Abstract
Treeline expansion is reported as a widespread response to rising temperatures, yet few studies have considered the impact of treeline advance on the diversity and function of high altitude systems. Evidence suggests that climate change is already having a negative impact on alpine diversity and is modifying functions such as carbon sequestration and nutrient cycling. Treeline advance is likely to further affect diversity and function, yet our understanding of the processes involved is limited. Here we review and synthesize literature that assesses the impact of treeline advance into treeless ecosystems. Using published literature, we explore to what extent treeline advance will lead to the displacement of alpine species and the fragmentation of alpine habitats. While large changes will be observed in the ecosystems above the current treeline as trees migrate, it is likely that these newly forested areas will deviate substantially from the established forests from which they have developed. Consequently, at the forest community level we investigate the potential for differential response speeds of typical forest plant species, and the potential for treeline advance to lead to community disassembly. Given that changes in species presence and abundance can alter the functional composition of plant communities, we explore the potential for shifts in tree distribution to lead to changes in carbon storage, nutrient cycling, and hydrological properties of ecosystems. Despite typically being intensively studied regions, the likely impact of forest expansion above the current mountain treeline has received relatively little attention and so we identify key knowledge gaps that should act as priorities for future research in mountain systems.
Introduction
Recent and historical changes in species distributions in response to environmental change are well documented in the scientific literature. In recent decades, investigation of the impact of current changes in climate has been a major focus in biogeographical studies (CitationParmesan, 2006; CitationParmesan and Yohe, 2003; CitationWalther, 2003). Climate conditions play an important role in determining the limits to species distributions. Consequently, when climatic conditions change rapidly, it is often observed that species distributions can respond rapidly, for example, by migration to new areas that have only recently become climatically favorable for survival (CitationPauli et al., 1996; CitationLenoir et al., 2008) or through range retraction elsewhere (CitationBeckage et al., 2008; CitationKelly and Goulden, 2008). These changes in distribution will continue into the future as climate continues to warm (CitationIPCC, 2007, Citation2013).
Upward elevational migration of mountain treelines and latitudinal advance toward the poles (treeline shifts) represent a well-studied and ecologically highly important example of species distributional change occurring across the globe. Many authors have discussed the factors responsible for controlling treeline position and the phenomenon and mechanisms of treeline advance; the majority of alpine and polar latitudinal treelines are showing at least some response to climate change (CitationHarsch et al., 2009). However, despite the widespread nature of this biotic response to changing climate, little attention has been devoted to gaining an understanding of the impacts that this change in species distribution is likely to have in ecosystems occurring at and above the current tree limit. Forest advance into alpine areas will have profound effects on ecosystem structure and function. Trees are a key structural feature of the landscapes in which they occur and determine the soil and light environment and microclimate conditions experienced by the biotic communities associated with them, and also on the wider climate system through their functional roles in ecosystem processes such as carbon sequestration and hydrology.
Treeline position is strongly dependent on temperature (CitationTranquillini, 1979; CitationKörner, 1998; CitationJobbágy and Jackson, 2000; CitationMacDonald et al., 2008), although other factors such as precipitation levels and drought (CitationDaniels and Veblen, 2004), nutrient availability (CitationSveinbjörnsson et al., 1992), and orographic and anthropogenic influences also play a significant role in treeline position (CitationHoltmeier and Broll, 2005; CitationWieser, 2007). Treelines are, therefore, very sensitive to temperature increases associated with anthropogenic climate change, and they can provide early indications of the responses to be expected elsewhere in forest ecosystems. Climate change scenarios for 2100 predict a mean global temperature increase in the range of 0.3-4.8 °C compared with 1985-2005 mean values (CitationIPCC, 2013).
The response of treelines to warming climate via an upward range expansion is widespread (CitationSuarez et al., 1999; CitationKullman, 2002; CitationLloyd and Fastie, 2002, Citation2003; CitationMoiseev and Shiyatov, 2003; CitationPeñuelas and Boada, 2003; CitationShiyatov et al., 2005, Citation2007; CitationBaker and Moseley, 2007; CitationDevi et al., 2008; CitationBeckage et al., 2008; CitationKharuk et al., 2009, Citation2010). A recent global meta-analysis by Harsch et al. (Citation2009) found evidence for a treeline advance at 52% of studied sites, and in cases where no actual advance has been documented (e.g., CitationMasek, 2001) treelines are often still responding to climate through changes in growth (CitationVillalba and Veblen, 1997), growth form (CitationLescop-Sinclair and Payette, 1995), or density (CitationLescop-Sinclair and Payette, 1995; CitationSziecz and MacDonald, 1995; CitationCamarero and Gutiérrez, 2004; CitationLiang et al., 2011).
However, rising temperatures at the regional scale will not necessarily lead to treeline expansion. Steep slopes and lack of suitable substrate can be important limiting factors for treeline advance at high altitudes (CitationBatllori et al., 2009a; CitationMacias-Fauria and Johnson, 2013). Where appropriate substrates occur, microsite factors determining temperature and soil moisture conditions are extremely important in allowing for the successful establishment of tree seedlings (CitationCui and Smith, 1991; CitationGobbi and Schlichter, 1998; CitationLee et al., 2004; CitationCatorci et al., 2012). Establishment success can also be dependent on the degree of shelter due to microtopographic variation (CitationResler et al., 2005), surrounding ground-level vegetation (CitationGermino et al., 2002; CitationSmith et al., 2003; CitationBader et al., 2008; CitationMamet and Kershaw, 2012), and the presence of krummholz (Hättenschwiler and CitationSmith, 1999; CitationGermino and Smith, 2001; CitationBatllori et al., 2009b), rocks, and boulders (CitationResler et al., 2005). This sheltering effect can result in positive feedback (CitationBader et al., 2008), whereby establishment of tree seedlings above the treeline promotes further establishment by creating more favorable microclimate conditions (CitationSmith et al., 2003; CitationBekker, 2005; CitationResler et al., 2005).
Consequently, there will be considerable variability in the response of treelines to changes in climate at both the local and regional scale (CitationHofgaard et al., 2013), with some treelines remaining stable and lagging climate changes while others advance (CitationLloyd, 2005). This variability has important consequences for the diversity and function of ecosystems above the treeline, since rising temperatures in alpine regions will not always be associated with treeline advance. While experimental work assessing impacts of elevated temperatures on alpine and tundra vegetation have been conducted (CitationChapin et al., 1995; CitationWalker et al., 2006) relatively few studies have looked directly at the impact of treeline advance on the invaded ecosystems. In the following sections of this review, we discuss the consequences of treeline expansion for the structure and function of ecosystems, using evidence from direct assessments of changes occurring in advancing treeline ecotones, experimental manipulation of ecosystems beyond the treeline, predictions from modeling studies, and indirect evidence from other ecosystems. We conclude by outlining important gaps in our knowledge in this area. Specifically, we discuss impacts on biodiversity through an investigation of the displacement of alpine communities, alterations to microclimate associated with changes in tree cover, and the influence of individualistic responses on community structure. We then move on to explore impacts on ecosystem function, specifically carbon and nitrogen dynamics, hydrology, and slope stabilization (see for a visual summary). We aim for a global discussion of these topics, although most of the literature cited comes from studies of temperate and arctic regions, as this has been where most relevant research has been focused. While we explore the likely impacts of treeline shifts on biodiversity and ecosystem function, it is not our aim to exhaustively review the literature on treeline advance, which has been done previously. For detailed reviews on treeline positions, shifts, and their causes see Rochefort et al. (Citation1994), Körner (Citation1998), Harsch et al. (Citation2009), Grace et al. (Citation2002), Körner and Paulsen (Citation2004), and Holtmeier and Broll (Citation2005).
Biodiversity Impacts
High altitude areas can be disproportionately important for their biodiversity; high levels of habitat heterogeneity and isolation allow for the development of high levels of species endemism, species richness, and the retention of many rare species (CitationEssl et al., 2009; CitationKörner, 2003). However, mountainous areas are very sensitive to the effects of climate change and are also predicted to experience higher than average increases in temperature (CitationPauli et al., 1996; CitationDirnböck et al., 2011; CitationIPCC, 2007).
Climate change will have diverse effects on plant communities, for example, advancing phenological events, extending growing season length, and altering plant productivity (CitationPeñuelas et al., 2002; CitationWalther et al., 2002; CitationParmesan and Yohe, 2003; CitationParmesan, 2006). Changes in climate affect plant distribution (CitationWalther, 2003; CitationLenoir et al., 2008) and competitive dynamics (CitationCallaway et al., 2002). Changes to plant communities may be particularly evident in mountainous or high latitude regions where the presence or absence of species is often determined by relatively low threshold temperatures (CitationKörner, 2003; CitationKullman, 2007). The upslope migration of forests can be a threat for many plant species due to out-competition for space or substrate (CitationGrabherr et al., 1994). It is, therefore, probable that the upward migration of vegetation zones will eventually lead to species loss and a reduction in diversity through the removal of specialized species with small niche tolerances and an increase in more widespread species from lower altitudes (CitationJump et al., 2012).
Displacement of Alpine Communities
Treeline advance leads to a reduction in the area available for alpine and nival species and can cause the fragmentation of remaining habitat. Forest expansion in the Urals has already reduced the area of alpine grassland and heath by around 10%-30% (CitationMoiseev and Shiyatov, 2003), and in the Mediterranean advancing shrub and broom have displaced nival vegetation (CitationGarcía-Romero et al., 2010). In Glacier National Park, Arizona forest encroachment has led to a reduction in diversity; open areas have four to five times higher diversity than areas with high tree cover but are much reduced in size as tree regeneration increases (CitationMoore and Huffman, 2004).
A number of predictive modelling studies have come to similar conclusions; general circulation model scenarios coupled with ordinal regression models of alpine vegetation responses in Austria show that expansion of Pinus mugo is likely to occur at the expense of alpine habitat (CitationDirnböck et al., 2003). Moen et al. (Citation2004) simulated treeline advance on Swedish mountains using climate predictions and a digital elevation model and found that the predicted degree of treeline advance led to a severe reduction in alpine areas: even a conservative estimate of a 100 m rise in treeline position would reduce the occurrence of alpine heath by 41%, and the majority of alpine areas remaining would be on scree slopes and boulder areas. Cliffs and rocky areas act as refugia for alpine species during periods of tree expansion, allowing alpine species to persist in areas with forest cover (CitationBruun and Moen, 2003); such areas could become increasingly important as treelines advance and further reduce alpine habitat. However, open refuges in newly expanding forest that are unsuitable for forest development will be inadequate to maintain the full range of species currently found in alpine areas since they will be dramatically different in terms of area, microclimate, and substrate quality compared with the range of existing alpine habitats.
Accordingly, alpine plant extinctions have been predicted. In New Zealand, substantial loss of species is expected under a scenario of a 3 °C rise in temperature and an associated 500 m rise in treeline, because the already fragmented alpine habitat will be disturbed by treeline advance and 93% of alpine habitat islands will likely be lost (CitationHalloy and Mark, 2003). Dirnböck et al. (Citation2011), combining the distribution of endemic species in the Austrian Alps with a model projecting forest expansion under different climate scenarios, found that, even with conservative estimations of climate change, the area of alpine habitat lost to forest was very high and that areas of high endemism suffered disproportionately.
Ground-level non-vascular plants such as bryophytes and lichenized fungi occurring beyond the treeline may be especially at risk from forest encroachment. They will face increased competition for light resources where elevated temperatures result in increased plant growth and/or the establishment of more competitive species from lower altitudes (CitationTrivedi et al., 2008). For alpine communities dominated by non-vascular plants, such as Racomitrium heath, this is likely to result in their out-competition and replacement by faster growing plant species. This effect has already been reported for Racomitrium heath in response to changes associated with nitrogen deposition in Scotland (Citationvan der Wal et al., 2005). Sphagnum spp. occurring above treeline in peat-lands in both mountain and boreal areas could also be at risk from forest advance, as can be seen from their response to afforestation by modern management and to past changes in treeline position (CitationDudova et al., 2012). However, direct out-competition of such communities by forest species is unlikely since they typically occur well beyond the treeline under current conditions.
Trees substantially modify their surrounding environment (CitationHoltmeier and Broll, 2005) and play a critical role in determining the identity of co-occurring plant species. Soil temperatures are lower during summer and higher during winter under tree canopies compared with open sites (CitationJennings et al., 1999; CitationKörner, 1998; CitationKammer et al., 2009), and dense canopies can almost completely prevent the penetration of photosynthetically active radiation to understory level (CitationCanham and Burbank, 1994). The transmission of light through the forest canopy is the most important factor limiting understory plant diversity and productivity (CitationHart and Chen, 2006), such that a positive relationship is often found between understory light levels and plant cover and diversity and a negative one with canopy density (e.g., CitationGrytnes, 2000; CitationDoležal and Šrutek, 2002; CitationCoop et al., 2010). Forest development also modifies factors including soil, nutrient content, pH, and soil moisture (CitationAugusto et al., 2002), all of which can impact the presence and abundance of understory species. The environmental modifications associated with tree establishment will, therefore, substantially modify plant communities as forest cover expands at high latitudes and altitudes. The influence of tree establishment extends well beyond the forest edge; Hofgaard (Citation1997) found a high turnover of species across the treeline ecotone, and both Hofgaard and Wilmann (Citation2002) and Camarero and Gutiérrez (Citation2002) found a positive relationship between field layer diversity and distance from the treeline, and a negative relationship with tree cover. The commonality of evidence showing that alpine species are unable to survive below forest cover has important implications for diversity changes associated with treeline shifts, and displacement of alpine and nival species is therefore largely inevitable as treelines migrate upward.
Increased density in high elevation forest below and at treeline will also lead to changes in microclimate that are likely to drive changes in the composition of existing forest understory communities, as seen in experimental work manipulating the density of managed stands (CitationThomas et al., 1999; CitationAres et al., 2010). A higher density of trees throughout the treeline ecotone will reduce light levels and increase sheltering effects, reducing the probability of persistence of alpine species co-occurring in the woodland/grassland mosaics that often occur at high altitudes. However, many treelines were higher during the early Holocene, and Hofgaard and Wilmann (Citation2002) found that some species occurring in plant communities above the treeline were indicative of past treeline position. An advance of the treeline was, therefore, less likely to dramatically change the field layer composition in such areas as it was pre-adapted to a higher forest position. Consequently, increased treeline elevation should not be viewed as an indication of the definite loss of species that currently exist in unforested areas at higher altitudes, though greater ecological information is needed for species in such areas in order to better predict those most likely to be displaced.
Differential Migration Rates and Community Disassembly
Species display individualistic responses to climatic change (CitationHuntley, 1991; CitationPauli et al., 1996; CitationHansen et al., 2001) and will, therefore, migrate at different rates with climate warming. Such differential responses are detected both within and between plant functional types. Tree species co-occurring in the same forests can show different responses to change (CitationRasaba et al., 2013); for example, in southern Siberia (CitationKharuk et al., 2009) and the Alps (CitationMotta and Nola, 2001), pine species show a greater response of growth and regeneration to temperature and are beginning to replace larch species as dominant. Similarly, proportions of tree species have been altered in the Northern Urals due to birch showing higher responses than pine or larch (CitationKapralov et al., 2006) and in Vermont where northern hardwood species are replacing boreal species that are suffering from high mortality rates as the climate warms (CitationBeckage et al., 2008).
Upslope expansion of species on the sub-Antarctic Marion Island led to community changes because more than half of the species studied were unable to move upslope in pace with climatic changes, whereas the remainder demonstrated high migration rates; an average of 1.8 m yr-1 change in altitudinal limit (Citationle Roux and McGeoch, 2008). Species responded individualistically to warming and nutrient addition treatments in alpine sites in Norway (CitationKlanderud, 2008); grass species generally responded positively, whereas many forb species, mosses, sedges, and lichens had negative responses (species of large stature responded well, but small species showed a decline with treatment), leading to changes in community composition. As Pigott and Huntley (Citation1978) identified, this interspecific variation in sensitivity to temperature can alter the competitive balance of communities, potentially leading to greater changes in species composition than would be expected based solely on individual species responses.
The migration rate of forest herbs is very different from that of tree species; migration rates of common European forest herbs are around 0.3-0.5 m yr-1 (CitationBrunet and Von Oheimb, 1998). This contrasts sharply with the much higher rates of around 100 m yr-1 suggested for tree species (CitationMcLachlan et al., 2005). Understory plants tend to produce fewer seeds and have slower migration rates than tree species (CitationRoberts, 1989) and are often dispersal limited (CitationMatlack, 1994; CitationCain et al., 1998), so it is likely that understory species will not be able to respond quickly to changes in treeline position where advance is occurring rapidly. Epiphytic lichens and non-vascular plants such as bryophytes are also likely to have different migration and colonization rates from trees. Forest lichens and bryophytes have been shown to be very sensitive to forest disturbance (CitationJohansson, 2008) and to have low growth and establishment rates. The negative impact of disturbance could be due to dispersal limitations (CitationSillett et al., 2000), establishment limitation (CitationKuusinen and Siitonen, 1998), or because of high sensitivity to microclimate (CitationSillett et al., 1994). Dispersal ranges and colonization rates are very variable among lichen species, and those with low colonization rates require more stand continuity (CitationRuete et al., 2014). Studies suggest that epiphytic lichens can be slow to respond to changes in forest conditions (CitationJohansson et al., 2013), with current lichen distributions often reflecting past, rather than current forest conditions (CitationEllis and Coppins, 2007, Citation2009).
The logical consequence of individualistic species responses is that community composition will change, and new assemblages will form. There is evidence of this from Quaternary migration rates, inferred from isochrones (CitationHuntley, 1991) and isopoll maps (CitationHuntley, 1990) showing that past compositional changes in forests led to the formation of communities with no modern analogue. Edwards et al. (Citation2005) also provided evidence from early Holocene (13,000–10,000 yr B.P.) fossil pollen, showing that during this time, the structure and functioning of communities was very different from that of assemblages found in the same areas today. Studies of fossilized packrat middens in the southwestern United States (CitationVan Devender and Spaulding, 1979), together with macrofossil investigations of the tree flora of the Swedish Scandes (CitationKullman, 1998) and forest composition in the Pacific Northwest (CitationSea and Whitlock, 1995) during the early Holocene, confirm the generality of these findings of community breakup and change in response to past warming periods.
Consequences of Response Lags for Biodiversity
Both modelling (CitationChapin and Starfield, 1997; CitationBugmann and Pfister, 2000) and dendro-ecological studies have suggested that a lag of at least several hundred years is likely to occur between a change in climatic conditions and the subsequent development of forests at higher altitudes or latitudes. This lag is thought to be due to limitations in seed availability and establishment probability, disturbance events, and variations in tree growth rates. Furthermore, this lag varies both spatially and temporally due to local site conditions such as the presence of permafrost, krummholz, or high wind exposure (CitationLloyd, 2005). The critical factor that will determine how a lag in the response of vegetation to climate will affect biodiversity and community composition is the difference in response rate between different species and vegetation types. If, due to greater seed production and higher dispersal distances, trees respond more rapidly to increased temperatures than alpine plants, the likelihood of survival of many alpine species will be low. However, evidence exists that forests may be less responsive to interannual changes in climate because of the buffering effect of the canopy (CitationLenoir et al., 2008) and are often more influenced by long-term climate trends rather than interannual variation. In contrast, alpine vegetation is responsive to these short-term changes (CitationKullman, 2007) and is therefore likely to be able to respond faster, although more variably, to climatic change. The lag in treeline response to climate is likely to have positive implications for the survival of alpine communities, at least in the short term, as they might show increased response rates compared with forest understory communities and be able to migrate to areas of suitable habitat before they are displaced by advancing treelines.
Ecosystem Function and Services
High altitude ecosystems also play crucial roles in many ecosystem functions that will be impacted directly as temperatures rise, but also indirectly as plant species and life-forms from lower altitudes migrate upward and increase in abundance. High altitude forests provide important ecosystem services, including carbon sequestration and storage (CitationPeng et al., 2009; CitationWhite et al., 2000), slope stabilization, and erosion prevention (CitationStoffel et al., 2006; CitationSchönenberger et al., 2005), and they play key roles in water and nutrient cycling (CitationDirnböck and Grabherr, 2000). There is already a large body of evidence to suggest that the response of forests to climatic change will have an impact on associated ecosystem functions (CitationSaxe et al., 2001), although relatively little research has been conducted on the specific impacts of treeline advance in alpine systems. In the following sections, we discuss the likely consequences of treeline advance for some of the fundamental processes in which forests are involved.
Carbon Sequestration and Storage
Given the fundamental dependence of processes such as photosynthesis, respiration, and soil decomposition rates on temperature and CO2 concentrations (CitationSaxe et al., 2001; CitationXu et al., 2012), climate change will modify plant photosynthetic assimilation rates, and hence growth and production, as CO2 concentration and temperature continue to rise (CitationGriffis et al., 2003; CitationKallarackal and Roby, 2012). However, indefinite increases in productivity are not expected since studies show that the response of plant productivity to rising CO2 saturates (CitationNabuurs et al., 2013). Forests are major carbon stores both in plant biomass and in soils (CitationHyvönen et al., 2007), and rising temperatures in high altitude forests could result in increased CO2 sequestration, growth, and plant biomass (CitationDevi et al., 2008; CitationFan et al., 2009). In a recent meta-analysis, Lin et al (Citation2010) reported that biomass increased in all plant types with warming but that woody species showed the greatest response. As rising temperatures drive tree range expansion and increases in woody biomass at and above the current the treeline, carbon accumulation at treeline will increase, although such increases may be accompanied by carbon release from low altitude forests and the overall balance remains unclear (CitationZierl and Bugmann, 2007). There is, however, considerable evidence that increased growth and biomass at many treelines is increasing their ability to act as carbon sinks—for example, Lopatin et al (Citation2006) in Russia, Tømmervik et al. (Citation2009) in Norway, and from the many recent studies of increased tree growth, density, and forest expansion (CitationDevi et al., 2008; CitationKharuk et al., 2009; CitationKullman, 2002; CitationVillalba and Veblen, 1997). Modelled changes in vegetation and net primary productivity (NPP) in China under various climate warming scenarios show that a replacement of alpine vegetation by forest trees and shrubs occurs alongside overall increases in net photosynthesis (CitationWang, 2013), although such changes are expected to be highly spatially variable.
Increased growing season temperatures can combine with an increase in growing season length due to warmer spring and/or milder winter temperatures to increase net ecosystem production (NEP) (CitationGriffis et al., 2003; CitationBarr et al., 2007). However, the relationship between growing season and NEP can be complicated at high altitude. For example, there was a negative correlation between growing season length and NEP in subalpine forests in the Western United States due to a decrease in water availability because of decreased snowfall and earlier snowmelt (CitationHu et al., 2010). Similar results have been reported by other authors, including transitory increases in productivity that are then offset by higher temperatures and/or low water availability later in the season (CitationMonson et al., 2002; CitationAngert et al., 2005; CitationDelpierre et al., 2009). The impact of growing season and temperature changes on carbon sequestration at the treeline will therefore be influenced by other factors and is likely to vary between regions.
Increased temperature is also likely to elevate soil respiration, which represents a major component of the carbon cycle. A meta-analysis by Rustad et al. (Citation2001) found that an increase of temperature in the range 0.3–6 °C would increase soil respiration by as much as 20%, with the largest increases occurring in forested systems. This has major implications for carbon sequestration and ecosystem carbon balance (CitationSjögersten and Wookey, 2002). Soils in colder regions, such as treeline and alpine areas are especially sensitive to warming (CitationSjögersten et al., 2011). The response can be due to the direct effect of temperature on microbial activity; experimental warming of alpine soils in Switzerland led to a greater microbial use of older soil organic carbon, potentially reducing long-term C storage (CitationStreit et al., 2014). Indirect effects of changes in ecosystem properties and functions can also be important; such as the change across the forest ecotone when treelines advance with subsequent changes in microenvironment and litter inputs.
Changes in vegetation cover at the treeline have been shown to have an impact on soil carbon storage and on the quality of soil organic matter (SOM). Kammer et al. (Citation2009) found that tundra soils contained significantly more carbon in organic layers, but less in the mineral horizon compared with forest soils. Total stocks of carbon did not vary significantly, so treeline advance was likely to have little impact on soil carbon pools. Soil respiration rates were found to vary across the mountain birch forest-tundra ecotone in Abisko, Sweden (CitationSjögersten and Wookey, 2002), with higher rates occurring in forest soils, and in tundra soils transplanted to forest sites, than in tundra sites. Consequently, an expansion of forest into tundra in response to warming could lead to increased soil respiration, at least initially (CitationRustad et al., 2001), potentially reducing the impact of higher carbon storage in plant biomass.
Evidence of this offsetting of carbon sequestration exists from a study of forest expansion into tundra in northwest Alaska (CitationWilmking et al., 2006) where it was found that conversion of tundra into forest led to a net loss of ecosystem carbon; increases in soil respiration completely offset the increase in above ground biomass. This effect was also reported by Hartley et al. (Citation2012), who found that soil carbon stocks were lower in mountain birch forest than in nearby tundra sites, by an amount that offset the increase in aboveground biomass, and that the establishment of birch led to a loss of soil carbon in tundra.
These studies are in agreement with simulations conducted by Peng et al. (Citation2009), who modelled the effects of climate change on forest C dynamics in northeastern China. However, Steltzer (Citation2004) proposed that expansion of spruce into tundra would lead to increases in the carbon storage of the system due to the higher NPP values of forest compared with tundra and because of the possibly lower decomposition values, given the lower litter quality of trees and the cooling effect of shading. This study found that soil carbon accumulated under spruce trees as the trees aged. Although in contrast with the findings of the studies above, the work of Steltzer (Citation2004) suggests the need for observational and experimental timescales that are long enough to fully capture the range of processes that can occur during natural stand development.
The relationship between tree cover and soil carbon storage may be very different depending on whether altitudinal or latitudinal treeline advance is considered; in the case of latitudinal expansion, trees are invading tundra sites with a high proportion of carbon contained within soils (CitationTarnocai et al., 2009), conversely, in altitudinal treelines the soils of the invaded alpine areas tend to be thinner with lower carbon content (CitationMichaelson et al., 1996; CitationKörner, 1998) and, because trees tend to preferentially regenerate on mineral soils in these areas, it is probable that tree expansion into alpine zones will increase the carbon content. Due to the importance of carbon storage in high latitude tundra sites, the majority of studies investigating the impacts of treeline advance on carbon dynamics have been concentrated in these systems; more studies in alpine ecosystems are needed to explore the impact of treeline advance on soil carbon properties and the balance between carbon sequestration and emissions. Increased focus on mountain systems will allow us to better identify how altitudinal and latitudinal treelines differ in this respect.
Nitrogen Content and Availability
Soil decomposition rates, and the amount of SOM, affect nitrogen availability (CitationSpargo et al., 2011), and because nitrogen is frequently limiting to plant growth in high latitude/altitude areas (CitationRustad et al., 2001), it is important to consider how its availability may be affected both by climate change and by treeline advance. It is clear that increasing temperatures can directly alter nutrient cycling and potentially increase availability (CitationButler et al., 2012; CitationRustad et al., 2001), but the effects of climate warming on N cycling are likely to be complex. For example, recent evidence suggests that warming leads to increases in the amount of N fixed by cryptogams in arctic areas (CitationLett and Michelsen, 2014) but that reduced snow cover could reduce rates of decomposition and release of N from plant litter (CitationWu et al., 2014). It is also possible that an advance of treeline could have more impact than warming on N availability (CitationSjögersten and Wookey, 2005).
Forest soils at the mountain birch-tundra treeline in Fennoscandia had significantly higher ammonification and mineralization rates, and higher N availability than did tundra soils, so the expansion of birch forest in the study areas could have significant impacts on N cycling (CitationSjögersten and Wookey, 2005). Similar results have been obtained by Davis et al. (Citation1991) in Fennoscandia and Sveinbjörnsson et al. (Citation1995) in Alaska, where ammonium concentrations were found to be higher within the forest areas than at the treeline and krummholz zones, though Hartley et al. (Citation2012) provided a counter-example.
Plants from lower altitudes tend to have lower N contents (CitationReich and Oleksyn, 2004) so the upslope migration of lower altitude plants as temperatures rise could decrease the N inputs from plant litter. However, the concentration of N in shrubs growing within a forest was found to be higher than those from tundra sites (CitationKaarlejärvi et al., 2012), so the converse could be true, with treeline advance leading to an increase in plant N levels. Lower N levels in needles and soil have been found at Arctic treeline sites and attributed to lower microbial activity at low temperatures (CitationMcNown and Sullivan, 2013). N availability reduced with elevation at Patagonian treelines and there was a change in microbial communities from bacteria to fungal dominated. Changes in forest cover and composition influence both microbial communities and N availability through litter inputs (CitationThébault et al., 2014).
Lower nutrient availabilities at the approach to and beyond treeline have been hypothesized to account for the increase in fine root biomass that has been observed at treeline compared with lower elevation forest (CitationHertel and Scholing, 2011), such that increased N availability through vegetation and temperature change at the treeline would likely lead to alterations in biomass partitioning. A positive feedback mechanism whereby warming increased tree establishment, which then increases nitrogen content and subsequent tree establishment, is possible (CitationSjögersten and Wookey, 2005; CitationSundqvist et al., 2011).
Evidence is mixed on the likely effects of treeline advance for N cycling and availability. As with C dynamics, most research has focused on latitudinal treelines; current evidence suggests that for N similar patterns of change will be observed at advancing altitudinal and latitudinal treelines, and N tends to be limiting at both (CitationRustad et al., 2001; CitationThébault et al., 2014). However, there is little direct evidence of the effect of altitudinal treeline advance, and since C and N are closely linked, because N is stored in soil organic matter (CitationThébault et al., 2014), more research is required to fully understand the impacts of altitudinal treeline advance on N availability and mineralization.
Hydrology
Mountain areas are extremely important in terms of controlling the volume and quality of water provision; plant cover and associated soil properties have a major influence on this service (CitationDirnböck and Grabherr, 2000). Plant cover can reduce flood flow but also increases infiltration into the soil and water table and hence will augment low flow and provide water supply throughout the dry season (CitationDirnböck et al., 2003). Treeline advance into previously treeless areas has the potential to significantly impact downslope hydrology since the development of krummholz and montane forest in grasslands increases the water absorption and retention capacities of soils (CitationDirnböck and Grabherr, 2000). Furthermore, the impacts of forests on hydrological properties can have implications for human societies because the degree of forest cover has been shown to affect sediment load and therefore water quality (CitationDouglas et al., 1992). However, afforestation may lead to reductions in stream flow due to reduced runoff and reduced but more predictable water availability downstream (CitationMcVicar et al., 2007). In areas where water supply is already limited this could produce major problems, yet our current understanding of the impact of treeline advance on hydrology, especially in mountain areas, is limited by lack of direct research.
Slope Stabilization and Avalanche/Rockfall Prevention
Mountain forests also play a highly important role in slope stabilization and the prevention of landslides and avalanches (CitationBebi et al., 2001), and the anthropogenic lowering of the treeline due to land use in regions such as the European Alps is believed to have led to increased avalanche risk (CitationLi and Yang, 2004). Modelling of avalanche risk based on forest cover found that the risk of extreme events depends on degree of forest cover and forest structural features (CitationTeich and Bebi, 2009), with avalanche risk being significantly reduced by the presence and high cover of structurally complex undisturbed forest. Wehrli et al. (Citation2006) combined a forest dynamic model (ForClim) with a model of rockfall risk (RockFor) in order to investigate the role of forests in preventing rockfall, and the degree to which this was affected by forest dynamics. It was found that high stand density, as well as high regeneration, improved the ability of forests to protect against small rocks but that forests were limited in their ability to prevent the damage caused by large rock displacement. Model simulations by Dorren et al. (Citation2005) produced similar results; an absence of forest would lead to a greater impact of rockfall events, whereas an increase in tree density improved the protective function of forested areas.
Conclusion
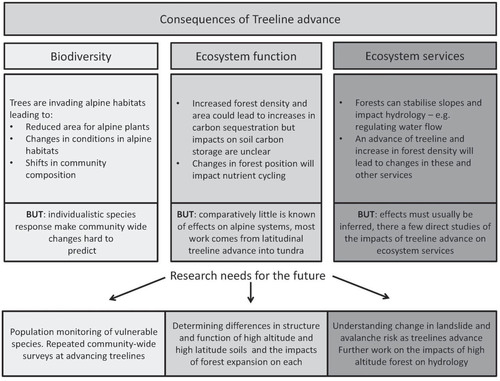
Treelines are advancing upward and toward the poles in response to rising temperatures worldwide (CitationHarsch et al., 2009). Although these changes in distribution and the reasons for them are relatively well investigated, much less attention has been focused on the impacts of treeline advance for ecosystems beyond the current treeline. Research discussed herein suggests that the upward migration of forests will have major consequences for biodiversity (), eventually leading to reductions in alpine diversity (CitationMoore and Huffman, 2004; CitationHalloy and Mark, 2003). However, this process is highly unlikely to occur as a simple replacement of one ecosystem by another. Rather, newly formed communities are likely to lack historical precedent, retaining some components of former vegetation types due to differential migration rates of individual species and variation in their tolerance of shading in the forest understory. Evidence from assessments of responses to past periods of temperature change indicates that species have responded to environmental fluctuations in the past in a similar way, with the breakdown of communities and formation of novel assemblages (CitationEdwards et al., 2005; CitationVan Devender and Spaulding, 1979). Such changes, and the possible impacts that they will have on the surrounding communities, represent a natural continuous process because treeline position and vegetation composition are dynamic. However, the current rate of climatic warming is unprecedented (CitationIPCC, 2013) and is therefore likely to drive larger and more variable impacts on species migration and community response compared to that which has occurred in the past. In addition to impacts on diversity across the treeline ecotone and in the alpine zone above, ecosystem functions will be affected in a multitude of ways () such as alterations to net ecosystem productivity and carbon storage (CitationGriffis et al., 2003; CitationRustad et al., 2001), nutrient cycling (CitationSjögersten and Wookey, 2005), and hydrology (CitationDirnböck and Grabherr, 2000). However, at present there is insufficient information available, particularly from mountain regions to make reliable predictions on the direction and magnitude of effects in most cases. Mountain regions present a particular challenge in this respect as their varied topography can result in ecosystems showing a wide diversity of responses in a relatively small area.
Future Research
From a biodiversity perspective, knowledge is particularly lacking in the diversity of recently colonized areas of high altitude forest compared to long established areas and in the migration rates of forest understory and epiphytic species. Given the importance of alpine areas for biodiversity, and because of the high endemism rates, more field-based research on the impacts of treeline expansion on alpine species' survival and migration is required. An important aspect of such research should be the determination of the balance between competition and abiotic factors such as habitat availability and climate in determining the presence or absence of key species. Given the highly heterogeneous landscapes of mountain regions, such knowledge would allow us to substantially improve local scale modelling of species distributions to better forecast species loss or retention.
In terms of ecosystem function, research is required to assess the spatial heterogeneity of impacts of treeline advance for carbon and nutrient cycling. The imbalance in our understanding of tree invasion into arctic and alpine areas is particularly important to redress given feedbacks of such changes in vegetation and soil properties to the global climate system. Furthermore, in order to better predict the effects of forest expansion on ecosystem carbon storage, a better understanding of the relationship between above- and belowground processes is required across the alpine treeline ecotone.
Treeline inertia in response to climate could modify the degree of feedback that occurs with climate, and this phenomenon produces significant uncertainties in models attempting to simulate the consequence of treeline advance for climate feedbacks. Further field research on rates and time courses of treeline advance in arctic and alpine areas will contribute vital information that can reduce this uncertainty.
The advance of treelines into previously treeless ecosystems will continue as the climate continues to warm. The consequences of such advance for biodiversity and ecosystem function will be felt over ever-greater areas. To date, the majority of investigations of the consequences of treeline advance have been focused on northern tundra ecosystems, which seems intuitively sensible given both the expanse of tundra and boreal forest, with a consequently large potential to influence climate, and the far larger predicted rate of treeline advance (distances of kilometers rather than tens of meters) compared with alpine sites. However, given that mountain ecosystems cover a major proportion of the Earth's surface (somewhere between 20%-24% of total land surface) (CitationIPCC, 2007), it is essential to ensure that data availability on ecosystem response to change in high altitude and high latitude regions is comparable. On the broadest scale, an assessment of the comparability of such data from altitudinal and latitudinal systems is lacking, and is necessary if we are to fully understand the interactions between spatial scale and heterogeneity and ecological processes over altitudinal and latitudinal gradients (CitationJump et al., 2009). A clear understanding of the potential for data transferability between these systems would be highly advantageous for more accurate modelling of ecosystem responses to current and future environmental changes.
Acknowledgments
Financial support for this work was provided by the U.K. Natural Environment Research Council, National Science Council (Taiwan), Royal Society, and Royal Society of Edinburgh. We thank James Juvik and the conference funders for supporting our participation in the international symposium on tropical alpine and sub-alpine ecosystems and the opportunity to contribute to this special issue.
References Cited
- Angert, A. , Biraud, S. , Bonfils, C. , Henning, C. C. , Buermann, W. , Pinzon, J. , Tucker, C. J. , and Fung, I. , 2005: Drier summers cancel out the CO2 uptake enhancement induced by warmer springs. Proceedings of the National Academy of Sciences of the United States of America , 102: 10823–10827.
- Ares, A. , Neill, A. R. , and Puettmann, K. J. , 2010: Understory abundance, species diversity and functional attribute response to thinning in coniferous stands. Forest Ecology and Management , 260: 1104–1113.
- Augusto, L. , Ranger, J. , Binkley, D. , and Rothe, A. , 2002: Impact of several common tree species of European temperate forests on soil fertility. Annals of Forest Science , 59: 233–253.
- Bader, M. Y. , Rietkerk, M. , and Bregt, A. K. , 2008: A simple spatial model exploring positive feedbacks at tropical alpine treelines. Arctic, Antarctic, and Alpine Research , 40: 269–278.
- Baker, B. B. , and Moseley, R. K. , 2007: Advancing treeline and retreating glaciers: implications for conservation in Yunnan, P.R. China. Arctic, Antarctic, and Alpine Research , 39: 200–209.
- Barr, A. G. , Black, T. A. , Hogg, E. H. , Griffis, T. J. , Morgenstern, K. , Kljun, N. , Theede, A. , and Nesic, Z. , 2007: Climatic controls on the carbon and water balances of a boreal aspen forest, 1994–2003. Global Change Biology , 13: 561–576.
- Batllori, E. , Blanco-Moreno, J. M. , Ninot, J. M. , Gutiérrez, E. , and Carrillo, E. , 2009a: Vegetation patterns at the alpine treeline ecotone: the influence of tree cover on abrupt change in species composition of alpine communities. Journal of Vegetation Science , 20: 814–825.
- Batllori, E. , Camarero, J. J. , Ninot, J. M. , and Gutiérrez, E. , 2009b: Seedling recruitment, survival and facilitation in alpine Pinus uncinata tree line ecotones. Implications and potential responses to climate warming. Global Ecology and Biogeography , 18: 460–472.
- Bebi, P. , Kienast, F. , and Schönenberger, W. , 2001: Assessing structures in mountain forests as a basis for investigating the forests' dynamics and protective function. Forest Ecology and Management , 145: 3–14.
- Beckage, B. , Osborne, B. , Gavin, D. G. , Pucko, C. , Siccama, T. , and Perkins, T. , 2008: A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proceedings of the National Academy of Sciences , 105: 4197–4202.
- Bekker, M. F. , 2005: Positive feedback between tree establishment and patterns of subalpine forest advancement, Glacier National Park, Montana, U.S.A. Arctic, Antarctic, and Alpine Research , 37: 97–107.
- Brunet, J. , and Von Oheimb, G. , 1998: Migration of vascular plants to secondary woodlands in southern Sweden. Journal of Ecology , 86: 429–438.
- Bruun, H. H. , and Moen, J. , 2003: Nested communities of alpine plants on isolated mountains: relative importance of colonization and extinction. Journal of Biogeography , 30: 297–303.
- Bugmann, H. , and Pfister, C. , 2000: Impacts of inter annual climate variability on past and future forest composition. Regional Environmental Change , 1: 112–125.
- Butler, S. M. , Melillo, J. M. , Johnson, J. E. , Mohan, J. , Steudler, P. A. , Lux, H. , Burrows, E. , Smith, R. M. , Vario, C. L. , Scott, L. , Hill, T. D. , Aponte, N. , and Bowles, F. , 2012: Soil warming alters nitrogen cycling in a New England forest: implications for ecosystem function and structure. Oecologia , 168: 819–828.
- Cain, M. L. , Damman, H. , and Muir, A. , 1998: Seed dispersal and the Holocene migration of woodland herbs. Ecological Monographs , 68: 325–347.
- Callaway, R. M. , Brooker, R. W. , Choler, P. , Kikvidze, Z. , Lortiek, C. J. , Michalet, R. , Paolini, L. , Pugnaireq, F. I. , Newingham, B. , Aschehoug, E. T. , Armasq, C. , Kikodze, D. , and Cook, B. J. , 2002: Positive interactions among alpine plants increase with stress. Nature , 417: 844–848.
- Camarero, J. J. , and Gutiérrez, E. , 2002: Plant species distribution across two contrasting treeline ecotones in the Spanish Pyrenees. Plant Ecology , 162: 247–257.
- Camarero, J. J. , and Gutiérrez, E. , 2004: Pace and pattern of recent treeline dynamics: response of ecotones to climatic variability. Climatic Change , 63: 181–200.
- Canham, C. D. , and Burbank, D. H. , 1994: Causes and consequences of resource heterogeneity in forests: interspecific variation in light variation by canopy trees. Canadian Journal of Forest Research , 24: 337–349.
- Catorci, A. , Scapin, W. , Tardella, M. F. , and Vitanzi, A. , 2012: Seedling survival and dynamics of upper timberline in Central Apennines. Polish Journal of Ecology , 60: 79–94.
- Chapin, F. S. , and Starfield, A. M. , 1997: Time lags and novel ecosystems in response to transient climatic change in Arctic Alaska. Climatic Change , 35: 449–461.
- Chapin, F. S. , Shaver, G. R. , Giblin, A. E. , Nadelhoffer, K. J. , and Laundre, J. A. , 1995: Responses of arctic tundra to experimental and observed changes in climate. Ecology , 76: 694–711.
- Coop, J. D. , Massatti, R. T. , and Schoettle, A. W. , 2010: Subalpine vegetation pattern three decades after stand-replacing fire: effects of landscape context and topography on plant community composition, tree regeneration, and diversity. Journal of Vegetation Science , 21: 472–487.
- Cui, M. , and Smith, W. K. , 1991: Photosynthesis, water relations and mortality in Abies Zasiocarpa seedlings during natural establishment. Tree Physiology , 8: 37–46.
- Daniels, L. D. , and Veblen, T. T. , 2004: Spatiotemporal influences of climate on altitudinal treeline in Northern Patagonia. Ecology , 85: 1284–1296.
- Davis, J. , Schober, A. , Bahn, M. , and Sveinbjörnsson, B. , 1991: Soil carbon and nitrogen turnover at and below the elevational treeline in Northern Fennoscandia. Arctic and Alpine Research , 23: 279–286.
- Delpierre, N. , Soudani, K. , François, C. , Köstner, B. , Pontailler, J. Y , Nikinmaa, E. , Misson, L. , Aubinet, M. , Bernhofer, C. , Granier, A. , Grünwald, T. , Heinesch, B. , Longdoz, B. , Ourcival, J. M. , Rambal, S. , Vesala, T. , and Dufrêne, E. , 2009: Exceptional carbon uptake in European forests during the warm spring of 2007: a data-model analysis. Global Change Biology , 15: 1455–1474.
- Devi, N. , Hagedorn, F. , Moiseev, P. , Bugmann, H. , Shiyatov, S. , Mazepa, V. , and Rigling, A. , 2008: Expanding forests and changing growth forms of Siberian larch at the Polar Urals treeline during the 20th century. Global Change Biology , 14: 1581–1591.
- Dirnböck, T. , and Grabherr, G. , 2000: GIS assessment of vegetation and hydrological change in a high mountain catchment of the Northern Limestone Alps. Mountain Research and Development , 20: 172–179.
- Dirnböck, T. , Dullinger, S. , and Grabherr, G. , 2003: A regional impact assessment of climate and land-use change on alpine vegetation. Journal of Biogeography , 30: 401–417.
- Dirnböck, T. , Essl, F. , and Rabitsch, W. , 2011: Disproportional risk for habitat loss of high-altitude endemic species under climate change. Global Change Biology , 17: 990–996.
- Doležal, J. , and Šrutek, M. , 2002: Altitudinal changes in composition and structure of mountain-temperate vegetation: a case study from the Western Carpathians. Plant Ecology , 158: 201–221.
- Dorren, L. K. A. , Berger, F. , le Hir, C. , Mermin, E. , and Tardif, P. , 2005: Mechanisms, effects and management implications of rockfall in forests. Forest Ecology and Management , 215: 183–195.
- Douglas, I. , Spencer, T. , Greer, T. , Bidin, K. , Sinun, W. , and Meng, W. W. , 1992: The impact of selective commercial logging on stream hydrology, chemistry and sediment loads in the Ulu Segama Rain Forest, Sabah, Malaysia. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences , 335(1275): 397–406.
- Dudova, L. , Hajkova, P. , Buchtova, H. , and Opravilova, V. , 2012: Formation, succession and landscape history of Central-European summit raised bogs: a multiproxy study from the Hruby Jesenik Mountains. The Holocene , 23: 230–242.
- Edwards, M. E. , Brubaker, L. B. , Lozhkin, A. V. , and Anderson, P. M. , 2005: Structurally novel biomes: a response to past warming in Beringia. Ecology , 86: 1696–1703.
- Ellis, C. J. , and Coppins, B. , 2007: 19th century woodland structure controls stand-scale epiphyte diversity in present-day Scotland. Diversity and Distributions , 13: 84–91.
- Ellis, C. J. , and Coppins, B. , 2009: Quantifying the role of multiple landscape-scale drivers controlling epiphyte composition and richness in a conservation priority habitat (juniper scrub). Biological Conservation , 142: 1291–1301.
- Essl, F. , Staudinger, M. , Stöhr, O. , Schratt-Ehrendorfer, L. , Rabitsch, W. , and Niklfeld, H. , 2009: Distribution patterns, range size and niche breadth of Austrian endemic plants. Biological Conservation , 142: 2547–2558.
- Fan, Z.-X. , Bräuning, A. , Cao, K.-F. , and Zhu, S. D. , 2009: Growth-climate responses of high-elevation conifers in the central Hengduan Mountains, southwestern China. Forest Ecology and Management , 258: 306–313.
- García-Romero, A. , Muñoz, J. , Andrés, N. , and Palacios, D. , 2010: Relationship between climate change and vegetation distribution in the Mediterranean mountains: Manzanares Head valley, Sierra De Guadarrama (Central Spain). Climatic Change , 100: 645–666.
- Germino, M. J. S. , and Smith, W. K. , 2001: Relative importance of microhabitat, plant form, and photosynthetic physiology to carbon gain in two alpine herbs. Functional Ecology , 15: 243–251.
- Germino, M. J. S. , Smith, W. K. , and Resor, A. C. , 2002: Conifer seedling distribution and survival in an alpine-treeline ecotone. Plant Ecology , 162: 157–168.
- Gobbi, M. , and Schlichter, T. , 1998: Survival of Austrocedrus chilensis seedlings in relation to microsite conditions and forest thinning. Forest Ecology and Management , 111: 137–146.
- Grabherr, G. , Gottfreid, M. , and Pauli, H. , 1994: Climate effects on mountain plants. Nature , 369: 449.
- Grace, J. , Berninger, F. , and Nagy, L. , 2002: Impacts of climate change on the tree line. Annals of Botany , 90: 537–544.
- Griffis, T. J. , Black, T. A. , Morgenstern, K. , Barr, A. G. , Nesic, Z. , Drewitt, G. B. , Gaumont-Guay, D. , and McCaughey, J. H. , 2003: Ecophysiological controls on the carbon balances of three southern boreal forests. Agricultural and Forest Meteorology , 117: 53–71.
- Grytnes, J. A. , 2000: Fine-scale vascular plant species richness in different alpine vegetation types: relationships with biomass and cover. Journal of Vegetation Science , 11: 87–92.
- Halloy, S. , and Mark, A. , 2003: Climate-change effects on alpine plant biodiversity: a New Zealand perspective on quantifying the threat. Arctic, Antarctic, and Alpine Research , 35: 248–254.
- Hansen, A. J. , Neilson, R. P. , Dale, V. H. , Flather, C. H. , Iverson, L. R. , Currie, D. J. , Shafer, S. , Cook, R. , and Bartlein, P. J. , 2001: Global change in forests: responses of species, communities, and biomes. Bioscience , 51: 765–779.
- Harsch, M. A. , Hulme, P. E. , McGlone, M. S. , and Duncan, R. P. , 2009: Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters , 12: 1040–1049.
- Hart, S. A. , and Chen, H. Y. H. , 2006: Understory vegetation dynamics of North American boreal forests. Critical Reviews in Plant Sciences , 25: 381–397.
- Hartley, I. P. , Garnett, M. H. , Sommerkorn, M. , Hopkins, D. W. , Fletcher, B. J. , Sloan, V. L. , Phoenix, G. K. , and Wookey, P. A. , 2012: A potential loss of carbon associated with greater plant growth in the European Arctic. Nature Climate Change, advance online publication.
- Hättenschwiler, S. , and Smith, W. K. , 1999: Seedling occurrence in alpine treeline conifers: a case study from the central Rocky Mountains, USA. Acta Oecologica , 20: 219–224.
- Hertel, D. , and Scholing, D. , 2011: Norway spruce shows contrasting changes in below- versus above-ground carbon partitioning towards the alpine tree line: evidence from a Central European case study. Arctic Antarctic and Alpine Research , 43: 46–55.
- Hofgaard, A. , 1997: Inter-relationships between treeline position, species diversity, land use and climate change in the Central Scandes Mountains of Norway. Global Ecology and Biogeography Letters , 6: 419–429.
- Hofgaard, A. , and Wilmann, B. , 2002: Plant distribution pattern across the forest-tundra ecotone: the importance of treeline position. Ecoscience , 9: 375–385.
- Hofgaard, A. , Tommervick, H. , Rees, G. , and Hanssen, F. , 2013: Latitudinal forest advance in northernmost Norway since the early 20th century. Journal of Biogeography , 40: 938–949.
- Holtmeier, F. K. , and Broll, G. , 2005: Sensitivity and response of northern hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Global Ecology and Biogeography , 14: 395–410.
- Hu, J. , Moore, D. J. P. , Burns, S. P. , and Monson, R. K. , 2010: Longer growing seasons lead to less carbon sequestration by a subalpine forest. Global Change Biology , 16: 771–783.
- Huntley, B. , 1990: European post-glacial forests: compositional changes in response to climatic change. Journal of Vegetation Science , 1: 507–518.
- Huntley, B. , 1991: How plants respond to climate change: migration rates, individualism and the consequences for plant communities. Annals of Botany , 67: 15–22.
- Hyvönen, R. , Ågren, G. I. , Linder, S. , Persson, T. , Cotrufo, M. F. , Ekblad, A. , Freeman, M. , Grelle, A. , Janssens, I. A. , Jarvis, P. G. , Kellomäki, S. , Lindroth, A. , Loustau, D. , Lundmark, T. , Norby, R. J. , Oren, R. , Pilegaard, K. , Ryan, M. G. , Sigurdsson, B. D. , Strömgren, M. , Van Oijen, M. , and Wallin., G. , 2007: The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytologist , 173: 463–480.
- IPCC, 2007: Climate Change 2007: Impacts, Adaptation and Vulnerability. Parry, M. L. , Canziani, O. F. , Palutikof, J. P. , van der Linden, P. J. , and Hanson C. E. (eds.), Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, U.K., and New York: Cambridge University Press.
- IPCC, 2013: Climate Change 2013: The Physical Science Basis. Stocker, T. F. , Qin, D. , Plattner, G. K. , Tignor, M. , Allen, S. K. , Boschung, J. , Nauels, A. , Xia, Y. , Bex, V. , and Midgley, P. M. (eds.), Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, U.K., and New York: Cambridge University Press, 1535 pp.
- Jennings, S. B. , Brown, N. D. , and Shiel, D. , 1999: Assessing forest canopies and understory illumination: canopy closure, canopy cover and other measures. Forestry , 72: 59–73.
- Jobbágy, E. G. , and Jackson, R. B. , 2000: Global controls of forest line elevation in the northern and southern hemispheres. Global Ecology & Biogeography , 9: 253–268.
- Johansson, P. , 2008: Consequences of disturbance on epiphytic lichens in boreal and near boreal forests. Biological Conservation , 141: 1933–1944.
- Johansson, V. , Ranius, T. , and Snall, T. , 2013: Epiphyte metapopulation persistence after drastic habitat decline and low tree regeneration: time-lags and effects of conservation actions. Journal of Applied Ecology , 50: 414–422.
- Jump, A. S. , Mátyás, C. , and Peñuelas, J. , 2009: The altitude-for-latitude disparity in the range retractions of woody species. Trends in Ecology and Evolution , 24(12): 694–701.
- Jump, A. S. , Huang, T. J. , and Chou, C. H. , 2012: Rapid altitudinal migration of mountain plants in Taiwan and its implications for high altitude biodiversity. Ecography , 35: 204–210.
- Kaarlejärvi, E. , Baxter, R. , Hofgaard, A. , Hytteborn, H. , Khitun, O. , Molau, U. , Sjögersten, S. , Wookey, P. , and Olofsson, J. , 2012: Effects of warming on shrub abundance and chemistry drive ecosystem-level changes in a forest-tundra ecotone. Ecosystems , 15: 1219–1233.
- Kallarackal, J. , and Roby, T. J. , 2012: Responses of trees to elevated carbon dioxide and climate change. Biodiversity and Conservation , 21: 1327–1342.
- Kammer, A. , Hagedorn, F. , Shevchenko, I. , Leifeld, J. , Guggenberger, G. , Goryacheva, T. , Rigling, A. , and Moiseev, P. , 2009: Treeline shifts in the Ural Mountains affect soil organic matter dynamics. Global Change Biology , 15: 1570–1583.
- Kapralov, D. S. , Shiyatov, S. G. , Moiseev, P. A. , and Fomin, V. V. , 2006: Changes in the composition, structure, and altitudinal distribution of low forests at the upper limit of their growth in the Northern Ural Mountains. Russian Journal of Ecology , 37: 367–372.
- Kelly, A. E. , and Goulden, M. L. , 2008: Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences , 105(33): 11823–11826.
- Kharuk, V. I. , Ranson, K. J. , Im, S. T. , and Dvinskaya, M. L. , 2009: Response of Pinus sibirica and Larix sibirica to climate change in southern Siberian alpine forest-tundra ecotone. Scandinavian Journal of Forest Research , 24: 130–139.
- Kharuk, V. I. , Ranson, K. J. , Im, S. T. , and Vdovin, A. S. , 2010: Spatial distribution and temporal dynamics of high-elevation forest stands in southern Siberia. Global Ecology and Biogeography , 19: 822–830.
- Klanderud, K. , 2008: Species-specific responses of an alpine plant community under simulated environmental change. Journal of Vegetation Science , 19: 363–372.
- Körner, C. , 1998: A re-assessment of high elevation treeline positions and their explanation. Oecologia , 115: 445–459.
- Körner, C. , 2003: Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Second edition. Berlin: Springer, 349 pp.
- Körner, C. , and Paulsen, J. , 2004: A world-wide study of high altitude treeline temperatures. Journal of Biogeography , 31: 713–732.
- Kullman, L. , 1998: Non-analogous tree flora in the Scandes Mountains, Sweden, during the early Holocene-macrofossil evidence of rapid geographic spread and response to palaeoclimate. Boreas , 27: 153–161.
- Kullman, L. , 2002: Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. Journal of Ecology , 90: 68–77.
- Kullman, L. , 2007: Tree line population monitoring of Pinus sylvestris in the Swedish Scandes, 1973–2005: implications for tree line theory and climate change ecology. Journal of Ecology , 95: 41–52.
- Kuusinen, M. , and Siitonen, J. , 1998: Epiphytic lichen diversity in old-growth and managed Picea abies stands in southern Finland. Journal of Vegetation Science , 9: 283–292.
- Lee, C. S. , Kim, J. H. , Yi, H. , and You, Y. H. , 2004: Seedling establishment and regeneration of Korean red pine (Pinus densiflora S. et Z.) forests in Korea in relation to soil moisture. Forest Ecology and Management , 199: 423–432.
- Lenoir, J. , Gegout, J. C. , Marquet, P. A. , de Ruffray, P. , and Brisse, H. , 2008: A significant upward shift in plant species optimum elevation during the 20th century. Science , 320: 1768–1771.
- le Roux, P. C. , and McGeoch, M. A. , 2008: Rapid range expansion and community reorganization in response to warming. Global Change Biology , 14: 2950–2962.
- Lescop-Sinclair, K. , and Payette, S. , 1995: Recent advance of the Arctic treeline along the Eastern Coast of Hudson Bay. Journal of Ecology , 83: 929–936.
- Lett, S. , and Michelsen, A. , 2014: Seasonal variation in nitrogen fixation and effects of climate change in a subarctic heath. Plant and Soil , 379: 193–204.
- Li, M.-H. , and Yang, J. , 2004: Effects of microsite on growth of Pinus cembra in the subalpine zone of the Austrian Alps. Annals of Forest Science , 61: 319–325.
- Liang, E. , Wang, Y. , Eckstein, D. , and Luo, T. , 2011: Little change in the fir tree-line position on the southeastern Tibetan Plateau after 200 years of warming. New Phytologist , 190: 760–769.
- Lin, D. , Xia, J. , and . Wan, S. , 2010: Climate warming and biomass accumulation of terrestrial plants: a meta-analysis. New Phytologist , 188: 187–198.
- Lloyd, A. H. , 2005: Ecological histories from Alaskan tree lines provide insight into future change. Ecology , 86: 1687–1695.
- Lloyd, A. H. , and Fastie, C. L. , 2002: Spatial and temporal variability in the growth and climate response of treeline trees in Alaska. Climatic Change , 52: 481–509.
- Lloyd, A. H. , and Fastie, C. L. , 2003: Recent changes in treeline distribution and structure in interior Alaska. Ecoscience , 10(2): 176–185.
- Lopatin, E. , Kolström, T. , and Spiecker, H. , 2006: Determination of forest growth trends in Komi Republic (northwestern Russia): combination of tree-ring analysis and remote sensing data. Boreal Environment Research , 11: 341–353.
- MacDonald, G. M. , Kremenetski, K. V. , and Beilman, D. W. , 2008: Climate change and the northern Russian treeline zone. Philosophical Transactions of the Royal Society-Biology , 363: 2285–2299.
- Macias-Fauria, M. , and Johnson, E. A. , 2013: Warming-induced upslope advance of subalpine forest is severely limited by geomorphic processes. Proceedings of the National Academy of Sciences , 110(20): 8117–8122.
- Mamet, S. D. , and Kershaw, G. P. , 2012: Subarctic and alpine tree line dynamics during the last 400 years in north-western and central Canada. Journal of Biogeography , 39: 855–868.
- Masek, J. G. , 2001: Stability of boreal forest stands during recent climate change: evidence from Landsat satellite imagery. Journal of Biogeography , 28: 967–976.
- Matlack, G. R. , 1994: Plant species migration in a mixed-history forest landscape in eastern North America. Ecology , 75: 1491–1502.
- McLachlan, J. S. , Clark, J. S. , and Manos, P. S. , 2005: Molecular indicators of tree migration capacity under rapid climate change. Ecology , 86: 2088–2098.
- McNown, R.W. , and Sullivan, P. F. , 2013: Low photosynthesis of treeline white spruce is associated with limited soil nitrogen availability in the Western Brooks Range, Alaska. Functional Ecology , 27: 672–683.
- McVicar, T. R. , Li, L. , Van Niel, T. G. , Zhang, L. , Li, R. , Yang, Q. , Zhang, X. , Mu, X. , Wen, Z. , Liu, W. , Zhao, Y. A. , Liu, Z. , and Gao, P. , 2007: Developing a decision support tool for China's re-vegetation program: simulating regional impacts of afforestation on average annual streamflow in the Loess Plateau. Forest Ecology and Management , 251: 65–81.
- Michaelson, G. J. , Ping, C. L. , and Kimble, J. M. , 1996: Carbon storage and distribution in tundra soils of Arctic Alaska, U.S.A. Arctic and Alpine Research , 28: 414–424.
- Moen, J. , Aune, K. , Edenius, L. , and Angerbjorn, A. , 2004: Potential effects of climate change on treeline position in the Swedish mountains. Ecology and Society , 9: 16–26.
- Moiseev, P. A. , and Shiyatov, S. G. , 2003: Vegetation dynamics at the treeline ecotone in the Ural highlands, Russia. In Nagy, L. , Grabherr, G. , Körner, C. , and Thompson, D. B. A. (ed.), Alpine Biodiversity in Europe-A Europe-wide Assessment of Biological Richness and Change. Berlin: Springer, Ecological Studies 167, 423–435.
- Monson, R. K. , Turnipseed, A. A. , Sparks, J. P. , Harley, P. C. , Scott-Denton, L. E. , Sparks, K. , and Huxman, T. E. , 2002: Carbon sequestration in a high-elevation, subalpine forest. Global Change Biology , 8: 459–478.
- Moore, M. , and Huffman, D. , 2004: Tree encroachment on meadows of the North Rim, Grand Canyon National Park, Arizona, U.S.A. Arctic, Antarctic, and Alpine Research , 36: 474–483.
- Motta, R. , and Nola, P. , 2001: Growth trends and dynamics in sub-alpine forest stands in the Varaita Valley (Piedmont, Italy) and their relationships with human activities and global change. Journal of Vegetation Science , 12: 219–230.
- Nabuurs, G-J. , Linder, M. , Verkerk, P. , Gunia, K. , Deda, P. , Michalak, R. , and Grassi, G. , 2013: First signs of carbon sink saturation in European forest biomass. Nature Climate Change , 3: 792–796.
- Parmesan, C. , 2006: Ecological and evolutionary responses to recent climate change. Annual Reviews in Ecology, Evolution and Systematics , 37: 669.
- Parmesan, C. , and Yohe, G. , 2003: A globally coherent fingerprint of climate change impacts across natural systems. Nature , 421: 37–42.
- Pauli, H. , Gottfreid, M. , and Grabherr, G. , 1996: Effects of climate change on mountain ecosystems-upward shifting of alpine plants. World Resource Review , 8: 382–390.
- Peng, C. , Zhou, X. , Zhao, S. , Wang, X. , Zhu, B. , Piao, S. , and Fang, J. , 2009: Quantifying the response of forest carbon balance to future climate change in northeastern China: model validation and prediction. Global and Planetary Change , 66: 179–194.
- Peñuelas, J. , and Boada, M. , 2003: A global change-induced biome shift in the Montseny Mountains (NE Spain). Global Change Biology , 9: 131–140.
- Peñuelas, J. , Filella, I. , and Comas, P. , 2002: Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Global Change Biology , 8: 531–544.
- Pigott, C. D. , and Huntley, J. P. 1978. Factors controlling the distribution of Tilia cordata at the northern limits of its geographical range. I. Distribution in north-west England. New Phytologist , 81: 429–441.
- Rabasa, S. G. , Granda, E. , Banavides, R. , Kunstler, G. , Espelta, J. M. , Ogaya, R. , Peñuelas, J. , Scherer-Lorenzen, M. , Gil, W. , Grodki, W. , Ambrozy, S. , Bergh, J. , Hodar, J. , Zamora, R. , and Valladares, F. , 2013: Disparity in elevational shifts of European trees in response to recent climate warming. Global Change Biology , 19: 2490–2499.
- Reich, P. B. , and Oleksyn, J. , 2004: Global patterns of plant leaf N and P in relation to temperature and latitude. Proceedings of the National Academy of Sciences , 101(30): 11001–11006.
- Resler, L. M. ,. Butler, D. R. , and Malanson, G. P. , 2005: Topographic shelter and conifer establishment and mortality in an alpine environment, Glacier National Park, Montana. Physical Geography , 26: 112–125.
- Roberts, L. , 1989. How fast can trees migrate? Science , 243: 735–737.
- Rochefort, R. M. , Little, R. L. , Woodward, A. , and Peterson, D. L. , 1994: Changes in sub-alpine tree distribution in western North America: a review of climatic and other causal factors. The Holocene , 4: 89–100.
- Ruete, A. , Fritz, Ö. , Snäll, T. , and Salguero-Gómez, R. , 2014: A model for non-equilibrium metapopulation dynamics utilizing data on species occupancy, patch ages and landscape history. Journal of Ecology , 102: 678–689.
- Rustad, L. E. , Campbell, J. L. , Marion, G. M. , Norby, R. J. , Mitchell, M. J. , Hartley, A. E. , Cornelissen, J. H. C. , and Gurevitch, J. , 2001: A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia , 126: 543–562.
- Saxe, H. , Cannell, M. G. R. , Johnsen, B. , Ryan, M. G. , and Vourlitis, G. , 2001: Tree and forest functioning in response to global warming. New Phytologist , 149: 369–399.
- Schönenberger, W. , Noack, A. , and Thee, P. , 2005: Effect of timber removal from windthrow slopes on the risk of snow avalanches and rockfall. Forest Ecology and Management , 213(1–3): 197–208.
- Sea, D. S. , and Whitlock, C. , 1995: Postglacial vegetation and climate of the Cascade Range, central Oregon. Quaternary Research , 43: 370–381.
- Shiyatov, S. G. , Terent'ev, M. M. , and Fomin, V. V. , 2005: Spatiotemporal dynamics of forest-tundra communities in the polar Urals. Russian Journal of Ecology , 36: 69–75.
- Shiyatov, S. , Terent'ev, M. , Fomin, V. , and Zimmermann, N. , 2007: Altitudinal and horizontal shifts of the upper boundaries of open and closed forests in the Polar Urals in the 20th century. Russian Journal of Ecology , 38: 223–227.
- Sillett, S. C. , 1994: Growth rates of two epiphytic cyanolichen species at the edge and in the interior of a 700-year-old Douglas fir forest in the Western Cascades of Oregon. The Bryologist , 97: 321–324.
- Sillett, S. C. , McCune, B. , Peck, J. E. , Rambo, T. R. , and Ruchty, A. , 2000: Dispersal limitations of epiphytic lichens result in species dependent on old-growth forests. Ecological Applications , 10: 789–799.
- Sjögersten, S. , and Wookey, P. A. , 2002: Climatic and resource quality controls on soil respiration across a forest-tundra ecotone in Swedish Lapland. Soil Biology and Biochemistry , 34: 1633–1646.
- Sjögersten, S. , and Wookey, P. A. , 2005: The role of soil organic matter quality and physical environment for nitrogen mineralization at the forest-tundra ecotone in Fennoscandia. Arctic, Antarctic, and Alpine Research , 37: 118–126.
- Sjögersten, S. , Alewell, C. , Cecillon, L. , Hagedorn, F. , Jandl, R. , Leifeld, J. , Martinsen, V. , Schindlbacher, A. , Sebastià, M.-T. , and Van Miegroet, H. , 2011: Mountain soils in a changing climate-Vulnerability of carbon stocks and ecosystem feedbacks. In Jandl, R. , Rodeghiero, M. , and Olsson, M. (eds.), Soil Carbon in Sensitive European Ecosystems. West Sussex, U.K.: John Wiley & Sons, 118–148.
- Smith, W. K. , Germino, M. J. , Hancock, T. E. , and Johnson, D. M. , 2003: Another perspective on altitudinal limits of alpine timberlines. Tree Physiology , 23: 1101–1112.
- Spargo, J. , Cavigelli, M. , Mirsky, S. B. , Maul, J. E. , and Meisinger, J. J. , 2011: Mineralizable soil nitrogen and labile soil organic matter in diverse long-term cropping systems. Nutrient Cycling in Agroecosystems , 90(2): 253–266.
- Steltzer, H. , 2004: Soil carbon sequestration with forest expansion in an Arctic forest-tundra landscape. Canadian Journal of Forest Research , 34: 1538–1542.
- Stoffel, M. , Wehrli, A. , Kühne, R. , Dorren, L. K. A. , Perret, S. , and Kienholz, H. , 2006: Assessing the protective effect of mountain forests against rockfall using a 3D simulation model. Forest Ecology and Management , 225(1–3): 113–122.
- Streit, K. , Hagedorn, F. , Hiltbrunner, D. , Portmann, M. , Saurer, M. , Buchmann, N. , Wild, B. , Richter, A. , Wipf, S. , and Siegwolf, R. T. W. , 2014: Soil warming alters microbial substrate use in alpine soils. Global Change Biology , 20: 1327–1338.
- Suarez, F. , Binkley, D. , and Kaye, M. W. , 1999: Expansion of forest stands into tundra in the Noatak National Preserve, northwest Alaska. Ecoscience , 6: 465–470.
- Sundqvist, M. K. , Giesler, R. , Graae, B. J. , Wallander, H. , Fogelberg, E. , and Wardle, D. A. , 2011: Interactive effects of vegetation type and elevation on aboveground and belowground properties in a subarctic Tundra. Oikos , 120: 128–142.
- Sveinbjörnsson, B. , Nordel, O. , and Kauhanen, H. , 1992: Nutrient relations of mountain birch growth at and below the elevational tree-line in Swedish Lapland. Functional Ecology , 6: 213–220.
- Sveinbjörnsson, B. , Davis, J. , Abadi, W. , and Butler, A. , 1995: Soil carbon and nitrogen mineralization at different elevations in the Chugach Mountains of south-central Alaska, U.S.A. Arctic and Alpine Research , 27: 29–37.
- Sziecz, J. M. , and MacDonald, G. M. , 1995: Recent white spruce dynamics at the subarctic alpine treeline of north-western Canada. Journal of Ecology , 83: 873–885.
- Tarnocai, C. , Canadell, J. G. , Schuur, E. A. G. , Kuhry, P. , Mazhitova, G. , and Zimov, S. , 2009: Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochemical Cycles , 23: http://dx.doi.org/10.1029/2008GB003327.
- Teich, M. , and Bebi, P. , 2009: Evaluating the benefit of avalanche protection forest with GIS-based risk analyses-A case study in Switzerland. Forest Ecology and Management , 257: 1910–1919.
- Thébault, A. , Clément, J.-C. , Ibanez, S. , Roy, J. , Geremia, R. A. , Pérez, C. A. , Buttler, A. , Estienne, Y. , and Lavorel, S. , 2014: Nitrogen limitation and microbial diversity at the treeline. Oikos , 123: 729–740.
- Thomas, S. C. , Halpern, C. B. , Falk, D. A. , Liguori, D. A. , and Austin, K. A. , 1999: Plant diversity in managed forests: understory responses to thinning and fertilization. Ecological Applications , 9: 864–879.
- Tømmervik, H. , Johansen, B. , Riseth, J. Å. , Karlsen, S. R. , Solberg, B. , and Høgda, K. A. , 2009: Above ground biomass changes in the mountain birch forests and mountain heaths of Finnmarksvidda, northern Norway, in the period 1957–2006. Forest Ecology and Management , 257: 244–257.
- Tranquillini, W. , 1979: Physiological Ecology of the Alpine Timberline. Tree Existence at High Altitudes with Special Reference to the European Alps. Berlin: Springer, Ecological Studies 31.
- Trivedi, M. R. , Morecroft, M. D. , Berry, P. M. , and Dawson, T. P. , 2008: Potential effects of climate change on plant communities in three montane nature reserves in Scotland, UK. Biological Conservation , 141(6): 1665–1675.
- van der Wal, R. , Pearce, I. S. K. , and Brooker, R. W. , 2005: Mosses and the struggle for light in a nitrogen-polluted world. Oecologia , 142: 159–168.
- Van Devender, T. R. , and Spaulding, W. G. , 1979: Development of vegetation and climate in the southwestern United States. Science , 204: 701–710.
- Villalba, R. , and Veblen, T. T. , 1997: Regional patterns of tree population age structures in Northern Patagonia: climatic and disturbance influences. Journal of Ecology , 85: 113–124.
- Walker, M. D. , Wahren, C. H. , Hollister, R. D. , Henry, G. H. R. , Ahlquist, L. E. , Alatalo, J. M. , Bret-Harte, M. S. , Calef, M. P. , Callaghan, T. V. , Carroll, A. B. , Epstein, H. E. , Jónsdóttir, I. S. , Klein, J. A. , Magnússon, B. ó. , Molau, U. , Oberbauer, S. F. , Rewa, S. P. , Robinson, C. H. , Shaver, G. R. , Suding, K. N , Thompson, C. C. , Tolvanen, A. , Totland, Ø. , Turner, P. L. , Tweedie, C. E. , Webber, P. J. , and Wookey, P. A. , 2006: Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences of the United States of America , 103: 1342–1346.
- Walther, G. R. , 2003: Plants in a warmer world. Perspectives in Plant Ecology, Evolution and Systematics , 6: 169–185.
- Walther, G. R. , Post, E. , Convey, P. , Menzel, A. , Parmesan, C. , Beebee, T. J. C. , Fromentin, J. M. , and Hoegh-Guldberg, O. B. F. , 2002: Ecological responses to recent climate change. Nature , 416: 389–395.
- Wang, H. , 2013: A multi-model assessment of climate change impacts on the distribution and productivity of ecosystems in China. Regional Environmental Change , 14: 133–144.
- Wehrli, A. D. , Dorren, L. K. A. , and Berger, F. 2006. Modelling long-term effects of forest dynamics on the protective effect against rockfall. Forest Snow and Landscape Research , 80: 57–76.
- White, A. , Cannell, M. G. R. , and Friend, A. D. , 2000: The high-latitude terrestrial carbon sink: a model analysis. Global Change Biology , 6: 227–245.
- Wieser, G. , 2007: Global change at the upper timberline. In Wieser, G. , and Tausz, M. (eds.), Trees at Their Upper Limit. Dordrecht, Netherlands: Springer, 197–207.
- Wilmking, M. , Harden, J. , and Tape, K. , 2006: Effect of tree line advance on carbon storage in NW Alaska. Journal of Geophysical Research , 111: 1–10.
- Wu, Q. Q. , Wu, F. Z. , Yang, W. Q. , Zhao, Y. Y. , He, W. , and Tan, B. , 2014: Foliar litter nitrogen dynamics as affected by forest gap in the alpine forest of Eastern Tibet Plateau. PloS One , 9(5): 1–14.
- Xu, Z. F. , Hu, T. X. , and Zhang, Y. B. , 2012: Effects of experimental warming on phenology, growth and gas exchange of treeline birch (Betula utilis) saplings, Eastern Tibetan Plateau, China. European Journal of Forest Research , 131: 811–819.
- Zierl, B. , and Bugmann, H. , 2007: Sensitivity of carbon cycling in the European Alps to changes of climate and land cover. Climatic Change , 85: 195–212.