Abstract
In this study, we analyzed the effect of topography, particularly slope, on the expansion rates and population dynamics of Echinospartum horridum at small spatial and temporal scales in the grassland communities of Ordesa–Monte Perdido National Park (OMPNP) (NE Spain). E. horridum is a thorny cushion dwarf, endemic of Spain and south of France, forming dense mono-specific large patches difficult to be penetrated by other plants and large herbivores once it is established. Between 2005 and 2007, we collected demographic parameters from 300 marked plants of E. horridum that were distributed in the center and the edge of four patches. At the patch edge, two of the four patches had a high slope (≥10°) and two had a low slope (<10°), whereas at the patch centers the slope was high (18°–30°). To calculate invasion speed, we use aerial photographs from 1981 and 2003. Plants in the center of patches had lower growth rates, fewer flowers, and higher crown death rates than did the plants at edge of patches. Slope influenced significantly invasion rates: the speed of expansion was lowest on gentle slopes, probably because of competition with grass. The speed of diffusion from 1981 to 2003 varied from 2.09 m yr−1 on the steep slopes to 1.93 m yr−1 on the shallow slope. Plant dynamics at patch edges suggest that colonization by E. horridum will continue in the grasslands of the OMPNP.
Introduction
The widespread shift in growth form dominance from grasslands to shrublands is one of the most important threats to the conservation of grasslands in many mountainous regions of the world (CitationGrace et al., 2002; CitationWearne and Morgan, 2001; CitationMacDonald et al., 2000; CitationOlsson et al., 2000; CitationDullinger et al., 2003) and can have negative effects on livestock production, wildlife, and biodiversity (CitationSchlesinger et al., 1990) by altering facilitative-competitive interactions (Choler et al., 2001; CitationCallaway et al., 2002). The shift involves the impenetrable and irremediable growth of woody species within a matrix of grasslands and differs from the shrub encroachment that occurs in savanna ecosystems (CitationArcher et al., 1988; CitationBrown and Carter, 1988; CitationAguiar et al., 1996; CitationRoques et al., 2001; CitationBriggs et al., 2005), where water and nutrients limitations constrain woody cover and allow grass to coexist (CitationSankaran et al., 2005). In Western Europe, shrub expansion into subalpine grasslands is the result of vegetation successional change after anthropomorphic deforestation where the effect of climate change is even limited (Gehrig-Fasel et al., 2007), and disturbance (livestock and fire) is required to maintain grasslands (CitationSankaran et al., 2005).
Since at least the Middle Ages, alpine and subalpine grasslands in the mountains of Europe have been used for seasonal farming (CitationMontserrat-Recoder and Fillat, 1990; CitationBätzing, 1991), which has had significant effects on subalpine vegetation (CitationEllenberg, 1988), giving place to a high biological diversity of these semi-natural grasslands (CitationEriksson et al., 1995; CitationPärtel et al., 1999). Typically, high shrub cover is undesirable in grasslands because it has negative effects on livestock production through a reduction in grass production (CitationScholes and Archer, 1997), or it increases the difficulty of managing livestock (CitationPyke and Archer, 1991). Repeated physical disturbances of shrubs and trees (CitationGrace, 1999; CitationCousins and Eriksson, 2001) and management activities such as fire and grazing (CitationKörner, 1999; CitationLeague and Veblen, 2006; CitationHoltmeier and Broll, 2005; CitationBartolomé et al., 2005) maintain those grassland communities. As those activities declined over the last century, shrubs colonized alpine grasslands, which has produced a dense shrub cover (CitationGarcia-Ruiz et al., 1996; CitationAustrheim et al., 1999; CitationMacDonald et al., 2000; CitationTasser and Tappeiner, 2002; CitationJulien et al., 2006; CitationBatllori and Gutiérrez, 2008). The post-abandonment successional displacement has reduced plant species diversity (CitationJackson et al., 2002; CitationDullinger et al., 2003). Nevertheless, the patterns, dynamics, and rates of shrub colonization in semi-natural grasslands in response to changes in land use are not well studied (CitationBartolomé et al., 2005).
Despite numerous studies on the mechanisms of woody plant encroachment and grass-tree interactions (CitationSkarpe, 1990; CitationWiegand et al., 2006; CitationSankaran et al., 2005), the underlying mechanisms are not well understood (CitationSankaran et al., 2004; CitationWard, 2005). Environmental factors interact with management practices to influence establishment and expansion processes (CitationRouget and Richardson, 2003; CitationJulien et al., 2006), but the study of responses to climate change at the regional and finer scales requires a more involved approach than is required at the global scale (e.g., CitationTrivedi et al., 2008). Despite the virtues of understanding the patterns and dynamics of shrub colonization in aid of the preservation of biodiversity in protected areas, the subject has received limited study (CitationBartolomé et al., 2005). After land abandonment, the topography of the area strongly influences the rate of vegetation succession. Plant species richness varies in response to topography factors such as solar radiation and slope angle (CitationPykälä et al., 2005; CitationBennie et al., 2006). For example, slope can affect shrub-grass interactions because species differ in their root structure. Grass species that have shallow roots are well adapted to shallow slopes or gentle slopes because there is easier access to water in the upper soil layers, whereas species that have deep roots, such as shrubs, can survive on steep slopes (CitationJackson et al., 1996; CitationJurena and Archer, 2003). When shrubs expand into grasslands, they can alter many aspects of the environment that lead to a reduction in substrate quality (CitationPolley et al., 1997; CitationMontané et al., 2007, and references therein). In addition, grass can reduce the emergence, growth, and survival of woody seedlings (CitationGordon et al., 1989; CitationMartinez and Fuentes, 1993; CitationMcPherson, 1993; CitationRiginos, 2009), which can influence the size and density of shrub cover (CitationArcher et al., 1995).
In the Central Pyrenees, where the average annual precipitation is >1500 mm (CitationBarrio et al., 1990), human disturbances, such as livestock and fire, are required to maintain grasslands (CitationSankaran et al., 2005). Thus, traditional land uses practices prevented alpine and subalpine grasslands from shrub encroachment (CitationLasanta et al., 2006), but a reduction in livestock farming at the end of the 20th century has lead to an irrevocable shrub-colonization process (CitationPallaruelo, 1993; CitationManrique et al., 1999; CitationMottet et al., 2006). In the Central Pyrenees, Echinospartum horridum (Vahl) Rothm, a thorny cushion dwarf, is one of the species that plays a significant role in shrub encroachment. E. horridum is highly invasive on the southern slopes, where it colonizes subalpine grasslands, one of the important ecosystems for plant diversity (CitationBenito, 2006). Its thorns, which reduce the damage caused by herbivores, and its great colonizing ability contribute to the significant invasive nature of E. horridum (CitationMarinas et al., 2004) and its remarkably rapid spread in recent decades (CitationMontserrat-Recoder et al., 1984; CitationPérez-Cabello and Ibarra, 2004). In addition, as a leguminous plant, E. horridum fix more atmospheric nitrogen than grass (CitationMontserrat-Recoder et al., 1984), which increases its competitiveness in colonizing grasslands (CitationKochy and Wilson, 2000), especially at high elevations, where N is limited (CitationJacot et al., 2000). E. horridum forms large, dense, monospecific patches that can cover several hectares, where only a few other plant species survive within small gaps. It is not well understood, however, how topography can affect grass-shrub interactions community. The aim of this study was to assess the impacts of slope on the growth and reproduction of E. horridum in the subalpine grasslands of Ordesa–Monte Perdido National Park (OMPNP), Central Pyrenees, Spain. The study examined the demographic parameters associated with age structure, population density, and ecological conditions (CitationCrawley, 1986), which can provide the best predictions of colonization over time (CitationPrevosto et al., 2004). The following questions were addressed:
Does location within a patch (center vs. edge) influence the demography and reproduction of E. horridum?
Does the slope at the patch periphery affect the demographics of E. horridum?
Has slope influenced the expansion rate over the last 25 yrs?
We expected that that plants in the center and those on the edge would differ in their demographics because plants in the interior of a patch experience high population densities and low competition from grass, whereas plants at the periphery are exposed to relatively low E. horridum densities and greater competition from grass. Furthermore, we predicted that slope would have differential effects at the center and edge of the patches: higher resistance by grass to encroachment on low slopes because they are better adapted to flat terrain, which leads to a reduction in colonization rates.
Methods
STUDY AREA
The study was conducted in OMPNP, Central Pyrenees, Spain. OMPNP has been a protected area (15,600 ha) since 1918 for the Ordesa Canyon (2100 ha) and, in 1982, it assumed its current limits. More than 75% of the park is alpine and subalpine grassland. In the Pyrenees, the subalpine grasslands are secondary plant communities that replaced native forest and have been grazed continuously for at least 500 yrs (CitationMontserrat-Recoder and Fillat, 1990).
The climatic conditions in the study area are influenced by several factors: the mountain continental climate is modulated by the westward Atlantic winds, whereas the climate farther south is Mediterranean. At the Goriz weather station (42°40′N, 00°02′E; 2215 m a.s.l.), average annual rainfall has been 1735 mm (1981–2006). Snow cover persists from early November to late May. The mean annual temperature is ∼5 °C, but with daily temperatures between 25 °C and −21 °C.
The populations of E. horridum were in four patches, which were 75.4 ha to 267.5 ha in size, at similar elevations (1765 m to 1980 m), and had southern orientations. At the patch center, the slope ranged from 18° to 30°. The slopes at the edge of patches were classified as either high (12°–13°) or low (5°–8°).
STUDY SPECIES
E. horridum (Vahl.) Rothm. (Fabaceae) is a thorny cushion dwarf that is endemic to the central Spanish Pyrenees and southern France. It is a strictly calcicolous plant (CitationAparicio et al., 2002) that reproduces sexually and asexually. The inflorescence is apical, with two opposite yellow flowers, and the plant produces sparsely sericeous legumes. The flowering period occurs in mid-July. Sexual reproduction produces persistent seed banks (CitationAparicio and Guisande, 1997), which confer the capacity for good post-disturbance establishment (CitationPrevosto et al., 2004). Creeping asexual reproduction is through the clonal propagation of decumbent branches, which root at nodes along the stem. Newly rooted stems can break off and become independent clonal fragments. Aged shrubs can split into clonal fragments after a central portion of the plant dies. In this study, we did not differentiate between clonal fragments (originated from nodes) and a genet (originated from seeds). In both cases, individual plants were connected networks of stems and ramets. Clonal fragments allow the plant to maintain and increase its cover, which suppresses the establishment of other species. Because they out-compete neighbors, clonal propagation favors the persistence of the plant in dense coverage where seedling establishment is unusual (CitationBierzychudek, 1985), which has led to the formation of monospecific patches of E. horridum in the study area.
DATA COLLECTION
Age and Crown Size of E. horridum
To detect a relationship between plant age and crown size in E. horridum, we measured crown diameter and the number of annual rings. We selected randomly 76 individual plants from the center and the periphery of the four patches. Before we removed the individual by cutting the principal trunk, we measured crown circumference and the largest diameter. Each trunk section was polished using sandpaper of progressively finer grades until rings were clearly visible. In the laboratory, plant age was estimated by counting the number of annual rings along two radii in each trunk section using at ×3-magnification stereomicroscope (Leica MZ 125, Wetzlar, Germany). As in other species of the genus, the wood of E. horridum was semi-ring-porous (CitationSchweingruber, 1990) and, in most of the samples, ring boundaries were distinctive. In addition, the wood had dendritic vessel groups (). When different ring counts were obtained from the same individual, the rounded-up mean of the minimum and maximum counts was used as the estimate of plant age (CitationDietz and von Arx, 2005). Three size classes were defined based on the relationship between crown diameter and the number of rings: crown diameter <20 cm (Class I), crown diameter 20–50 cm (Class II), and crown diameter >50 cm (Class III). The first size class corresponds with seedlings, the second class is young reproductive plants, and the third class is adult plants.
Vital Rates and Body Condition
In the first week of June 2005, we marked 75 plants in each of the four patches (30 and 45 from the center and from the edge of the patch, respectively. Of the 30 plants marked in the center of each patch, 10 were in Class I, 10 were in Class II, and 10 were in Class III. Of the 45 plants marked at the patch edge, 15 were in Class I, 15 were in Class II, and 15 were in Class III. Between the last week of May and the first week of June in 2005, 2006, and 2007, we measured the largest crown diameter and its perpendicular of all of the plants. In the middle of July in 2005, 2006, and 2007, we recorded the numbers of flowers and estimated the proportion of the standing plant that was dead. To estimate the recruitment of each plant, in June 2005 and June 2007, we recorded the size and the nearest neighbor distance of each juvenile plant around adult plants. Recruitment was estimated based on the plants at the edge of patches because the high density of adult plants in the center of the patches precluded attributing offspring to a specific parent plant.
To estimate population density, we used equal-stratified random sampling. We performed 24 sample plots of 10 × 10 m each, distributed within the four patches of E. horridum (6 per patch). Variation in slope was greater at the edge of a patch than in the center of the patch; therefore, we took two samples from the center and four samples from the edge of the four patches. We estimated the number of plants of each class size in each of the 24 sample plots.
We excavated eight E. horridum plants complete with their systems of ramets connected by decumbent stems. We identified each ramet (a unit consisting of shoot and root systems) and aged them using the annual growth rings in stem cross sections. We measured decumbent stem length between the apical root and each ramet.
Patch Growth
The patches of E. horridum were quantified based on digitized orthorectified aerial photographs taken in 1981 and 2005. To calculate the expansion of E. horridum patches, we measured the distance between the front line in 1981 and 2005, along 24 linear transects (6 per patch).
The advances of E. horridum along the frontal wave were of interest; therefore, transects were laid out along the slope line. In addition, in June 2005, we recorded E. horridum cover using the Line-Intercept Method. We randomly assigned 16 transects along the slope line (four transects per patch) from the base to the top of the patch. Patch width along transects varied between 180 m and 750 m.
DATA ANALYSIS
Estimates of Vital Rates and Body Condition
The probability of surviving one year was calculated for the marked plants using the exponential survival function,
which has a single parameter, ρ, the death rate per unit time, t. The death rate depends on the age of the plant, x, according to this function,
where alpha is the natural logarithm of death rate at birth (age equal to zero) and beta is the rate of increase of the logarithm of death rate with age. We calculated the parameters α and β using maximum likelihood estimations from the binomial distribution.
To calculate the proportion of individuals surviving from birth through to a given life history, we estimated the survivorship function by multiplying the proportion of individuals surviving through each previous life-history stage, following the Kaplan-Meier Method (CitationMcCallum, 2000). Confidence intervals for the exponential survival function were calculated using Gauss-Newton methods (SAS 9.1; CitationSAS Institute, 2004).
Floral production was calculated as the average number of flowers produced in July 2005, 2006, and 2007 by the marked individuals. The seedling kernel dispersal rate was estimated from the occurrence of seedlings and the distance to the nearest neighbor adult plant at the patch edge. We assumed that the expected number of recruits produced by E. horridum is given by the equation
(CitationRibbens et al., 1994; CitationClark et al., 1998), where x is the distance from the mother plant.
The crown area, A, was evaluated under the assumption that the plants are ellipsoidal using the Major Diameter, d, and its perpendicular, d:
The crown growth rate, G, was calculated as the difference between crown area in 2006 and 2005 divided by the crown area.
The crown death rate was calculated as the average proportion of standing death crown in 2005 and 2006. Dead branches remain standing for at least one year, and can be easily recognized by the yellow color vis-à-vis the green of the living part of the plant. We estimated visually the proportion of death crown following the Daubenmire Method (CitationDaubenmire, 1959).
Clonal propagation was calculated from four plants at the patch edge and four at the patch center. Decumbent stem elongation between the main root and the first ramet was calculated assuming exponential growth as
where S is decumbent stem, t is time, and r is decumbent stem net growth rate (CitationCain et al., 1995). We calculated the net growth rate as follows:
where St and S0
are the lengths between the initial and final stem length, and Δt is the time elapsed between observations.
Estimates of Patch Growth
For each patch, we analyzed the E. horridum density g(x) with distance, x, from the patch center to the external border. The density gradient, α, was estimated from the normalized organism density-distance equation
which represents the density of plants with distance x from the starting point of release, and α is the density gradient (CitationEdmonston and Davies, 1978). Non-linear adjustments were performed following the Gauss-Newton Method (SAS 9.1; CitationSAS Institute, 2004).
Assuming exponential growth and the absence of constraints for growth and expansion at the patch edge, patch size at time t (nt
) can be modeled by the following equation:
where nt
is the patch frontal length in 2005, N0
is the patch frontal length in 1981, and r is the population growth rate.
Estimates of the diffusion coefficient (d) and velocity of diffusion (c) were calculated under the assumption of no drift and following a Gaussian normal dispersal kernel (CitationKareiva, 1982, Citation1983), as
where
is the mean squared displacement at time t, and
where r is the population growth rate.
Results
Age and Crown Size of E. horridum
To model individual growth, we used the Richards Function (CitationRichards, 1959)
where y is crown diameter, A is asymptotic size, k is intrinsic growth rate, b is the growth constant, and t is age (number of rings). Based on the non-linear adjustment using the Gauss-Newton Method, A = 79.29 ± 9.51; k = 0.238 ± 0.058; b = −19.44 ± 9.47 (F
3, 72 = 195.67, P < 0.0001). The longest diameter of the crown was used to establish the size-age relationship. Age classes were derived from the equation
Class I corresponded to plants younger than 8 yr and a main crown diameter <20 cm. Class II included plants whose ages ranged from 8 to 15 yr and their longest crown diameter was 20–50 cm. Class III included plants older than 15 yrs and a crown diameter >50 cm.
Effects of Location within a Patch on E. horridum Demographics
To assess whether position in a patch influenced plant performance, we evaluated plants living at the center and plants living at the edge of patches (). In the twenty-four 10-m × 10-m quadrates, we recorded 3569 individual plants (density = 1.49 ind m−2), with densities in patches ranging from 1.36 ind m−2 to 1.78 ind m−2. Age-class structure differed between the center and the edge of a patch. At the center of the patches, 55.6% of the plants were Class III, whereas at the edges, 36.1% were in this age class (Goodness-of-Fit Test: G = 6.76, P < 0.01). Plants in Age Class I were more common at the edges (34.89%) than in the center (20.41%) of patches (G = 277.90, P < 0.001), and plants in Class II was more common at the edge (29.04%) than in the center (23.98%) of patches (G = 129.80, P < 0.001). The results from the 1000 bootstrap randomizations confirmed those differences of patterns.
The survival of E. horridum varied as a function of age (x), where the death rate ρ(x) best fit the equation
The residual deviance of the maximum likelihood estimation was 22.50, which was lower than expected based on the chi-square distribution (χ2
20, 0.05 = 31.41). E. horridum had a high mortality rate in the early years of life, but rates stabilized after age 12 (). Survival probability increased as plants aged.
FIGURE 2 (A) Graphical representation of the proportion (p) of E. horridum plants that survived and age; (B) the proportion of individuals that survived from birth to a given age from the study area in Ordesa–Monte Perdido National Park, Spain.
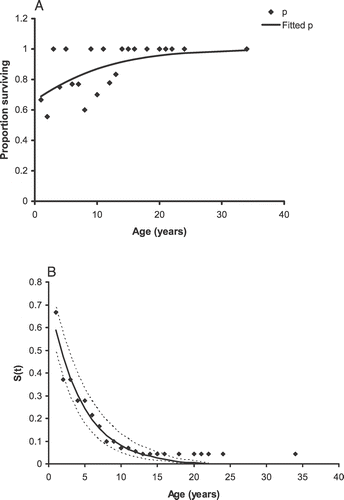
The proportion of plants that survived P, i.e., number of living individuals at time t + 1 divided by the number of individual marked at time t (nt +1/nt ) and the fitted P obtained from the exponential survival function eρ /(1 + eρ ) are present in , and it shows that after 12 yrs of age most individuals survive.
The proportion of individuals that survived from birth to a given age declined rapidly during the first years of life and fit the equation,
F
2, 20 = 328.62, P < 0.0001, where 0.22 is the death rate ().
Location within a patch (edge or center) had a significant influence on plant demographics. Plants that lived at the edge of the patch produced more flowers (F 1,298 = 19.36, P < 0.0001), had higher crown growth rates (F 1,298 = 10.68, P < 0.001), and lower mortality (crown death rate) than did those that lived in the center of the patch (F 1,298 = 35.49, P < 0.0001) ().
FIGURE 3 Mean (±se) number of flowers, crown growth rate, and the proportion of death crown rate of E. horridum plants collected from the edge and the center of patches in Ordesa–Monte Perdido National Park, Spain.
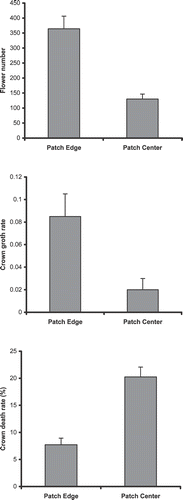
The responses of plants to position within the patch differed among age classes (), which had an effect on the population dynamics of plants living at the edge or in center of patches. As expected, at the edge of patches, the number of seedlings and plant age were positively correlated. Seedling establishment in the center of patches was not evaluated. The increase in flower production with age was similar at the edges and in the center of patches. Crown growth rate increased most dramatically in plants in the youngest age class and position within a patch had no significant effect on the crown growth rate of plants in the oldest age class. Crown death rate was lowest at the edge of patches and was lowest in Age Class II. Plants in the center of patches had the highest proportions of crown death and the effect was most intense among younger plants.
TABLE 1 Observed and expected proportions of three age classes of E. horridum at the center and the edge of patches in the grasslands of Ordesa–Monte Perdido National Park, Spain. Class I: <8 years old, Class II: 8–15 years old, and Class III: >15 years). Expected values derived from a bootstrap randomization. Significance values from t-test with 23 degrees of freedom are: * P < 0.05, ** P < 0.01.
TABLE 2 Means ± SE values of number of flowers per plant, annual crown growth rate, proportion of crown death, and the one-way ANOVA test of the effects of location within a patch for three age classes of E. horridum in Ordesa–Monte Perdido National Park, Spain. Significance values from t-test are: *** P < 0.001 ** P < 0.01, * P < 0.05, no asterisks indicate P > 0.05.
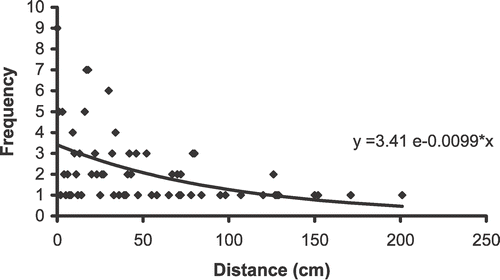
Seedlings became established close to conspecific neighbors; most of the seedlings <10 yrs of age were established within 50 cm of the adult plant. The number of seedlings decreased with an increase in the distance from the plant and their frequency distribution fit significantly a negative exponential function (F 2,59 = 66.57, P < 0.001) ().
FIGURE 4 Graphical representation of the frequency of E. horridum seedlings established as a function of the distance (cm) from the adult plant in Ordesa–Monte Perdido National Park, Spain.
Decumbent stem elongation between the main root and the first-generation ramets (n = 39) was 65.05 ± 5.66 mm. The time elapsed for the production of the first ramet was 11 ± 2 yrs (n = 38). Plants at the edge and those in the center of patches did not differ significantly in their stem elongation rates and the generation times for the first-generation ramets (F 1,37 = 1.18, NS, for stem elongation; F 1,36 = 0.01, NS for first-generation time elapsed). Second-generation ramets were produced after 5 ± 1 yrs (n = 34) and an average stem elongation of 100.53 ± 9.30 mm (n = 30), but stem elongation rates (F 1,32 = 1.06, NS) and generation times for second-generation ramets (F 1,28 = 2.33, NS) did not differ significantly between plants at the edge and those in the center of patches. Plants at the edge produced significantly (χ2 = 10.8, P < 0.001) more second-order ramets (24) than did plants in the center (6) of patches.
Clonal growth rate of first-order ramets (S01
) fit the equation
(F
1, 36 = 7.50, P < 0.01), whereas the growth rate of second-order ramets fit the equation
(F
1, 28 = 74.54, P < 0.0001).
Effect of Slope on E. horridum Demographics
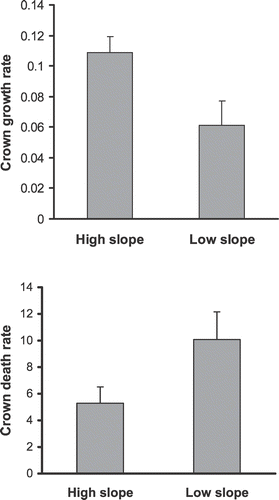
To confirm whether slope had a significant effect on plant demographics, we evaluated separately plants that lived in the center and plants that lived at the edge of patches, where steep and shallow slopes occurred. At the edge of patches, plants on steep slopes had significantly higher crown growth rates (F 1,178 = 5.95, P < 0.05) and lower crown death rate (F 1,178 = 3.90, P < 0.05) than did the plants on shallow slopes (), but slope did not have a significant effect on flower production or seedling establishment. Among the youngest plants only, crown growth rate was significantly (F 1,62 = 5.48, P < 0.05) higher among plants on steep slopes (0.158 ± 0.024) than among plants on shallow slopes (0.047 ± 0.040). Among the plants in Age Class II, crown death rate was significantly higher (F 1,58 = 6.01, P < 0.05) for the plants on shallow slopes (1.81 ± 0.97) than for plants on steep slopes (7.86 ± 2.33). Among the plants in the oldest age class, slope did not have a significant effect on crown growth rate or crown death rate.
Patch Growth
The change in patch density with an increase in the distance from a patch edge fit significantly the normalized density function
(). The steeper the slope, S, the higher the density gradient (α). Thus, the distribution of E. horridum was most dispersed on the shallowest slopes and the data fit significantly (F
2,4 = 26.33, P < 0.05) a Holling Type III functional response (CitationHolling, 1959)
(), where parameter A (mean = 0.038 ± 0.021) is the maximum density gradient, and parameter h (mean = 9.49 ± 5.51) is the saturation constant; i.e., the slope at which the density gradient is half of its maximum.
FIGURE 6 Graphical representation of the variation in the density gradient of the patch as a function of the slope at the patch edge in Ordesa–Monte Perdido National Park, Spain.
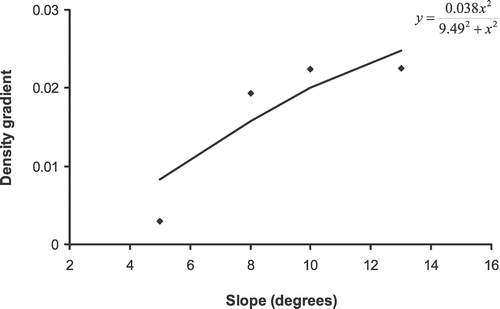
TABLE 3 Variation in E. horridum density G(x) with distance from the patch edge (x) after normalization, for the four patches analyzed in Ordesa–Monte Perdido National Park, Spain, based on the equation , where α is the density gradient (CitationEdmonston and Davies, 1978). F statistic, n1
and n2
degrees of freedom, and significance P values are obtained from the non-linear adjustment (Gauss–Newton method) between density G(x) and distance (x). Patch size is shown as width (direction perpendicular to the maximum slope) by length (direction along the slope).
Average annual growth rate of the four E. horridum patches was 0.008 ± 0.001 yr−1, and attained 0.20 ± 0.04 over a 24-yr period. Slope of the terrain favored the E. horridum growth rate (F 1,22 = 8.87, P < 0.01). Plants on steep slopes had a mean (± SE) annual growth rate (0.013 ± 0.003) that was significantly higher than the rate for plants on shallow slopes (0.004 ± 0.0006). The mean (± SE) growth rate over 24 yr was 0.31 ± 0.07 and 0.09 ± 0.01 on the high and low slopes, respectively.
On the steep-sloped sites, the diffusion coefficient D(24) was 83.9 m2 yr−1, with upper and lower 1-SE limits of 79.5 and 87.3, respectively. On the shallow-sloped sites, D(24) was 234.0 m2 yr−1 (286.9, 186.4, 1-SE upper and lower limits, respectively). The velocities of diffusion (c) were 2.09 m yr−1 and 1.93 m yr−1 at steep-sloped and shallow-sloped sites, respectively.
Discussion
EFFECTS OF LOCATION WITHIN A PATCH ON E. horridum DEMOGRAPHICS
In the subalpine grasslands of OMPNP, location within a patch had a significant effect on the age distribution of E. horridum plants. In the center of patches, the majority (55.6%) of plants were older than 15 yrs and a small proportion (20.4%) were young plants, whereas at the edge of patches, the proportions of the three were similar. CitationEriksson (1993) suggested that intraspecific competition might be responsible for the smaller proportion of young plants in the center of a patch. At the edge of patches, the relatively high proportion of seedlings and plants aged 8 to 15 yrs (despite the high potential for competition with grasses) reflected the colonization dynamics of this species.
In our study, plants at the edge of patches were in better general condition, produced more flowers, and had higher growth and lower mortality than did the plants in the center of patches. Other studies also reported high seed production in colonizing species (CitationWilliamson, 1996; CitationJulien et al., 2006).
In E. horridum flower production increased with the age of the plants, which led to a greater production of seedlings associated with the older plants at the edge of patches. Seedling was the stage most susceptible to death but, if they survive, they were the plants that had the highest growth rates. The mortality rate was highest among the plants younger than 12 yrs. Similarly, CitationWeppler et al. (2006) found that seedlings and young plants experienced the highest mortality because the survival probability is influenced by plant size (CitationRiba et al., 2002). Seedling recruitment is an important component of a shrub invasion and this stage can be the most critical, particularly where a thick grass cover surrounds seedlings because grasses are superior competitors to shrub seedlings (CitationKochy and Wilson, 2000).
E. horridum produced first-generation ramets after ∼10–12 yrs of age and at a distance of 60–70 cm from the main root, and second-generation ramets appeared 4–5 yrs later and 90–100 cm away from the main root. Decumbent stem elongation and the time elapsed for the production of first- and second-generation ramets did vary significantly with location in a patch. Others have found that clonal propagation can be highly variable and habitat does influence significantly ramet production (CitationDamman and Cain, 1998). In our study, the presence of grass did not appear to affect ramet production. In E. horridum, clonal reproduction is associated with shrub persistence, rather than with an increase in cover. Plants at the edge had more second-order ramets than did the plants in the center of patches. Plants in areas that have abundant resources produce more ramets than do plants in less favorable areas (CitationCain, 1994; CitationVan Kleunen and Fischer, 2001; CitationNesmith et al., 2006). We found that intra-species competition had a more important effect on the performance of E. horridum than did grass-shrub competition. Flower production and clonal growth were higher among the plants at the edge than among plants in the center of patches. Those parameters were affected similarly because the factors that influenced sexual reproduction (intraspecific competition and location) seemed to affect asexual reproduction in the same way: growth rates were highest at the edge where intraspecific competition is lower than it is in the center of a patch (see also CitationDamman and Cain, 1998).
EFFECT OF SLOPE ON E. horridum DEMOGRAPHICS
As we predicted, the slope of the terrain appeared to influence significantly the colonization by E. horridum plants in the OMPNP. Colonization was fastest on the steepest slopes, where the plants appeared in better condition than did the plants from sites that had shallow slopes. The plants on steep slopes had higher growth rates and lower death rates than did the plants on shallow slopes, and seedlings and young plants (Age Class I) were more strongly affected than were adult plants. Plants in Class I had the highest growth rates and plants in Class II had lower death rates on the steepest slopes. Competition between E. horridum and grasses probably is most intense in areas that have shallow slopes (CitationAlados et al., 2006; CitationDalaka and Sgardelis, 2006) and, in our study, younger plants seemed to be the age class most strongly affected, and seedling establishment was not significantly affected by slope. In our study and that of CitationRiba et al., (2002), growth and death rates were affected by site characteristics, but flower production and seedling establishment were not influenced by site.
PATCH GROWTH
Slope influenced the growth of the patch over a 24-yr period: at shallow-sloped sites, the expansion rate (1.93 m2 yr−1) was lower than it was at steep-sloped sites (2.09 m2 yr−1). We suspect that competition with grasses at sites where the slope is shallow might have limited the rate of expansion. The roots of grasses are better adapted to flat areas than are those of shrubs (CitationPolley et al., 1997; CitationGuerrero-Campo et al., 1999; CitationAlados et al., 2006), which might give grass a competitive advantage, improving its resistance to shrub invasion, and moderating the expansion rate of E. horridum.
In summary, although in our study area, with high average rainfall, livestock pressure and fire are needed to maintain grasslands (CitationSankaran et al., 2005), the higher competitive ability of grasses in low-slope areas play an important role in the persistence of subalpine grasslands. The slope of the terrain had a significant effect on the invasion of grasslands by the shrub E. horridum which may be due to the intense competition between shrubs and grass at low-slope areas retarded the expansion of E. horridum, and invasion rates were highest on the steepest sites. Indeed, plants at the edge had higher productivity, growth rates, flower production, and seedling production than did plants in the center of patches. Clonal reproduction, a means of persistence, produced dense, monospecific patches of E. horridum, which did not vary with slope.
At the time of our study, there were recent reductions in livestock farming and the controlled burning by shepherds, and colonization by E. horridum was in the initial stage. In time, however, the rate of invasion probably will decrease when shrubs reach the forest line. In terms of management, the most appropriate practice might be a grazing of the seedlings by, for instance, reintroduced goats, which have a larger preference than sheep for woody species, being able to forage on spiny legumes such as E. horridum (CitationPapachristou et al., 2005).
As others (CitationCrawley, 1986; CitationSheppard et al., 2002) have suggested, demographic analyses at the early stage of colonization should allow a comparison of the invasion rates across a range of habitats, which would provide an estimate of the invasion dynamic and of the life cycle stage more sensitive to management directives.
Acknowledgments
We gratefully acknowledge the support of the Spanish Science and Innovation Ministry (PN-MCI) (CGL2008-00655/BOS) and Spanish Environmental Ministry (MMA 002/2007). The Ordesa-Monte Perdido National Park (OMPNP) provided logistic infrastructures and free access to the national park. We thank Beatriz Bueno, Elena Lahoz, Jesus Martinez, and Angel De Frutos for assistance in collecting field data, and Fernando Mauleón for assistance with the orthorectification of the aerial photographs. We thank Bruce MacWhirter for critically reading and providing helpful suggestions on the manuscript. Camarero acknowledges the support of Foundation “Aragón I+D.”
References Cited
- Aguiar, M. R. , J. M. Paruelo , O. E. Sala , and W. K. Lauenroth . 1996. Ecosystem responses to changes in plant functional type composition: an example from the Patagonian steppe. Journal of Vegetation Science 7:381–390.
- Alados, C. L. , R. Gotor , P. Ballester , D. Navas , J. M. Escos , T. Navarro , and B. Cabezudo . 2006. Association between competition and facilitation processes and vegetation spatial patterns in alpha steppes. Biological Journal of the Linnaean Society 87:103–113.
- Aparicio, A. and R. Guisande . 1997. Replenishment of the endangered Echinospartum algibicum (Genisteae, Fabaceae) from the soil seed bank. Biological Conservation 81:267–273.
- Aparicio, A. , R. G. Albaladejo , and G. L. Ceballos . 2002. Genetic differentiation in silicicolous Echinospartum (Leguminosae) indicated by allozyme variability. Plant Systematics and Evolution 230:189–201.
- Archer, S. , C. Scifres , D. Bassham , and R. Maggio . 1988. Autogenic succession in a subtropical savanna: conversion of grassland to thorn woodland. Ecological Monographs 58:111–127.
- Archer, S. , D. S. Schimel , and E. A. Holland . 1995. Mechanisms of shrubland expansion—Land-use, climate or CO2. Climatic Change 29:91–99.
- Austrheim, G. , E. Gunilla , A. Olsson , and E. Grontvedt . 1999. Land-use impact on plant communities in semi-natural sub-alpine grasslands of Budalen, central Norway. Biological Conservation 87:369–379.
- Barrio, G. , J. Creus , and J. Puidefábregas . 1990. Thermal seasonality of the high mountain belts of the Pyrenees. Mountain Research and Development 10:227–233.
- Bartolomé, J. , Z. G. Lopez , M. J. Broncano , and J. Plaixats . 2005. Grassland colonization by Erica scoparia (L.) in the Montseny Biosphere Reserve (Spain) after land-use changes. Agriculture Ecosystems and Environment 111:253–260.
- Batllori, E. and E. Gutiérrez . 2008. Regional tree line dynamics in response to global change in the Pyrenees. Journal of Ecology 96:1275–1288.
- Bätzing, W. 1991. Die Alpen. Entstehung und Gefährdung einer europäischen Kulturlandschaft. München, Germany C. H. Beck.
- Benito, J. L. 2006. Vegetación del Parque Nacional de Ordesa y Monte Perdido (Sobrarbe, Pirineo Central Aragonés. Zaragoza, Spain Publicaciones del Consejo de Protección de la Naturaleza de Aragón.
- Bennie, J. , M. O. Hill , R. Baxter , and B. Huntley . 2006. Influence of slope and aspect on long-term vegetation changes in British chalk grasslands. Journal of Ecology 94:355–368.
- Bierzychudek, P. 1985. Patterns in plant parthenogenesis. Cellular and Molecular Life Sciences 41:1255–1264.
- Briggs, J. M. , A. K. Knapp , J. M. Blair , J. L. Heisler , G. A. Hoch , M. S. Lett , and K. McCarron . 2005. An ecosystem in transition: woody plant expansion into mesic grassland. BioScience 55:243–254.
- Brown, J. R. and J. Carter . 1988. Spatial and temporal patterns of exotic shrub invasion in an Australian tropical grassland. Landscape Ecology 13:93–102.
- Cain, M. L. 1994. Consequences of foraging in clonal plant-species. Ecology 75:933–944.
- Cain, M. L. , S. W. Pacala , J. A. Silander Jr , and M. J. Fortin . 1995. Neighborhood models of clonal growth in the white clover Trifolium . The American Naturalist 145:888–917.
- Callaway, R. M. , R. W. Brooker , P. Choler , Z. Kikvidze , C. J. Lortie , R. Michalet , L. Paolini , F. I. Pugnaire , B. Newingham , E. T. Aschehoug , C. Armas , D. Kikodze , and B. J. Cook . 2002. Positive interactions among alpine plants increase with stress. Nature 417:844–848.
- Clark, J. S. , E. Macklin , and L. Wood . 1998. Stages and spatial scales of recruitment limitation in southern Appalachian forests. Ecological Monographs 68:213–235.
- Cousins, S. A. and Á Eriksson . 2001. The occurrence of plant biodiversity in a hemiboreal landscape: the effects of habitat and history. Ecography 24:461–469.
- Crawley, M. J. 1986. The population biology of invaders. Philosophical Transactions of the Royal Society of London Series B–Biological Sciences 314:711–731.
- Dalaka, A. and S. Sgardelis . 2006. Life strategies and spatial arrangement of grasses in a Mediterranean ecosystem in Greece. Grass and Forage Science 61:218–231.
- Damman, H. and M. L. Cain . 1998. Population growth and viability analyses of the clonal woodland herb, Asarum canadense . Journal of Ecology 86:13–26.
- Daubenmire, R. 1959. A canopy-coverage method of vegetational analysis. Northwest Science 33:43–64.
- Dietz, H. and G. von Arx . 2005. Climatic fluctuation causes large-scale synchronous variation in radial increments of the main roots of northern hemisphere forbs. Ecology 86:327–333.
- Dullinger, S. , T. Dirnböck , J. Greimler , and G. Grabherr . 2003. A resampling approach for evaluating effects of pasture abandonment on subalpine plant species diversity. Journal of Vegetation Science 14:243–252.
- Edmonston, B. and O. Davies . 1978. Interpreting the negative exponential density gradient. Journal of the Royal Statistical Society, Series A 141:235–241.
- Ellenberg, H. 1988. Vegetation Ecology of Central Europe. Cambridge, New York Cambridge University Press.
- Eriksson, Á , O. Eriksson , and H. Berglund . 1995. Species abundance patterns of plants in Swedish semi-natural pastures. Ecography 18:310–317.
- Eriksson, O. 1993. Dynamics of genets in clonal plants. Trends in Ecology and Evolution 8:313–316.
- Garcia-Ruiz, J. M. , T. Lasanta , P. Ruiz-Flano , L. Ortigosa , S. White , C. Gonzalez , and C. Marti . 1996. Land-use changes and sustainable development in mountain areas: a case study in the Spanish Pyrenees. Landscape Ecology 11:267–277.
- Gordon, D. R. , J. M. Welker , J. W. Menke , and K. J. Rice . 1989. Competition for soil water between annual plants and blue oak ( Quercus douglasii ) seedlings. Oecologia 79:533–541.
- Grace, J. , F. Berninger , and L. Nagy . 2002. Impacts of climate change on the tree line. Annals of Botany 90:537–544.
- Grace, J. B. 1999. The factors controlling species density in herbaceous plant communities: an assessment. Perspectives in Plant Ecology, Evolution and Systematics 2:1–28.
- Guerrero-Campo, J. , F. Alberto , J. Hodgson , J. M. Garcia-Ruiz , and G. Montserrat-Marti . 1999. Plant community patterns in a gypsum area of NE Spain. I. Interactions with topographic factors and soil erosion. Journal of Arid Environments 41:401–410.
- Holling, C. S. 1959. The components of predation as revealed by a study of small mammal predation of the European pine sawfly. Canadian Entomologist 91:293–320.
- Holtmeier, F. K. and G. Broll . 2005. Sensitivity and response of northern hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Global Ecology and Biogeography 14:395–410.
- Jackson, R. B. , J. Canadell , J. R. Ehleringer , H. A. Mooney , O. E. Sala , and E. D. Schulze . 1996. A global analysis of root distributions for terrestrial biomes. Oecologia 108:389–411.
- Jackson, R. B. , J. L. Banner , E. G. Jobbagy , W. T. Pockman , and D. H. Wall . 2002. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418:623–626.
- Jacot, K. A. , A. Lüscher , J. Nösberger , and U. A. Hartwig . 2000. The relative contribution of symbiotic N2 fixation and other nitrogen sources to grassland ecosystems along an altitudinal gradient in the Alps. Plant Soil 225:201–211.
- Julien, M. P. , D. Alard , and G. Balent . 2006. Patterns of ash ( Fraxinus excelsior L.) colonization in mountain grasslands: the importance of management practices. Plant Ecology 183:177–189.
- Jurena, P. N. and S. Archer . 2003. Woody plant establishment and spatial heterogeneity in grasslands. Ecology 84:907–919.
- Kareiva, P. 1982. Experimental and mathematical analysis of herbivore movement: quantifying the influence of plant spacing and quality on foraging discrimination. Ecological Monographs 52:261–282.
- Kareiva, P. 1983. Local movements in herbivorous insects: applying a passive diffusion model to mark-recapture field experiments. Oecologia 57:322–327.
- Kochy, M. and S. D. Wilson . 2000. Competitive effects of shrubs and grasses in prairie. Oikos 91:385–395.
- Körner, C. 1999. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Berlin, Germany Springer-Verlag.
- Lasanta, T. , J. C. González-Hidalgo , S. M. Vicente-Serrano , and E. Sferi . 2006. Using landscape ecology to evaluate an alternative management scenario in abandoned Mediterranean mountain areas. Landscape and Urban Planning 78:101–114.
- League, K. and T. Veblen . 2006. Climatic variability and episodic Pinus ponderosa establishment along the forest-grassland ecotones of Colorado. Forest Ecology and Management 228:98–107.
- MacDonald, D. , J. R. Crabtree , G. Wiesinger , T. Dax , N. Stamou , P. Fleury , J. G. Lazpita , and A. Gibon . 2000. Agricultural abandonment in mountain areas of Europe: environmental consequences and policy response. Journal of Environmental Management 59:47–69.
- Manrique, E. , A. M. Olaizola , A. Bernués , M. T. Maza , and A. Sáez . 1999. Economic diversity of farming systems and possibilities for structural adjustment in mountain livestock farms. Options Mediterranénennes 65:81–94.
- Marinas, A. , R. García-González , A. Aldezabal , S. Palacio , and D. Gómez-García . 2004. Interés ecológico y pastoral del erizón ( Echinospartum horridum (Vahl) Rothm.). In García-Criado, B. , A. García-Ciudad , B. R. Vázquez de Aldana , and I. Zabalgogeazcoa . (eds.). Salamanca, Spain XLIV Reunión Científica de la Sociedad Española para el Estudio de los Pastos. 117–122.
- Martinez, E. and E. Fuentes . 1993. Can we extrapolate the California model of grassland-shrubland ecotone? Ecological Applications 3:417–423.
- McCallum, H. 2000. Population Parameters: Estimation for Ecological Models. Oxford, United Kingdom Blackwell Scientific Publications.
- McPherson, G. R. 1993. Effects of herbivory and herb interference on oak establishment in a semi-arid temperate savanna. Journal of Vegetation Science 4:687–692.
- Montané, F. , P. Rovira , and P. Casals . 2007. Shrub encroachment into mesic mountain grasslands in the Iberian Peninsula: effects of plant quality and temperature on soil C and N stocks. Global Biogeochemical Cycles 21:GB4016.
- Montserrat-Recoder, P. and F. Fillat . 1990. The system of grassland management in Spain. In Breymeyer, A. I. (ed.). Managed Grasslands Regional Studies. Amsterdam, Netherlands Elsevier. 33–70.
- Montserrat-Recoder, P. , J. M. Montserrat-Martí , and G. Montserrat-Martí . 1984. Estudio de las comunidades de Echinospartum horridum en el Pirineo español. Acta Biologica Montana 4:249–257.
- Mottet, A. , S. Ladet , S. Coque , and A. Gibon . 2006. Agricultural land-use change and its drivers in mountain landscapes: a case study in the Pyrenees. Agriculture, Ecosystems and Environment 114:296–310.
- Nesmith, J. C. B. , D. E. Hibbs , and J. P. A. Shatford . 2006. Clonal growth patterns of creeping snowberry ( Symphoricarpos hesperius GN Jones) and trailing blackberry ( Rubus ursinus Cham. and Schlecht) in the western foothills of the Cascade Mountains, Oregon. Northwest Science 80:274–282.
- Olsson, E. G. A. , G. Austrheim , and S. N. Grenne . 2000. Landscape change patterns in mountains, land use and environmental diversity, mid-Norway 1960–1993. Landscape Ecology 15:155–170.
- Pallaruelo, S. 1993. Cuadernos de la trashumancia, número 6: Pirineo Aragonés. Madrid, Spain ICONA.
- Papachristou, T. G. , L. E. Dziba , and F. D. Provenza . 2005. Foraging ecology of goats and sheep on wooded rangelands. Small Ruminant Research 59:141–156.
- Pärtel, M. , R. Mändla , and M. Zobel . 1999. Landscape history of a calcareous (alvar) grassland in Hanila, western Estonia during the last three hundred years. Landscape Ecology 14:187–196.
- Pérez-Cabello, F. and P. Ibarra . 2004. Procesos de regeneración vegetal en comunidades incendiadas (Prepirineo Oscense). In Peña, J. L. , L. A. Longares , and M. Sánchez . (eds.). Geografía física de Aragón, Aspectos generales y temáticos. Zaragoza, Spain Universidad de Zaragoza e Institución Fernando el Católico. 153–162.
- Polley, H. W. , H. S. Mayeux , H. B. Johnson , and C. R. Tischler . 1997. Viewpoint: atmospheric CO2, soil water, and shrub/grass ratios on rangelands. Journal of Range Management 50:278–284.
- Prevosto, B. , A. Robert , and P. Coquillard . 2004. Development of Cytisus scoparius L. at stand and individual level in a mid-elevation mountain of the French Massif Central. Acta Oecologica 25:73–81.
- Pykälä, J. , M. Luoto , R. K. Heikkinena , and T. Kontula . 2005. Plant species richness and persistence of rare plants in abandoned semi-natural grasslands in northern Europe. Basic and Applied Ecology 6:25–33.
- Pyke, D. A. and S. Archer . 1991. Plant-plant interactions affecting plant establishment and persistence on revegetated rangeland. Journal of Range Management 44:550–557.
- Riba, M. , F. X. Pico , and M. Mayol . 2002. Effects of regional climate and small-scale habitat quality on performance in the relict species Ramonda myconi . Journal of Vegetation Science 13:259–268.
- Ribbens, E. , J. A. Silander Jr , and S. W. Pacala . 1994. Seedling recruitment in forests: calibrating models to predict patterns of tree seedling dispersion. Ecology 75:1794–1806.
- Richards, F. J. 1959. A flexible growth function for empirical use. Journal of Experimental Botany 10:290–300.
- Riginos, C. 2009. Grass competition suppresses savanna tree growth across multiple demographic stages. Ecology 90:335–340.
- Roques, K. G. , T. G. O'Connor , and A. R. Watkinson . 2001. Dynamics of shrub encroachment in an African savanna: relative influences of fire, herbivory, rainfall and density dependence. Journal of Applied Ecology 38:268–280.
- Rouget, M. and D. M. Richardson . 2003. Inferring process from pattern in plant invasions: a semi-mechanistic model incorporating propagule pressure and environmental factors. The American Naturalist 162:713–724.
- Sankaran, M. , J. Ratnam , and N. P. Hanan . 2004. Tree-grass coexistence in savannas revisited—Insights from an examination of assumptions and mechanisms invoked in existing models. Ecology Letters 7:480–490.
- Sankaran, M. , N. P. Hanan , R. J. Scholes , J. Ratnam , D. J. Augustine , B. S. Cade , J. Gignoux , S. I. Higgins , X. Le Roux , F. Ludwig , J. Ardo , F. Banyikwa , A. Bronn , G. Bucini , K. K. Caylor , M. B. Coughenour , A. Diouf , W. Ekaya , C. J. Feral , E. C. February , P. G. H. Frost , P. Hiernaux , H. Hrabar , K. L. Metzger , H. H. T. Prins , S. Ringrose , W. Seal , J. Tews , J. Worden , and N. Zambatis . 2005. Determinants of woody cover in African savannas. Nature 438:846–849.
- SAS Institute 2004. SAS/STAT User's Guide. Cary, North Carolina, U.S.A SAS Institute Inc.
- Schlesinger, W. H. , J. F. Reynolds , G. L. Cunningham , L. F. Huenneke , W. M. Jarrell , R. A. Virginia , and W. G. Whitford . 1990. Biological feedbacks in global desertification. Science 247:1043–1048.
- Scholes, R. J. and S. Archer . 1997. Tree-grass interactions in savannas. Annual Review of Ecology and Systematics 28:517–544.
- Schweingruber, F. H. 1990. Anatomy of European Woods: an Atlas for the Identification of European Trees, Shrubs, and Dwarf Shrubs. Bern, Switzerland Paul Haupt.
- Sheppard, A. W. , P. Hodge , Q. Paynter , and M. Rees . 2002. Factors affecting invasion and persistence of broom Cytisus scoparius in Australia. Journal of Applied Ecology 39:721–734.
- Skarpe, C. 1990. Shrub layer dynamics under different herbivore densities in an arid savanna, Botswana. Journal of Applied Ecology 27:873–885.
- Tasser, E. and U. Tappeiner . 2002. Impact of land use changes on mountain vegetation. Applied Vegetation Science 5:173–184.
- Trivedi, M. R. , P. M. Berry , M. D. Morecroft , and T. P. Dawson . 2008. Effects of climate and land-use change on the establishment and growth of cembran pine ( Pinus cembra L.) over altitudinal treeline ecotone in the Central Swiss Alps. Arctic, Antarctic, and Alpine Research 40:225–232.
- Van Kleunen, M. and M. Fischer . 2001. Adaptive evolution of plastic foraging responses in a clonal plant. Ecology 82:3309–3319.
- Ward, D. 2005. Do we understand the causes of bush encroachment in African savannas? African Journal of Range and Forage Science 22:101–105.
- Wearne, L. J. and J. W. Morgan . 2001. Recent forest encroachment into subalpine grasslands near Mount Hotham, Victoria, Australia. Arctic, Antarctic, and Alpine Research 33:369–377.
- Weppler, T. , P. Stoll , and J. Stocklin . 2006. The relative importance of sexual and clonal reproduction for population growth in the long-lived alpine plant Geum reptans . Journal of Ecology 94:869–879.
- Wiegand, K. , D. Saltzb , and D. Ward . 2006. A patch-dynamics approach to savanna dynamics and woody plant encroachment—Insights from an arid savanna. Perspectives in Plant Ecology, Evolution and Systematics 7:229–242.
- Williamson, M. 1996. Biological Invasions. London, United Kingdom Chapman & Hall.