.01) (). The higher CO2 concentrations in troughs are a result of higher root abundance and the higher microbial activity sustained by root exudates (CitationIlleris et al., 2003) along with the overall larger amounts of organic matter. In ridges, pore space CO2 concentrations increased with depth (892 ± 89 ppm at 30 cm [n = 8], 1298 ± 271 ppm at 60 cm [n = 4]), and exhibited a seasonal pattern with a maximum in mid-summer (1404 ppm at 30 and 1819 ppm at 60 cm), when soil temperature peaked ().
Figure 2 Seasonal pattern of CO2 in the soil pore space in ridges (at 20, 30, and 60 cm depth) and troughs (20 cm) and of CO2 in ambient air at South Mountain. (a) Concentrations, (b) δ13C ratios, and (c) Δ14C signatures. Symbols represent point measurements. Dashed line indicates day of leaf-out (18 June 2007).
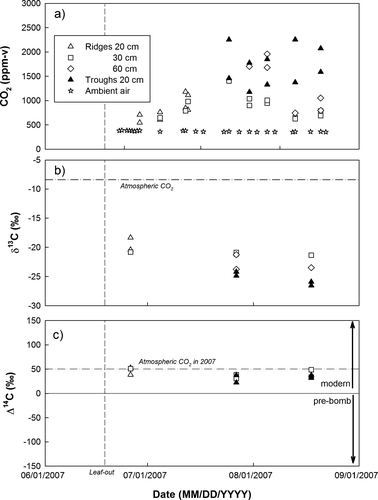
The 14C signature of pore space CO2 throughout the growing season was modern (1950 to 2007, Δ14C ≥ 0‰), but similar or depleted relative to the 14C signature of CO2 in ambient air (). This suggests that the respired CO2 was a mixture of C fixed during the bomb-period (1950 to 2007, Δ14C ≥ 0‰) and older C (pre-1950, Δ14C < 0‰). The 14C signature of pore space CO2 reached a minimum in mid-summer when CO2 concentrations and soil temperatures peaked. This decrease in 14C signature was not related to changes in the proportion of leakage of ambient CO2 into the wells: The δ13C signature of pore space CO2 at a given depth remained constant over time (). Instead, the decrease implies that the contribution of older C was largest during mid-summer. Measurements of CO2 in the soil pore space provide evidence that older C pools are being decomposed, and that decomposition rates increase as a function of soil temperature. It is unlikely that carbonates were the source of the observed 14C-depleted soil respiration flux, because carbonate concentrations were low, and a substantial contribution of carbonate-derived CO2 is not apparent in the δ13C ratios of pore space CO2 () or soil respiration ().
SOIL RESPIRATION
At both sites, soil respiration fluxes were higher from troughs than ridges (). In troughs, soil respiration showed a strong seasonal pattern, with a maximum in mid-summer when air and soil temperatures peaked (), especially at Polar Desert (). On the ridges, respiration fluxes were relatively constant over time (). Soil respiration was positively correlated with soil temperature (), except for ridges at South Mountain. The slightly lower respiration flux from the troughs of South Mountain compared to Polar Desert is likely related to lower plant abundance ().
Figure 3 Seasonal pattern of soil respiration (panels a and b) from the surface of troughs and ridges and of air and soil temperature (panels c and d) at South Mountain and Polar Desert in 2007. Dashed line indicates day of leaf-out. Panels e and f show the correlation of soil respiration to soil temperature at 5 cm depth.
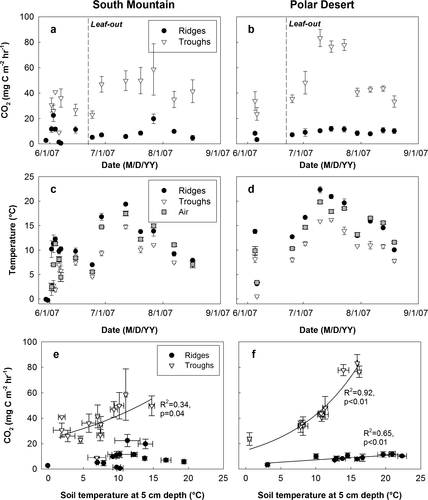
Before leaf-out (18 June 2007, day 168), soil respiration rates in troughs and ridges rapidly increased in response to thawing events ( = snow melt) and decreased during subsequent colder periods (). The most likely explanation for these repeated large, but short soil respiration events is that warming and the associated increase in soil moisture triggered the growth of microbes, while subsequent freezing and decline in soil moisture sharply reduced their activity. Microbial biomass turnover associated with freeze-thaw cycles may account for a significant proportion of annual soil respiration (CitationSchimel and Clein, 1996; CitationWallenstein et al., 2007). In contrast, it is unlikely that we observed the release of stored CO2 that had accumulated in soils during the winter. The snow pack at our site is thin (less than 25 cm; M. Rogers, personal communication, 2008) and biological activity is restricted by very cold soil temperatures (<−10 °C) and therefore low soil moisture—limiting both the production of CO2 and the formation of diffusive barriers such as ice lenses.
Soil respiration in troughs had modern 14C signatures (). Before leaf-out, soil respiration tended to be depleted in 14C relative to the 14C signatures of ambient CO2 as well as root and microbial respiration at 0–10 cm depth. As for soil pore pace CO2, these signatures between 0‰ and 50‰ indicate that the respired CO2 was a mixture of modern (1950 to 2007; Δ14C ≥ 0‰) and older C (pre-1950; Δ14C < 0‰). After leaf-out, 14C signatures of soil respiration were similar to those of CO2 in ambient air, root, and microbial respiration.
Figure 4 Seasonal pattern of the Δ14C signatures of soil, root respiration, and microbial respiration at South Mountain and Polar Desert in (a) troughs and (b) ridges. Soil respiration Δ14C (average ± SD, n = 3) are corrected for contributions of CO2 in ambient air based on observed δ13C ratios (panel c), using either the δ13C of root respiration (−28‰ in June, −26‰ in July and August) or the range of δ13C of microbial respiration (−19 to −29‰) as a proxy for the δ13C of soil respiration. (c) Seasonal pattern of observed δ13C ratios of soil, root respiration, and microbial respiration at South Mountain and Polar Desert. Symbols represent point measurements of soil or root respiration, horizontal bars indicate observed range of microbial respiration at 0–10 cm soil depth (average ± SE). Dash-dotted lines indicate Δ14C or δ13C ratios of CO2 in ambient air (50.34 ± 0.65‰, −8.37 ± 0.11; n = 7), and dashed line day of leaf-out.
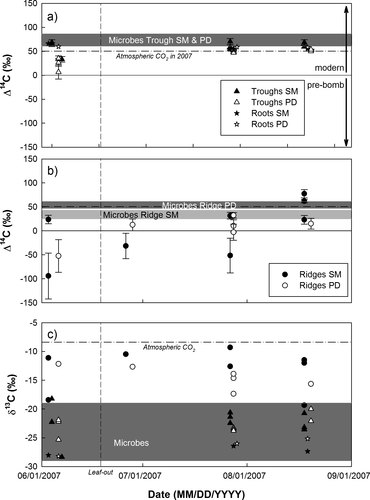
In ridges, the 14C signatures of soil respiration showed a similar seasonal pattern as in troughs, with more depleted signatures at the beginning of the growing season and more enriched signatures later in the season (). In comparison 14C signatures of soil respiration in ridges were more variable than in troughs. Also, ridge soil respiration originated from older C sources than in troughs—indicated both by depleted 14C signatures (pre-bomb C or mixture of bomb and pre-bomb C) and enriched 14C signatures (bomb C) relative to that of ambient CO2—and losses of older C could be detected throughout the entire growing season.
HOW MUCH DO ROOT AND MICROBIAL RESPIRATION CONTRIBUTE TO SOIL RESPIRATION?
Root-respired CO2 had a 14C signature of 58.8 ± 4.3‰ (Mean ± SD, n = 6) and there was no difference between the two sites (t-test, p = 0.90) (). The 14C signature of root respiration was thus enriched in 14C relative to CO2 in ambient air (50 ± 1‰; n = 7) by 4–14‰ (). Assuming that the 14C signature of CO2 in ambient air has been recently declining at a rate of 5.5‰ per year (CitationLevin et al., 2008), roots were respiring C that was fixed on average 1–3 years ago. The δ13C ratio of root respiration was typical for C3 vegetation (−27‰) ().
The 14C signature of microbial respiration from a given soil sample was similar during thawing and subsequent incubation at a range of temperatures (data not shown). Therefore, data was analyzed after averaging 14C signatures obtained during and after thawing. This insensitivity of 14C signatures to temperature during short-term incubations confirms earlier findings (CitationCzimczik and Trumbore, 2007).
The 14C signatures of microbial respiration exhibited large spatial variability (). In troughs, microbial respiration had a 14C signature of 74 ± 13‰ (Mean ± SD, n = 12), with no difference between sites (t-test, p = 0.08) (). Microbial respiration from troughs was thus enriched in 14C relative to root respiration and to CO2 in ambient air, suggesting that this C was on average older than the C respired by roots, but fixed after 1950 (Δ14C ≥ 0‰ = modern).
In ridges, the mean 14C signature of microbial respiration was 34 ± 9‰ at South Mountain and 54 ± 7‰ at Polar Desert (Mean ± SD, n = 4 [SMnt.] or 6 [PD]) (). This variability between locations probably reflects the observed high spatial variability of soil organic C pools (CitationHorwath et al., 2008) rather than a difference in C turnover. Microbial respiration from ridges at South Mountain was depleted in 14C relative to ambient CO2, but still modern—indicating that microorganisms were decomposing a mixture of recently fixed and older (pre-bomb) C. At Polar Desert, 14C signatures slightly enriched relative to ambient CO2 suggest that microorganisms were decomposing a mixture of modern C sources. The δ13C ratio of microbial respiration ranged from −19 to −29‰ ().
A clear-cut partitioning of the net soil respiration flux into its sources (root and microbial respiration at 0–10 cm depth) was not feasible in troughs or ridges, because the 14C signature of soil respiration was either lower (or higher) than the 14C signature of both root and microbial respiration or there was no clear difference between the 14C signatures of the two sources (). Clearly, future soil respiration partitioning approaches need to consider organic C pools within the entire active layer as potential sources for microbial respiration, and additional measurements are needed to better constrain the spatial variability of organic C sources.
ARE SOILS CURRENTLY LOSING OLD C?
Pore space 14CO2 measurements (made after leaf-out) indicate that decomposition of older C intensified with increasing soil temperature and a deepening active layer and peaked in mid-summer. In the soil respiration flux, however, losses of older C were most pronounced early in the season (before leaf-out) and became obscured by larger fluxes from plant roots and/or microorganisms decomposing relatively young C pools. This pattern has been observed before in northern soils (CitationWookey et al., 2002.; CitationHirsch et al., 2003; CitationCzimczik et al., 2006) and demonstrates the difficulty of detecting slow, but potentially sustained decomposition of older soil C pools in surface fluxes.
For spring, when the active layer is shallow, we can estimate the maximum contribution of decomposition of old organic C present at 0–10 cm (Fmicrobes
) to the observed soil respiration flux (FSR
) relative to root respiration (Froots
) using an isotope mass balance approach:
For this calculation, we used an average soil respiration flux of 10 or 30 mg C m−2 hr−1 in ridges or troughs (), the 14C signature of root respiration () and the oldest observed 14C signature of bulk organic matter (). For soil respiration to reach the observed 14C signatures between +3 and −106‰, the decomposition of bulk organic matter would have to contribute a maximum of 20–60% of the total flux—depending on the assumed age of the old C pool (). After leaf-out, the old C source must have contributed less.
Table 3 Estimated maximum contribution of microbial decomposition of bulk soil organic matter (SOM) to the soil respiration flux at the beginning of the growing season, assuming an active layer depth of <10 cm.
Accelerated warming and/or changes in winter and/or summer precipitation—all of which may lead to a deepening of the active layer (CitationLawrence et al., 2008a; CitationNowinski et al., 2010)—may result in the release of greater amounts of older soil C into the atmosphere (CitationSchuur et al., 2009). To estimate at what rate older C is being lost, soil C pools need to be better constrained, the actual C sources of the microbial population need to be assessed within the entire active layer, and soils need to be monitored over longer time periods.
Conclusions
Non-sorted, striped Cryosols in Northwest Greenland contain old C pools (Δ14C modern to −470‰), located within the top 10 cm of mineral soil. Before leaf-out, we estimated that older C pools (fixed by plants prior to 1950) contributed a maximum of 20–60% to the net soil respiration flux. After leaf-out, the contribution of older C pools to net soil respiration declined, but was still apparent in the soil respiration flux from barren ridges as well as in the pore space of mineral soils. Soil respiration in vegetated troughs became dominated by more recently fixed C (between 1950 and 2007). As air temperatures continue to warm in this region of the Arctic, we anticipate a deeper active layer, increased microbial degradation of older soil C pools, and a larger contribution of older C pools to the soil respiration flux.
Acknowledgments
We thank M. Rogers, H. Kristenson, and K. Nagel for their assistance in the field, X. Xu, M. Kosh, M. Spalding, and M. Castaňeda for their assistance in the lab, S. Trumbore for suggestions on the study design and manuscript, and Thule Air Base and the KCCAMS lab for logistical support. This work was funded by the NSF Biocomplexity grant (0508408) awarded to Welker and the G. Comer Foundation.
References Cited
- Bliss, L. C. and N. V. Matveyeva . 1992. Circumpolar arctic vegetation. In Chapin III, F. S. , R. L. JeVeries , J. F. Reynolds , G. R. Shaver , J. Svoboda , and E. W. Chu . (eds.). Arctic Ecosystems in a Changing Climate: an Ecophysiological Perspective. San Diego Academic Press. 59–98.
- Box, J. E. 2002. Survey of Greenland instrumental temperature records: 1873–2001. International Journal of Climatology 22:1829–1847.
- Czimczik, C. I. and S. E. Trumbore . 2007. Short-term controls on the age of microbial carbon sources in boreal forest soils. Journal of Geophysical Research 112:article G03001.
- Czimczik, C. I. , S. E. Trumbore , M. S. Carbone , and G. C. Winston . 2006. Changing sources of soil respiration with time since fire in a boreal forest. Global Change Biology 12:1–15.
- Gaudinski, J. , S. E. Trumbore , E. A. Davidson , and S. Zheng . 2000. Soil carbon cycling in a temperate forest: radiocarbon-based estimates of residence times, sequestration rates and partitioning of fluxes. Biogeochemistry 1:33–69.
- Hinzman, L. D. , N. D. Bettez , W. R. Bolton , F. S. Chapin , M. B. Dyurgerov , C. L. Fastie , B. Griffith , R. D. Hollister , A. Hope , H. P. Huntington , A. M. Jensen , G. J. Jia , T. Jorgenson , D. J. Kane , D. R. Klein , G. Kofinas , A. H. Lynch , A. H. Lloyd , A. D. McGuire , F. E. Nelson , W. C. Oechel , T. E. Osterkamp , C. H. Racine , V. E. Romanovsky , R. S. Stone , D. A. Stow , M. Sturm , C. E. Tweedie , G. L. Vourlitis , M. D. Walker , D. A. Walker , P. J. Webber , J. M. Welker , K. S. Winker , and K. Yoshikawa . 2005. Evidence and implications of recent climate change in northern Alaska and other arctic regions. Climatic Change 72:251–298.
- Hirsch, A. I. , S. E. Trumbore , and M. L. Goulden . 2003. Direct measurement of the deep soil respiration accompanying seasonal thawing of a boreal forest soil. Journal of Geophysical Research 108:8221.
- Horwath, J. L. 2007. Quantification and Spatial Assessment of High Arctic Soil Organic Carbon Storage in Northwest Greenland PhD thesis. Department of Earth and Space Sciences, University of Washington, Seattle, Washington, U.S.A. 296 pp.
- Horwath, J. L. , R. S. Sletten , B. Hagedorn , and B. Hallet . 2008. Spatial and temporal distribution of soil organic carbon in nonsorted striped patterned ground of the High Arctic. Journal of Geophysical Research 113:article G03S07.
- Illeris, L. , A. Michelson , and S. Jonasson . 2003. Soil plus root respiration and microbial biomass following water, nitrogen, and phosphorus application at a high arctic semi desert. Biogeochemistry 65:15–29.
- Jones, M. H. , J. T. Fahnestock , P. D. Stahl , and J. M. Welker . 2000. A note on summer CO2 flux, soil organic matter, and soil microbial biomass from different high arctic ecosystem types in northwestern Greenland. Arctic, Antarctic, and Alpine Research 32:104–106.
- Kessler, M. A. and B. T. Werner . 2003. Self organization of sorted pattern ground. Science 299:380–383.
- Lawrence, D. M. , A. G. Slater , V. E. Romanovsky , and D. J. Nicolsky . 2008a. The sensitivity of a model projection of near-surface permafrost degradation to soil column depth and representation of soil organic matter. Journal of Geophysical Research 113:article F02011.
- Lawrence, D. M. , A. G. Slater , R. A. Tomas , M. M. Holland , and C. Deser . 2008b. Accelerated arctic land warming and permafrost degradation during rapid sea ice loss. Geophysical Research Letters 35:article L11506.
- Levin, I. , S. Hammer , B. Kromer , and F. Meinhardt . 2008. Radiocarbon observations in atmospheric CO2: determining fossil fuel CO2 over Europe using Jungfraujoch observations as background. Science of the Total Environment 391:211–216.
- Mastepanov, M. , C. Sigsgaard , E. Dlugokencky , S. Houweling , L. Ström , M. Tamstorf , and T. Christensen . 2008. Large tundra methane burst during onset of freezing. Nature 456:628–630.
- Michaelson, G. J. , C. L. Ping , and J. M. Kimble . 1996. Carbon storage and distribution in tundra soils of arctic Alaska, U.S.A. Arctic, Antarctic, and Alpine Research 28:414–424.
- Nordstroem, C. , H. Soegaard , T. R. Christensen , T. Friborg , and B. U. Hansen . 2001. Seasonal carbon dioxide balance and respiration of a high-arctic fen ecosystem in NE-Greenland. Theoretical and Applied Climatology 70:149–166.
- Nowinski, N. S. , L. Taneva , S. E. Trumbore , and J. M. Welker . 2010. Decomposition of old organic matter as a result of deeper active layers in a snow depth manipulation experiment. Oecologia doi 10.1007/s00442-009-1556-x.
- Oechel, W. C. , G. L. Vourlitis , S. J. Hastings , and S. A. Bochkarev . 1995. Change in arctic CO2 flux over two decades: effects of climate change at Barrow, Alaska. Ecological Applications 5:846–855.
- Schimel, J. P. and J. S. Clein . 1996. Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biology & Biochemistry 28:1061–1066.
- Schimel, J. P. , J. T. Fahnestock , G. Michaelson , C. Milkan , C. L. Ping , V. E. Romanovsky , and J. M. Welker . 2006. Cold-season production of CO2 in arctic soils: can laboratory and field estimates be reconciled through a simple modeling approach? Arctic, Antarctic, and Alpine Research 38:249–256.
- Schuur, E. , J. Vogel , K. Crummer , H. Lee , J. Sickman , and T. Osterkamp . 2009. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature 459:556–559.
- Sitch, S. , A. D. McGuire , J. Kimball , N. Gedney , J. Gamon , R. Engstrom , A. Wolf , Q. Zhuang , J. S. Clein , and K. C. McDonald . 2007. Assessing the carbon balance of circumpolar arctic tundra using remote sensing and process modeling. Ecological Applications 17:213–234.
- Southon, J. R. and G. M. Santos . 2007. Life with MC-SNICS part II: recent ion source development at the Keck Carbon Cycle AMS Facility. Nuclear Instruments and Methods in Physics Research B 259:88–93.
- Stuiver, M. and H. A. Polach . 1977. Discussion: reporting of 14C data. Radiocarbon 19:355–363.
- Sullivan, P. F. , S. J. T. Arens , R. A. Chimner , and J. M. Welker . 2008a. Temperature and microtopography interact to control carbon cycling in a high arctic fen. Ecosystems 11:61–76.
- Sullivan, P. F. , J. M. Welker , H. Steltzer , R. S. Sletten , B. Hagedorn , S. J. T. Arens , and J. L. Horwath . 2008b. Energy and water additions give rise to simple responses in plant canopy and soil microclimates of a high arctic ecosystem. Journal of Geophysical Research 113:article G03S08.
- Tarnocai, C. , J. G. Canadell , E. A. G. Schuur , P. Kuhry , G. Mazhitova , and S. Zimov . 2009. Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochemical Cycles 23:article GB2023.
- Trumbore, S. E. 2006. Carbon respired by terrestrial ecosystems—Recent progress and challenges. Global Change Biology 12:141–153.
- Walker, D. A. , M. K. Raynolds , F. J. A. Daniëls , E. Einarsson , A. Elvebakk , W. A. Gould , A. E. Katenin , S. S. Kholod , C. J. Markon , E. S. Melnikov , N. G. Moskalenko , S. S. Talbot , and B. A. Yurtsev . the other members of the CAVM Team 2005. The circumpolar Arctic vegetation map. Journal of Vegetation Science 16:267–282.
- Walker, D. A. , H. E. Epstein , and J. M. Welker . 2008. Introduction to special section on biocomplexity of arctic tundra ecosystems. Journal of Geophysical Research 113:article G03S00.
- Wallenstein, M. D. , S. McMahon , and J. P. Schimel . 2007. Bacterial and fungal community structure in arctic tundra tussock and shrub soils. FEMS Microbiology Ecology 59:428–435.
- Welker, J. M. , J. T. Fahnestock , G. H. R. Henry , K. W. O'dea , and R. A. Chimner . 2004. CO2 exchange in three Canadian high arctic ecosystems: response to long-term experimental warming. Global Change Biology 10:1981–1995.
- Wookey, P. A. , R. A. Bol , C. J. Caseldine , and D. D. Harkness . 2002. Surface age, ecosystem development, and C isotope signatures of respired CO2 in an alpine environment, North Iceland. Arctic, Antarctic, and Alpine Research 34:76–87.
- Xu, X. , S. E. Trumbore , S. Zheng , J. R. Southon , K. E. McDuffee , M. Luttgen , and J. C. Liu . 2007. Modifying a sealed tube zinc reduction method for preparation of AMS graphite targets: reducing background and attaining high precision. Nuclear Instruments and Methods in Physics Research B 259:320–329.