Abstract
Alpine areas of the tropical Andes constitute the largest of all tropical alpine regions worldwide. They experience a particularly harsh climate, and they are fragmented into tropical alpine islands at various spatial scales. These factors generate unique patterns of continental insularity, whose impacts on biodiversity remain to be examined precisely. By reviewing existing literature and by presenting unpublished data on beta-diversity and endemism for a wide array of taxonomic groups, we aimed at providing a clear, overall picture of the isolation-biodiversity relationship in the tropical alpine environments of the Andes. Our analyses showed that (1) taxa with better dispersal capacities and wider distributions (e.g., grasses and birds) were less restricted to alpine areas at local scale; (2) similarity among communities decreased with spatial distance between isolated alpine areas; and (3) endemism reached a peak in small alpine areas strongly isolated from main alpine islands. These results pinpoint continental insularity as a powerful driver of biodiversity in the tropical High Andes. A combination of human activities and warming is expected to increase the effects of continental insularity in the next decades, especially by amplifying the resistance of the lowland matrix that surrounds tropical alpine islands.
Introduction
Tropical alpine environments (TAEs) represent as much as 10% of alpine systems worldwide and display unique environmental characteristics among alpine regions (CitationRundel et al., 1994; CitationKörner, 2003). Among them, low seasonality, inverted rainfall gradients above 3000–3500 m, and the high frequency of freeze-thaw cycles (see CitationAnthelme and Dangles, 2012) set up a peculiar pool of environmental conditions that have shaped the evolution of species assemblages substantially different from those that live in extratropical alpine systems (CitationHedberg and Hedberg, 1979; CitationRamsay and Oxley, 1997; CitationNavas, 2003; CitationJacobsen et al., 2010). For instance, plant growth forms like giant rosettes, giant cushions (, parts A and C), and tussock grasses are more abundant under the tropics than anywhere else in alpine regions (CitationHedberg and Hedberg, 1979; CitationKörner, 2003). Another specificity of TAE is that they develop at higher elevation than any other alpine ecosystem (CitationKörner, 2003), maybe with the exception of dry subtropical regions (CitationHalloy, 1991; CitationMacek et al., 2012). This argument has been commonly cited to generate a higher isolation of alpine systems under the tropics, with fragmented TAE constituting an archipelago of continental islands (CitationSmith and Young, 1987; CitationLuteyn, 1999; CitationHughes and Eastwood, 2006; CitationSklenář et al., 2014).
Yet, the effects of such pronounced continental insularity on biodiversity patterns and dynamics are not straightforward. Some authors argue that continental insularity is one of the main drivers of the high proportion of endemism observed in TAE (CitationLuteyn, 1999), especially because topographical barriers may be more effective than in extratropical regions (CitationJanzen, 1967; CitationRichter et al., 2009). Other authors tend to reduce its influence because the lowlands located between TAE are not only a matrix resistant to the dispersion of alpine taxa, but they may act instead as a source of colonization during climatic fluctuations (e.g., CitationSmith and Young, 1987). The extent to which continental insularity explains patterns of diversity in tropical alpine systems in comparison with oceanic insularity is a topical question that has been poorly addressed so far and certainly deserves more attention given the high levels of biodiversity found in TAE and their high sensitivity to current global changes (CitationLuteyn, 1999; CitationBradley et al., 2006).
FIGURE 1. The elements of biodiversity characteristic of the tropical High Andes. (A) Espeletia pycnophylla (páramo El Angel, Ecuador), a giant rosette-like species adapted to the harsh climate of the páramo; (B) Theristicus melanopis (páramo del Antisana, Ecuador), a bird restricted to high-elevation wetlands; (C) Azorella compacta (Sajama National Park, Bolivia), a giant cushion-like species adapted to the dry climate of the puna; and (D) Telmantobius culeus (Bolivia), a frog endemic to Titicaca Lake. Photos taken by Olivier Dangles.

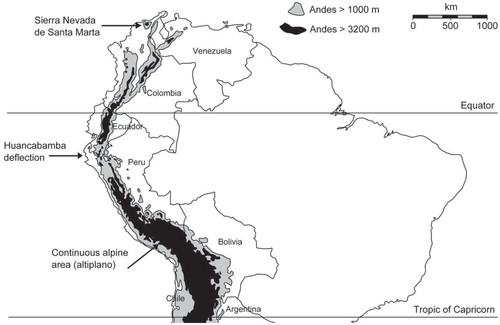
The tropical High Andes represent an ideal study region to examine the effects of continental insularity on biodiversity patterns for several reasons. First, they are particularly representative of TAEs as they gather more than 90% of their area worldwide (CitationJacobsen, 2008a). Second, they shelter a wide latitudinal range of environmental conditions from the humid páramos in the north to the dry puna in the south. Third, they display both highly fragmented alpine areas, as in Colombia and Venezuela (CitationVuilleumier, 1970), and continuous alpine areas in the central Andes, as found in the Peruvian and Bolivian altiplano (). Also, by encompassing Colombia, Venezuela, Ecuador, Peru, Bolivia, North Chile, and North Argentina, tropical Andes are the largest tropical mountain range worldwide, providing opportunities for tropical alpine biodiversity to diverge not only because of habitat fragmentation, but also because of the effects of geographical distance along a continuous topographical unit (CitationHughes and Eastwood, 2006; CitationSklenář et al., 2014).
Continental insularity in the tropical Andes not only is a matter of single isolated summits but also is effective at a coarser, regional scale, with entire mountain ranges being separated from the main cordillera (e.g., Cordillera del Condor in Ecuador, Cordillera Apolobamba in Bolivia, and Sierra Nevada de Santa Marta in Colombia). The most important interruption of Andean tropical alpine regions is the Huancabamba deflection in northern Peru (; CitationLauer, 1968; CitationSimpson, 1975). There, both the Eastern and the Central Cordillera are entirely interrupted and the only mountain chain providing continuous elevation above 2150 m a.s.l. is the very dry mountain range of the Western Cordillera, which may have contributed to increasing contemporary isolation between the southern and the northern tropical Andes, thus being likely a driver of dissimilarities in floristic elements between these two regions (CitationSmith and Cleef, 1988; CitationSklenář et al., 2011). However, current patterns of community dissimilarity between Central and Northern Andes are also indebted to more ancient biogeographical processes, including the effects of a former lowland corridor invaded by wetlands, termed the “Western Andean Portal” at this location (CitationAntonelli and Sanmartín, 2011).
The tropical High Andes display three main features—harsh environmental conditions, large spatial extent, and strong habitat fragmentation—that are expected to greatly influence the organization of biodiversity at both local and regional scales. By analyzing variations in beta-diversity of different plant and animal groups at various spatial scales, the objective of our study is to describe general patterns in the organization and endemism of natural communities in the alpine areas of the tropical High Andes. Beta-diversity among sampling sites is indeed an important criterion for obtaining adequate representation of regional biodiversity (CitationKattan et al., 2006), an issue that has been poorly considered at the scale of the tropical Andes (CitationKessler et al., 2011). We specifically intended to answer the following questions: (1) What proportions of the taxa that live in tropical alpine highlands are strictly restricted to this environment? (2) How does spatial distance between pairs of highlands affect community similarity? (3) How does the spatial configuration of tropical highlands influence the number of endemic species? It is not the objective of this paper to discuss the biogeographical/historical reasons for the present patterns of biodiversity found in tropical alpine islands of the Andes. Rather, our “ecological” approach, by providing an original insight from a different angle, may be used by biogeographers to provide a synthetic overview on these patterns.
Methods
STUDY AREA
The study area was limited to the tropical Andes sensu stricto, from Colombia (Sierra Nevada de Santa Marta; 11°N) to the Southern tropics (23°S; ), thus excluding the tropical alpine regions of Central America. Most of the literature considers that the lower limit of TAE in the Andes occur between 3000 m and 3500 m a.s.l. (CitationVuilleumier, 1970; CitationSmith and Cleef, 1988; CitationRundel et al., 1994; CitationLuteyn, 1999; CitationKörner, 2003). We arbitrary used the elevation 3200 m as the lower limit of TAE, and focused on open environments in our review. However, given that Andean forest in some places develop at higher elevation, we eliminated data collected in mountain forests above 3200 m. As an exception, distribution data for terrestrial arthropods were only available from 3400 m upward (CitationMoret, 2005, unpublished data), which sets the lower limit of alpine areas for this group.
DATABASE
Our database was extracted from the literature and unpublished data, and each study contributed one or several analyses (). Data in the literature had to fulfil two criteria: (1) to be located in tropical alpine regions of the Andes, and (2) to provide clear indices of beta-diversity, or at least raw data, to be considered in our meta-analyses. Among plants, information was provided for spermatophytes (wetland, terrestrial, and the specific group of grasses), pteridophytes, and bryophytes. Among animals, information was provided for aquatic (trichopteran) and terrestrial arthropods (carabids), frogs, reptiles, birds, and mammals. Most data were provided at species level, but some relevant data at genera level were also included in the analyses ().
We then performed three types of analyses. First, at local/country scale we calculated the proportion of taxa restricted to (or precinctive of) alpine areas as the total number of taxa found only above the lower elevational limit of the alpine area divided by the total number of taxa found in the alpine area. Depending on available data, this calculation was applied along specific elevation transects or along elevation transects at country scale (see ).
Second, at regional scale we plotted the community similarity vs. geographical distance relationship among pairs of alpine areas separated by a non-alpine lowland matrix for six taxa in Bolivia (3) and in Ecuador (3; see number of sites and matrices in ). Community similarity was quantified using the Sørensen's index of similarity S between two alpine communities as follows:
where A is the number of species observed in one community, B is the number of species found in another community, and C is the number of species shared by the two communities. The distance between alpine areas was measured using the free software imageJ (http://rsb.info.nih.gov/ij). When the alpine area was clearly dominated by a single summit, we chose this summit as the starting/ending point for the spatial distance. However, when no single summit was easily identified (e.g., Nudo de Cajas, Ecuador) or when several summits of similar importance coexisted in the same area (e.g., Antisana and Cotopaxi, Ecuador), then we selected the center or the equidistance between the summits of the alpine area to perform our measurement.
Third, we compiled data on “strict” endemic species of several alpine areas for three studies that provided particularly clean data (plants and birds in Colombia; Arthropods in Ecuador; 14, 15, and 15 sites, respectively, see ). Strict endemism occurred as soon as one species was restricted to only one alpine area in the whole study area. Strict endemism was put in relation with the spatial extent of each alpine area (ranging from 0.73 km2 to 3487 km2; see ) and with the distance to nearest large alpine area (minimum 200 km2 for plants and birds; 100 km2 for carabid beetles), two of the best predictors of strict endemism as described in the literature (CitationVuilleumier, 1970; CitationSimpson, 1975). Note that for beetles (CitationMoret, 2005, unpublished data) the data set comes from Ecuador where alpine areas are much less fragmented than in Colombia (data on birds and plants; CitationVuilleumier, 1970; CitationSimpson, 1975). Therefore, to make taxonomic groups comparable, we lifted the lower limit of alpine areas of arthropods to 4000 m, which resulted in a similar pattern of habitat fragmentation.
The different nomenclatures used in each of the five countries sampled may generate lists of species composition that are not fully comparable (e.g., the genus Deyeuxia [Poaceae] in Bolivia is described as Calamagrostis in Peru). However, since our analyses did not compare species composition between countries, but rather within each country or each alpine site, this divergence did not affect our data.
STATISTICAL ANALYSES
The similarity—geographic distance relationship was fitted with linear regressions and mean values of similarity were compared across two-sample T-tests. The proportion of animal vs. plant precinctive taxa was also inferred across two-sample T-tests. The relation between strict endemism and the two variables “area” and “distance to nearest large alpine area” was tested for each taxonomic group with stepwise regression (alpha to enter = 0.15; alpha to exit = 0.15; R 2 adjusted when two explaining variables). Results are presented in the form of mean ± standard error. Extrapolation provided by three-dimensional surface plot for strict endemism used the distance method (distance power: 2). All analyses were performed using MINITAB 15.
Results
BIODIVERSITY SPECIFIC TO ALPINE AREAS ()
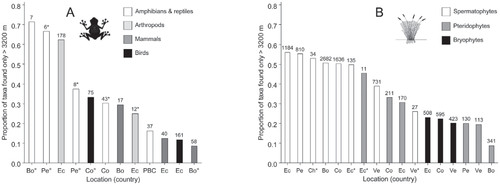
The overall mean proportion of taxa precinctive to alpine areas was 0.35 ± 0.03, ranging from 0.09 (mammals and pteridophytes in Bolivia) to 0.71 (frogs in Bolivia). No significant differences were observed between animal and plant taxa (two-samples T-tests: p > 0.05). Among animals, the most precinctive taxa were amphibians/reptiles (0.71 in Bolivia and 0.67 in Peru) and terrestrial arthropods (0.62 in Ecuador). In comparison, mammals and birds displayed lower precinctive scores, from 0.09 to 0.33. Among plants, the six most precinctive scores concerned spermatophytes, with proportions ranging from 0.50 to 0.56. The lowest value of precinctive taxa among spermatophytes was obtained for Poaceae (0.26). Pteridophytes were less restricted to alpine areas, but their proportion of precinctive taxa were highly variable (average value: 0.26 ± 0.05, ranging from 0.09 to 0.45). Bryophytes were overall the less precinctive taxon and had an average value of 0.22 ± 0.01 (three studies).
Local studies provided a larger proportion of precinctive taxa than studies at the scale of a whole country. This was observed (1) for amphibians where the three most precinctive studies were supported by local data, (2) for birds (local study: 0.33, study at country level: 0.12), and (3) for pteridophytes, with the only study at local scale providing higher precinctive proportion than any other study at country scale (0.45 versus an average value of 0.22). We observed no clear effect of latitude or country on precinctive scores (one-way ANOVAs on both variables: p > 0.05).
COMMUNITY SIMILARITY BETWEEN ALPINE AREAS: EFFECTS OF DISTANCE ()
The overall similarity between communities from disjointed alpine areas was highest for amphibians, terrestrial plants, and reptiles (mean value of Sørensen's indices: 0.41 ± 0.07, 0.35 ± 0.03, and 0.34 ± 0.06, respectively); intermediate in wetland plants (0.22 ± 0.03); and lowest in mammals and arthropods (0.14 ± 0.04 and 0.16 ± 0.02, respectively). Similarity between communities reduced significantly with spatial distance between alpine areas for each taxonomic group (R 2 ranging from 0.18 to 0.53; p ≤ 0.05), except for amphibians (R 2 = 0.05; p > 0.05). Among plants, average similarity between terrestrial plants exceeded similarity between wetland plants (0.35 ± 0.03 vs. 0.22 ± 0.03), but pairwise distance correlated better with similarity for terrestrial plants than for wetland plants (R 2 = 0.53 and R 2 = 0.28, respectively). Among animals, the best relationship between distance and community similarity was obtained with reptiles and carabids (R 2 = 0.47 and R 2 = 0.38, respectively; p ≤ 0.001), whereas distance explained 18% of variation in the similarity observed in mammal communities (p ≤ 0.05).
TABLE 1 Studies incorporated in the database for meta-analyses (See and for detailed information).
DRIVERS OF STRICT ENDEMISM IN ISOLATED ALPINE AREAS ()
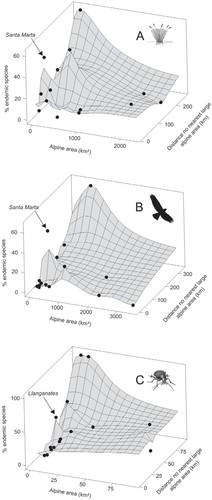
The average proportion of strict endemics in selected alpine areas was 0.32 ± 0.09 for carabid beetles (terrestrial arthropods) in Ecuador, 0.21 ± 0.05 for plants in Colombia, and 0.13 ± 0.05 for birds in Colombia (see ). In each group, the maximum proportion of strict endemism was observed (1) in the smallest alpine areas, and (2) at the most remote sites from nearest large alpine areas. Both variables explained 33% of the strict endemism in plants (stepwise regression; R 2 adjusted = 0.33, p ≤ 0.05). However, only the “distance to nearest large alpine area” term, and not “area,” had a significant effect on the observed number of strict endemics in carabids and birds (stepwise regressions; arthropods: R 2 = 0.61, p ≤⃒ 0.001; birds: R 2 = 0.47, p ≤ 0.01). In each group, a secondary peak of strict endemism was observed in small alpine areas with short distance to the nearest large alpine area. In plants and birds, this peak referred to data from the Sierra Nevada de Santa Marta (Colombia), while in carabids it was sustained by data from the páramo ‘Llanganates’; (Ecuador).
FIGURE 3. Proportion of taxa precinctive to tropical alpine areas in the Andes among the total number of taxa present in these areas. (A) animals (mammals, birds, reptiles/amphibians, arthropods: carabid beetles and aquatic trichopteran) and (B) plants (terrestrial and wetland spermatophytes, pteridophytes, bryophytes). All data at species level except of *genus level. Numbers on top of histograms refer to taxonomic richness. Co: Colombia; Ve: Venezuela; Ec: Ecuador; Pe: Peru; Bo: Bolivia; Ch: Chile; PBC: Peru + Bolivia + Chile. °Study conducted at a local level (e.g. elevation transect).
Discussion
CONTINENTAL INSULARITY AT VARIOUS SCALES EXPLAINS BIODIVERSITY PATTERNS
In contrast with patterns of plant species richness, which are poorly related to current degree of isolation and extent of tropical alpine areas of the Andes (see review in CitationSmith and Young, 1987), our data demonstrate that beta-diversity and endemism in the tropical alpine islands of the Andes are certainly dependent on the current degree of continental insularity, at various spatial scales.
At local/country scale, elevation shifts from the Andean forests to alpine areas (3000–3500 m) resulted in the existence of alpine communities with more than one-third of precinctive taxa (), thus revealing a strong environmental barrier between these two types of ecosystems. Contrastingly to other alpine environments, TAEs experience unusual abiotic constraints such as high frequency of freeze-thaw cycles, edaphic and atmospheric aridity overall stronger than in other alpine regions due to the absence of protective snow cover, and/or the inversion of precipitation gradients (see review in CitationAnthelme and Dangles, 2012). In some taxonomic groups (e.g., spermatophytes), these constraints are easily noticeable in the development of peculiar growth forms, such as giant rosettes of giant cushion-like species (, parts A and C), which are well adapted to these specific constraints (CitationMonasterio and Sarmiento, 1991; CitationKleier and Rundel, 2009; CitationAnthelme et al., 2012), and by physiological traits particularly resistant to low temperature and water stress (e.g., CitationRundel et al., 2003; CitationMacek et al., 2009; CitationSklenář et al., 2010; CitationAlmeida et al., 2013). Lower precinctive scores in pteridophytes and bryophytes may be indebted to the higher dispersal capacities of these taxa combined with an overall high tolerance and/or resistance to desiccation (CitationSakai and Larcher, 1987; CitationMuñoz et al., 2004; CitationAnthelme et al., 2011), which may permit species to occupy wider vertical distribution ranges. However, higher endemism in bryophytes observed in the northern Andes than in the central Andes (CitationChurchill, 2009) suggests that continental insularity also affects the diversity of this group. Concerning animals, while taxon richness generally decreases with elevation (CitationJacobsen, 2008b; CitationHerzog et al., 2011, and reference therein), the highland fauna, however, is not simply an attenuated version of the adjacent lowland fauna and many taxa are specific to high-elevation sites (e.g., the frogs of the genera Atelopus [CitationNavas, 2003], the rodent Thomasomys vulcani [CitationTirira, 2007], the bird Theristicus melanopis [CitationRidgely and Greenfield, 2006]; , part B). Moreover, the lower proportion of precinctive taxa in birds and mammals in comparison with amphibians, reptiles, and arthropods is likely due to group-specific differences in (1) mobility (CitationBrown and Lomolino, 1998) and (2) a tolerance to greater climatic constraints at higher elevation, both in favor of endotherm mammals and amphibians (CitationLaurance et al., 2011). From this viewpoint, carabids considered in our study are not fully representative of terrestrial arthropods, which may be expected to display overall lower proportions of precinctive taxa (CitationGonzalez and Engel, 2004). However, morphological adaptations such as wing atrophy or smaller body size are known from a number of tropical alpine insects, increasing their possibility to adapt to local conditions by finding sheltered microhabitats, but limiting their dispersion to adjacent lowlands (CitationSømme, 1989). Specialization to high elevation habitats can also occur in highly mobile organisms such as birds, for which ca. 15–20 species are limited to Polylepis woodlands above 3500 m a.s.l. in the Peruvian and Bolivian puna (e.g., Oreomanes fraseri, which feed exclusively on branches and trunks of Polylepis; CitationFjeldsa, 1993). However, from a methodological viewpoint, we must keep in mind that variation in the area sampled has a substantial influence on the proportion of precinctive taxa. The reduction of this proportion at country scale might be the result of a “compensation effect” with taxa precinctive on a single elevation gradient becoming ubiquitous at country scale.
FIGURE 4. Relationships between community similarity and spatial distance between pairs of alpine areas for (A) animals; (B) plants. R2 extracted from linear regressions for each taxonomic group. Similarity expressed with Sørensen's index.
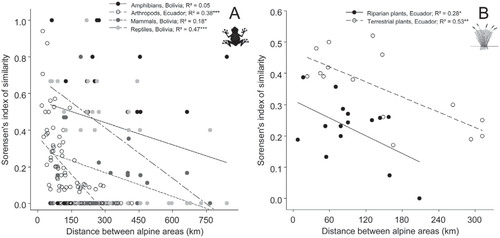
At regional scale, isolation between pairs of islands has frequently been cited to increase beta-diversity in the tropical High Andes (CitationSmith and Young, 1987; CitationLuteyn, 1999; CitationHughes and Eastwood, 2006). Our data corroborate this opinion for a large pool of taxonomic groups, with distance among tropical alpine islands being negatively correlated with the similarity between alpine communities (except for amphibians). In plants, terrestrial communities are without surprise more similar than communities restricted to humid ecosystems (wetland plants), which are much highly scattered in the High Andes. But the reduced influence of distance on the community similarity of the high Andean wetlands is an original result, which may deserve further investigation (existence of a relatively homogeneous wetland plant community at the scale of the tropical Andes?). Interestingly, when considering only the Poaceae family among terrestrial plants (which is one of the families with the most efficient propagule dispersion capacities across wind dispersal), similarity is much higher than in other studies on spermatophytes. Similarly, wind pollination capacities have been shown to reduce the effects of isolation by distance in the High Andes (CitationSchmidt-Lebuhn et al., 2007). Accordingly, seed dispersal and pollination capacity (by wind) are certainly major drivers of plant beta-diversity in isolated alpine islands of the Andes, as initially suggested by Hedberg (Citation1969). Among others, it explains why one of the most isolated alpine areas in the tropical Andes, the Sumaco Volcano in Ecuadorian Amazonia, is largely dominated by Poaceae and more generally speaking by plants with high seed dispersal capacities (CitationLøjtnant and Molau, 1983).
Among animals, the overall pattern of similarity between pairs of communities in the Bolivian puna may appear counterintuitive as (non-volant) mammals display a stronger similarity among alpine islands than amphibians and reptiles, which generally have lower dispersal capacities. As proposed by Tarifa et al. (Citation2007), such pattern can be explained by (1) a reduced species pool of amphibians and reptiles in the dry puna, (2) the occurrence of a few cosmopolitan species, and (3) a better adaptation of mammals to specific niches in alpine areas. However, distance-similarity relationships may differ in more humid, high-elevation locations of the tropical Andes, such as in the Colombian and Ecuadorian páramos, which are known to shelter site-specific amphibian communities (CitationNavas, 2003; CitationRon et al., 2012). Also, species of the frog genera Telmatobius (see , part D) are known to be highly endemic from specific locations, with species turnover taking place within a few tens or, at most, hundreds of kilometers (CitationDe La Riva et al., 2005). Overall, our knowledge of the factors influencing community similarity among alpine areas is likely to be influenced by the size of the species pool considered and also strongly limited by the patchiness of available information in the tropical Andes.
In addition to variation in elevation at local scale and distance between pairs of alpine islands, two other variables—“distance to nearest large alpine area” and to a lesser extent “alpine area”—were positively correlated with strict endemism for the three studied groups (arthropods, plants, birds). This finding supports our argumentation that the current degree of isolation of continental insularity in the tropical Andes is a strong driver of the organization of beta-diversity (CitationVuilleumier, 1970; CitationSimpson, 1975).
FIGURE 5. Proportion of strict endemism found in three taxonomic groups in each alpine area, in relation to the extent of the alpine area and the distance to the nearest large alpine area. (A) Plants in Colombia (>3200 m); (B) birds in Colombia (>3200 m); (C) arthropods (carabid beetles) in Ecuador (>4000 m). Black points represent the data used for extrapolation in the 3D surface plots.
CONTINENTAL INSULARITY DEPENDS ON THE RESISTANCE OF THE MATRIX
The existence of secondary peaks of strict endemism in relatively small areas such as the Sierra Nevada de Santa Marta, which is separated from the main Cordillera by a very low lowland matrix (, parts A and B), suggests that lowland matrices likely have a key influence on beta-diversity patterns. This is supported by various observations on TAE plant communities in East Africa (CitationHedberg, 1969), Ecuador (CitationLøjtnant and Molau, 1983), and Colombia (CitationSklenář and Balslev, 2005), and on arthropod communities in Ecuador (CitationMoret, 2005). In contrast to oceanic islands where the quality of the matrix is homogenous, continental insularity implies different degrees of matrix resistances among isolated alpine areas. As shown above, the resistance may primarily be dependent on elevation, with lowlands being barriers, but also opportunities for colonization, especially under the effects of large-scale climatic fluctuations (CitationSmith and Young, 1987; CitationSklenář et al., 2011). Nevertheless, other factors may influence the resistance of the continental matrix, among which are human activities such as the rapidly advancing agricultural frontier at higher elevations (CitationHedberg, 1969; CitationSklenář and Ramsay, 2001; CitationMorales and Sarmiento, 2002). Furthermore, this resistance differs among groups, as for plants it is usually lethal landing outside of TAE during dispersal event, while for animals it does not have to be.
MULTIPLE DRIVERS OF CONTINENTAL INSULARITY
The purpose of this study was to examine the effects of contemporary isolation by distance, area, and variation in elevation on the organization of biodiversity in the tropical Andes. However, apart from biogeographical and historical drivers, whose role on biodiversity organization in the High Andes has been amply documented (e.g., CitationFjeldsa, 1993; CitationRichter et al., 2009; CitationSklenář et al., 2011), other factors would certainly deserve more attention when trying to provide a synthetic explanation of observed diversity patterns in the tropical Andes. For example, local climatic variations, especially those observed between western and eastern sides of large Andean summits, are potential drivers of isolation (e.g., see dry and isolated “Arenal Grande” on the western side of Chimborazo, Ecuador; CitationMoret, 2009; CitationSklenář and Ramsay, 2001; CitationKessler et al., 2011). Also, the presence of large lakes (Lake Titicaca; ancient lake Minchin) was cited as a potential factor of isolation in the altiplano (CitationSimpson, 1975). As said before, human activities are expected to modify the degree of isolation of alpine areas by making the surrounding matrix more resistant to migration of natural species, but also by promoting the transport and introduction of species adapted to such disturbances (e.g., invasive species; CitationPauchard et al., 2009).
From this viewpoint, the secondary peaks of strict endemism that have been observed in several small alpine areas, despite their short distance to the nearest large alpine area (), deserve a special attention. For example, the Llanganates area in central Ecuador displays an outstanding 50% of strict endemic Carabid beetles above 4000 m (Moret, unpublished data), which cannot be explained by the existence of a topographical barrier. In this case, future studies should consider a combination of other factors, such as wet microclimate, absence of anthropogenic perturbation, specific soil properties, and most of all absence of Pleistocene and Holocene volcanic activity.
Finally, because of their high elevation, tropical alpine regions of the Andes are probably one of the terrestrial ecosystems that will face the highest warming up to 2100 (CitationBradley et al., 2006). In this context, species that occur in alpine areas may be especially prone to extinction under the effects of climate change because (1) they inhabit environments with a relatively low total colonizable area and have nowhere to migrate upward (CitationGosling et al., 2009), and (2) the rapid shift of the upper limits of alpine areas to higher elevation increases the isolation of contiguous viable populations (CitationJørgensen et al., 2011; CitationLarsen et al., 2011; CitationLaurance et al., 2011; CitationVelásquez-Tibatá et al., 2013). It must be taken into account, however, that species at higher elevations may be able to reduce their extinction risk by having possibly larger vertical distribution ranges (e.g., CitationHerzog et al., 2013). Further research in the line of that presented in this paper is urgently needed to better predict the response of the biodiversity of high mountains to rapid anthropogenic changes in the tropical Andes.
Acknowledgments
We warmly thank Carlos Boada, Michael Kessler, Santiago Ron, the Herbario Nacional de Bolivia (LPB), and the Missouri Botanical Garden for providing us with unpublished information that consistently improved our database. We are also grateful to James Juvik for inviting Fabien Anthelme to the international conference “Vulnerable Islands in the Sky: Science and Management of Tropical Island Alpine & Sub-alpine Ecosystems” held in Hawai‘i in August 2012. Macek was supported by MSMT LM2010009 CzechPolar.
References Cited
- Almeida, J. P. , Montúfar, R. , and Anthelme, F. , 2013: Patterns and origin of intraspecific functional variability in a tropical alpine species along an altitudinal gradient. Plant Ecology & Diversity , 6: 423–433.
- Anthelme, F. , and Dangles, O. , 2012: Plant-plant interactions in tropical alpine environments. Perspectives in Plant Ecology, Evolution and Systematics , 14: 363–372.
- Anthelme, F. , Abdoulkader, A. , and Viane, R. , 2011: Are ferns in arid environments underestimated? Contribution from the Saharan Mountains. Journal of Arid Environments , 75: 516–523.
- Anthelme, F. , Buendia, B. , Mazoyer, C. , and Dangles, O. , 2012: Unexpected mechanisms sustain the stress gradient hypothesis in a tropical alpine environment. Journal of Vegetation Science , 23: 62–72.
- Antonelli, A. , and Sanmartín, I. , 2011: Why are there so many plant species in the Neotropics? Taxon , 60: 403–414.
- Bradley, R. S. , Vuille, M. , Diaz, H. F. , and Vergara, W. , 2006: Threats to water supply in the tropical Andes. Science , 312: 1755–1756.
- Brown, J. H. , and Lomolino, M. V. , 1998: Biogeography. Sunderland, MA: Sinauer.
- Churchill, S. P. , 2009: Moss diversity and endemism of the Tropical Andes. Annals of the Missouri Botanical Garden , 96: 434–449.
- Cortez-Fernandez, C. , 2006: Variación altitudinal de la riqueza y abundancia relativa de los anuros del Parque Nacional y Área Natural de Manejo Integrado Cotapata. Ecología en Bolivia , 41: 46–64.
- De la Riva, I. , Aparicio, J. , and Ríos, J. N. , 2005: New species of Telmatobius (Anura: Leptodactylidae) from humid páramo of Peru and Bolivia. Journal of Herpetology , 39: 409–416.
- Fjeldsa, J. , 1993: The avifauna of the Polylepis woodlands of the Andean highlands: the efficiency of basing conservation priorities on patterns of endemism. Bird Conservation International , 3: 37–55.
- Gonzalez, V. H. , and Engel, M. S. , 2004: The tropical Andean bee fauna (Insecta: Hymenoptera: Apoidea), with examples from Colombia. Entomologische Abhandlungen , 62: 65–75.
- Gosling, W. D. , Hanselman, J. A. , Knox, C. , Valencia, B. G. , and Bush, M. B. , 2009: Long term drivers of change in Polylepis woodland distribution in the central Andes. Journal of Vegetation Science , 20: 1041–1052.
- Halloy, S. , 1991: Islands of life at 6000 m altitude-the environment of the highest autotrophic communities on Earth (Socompa Volcano, Andes). Arctic and Alpine Research , 23: 247–262.
- Hedberg, I. , and Hedberg, O. , 1979: Tropical-alpine life-forms of vascular plants. Oikos , 33: 297–307.
- Hedberg, O. , 1969: Evolution and speciation in a tropical high mountain flora. Biological Journal of the Linnean Society , 1: 135–148.
- Herzog, S. K. , Martínez, R. , Jørgensen, P. M. , and Tiessen, H. , 2011: Climate Change and Biodiversity in the Tropical Andes. São Josédos Campos, São Paulo, Brazil: Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE).
- Herzog, S. K. , Hamel-Leigue, A. C. , Larsen, T. H. , Mann, D. J. , Soria-Auza, R. W. , Gill, B. D. , Edmonds W. D. , and Spector, S. , 2013: Elevational distribution and conservation biogeography of phanaeine dung beetles (Coleoptera: Scarabaeinae) in Bolivia. PloS One , 8: e64963, doi: http://dx.doi.org/10.1371/journal.pone.0064963.
- Hughes, C. , and Eastwood, R. , 2006: Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences of the United States of America , 103: 10334–10339.
- Jacobsen, D. , 2000: Gill size of trichopteran larvae and oxygen supply in streams along a 4000 m gradient of altitude. Journal of the North American Benthological Society , 19: 329–343.
- Jacobsen, D. , 2008a: Tropical high-altitude streams. In Dudgeon, D. (ed.), Tropical Stream Ecology. Elsevier Science, 219–256.
- Jacobsen, D. , 2008b: Low oxygen pressure as a driving factor for the altitudinal decline in taxon richness of stream macroinvertebrates. Oecologia , 154: 795–807.
- Jacobsen, D. , and Terneus, E. , 2001: Aquatic macrophytes in cool aseasonal and seasonal streams: a comparison between Ecuadorian highland and Danish lowland streams. Aquatic Botany , 71: 281–295.
- Jacobsen, D. , Dangles, O. , Andino, P. , Espinosa, R. , Hamerlík, L. , and Cadier, E. , 2010: Longitudinal zonation of macroinvertebrates in an Ecuadorian glacier-fed stream: do tropical glacial systems fit the model? Freshwater Biology , 55: 1234–1248.
- Janzen, D. H. , 1967: Why mountain passes are higher in the Tropics? The American Naturalist , 101: 233–249.
- Jones, M. M. , Cicuzza, D. , Straaten, O. , Veldkamp, E. , and Kessler, M. , 2014: Determinants of fern and angiosperm herb community structure in lower montane rainforest in Indonesia. Journal of Vegetation Science , early online, http://dx.doi.org/10.1111/jvs.12181.
- Jørgensen, P. M. , Ulloa Ulloa, C. , Leon, B. , Leon-Yanez, S. , Beck, S. G. , Nee, M. , Zarucchi, J. L. , Celis, M. , Bernal, R. , and Gradstein, R. , 2011: Regional patterns of vascular plant diversity and endemism. In Herzog, S. K. , Martínez, R. , Jørgensen, P. M. , and Tiessen, H. (eds.), Climate Change and Biodiversity in the Tropical Andes. São Josédos Campos, São Paulo, Brazil: Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE), 192–203.
- Kattan, G. H. , Franco, P. , Saavedra-Rodriguez, C. A. , Valderrama, C. , Rojas, V. , Daniel, O. , and Jesus, M. , 2006: Spatial components of bird diversity in the Andes of Colombia: implications for designing a regional reserve system. Conservation Biology , 20: 1203–1211.
- Kessler, M. , 2002: The elevational gradient of Andean plant endemism: varying influences of taxon-specific traits and topography at different taxonomic levels. Journal of Biogeography , 29: 1159–1165.
- Kessler, M. , Grytnes, J. A. , Halloy, S. R. P. , Kluge, J. , Krömer, T. , Leon, B. , Macia, M. J. , and Young, K. R. , 2011: Gradients of plant diversity: Local patterns and processes. In Herzog, S. K. , Martínez, R. , Jørgensen, P. M. , and Tiessen, H. (eds.), Climate Change and Biodiversity in the Tropical Andes. São Josédos Campos, São Paulo, Brazil: Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE), 204–219.
- Kleier, C. , and Rundel, P. , 2009: Energy balance and temperature relations of Azorella compacta, a high-elevation cushion plant of the central Andes. Plant Biology , 11: 351–358.
- Körner, C. , 2003: Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems , Second edition. Berlin: Springer-Verlag.
- Krabbe, N. , Flórez, P. , Suárez, G. , Castaño, J. , Arango, J. D. , and Duque, A. , 2006: The birds of the páramo de Frontino, Western Andes of Colombia. Ornitología Colombiana , 4: 39–50.
- Larsen, T. H. , Brehm, G. , Navarrete, H. , Franco, P. , Gomez, H. , Mena, J. L. , Morales, V. , Argollo, J. , Blacutt, L. , and Canhos, V. , 2011: Range shifts and extinctions driven by climate change in the tropical Andes: synthesis and directions. In Herzog, S. K. , Martínez, R. , Jørgensen, P. M. , and Tiessen, H. , (eds.), Climate Change and Biodiversity in the Tropical Andes. São Josédos Campos, São Paulo, Brazil: Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE), 47–67.
- Lauer, W. , 1968: Problemas de la división fitogeográfica en América Central. In Troll, C. (ed.), Geo-ecology of the Mountainous Regions of the Tropical Americas. Bonn: Geographisches Institut, University of Bonn, 139–156.
- Laurance, W. F. , Useche, D. C. , Shoo, L. P. , Herzog, S. K. , Kessler, M. , Escobar, F. , Brehm, G. , Axmacher, J. C. , Chen, I. C. , Gamez, L. A. , Hietz, P. , Fiedler, K. , Pyrcz, T. , Wolf, J. , Merkord, C. L. , Cardelus, C. , Marshall, A. R. , Ah-Peng, C. , Aplet, G. H. , Arizmendi, M. D. , Baker, W. J. , Barone, J. , Bruhl, C. A. , Bussmann, R. W. , Cicuzza, D. , Eilu, G. , Favila, M. E. , Hemp, A. , Hemp, C. , Homeier, J. , Hurtado, J. , Jankowski, J. , Kattan, G. , Kluge, J. , Kromer, T. , Lees, D. C. , Lehnert, M. , Longino, J. T. , Lovett, J. , Martin, P. H. , Patterson, B. D. , Pearson, R. G. , Peh, K. S. H. , Richardson, B. , Richardson, M. , Samways, M. J. , Senbeta, F. , Smith, T. B. , Utteridge, T. M. A. , Watkins, J. E. , Wilson, R. , Williams, S. E. , and Thomas, C. D. , 2011: Global warming, elevational ranges and the vulnerability of tropical biota. Biological Conservation , 144: 548–557.
- Løjtnant, B. , and Molau, U. , 1983: Analysis of a virgin páramo plant community on Volcán Sumaco, Ecuador. Nordic Journal of Botany , 2: 567–574.
- Luteyn, J. L. , 1999: Páramos: a checklist of plant diversity, geographical distribution, and botanical literature. Memoirs of the New York Botanical Garden , 84: 1–278.
- Macek, P. , Macková, J. , and de Bello, F. , 2009: Morphological and ecophysiological traits shaping altitudinal distribution of three Polylepis treeline species in the dry tropical Andes. Acta Oecologica , 35: 778–785.
- Macek, P. , Klimeš, L. , Adamec, L. , Doležal, J. , Chlumská, Z. , de Bello, F. , Dvorský, M. , and Řeháková, K. , 2012: Plant nutrient content does not simply increase with elevation under the extreme environmental conditions of Ladakh, NW Himalaya. Arctic, Antarctic, and Alpine Research , 44: 62–66.
- Márquez, E. , Fariñas, M. R. , Briceño, B. , and Rada, F, 2004: Distribution of grasses along an altitudinal gradient in a Venezuelan páramo. Revista Chilena de Historia Natural , 77: 649–660.
- Monasterio, M. , and Sarmiento, L. , 1991: Adaptive radiation of Espeletia in the cold Andean tropics. Trends in Ecology & Evolution , 6: 387–391.
- Morales, J. , and Sarmiento, L. , 2002: Dinámica de los macroinvertebrados edáficos y su relación con la vegetación en una sucesión secundaria en el páramo Venezolano. Ecotropicos , 15: 99–110.
- Moret, P. , 2005: Los coleópteros Carabidae del páramo en los Andes del Ecuador. Sistemática, ecología y biogeografía. Quito, Ecuador: Pontificia Universidad Católica del Ecuador, Monografía 2.
- Moret, P. , 2009: Altitudinal distribution, diversity and endemicity of Carabidae (Coleoptera) in the páramos of Ecuadorian Andes. Annales de la Société Entomologique de France , 45: 500–510.
- Muñoz, J. , Felicísimo, Á. M. , Cabezas, F. , Burgaz, A. R. , and Martínez, I. , 2004: Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science , 304: 1144–1147.
- Navas, C. A. , 2003: Herpetological diversity along Andean elevational gradients: links with physiological ecology and evolutionary physiology. Comparative Biochemistry and Physiology , 133: 469–485.
- Novillo, A. , and Ojeda, R. A. , 2012: Diversity and distribution of small mammals in the South American Dry Andes. Austral Ecology , 37: 758–766.
- Pauchard, A. , Kueffer, C. , Dietz, H. , Daehler, C. C. , Alexander, J. , Edwards, P. J. , Arévalo, J. R. , Cavieres, L. , Guisan, A. , Haider, S. , Jakobs, G. , McDougall, K. L. , Millar, C. I. , Naylor, B. J. , Parks, C. G. , Rew, L. J. , and Seipel, T. , 2009: Ain't no mountain high enough: plant invasions reaching new elevations. Frontiers in Ecology and the Environment , 7: 479–486.
- Ramsay, P. M. , and Oxley, E. R. B. , 1997: The growth form composition of plant communities in the Ecuadorian páramos. Plant Ecology , 131: 173–192.
- Richter, M. , Diertl, K. H. , Emck, P. , Peters, T. , and Beck, E. , 2009: Reasons for an outstanding plant diversity in the tropical Andes of Southern Ecuador. Landscape Online , 12: 1–35.
- Ridgely, R. S. , and Greenfield, P. J. , 2006: Aves del Ecuador. Quito, Ecuador: Fundación Jocotoco y Academia de Ciencias de Philadelphia.
- Ron, S. R. , Coloma, L. A , Guayasamin, J. M. , and Yanez-Muñoz, M. H. , 2012: AmphibiaWebEcuador. Version 2012.0. Museo de Zoología, Pontificia Universidad Católica del Ecuador. http://zoologia.puce.edu.ec/Vertebrados/anfibios/AnfibiosEcuador.
- Rundel, P. W. , Smith, A. P. , and Meinzer, F. C. , 1994: Tropical Alpine Environments: Plant Form and Function. Cambridge University Press.
- Rundel, P. W. , Gibson, A. C. , Midgley, G. S. , Wand, S. J. E. , Palma, B. , Kleier, C. , and Lambrinos, J. , 2003: Ecological and ecophysiological patterns in a pre-altiplano shrubland of the Andean Cordillera in northern Chile. Plant Ecology , 169: 179–193.
- Sakai, A. , and Larcher, W. , 1987: Frost Survival of Plants. Responses and Adaptation to Freezing Stress. Berlin: Springer, Ecological Studies 62.
- Schmidt-Lebuhn, A. N. , Seltmann, P. , and Kessler, M. , 2007: Consequences of the pollination system on genetic structure and patterns of species distribution in the Andean genus Polylepis (Rosaceae): a comparative study. Plant Systematics and Evolution , 266: 91–103.
- Simpson, B. B. , 1975: Pleistocene changes in the flora of the high tropical Andes. Paleobiology , 1: 273–294.
- Sklenář, P. , and Balslev, H. , 2005: Superpáramo plant species diversity and phytogeography in Ecuador. Flora , 200: 416–433.
- Sklenář, P. , and Jørgensen, P. M. , 1999: Distribution patterns of páramo plants in Ecuador. Journal of Biogeography , 26: 681–692.
- Sklenář, P. , and Ramsay, P. M. , 2001: Diversity of zonal páramo plant communities in Ecuador. Diversity and Distribution , 7: 113–124.
- Sklenář, P. , Kučerová, A. , Macek, P. , and Macková. J. , 2010: Does plant height determine the freezing resistance in the páramo plants? Austral Ecology , 35: 929–934.
- Sklenář, P. , Dušková, E. , and Balslev, H. , 2011: Tropical and temperate: evolutionary history of páramo flora. The Botanical Review , 77: 71–108.
- Sklenář, P. , Hedberg, I. , and Cleef, A. M. , 2014: Island biogeography of tropical alpine floras. Journal of Biogeography , 41(2): 287–297, doi: http://dx.doi.org/10.1111/jbi.12212.
- Smith, A. P. , and Young, T. P. , 1987: Tropical alpine plant ecology. Annual Review of Ecology and Systematics , 18: 137–158.
- Smith, J. M. B. , and Cleef, A. M. , 1988: Composition and origin of the world's tropical alpine floras. Journal of Biogeography , 15: 631–645.
- Sømme, L. , 1989: Adaptations of terrestrial arthropods to the alpine environment. Biological Review , 64: 367–407.
- Soria, A. R. W. , and Kessler, M. , 2008: The influence of sampling intensity on the perception of the spatial distribution of tropical diversity and endemism: a case study of ferns from Bolivia. Diversity and Distributions , 14: 123–130.
- Tarifa, T. , Aparicio, J. , and Yensen, E. , 2007: Mammals, amphibians, and reptiles of the Bolivian High Andes: an initial comparison of diversity patterns in Polylepis woodlands. In Kelt, D. A. , Lessa, E. P. , Salazar-Bravo, J. , and Patton, J. L. (eds.), The Quintessential Naturalist: Honoring the Life and Legacy of Olivier P. Pearson. Berkeley: University of California Publications, 241–274.
- Tirira, D. G. , 2007: Guía de campo de los mamíferos del Ecuador. Quito, Ecuador: Ediciones Murciélago Blanco.
- Velásquez-Tibatá, J. , Salaman, P. , and Graham, C. H. , 2013: Effects of climate change on species distribution, community structure, and conservation of birds in protected areas in Colombia. Regional Environmental Change , doi: http://dx.doi.org/10.1007/s10113-012-0329-y.
- Villagran, C. , Armesto, J. J. , and Arroyo, M. T. K. , 1981: Vegetation in a high Andean transect between Turi and Cerro Leon in northern Chile. Vegetatio , 48: 3–16.
- Vuilleumier, F. , 1970: Insular biogeography in continental regions. 1. Northern Andes of South America. The American Naturalist , 104: 373–388.
- Wallace, R. , Gómez, H. , Porcel, Z. , and Rumiz D. , 2010: Distribución, ecología y conservación de los mamíferos medianos y grandes de Bolivia. Santa Cruz, Bolivia: Centro de Ecología Difusión Simón I. Patiño.
APPENDIX
TABLE A1 Raw data for analyses of Sorensen's similarity between communities, at regional scale (see also for data source).
Table
Table
Table
Table
Table
TABLE A2 data used for analyses on strict endemism (see also for data source).
Table