Abstract
The plant and insect communities of many summer pastures in the Swiss Alps are changing as they become encroached by woody plants. To understand the implications for overall biodiversity, we need to determine how different taxonomic groups respond to shrub encroachment. We investigated effects of encroachment upon species diversity of vascular plants, butterflies, and grasshoppers on two subalpine pastures (Mesocco and Guarda). On each site, we recognized a sequence of vegetation types representing a gradient of increasing shrub cover. The number of rare plant species was strongly affected by the degree of encroachment, with areas dominated by either dwarf shrubs or Alnus viridis having fewer species than areas of open grassland. In the Mesocco site, we also found differences in other measures of plant species richness (total species richness, number of herbaceous species) and in the number of grasshopper species. While plant richness was highest in grassland-dominated vegetation types, the species richness of grasshoppers was highest in types with a low to intermediate cover of dwarf shrubs. We found no effect of shrub cover upon butterfly species richness at either site. Biotic factors (shrub cover, grazing intensity, and also vegetation-related variables for the insect groups) explained a larger proportion of the variance in species composition of both plants and insects than did large-scale abiotic factors (altitude, aspect, and slope). Our results demonstrate that shrub encroachment is a threat to the biodiversity of subalpine grassland ecosystems. We recommend conservation actions that prevent extensive shrub encroachment but promote a mosaic of small areas at different successional stages.
Introduction
The term shrub encroachment refers to the spread of indigenous woody plant species into grassland (CitationVan Auken, 2009). Many reports suggest that encroachment is increasing in grassland and savanna ecosystems throughout the world because of changing land use and in response to climate change (CitationEldridge et al., 2011). The resulting transformation of grassland into woodland profoundly affects many ecosystem properties, including species diversity (CitationAnthelme et al., 2001; CitationFreléchoux et al., 2007), nutrient cycling (CitationArcher et al., 2001; CitationMcKinley et al., 2008), and water balance (CitationHuxman et al., 2005; CitationAlewell and Bebi, 2011).
Alpine summer pastures represent a habitat type in which encroachment is now common. Although these grasslands are mainly anthropogenic, they often support a high diversity of plant and animal species (CitationVäre et al., 2003) that have assembled over hundreds of years of extensive use (CitationBätzing, 2005). During the past century, however, the traditional forms of grazing management have ceased to be economically viable (CitationMacDonald et al., 2000; CitationChemini and Rizzoli, 2003; CitationTasser et al., 2007), and many summer pastures have either been abandoned or are now used less intensively (“agricultural marginalisation”: see CitationBaldock et al., 1996). These changes have set in train successional processes that lead ultimately to closed forest (CitationZoller et al., 1984; CitationSchütz et al., 2000). Indeed, forest cover in Switzerland has increased by at least one-third during the past 150 years (CitationBrändli, 2000), with most of this occurring on summer pastures (CitationBaur et al., 2006b; CitationStöcklin et al., 2007).
Above the tree line, and in certain topographic situations, dwarf shrubs form stable communities, whereas below the tree line they generally represent a successional stage from open grassland to forest (CitationBischof, 1984). However, even here a dense cover can prevent trees from establishing, so that dwarf shrub vegetation persists for decades or even centuries (CitationConnell and Slatyer, 1977; CitationPutz and Canham, 1992; CitationNiering, 2005). In addition, the speed and course of the succession may be affected by other factors such as altitude, local climate, aspect, and slope (CitationSchütz et al., 2000; CitationFreléchoux et al., 2007). One of the most abundant dwarf shrubs on Swiss alpine landscapes is Rhododendron ferrugineum, which is frequently accompanied by Vaccinium species to form the Rhododendro-Vaccinion association. Another is Juniperus communis subsp. alpina, which together with Arctostaphilos uva-ursi and Calluna vulgaris forms the Juniperion nanae association (CitationPoldini et al., 2004; CitationDelarze and Gonseth, 2008). On more moist and nutrient rich subalpine areas, both R. ferrugineum and J. communis subsp. alpina also associate with Alnus viridis (CitationBischof, 1984). Although less common in the lowlands, these shrub species have little conservational value in Swiss alpine habitats (CitationMoser et al., 2002).
In seeking to understand how land use change affects the species diversity of summer pastures, it is important to distinguish between responses due to an absence of grazing animals and those due to the spread of woody plants. In practice, however, most studies have been unable to make this distinction because they were conducted on abandoned pastures, where both processes are at work (CitationPykälä et al., 2005). In particular, studies have rarely examined the effects of dwarf shrubs upon biodiversity, although these shrubs may form dense stands on pastures that have been abandoned or are little used (CitationCavallero et al., 2007; CitationFreléchoux et al., 2007). Another limitation of previous studies of shrub encroachment is that they have rarely included both plant and insect taxa, even though this information would be very useful for management (CitationWallis de Vries et al., 2002). The few studies to investigate relationships between woody cover and species diversity in alpine landscapes have reported negative or hump-shaped patterns for plants and for some invertebrate taxa, whereby species diversity is greatest at no or an intermediate amount of woody cover (CitationAnthelme et al., 2001, Citation2007; CitationFreléchoux et al., 2007; CitationPornaro et al., 2013).
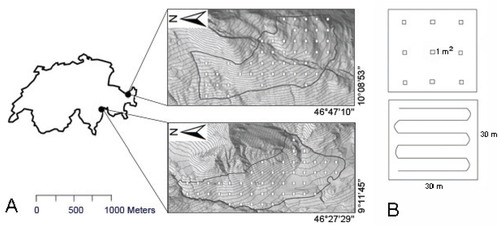
FIGURE 1. (A) The pasture areas in Guarda (top) and Mesocco (bottom) showing the boundaries and the systematically located plots. (B) The sampling design for plants (top) and insects (bottom).
We investigated the effects of dwarf shrub encroachment on the species richness and composition of vascular plants, butterflies, and grasshoppers on two subalpine pastures in the Swiss Alps. Aside from being important components of the biodiversity, these taxa are relatively easy to sample and identify, and react sensitively to changing environmental factors and agricultural practices (CitationMarini et al., 2009). In our study we posed three questions: (1) How do plant assemblages on subalpine summer pastures change along a gradient of increasing encroachment? (2) How does species richness of plants, butterflies, and grasshoppers change along this gradient? (3) How important are the effects of shrub cover and other biotic factors relative to abiotic or spatial explanatory variables in shaping plant, butterfly, and grasshopper communities on encroached summer pastures?
Material and Methods
STUDY SITES AND SAMPLING DESIGN
The study sites were two subalpine pastures near the villages of Mesocco (46°23′31″N, 9°13′58″E) and Guarda (46°46′33″N, 10°08′59″E) in the Canton of Grisons in southeastern Switzerland (, part A). Both cattle pastures are situated in marginal locations just above the present tree line (1762–2064 m elevation in Mesocco, and 2097–2410 m in Guarda), have a south-westerly aspect, and are approximately 1.5 km2 in area. Both have a long tradition of pastoral use between June and September (CitationWerthemann and Imboden, 1982) but are now less used and are being encroached by dwarf shrubs (J. Barbüda and A. Toscano, personal communication).
The mean annual precipitation (years 1961–1990) measured at weather stations close to the survey sites was 1864 mm at Mesocco and 693 mm at Guarda (CitationSchweizerische Meteorologische Anstalt, 2008). Given the underlying rocks, which are predominantly crystalline (CitationReinhard et al., 1962), acidic soil conditions prevail at both sites. The vegetation in both areas is a mosaic of grassland, mainly of Nardus stricta, and dwarf ericaceous shrubs (mainly Arctostaphylos uva-ursi, Calluna vulgaris, Rhododendron ferrugineum, Vaccinium myrtillus, and Vaccinium gaultheroides), and junipers (Juniperus communis subsp. alpina). Whereas these nutrient-poor N. stricta and shrubland vegetations dominate on steeper and remote areas of the pasture, nutrient-rich grasslands occur predominantly on flat areas and around buildings. In Mesocco there are also some areas encroached by the shrub Alnus viridis. A study of aerial photographs shows an increase in dwarf shrub cover by 31% in areas of the pasture at Mesocco between 1962 and 2005, despite the site never having been abandoned (CitationCrivelli, 2011). Mismanagement and low grazing pressure of more remote and steeper areas are possible reasons for this shrub increase.
SAMPLING OF SPECIES AND ENVIRONMENTAL DATA
On each pasture, 54 plots of 30 × 30 m (900 m2) were defined by systematic sampling (lower left corner of each plot placed at a randomly regular grid with a 155 m resolution). Plots with a slope steeper than 50° or those with more than 40% stones and rock were excluded, being unsuitable for cattle grazing. The remaining plots in the grid were all sampled, resulting in 49 plots at Mesocco and 48 plots at Guarda (, part A).
Data were collected between June and September 2010. To account for local heterogeneity, plants were surveyed in nine 1 m2 quadrats arranged regularly within the 900 m2 plot (, part B). The plant cover in the 1 m2 quadrats was estimated using the Braun-Blanquet scale, and the nomenclature follows Lauber and Wagner (Citation2001). We also recorded the presence of any additional species absent from the nine quadrats by searching the whole plot for 30 minutes. All plant species from the nine 1 m2 quadrats and the 30 minutes' walk were pooled to calculate the total number of plant species per plot. We recorded all adult butterflies (belonging to the Rhopalocera, Hesperiidae, and Zygaenidae) and grasshoppers (Ensifera and Caelifera) by traversing the 900 m2 plot in a serpentine transect (, part B; see CitationBalmer and Erhardt, 2000). Insects of both taxa were identified visually—and grasshoppers also acoustically—between 10:00 and 17:00 on sunny days with little or no wind. The butterfly survey was repeated five times, and the grasshopper survey twice. Nomenclature of butterflies followed Bühler-Cortesi (Citation2009) and that of grasshoppers followed Baur et al. (Citation2006a). For both taxa, data of different sampling times were pooled at the plot level prior to data analysis.
For each 900 m2 plot we recorded altitude, slope, aspect, and the percentage shrub cover. Moisture was disregarded in our study as an abiotic factor as the sites were relatively homogeneous in this respect. Further, both slope and aspect contribute to variation in insolation and are often used as surrogates for soil moisture spatial patterns (CitationTague, 2005). We also counted the cow-pats, using this number as a measure of grazing intensity.
STATISTICAL ANALYSES
To describe vegetation types of the two subalpine pastures, the plant cover data from the nine 1 m2 quadrats were classified by means of cluster analysis. Following the suggestion of Legendre and Gallagher (Citation2001), a Hellinger transformation was used for the plant cover data. The transformed cover data were then classified on the basis of k-means clustering using Euclidean distance.
One-way ANOVA was used to test for differences among vegetation types in various species measures: (1) total number of plant species per plot, hereafter plant species richness; (2) number of herbaceous plant species; (3) number of rare plant species, that is, species with an overall cover sum of ≤5% in the 9 × 1 m2 quadrats; (4) number of butterfly species, hereafter butterfly species richness; and (5) number of grasshopper species, hereafter grasshopper species richness. A Bonferroni correction was used to adjust p-values to avoid type-I errors leading to a critical p-value of p = 0.05 ≤ 5 = 0.01. Tukey's honest significance tests were then carried out to identify differences between vegetation types (p < 0.01).
To evaluate the impact of explanatory variables on the species composition of plants, butterflies, and grasshoppers, the abundance data were analyzed by variation partitioning with a redundancy analysis (RDA) (CitationBorcard et al., 1992). We related the data to three sets of explanatory variables: (1) biotic factors (shrub cover, its square term, grazing intensity; additionally for the insect groups: vegetation types, plant species richness); (2) abiotic factors (altitude, slope, aspect); and (3) spatial structure (characterized by principal coordinates of neighbor matrices [PCNM]). The PCNM method is based upon the orthogonal spectral decomposition of the spatial relationships among sampling sites and permits spatial patterns at different scales to be considered (CitationBorcard and Legendre, 2002). Because PCNM assumes the analysis of data in a regular grid, separate analyses had to be performed for the two sites. To fill a gap in the regular sampling grid in Guarda, a supplementary coordinate point was added before calculating the PCNMs (CitationBorcard and Legendre, 2002). The variables retained by the PCNM analysis were 25 for Mesocco and 26 for Guarda. A Hellinger transformation was performed on the cover data for plants and abundance data for butterflies and grasshoppers (CitationLegendre and Gallagher, 2001). First, for each species group, forward selection using the double stopping criteria was performed on each set of variables independently to retain only significant terms (CitationGuillaume Blanchet et al., 2008). Then, variation partitioning with RDA was used to determine how much of the variation in species assemblages was explained by pure (biotic, abiotic, spatial) and combined fractions (CitationBorcard et al., 1992). The significance of the pure fractions was then assessed under the reduced model. Adjusted R 2 (R 2 Adj) values were used as input for the variation partitioning to account for the different number of terms in the sets of variables (CitationPeres-Neto et al., 2006). Statistical analyses were performed with the packages “vegan” (CitationOksanen et al., 2012) and “PCNM” (CitationLegendre et al., 2012) of the statistical program R (CitationR Core Team, 2012).
Results
VEGETATION TYPES
The cluster analysis based upon plant species composition identified five vegetation types in Mesocco and four vegetation types in Guarda. These vegetation types can be characterized as follows ( and ):
Type 1 (a + b) subsumed plots in both pastures with very low shrub cover and many cow-pats (99.0 ± 20.1 and 67.0 ± 28.6 in Types 1a and 1b, respectively). The vegetation can be assigned to the phytosociological association Poion alpinae (CitationDelarze and Gonseth, 2008). In Mesocco (Type 1a) this vegetation type had a high cover of the grasses Festuca rubra, Phleum rhaeticum, and Agrostis capillaris often accompanied by Nardus stricta, whereas the most abundant species in Guarda (Type 1b) were Deschampsia caespitosa, F. rubra, Alchemilla vulgaris, and N. stricta.
Type 2b included plots in Guarda with a vegetation dominated by N. stricta, and which can be assigned to the Nardion association. The tufts of N. stricta were very vigorous, and few other species were present in high frequency. Similar to Type 1, this type was also characterized by low shrub cover and many cow-pats (109.5 ± 27.7).
Type 3 (a + b) comprised plots in both pastures with intermediate shrub cover and a mosaic of grassland and shrubs. The grassland vegetation was dominated by N. stricta, while the most prominent shrub was Juniperus communis subsp. alpina (hereafter J. communis). The Nardion and Juniperion nanae associations intermixed in these plots forming a mosaic of grassland with patchy distributed dwarf shrubs. Number of cow-pats in the vegetation Types 3a (34.1 ± 6.9) and 3b (25.6 ± 3.3) was rather low compared to Types 1 and 2.
Type 4 (a + b) comprised plots with vegetation dominated by J. communis. This vegetation type is characterized by high shrub cover and can be attributed to the Juniperion nanae association. In Mesocco (Type 4a), J. communis was frequently accompanied by Agrostis schraderiana, Rhododendron ferrugineum, and Alnus viridis. In Guarda (Type 4b), the most frequent accompanying species were the dwarf-shrubs Arctostaphylos uva-ursi, Calluna vulgaris, Vaccinium myrtillus, and Vaccinium gaultheroides. Very few cow-pats were found in plots with vegetation Types 4a (3.9 ± 2.6) or 4b (8.1 ± 3.1).
Type 5a was found only in Mesocco and was represented by plots with a complete dwarf shrub cover. No cow-pats were found in this vegetation type. The dominant species were J. communis and R. ferrugineum, but V. gaultheroides, C. vulgaris, and V. myrtillus were also frequent. The associations Juniperion nanae and Rhododendro-Vaccinion mixed in the plots dominated by Type 5a vegetation.
TABLE 1 Average (± SE) cover (%) for the most frequent plant species in the five vegetation types in Mesocco. Only species with mean percentage cover >2% in at least one type are shown in the table. Mean values >2% are shaded in gray, and those >5% are additionally highlighted in bold. Woody plant species are highlighted in bold. Biotic and abiotic factors for the vegetation types are summarized at the bottom of the table.
Type 6a comprised plots in Mesocco with a high cover of Alnus viridis and no cow-pats. This type can be assigned to the Alnenion viridis association. Other prominent species were Agrostis schraderiana, Calamagrostis villosa, Rubus idaeus, R. ferrugineum, Peucedanum ostruthium, and Chaerophyllum villarsii.
Types 1 to 5 occurred on rather dry sites and followed a gradient of increasing shrub encroachment, with increasing shrub cover and decreasing surface covered by grassland. In contrast to the other vegetation types, Type 6 was only present in particularly moist sites.
The vegetation types in Guarda and Mesocco presented differences in butterfly and grasshopper compositions. and display the most frequent species for each vegetation type.
PATTERNS OF SPECIES RICHNESS AND COMPOSITION
We recorded more species at Guarda than at Mesocco, with a total of 332 versus 318 plants, 52 versus 44 butterflies, and 14 versus 8 grasshoppers for the two sites, respectively. At both sites, we found significant variation in species richness among vegetation types, including in the number of rare plant species in Guarda, and in the numbers of vascular plants (all measures) and grasshoppers in Mesocco (one-way ANOVA; p-value < 0.01). Distinctions between the vegetation types revealed by Tukey's post hoc tests are reported in . In general, species richness of plants (all, herbaceous, and rare species) was higher in plots with a lower shrub cover, although in Guarda, number of all plant species and herbaceous species did not vary significantly according to vegetation type. Numbers of butterfly species did not vary significantly among vegetation types in either site. However, species richness of grasshoppers was significantly higher in Type 3a than Type 6a in Mesocco, but did not vary in Guarda.
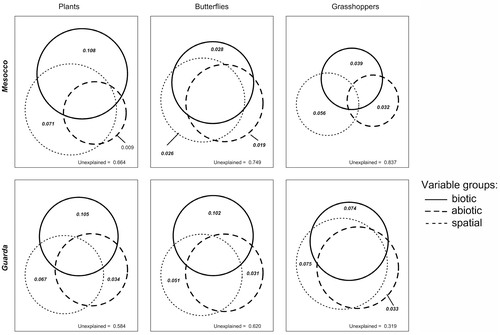
In the RDA analyses, the measured variables explained far more of the variation in species composition of plants, butterflies, and grasshoppers at Guarda than at Mesocco (38%–68.1% vs. 16.3%–33.6%; , Appendix A). Of this total variation, the fraction explained by biotic variables ranged from 10.9% to 32.1% (mean ± SE: 21.7 ± 3.1), and was significant for all groups at both sites. Abiotic variables explained between 2.7% and 19.6% (8.5 ± 2.1) of variation and showed a significant effect for all groups at Guarda, but not for plants at Mesocco. The fraction explained by spatial variables (PCNMs) was generally high, with values ranging between 10.4% and 34.4% (17.7 ± 3.2).
TABLE 2 Average (± SE) cover (%) for the most frequent plant species in the four vegetation types identified in Guarda. Only species with mean percentage cover > 2% in at least one type are shown in the table. Mean values >2% are shaded in grey and >5% are additionally highlighted in bold. Woody plant species are highlighted in bold. Biotic and abiotic factors for the vegetation types are summarized at the bottom of the table.
Discussion
The effect of shrubs on species diversity in abandoned or underused subalpine pastures has been investigated before, albeit usually focusing on plant diversity and rarely in the context of encroachment by dwarf shrubs (CitationAnthelme et al., 2001, Citation2007; CitationFreléchoux et al., 2007; CitationPornaro et al., 2013). In Mesocco, plant diversity, whether measured as plant species richness, number of herbaceous species, or number of rare species, was generally higher in grassland (Poion alpinae) and mosaic vegetation (Nardion with Juniperion nanae) than in dwarf shrub and A. viridis dominated stands; grasshopper diversity was highest in mosaic vegetation. In contrast, significant differences at the Guarda site were found only for the number of rare plant species, with grassland sites (Poion alpinae) supporting more species than sites of intermediate to high dwarf shrub cover. No significant variation in butterfly species richness was found among vegetation types. The variation in species composition of plants, butterflies, and grasshoppers explained by biotic and spatial variable sets was generally greater than that explained by large-scale abiotic environmental variables.
TABLE 3 Average (± SE) number of individuals for the most frequent butterfly and grasshopper species in the five vegetation types identified in Mesocco. Only species with mean number of individuals >1 in at least one type are shown in the table. Mean values >1 are shaded in grey and >3 for butterflies resp. >5 for grasshoppers are additionally highlighted in bold.
VEGETATION TYPES AND SUCCESSION
The patterns of encroachment at both sites were similar and probably result from the declining use of the pastures in recent decades. However, there were also important differences in plant species composition between the sites, which presumably reflect differing climatic and topographic conditions. For example, more rainfall may explain why R. ferrugineum and Alnenion viridis vegetation was found at the Mesocco site. On the other hand, the dominance of J. communis at Guarda is consistent with the drier, more continental conditions at this site.
Dominant plant species in areas assigned to vegetation Type 1 (a + b) were mainly grasses associated with relatively nutrient-rich pastures, such as Agrostis capillaris, Festuca rubra, Deschampsia caespitosa, and Phleum rhaeticum. In general, Festuca/Agrostis vegetation depends upon high grazing intensity (CitationWelch and Scott, 1995), which is confirmed by the high density of cow-pats in this vegetation type. The Nardion vegetation in Guarda also showed very low cover of shrub species and evidence of use by cattle. However, N. stricta is an unpalatable species that cattle tend to avoid (CitationWelch and Scott, 1995; CitationJewell et al., 2005), and its presence in an underused pasture may offer an opportunity for shrub species to establish. Indeed, both pastures had extensive mosaics of Juniperion nanae and Nardion associations (Type 3 a + b), suggesting that dwarf shrubs are more likely to spread into vegetation with N. stricta than into the Poion alpinae association. Further, following the modeling of Schütz et al. (Citation2000), N. stricta reaches a peak after F. rubra in the succession process, indicating that when Nardus is dominant, the succession is already advanced. The subsequent development of the mosaic into more dense shrub vegetation is probably driven by a positive feedback, as the shrubs are avoided by cattle while their spread is encouraged by reduced grazing (CitationGüsewell et al., 2005; CitationKohler et al., 2006). Furthermore, the ability of shrubs such as J. communis to develop “fertility islands” (CitationDeLuca and Zackrisson, 2007) gives them a competitive advantage in nutrient-poor pastures providing that the cattle density is not too high (CitationLivingston, 1972).
Based upon the species overlap and the increasing cover by dwarf shrubs from open grassland vegetation types (Poion alpinae and Nardion types) via mosaic vegetation to a stage of shrub dominance, we conclude that the vegetation types at each study site represent different successional stages (excluding the Alnenion viridis at Mesocco). This conclusion is also in accordance with the succession model of Schütz et al. (Citation2000). However, we found no evidence of the succession proceeding further to woodland. This is probably because the dense shrub cover inhibits the establishment of trees (CitationConnell and Slatyer, 1977), thereby enabling this seral vegetation type to persist for decades or even centuries.
In contrast to the other vegetation types, Type 6a, attributed to the Alnenion viridis association and only present at the Mesocco site, does not appear to be part of the same successional sequence. A. viridis is known to indicate moister sites (CitationWettstein, 2001) and because of its association with nitrogen-fixing symbionts can grow very vigorously even in nutrient-poor sites (CitationMallik et al., 1997). The accompanying herbaceous plants (e.g., Agrostis schraderiana, Calamagrostis villosa, Peucedanum ostruthium, and Chaerophyllum villarsii) are mainly fast-growing, shade-tolerant species that thrive in the moist, nitrogen-rich soils beneath A. viridis (CitationAnthelme et al., 2001; CitationWiedmer and Senn-Irlet, 2006). Soil moisture was not used as an abiotic factor in this study because it was assumed to be strongly related to slope and aspect, which are often used as surrogates for moisture conditions (CitationMoore et al., 1991; CitationWoodcock et al., 2002; CitationTague, 2005). The mean slope in plots with Type 6a vegetation is higher than for all other vegetation types. The plots colonized by Alnenion viridis might well be located on slide paths of snow avalanches, where this vegetation often occurs (CitationWiedmer and Senn-Irlet, 2006). This might explain the greater steepness and higher moisture of these sites. Nevertheless, the lack of cow-pats in plots with this vegetation type indicates an absence of grazing pressure, suggesting different plant interaction and competition patterns than for other vegetation types subjected to higher grazing intensity (CitationGraff et al., 2007). Indeed, herbaceous plant species accompanying A. viridis are rather competitive.
TABLE 4 Average (± SE) number of individuals for the most frequent butterfly and grasshopper species in the four vegetation types identified in Guarda. Only species with mean number of individuals >1 in at least one type are shown in the table. Mean values >1 are shaded in grey and >3 for butterflies resp. >5 for grasshoppers are additionally highlighted in bold.
FIGURE 2. Error bar plots (mean ± SE) displaying differences between the vegetation types identified in (A–E) Mesocco and (F–L) Guarda for various plant measures (plant species richness, number of herbaceous plant species, number of rare plant species), butterfly species richness, and grasshopper species richness. Vegetation types: 1a+b: Poion alpinae; 2b: Nardion; 3a+b: mosaic of Nardion and Juniperion nanae; 4a+b: Juniperion nanae; 5a: encroached areas with Juniperion nanae and Rhododendro-Vaccinion; 6a: Alnenion viridis. Significant differences between the vegetation types detected with Tukey's post-hoc tests (p < 0.01) are displayed as different lowercase letters at the top of the error bars.
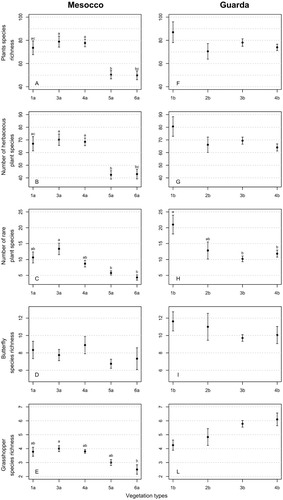
FIGURE 3. Results of variation partitioning with redundancy analysis (RDA) for the study sites Mesocco (n = 49) and Guarda (n = 48). Venn diagrams show the fractions of variance in species composition of plants, butterflies, and grasshoppers explained by pure biotic, abiotic, or spatial variables. Bold values indicate significant fractions. The unexplained variance is also reported in the single graphs. The reported fractions show adjusted R 2 to account for the different number of terms in the variable sets.
SHRUB ENCROACHMENT AND SPECIES DIVERSITY OF PLANTS, BUTTERFLIES, AND GRASSHOPPERS
The degree of shrub encroachment significantly affected the diversity of plants and grasshoppers in Mesocco, but only the number of rare plant species in Guarda. In Mesocco, the highly encroached plots (Type 5a) and those with Alnus viridis (Type 6a) had lower plant species richness and fewer herbaceous plant species than those with a mosaic of Nardion and Juniperion nanae (Type 3a) or with the Juniperion nanae association (Type 4a). Although shrub cover in plots dominated by A. viridis was not extremely high, plant species diversity was rather low, which is consistent with previous studies reporting Alnenion viridis plant communities to be particularly species-poor (CitationAnthelme et al., 2001, Citation2003; CitationFreléchoux et al., 2007). In Mesocco, encroached plots (Type 5a) had very low plant species richness, indicating that a high cover of dwarf shrubs has a negative effect upon plant diversity. Furthermore, herbaceous plant species occurring in highly encroached areas were mainly forest species such as Viola biflora and Oxalis acetosella, presumably because these can tolerate lower light conditions than most grassland species. In Guarda, however, where the shrub cover of the most encroached sites was lower than at Mesocco, we found no difference in plant species richness among vegetation types. These findings are in agreement with the model of Schütz et al. (Citation2000), which shows that grassland communities dominated by F. rubra or N. stricta are particularly rich in plant species.
We found no significant differences in butterfly species richness among vegetation types at either site (). One possible reason for this negative result is the greater mobility of butterflies compared not only to vascular plants but also to grasshoppers, which enables them to visit less suitable patches provided that adequate resources are present in the neighbourhood (CitationShreeve, 1995). However, habitat size and connectivity are known to be important for less mobile butterfly species, making such species particularly sensitive to habitat loss and fragmentation (CitationÖckinger et al., 2010). It is also possible, of course, that the scale considered was too small and/or the plots not big enough to detect differences in butterfly diversity among vegetation types. Regardless, the relatively lower number of species in vegetation types with higher shrub cover agrees with other authors that have reported that bushes and shrubs have a negative effect upon subalpine butterfly communities (CitationErhardt, 1985; CitationHohl, 2006). Another reason may be that some of the species occurring in encroached areas are either relatively undemanding in terms of habitat (CitationSamways et al., 2012) or even require more than one type of habitat to complete their life cycle. Indeed, in a study of butterfly communities on abandoned grasslands in northeast Spain, Stefanescu et al. (Citation2009) found that grassland specialists were replaced by common, more generalist species as succession proceeded. And in our study, at least one species, Callophrys rubi, needs both grassland and shrubland habitat patches, since adults feed in grassland but lay their eggs on dwarf shrubs.
In Mesocco, we found more grasshopper species in mosaic vegetation with Nardion and Juniperion nanae than in plots with Alnenion viridis association, while in Guarda grasshopper species richness tended to peak at intermediate shrub cover. Both findings are consistent with the study by Anthelme et al. (Citation2001) of an Alnus viridis succession in the French Alps, in which orthopteran biomass was much greater when the shrub formed a mosaic with grassland than when it became more dense. Assuming that the increasing shrub cover in our plots indicates a successional sequence, our results are also consistent with studies reporting highest orthopteran species richness in grassland and coastal heathland at early to mid-successional stages (CitationMarini et al., 2010; CitationSchirmel et al., 2011; CitationFartmann et al., 2012). At the other end, the slightly lower numbers of grasshopper species in vegetation types with very low shrub cover are probably linked to the preference of species such as Podisma pedestris or Metrioptera brachyptera for habitats rich in vegetation structure, including dwarf shrubs (CitationSzövényi, 2002; CitationBaur et al., 2006a).
EFFECT OF BIOTIC AND ABIOTIC FACTORS ON SPECIES COMPOSITION
Overall, biotic variables (related to shrub encroachment and plant diversity for the insect groups) explained significant amounts of variance in plant, butterfly, and grasshopper species composition at both sites, while abiotic factors explained much less. This suggests that shrub encroachment plays a major role in shaping species assemblages of plants and insects on summer pastures. Similarly, Rudmann-Maurer et al. (Citation2008) found that land use (mowing, grazing, abandonment) had a stronger impact on alpine grassland composition than abiotic factors and cultural tradition. Dullinger et al. (Citation2003) showed that patterns of Pinus mugo recruitment success depended mainly on propagule pressure and invasibility of the grassland community, but only marginally on abiotic habitat conditions. However, the joint fraction of variance explained by both biotic and abiotic factors was relatively large, suggesting that interactions between these two sets of variables also play an important role in shaping spatial species distributions. Grazing patterns highly depend on pasture topography, especially slope, which is an important parameter limiting movements of grazing animals (CitationGanskopp and Vavra, 1987). Conversely, grazing and shrub encroachment in turn alter small-scale abiotic site conditions such as soil nutrients and moisture or shading (CitationEl-Bana et al., 2002). Given the relatively small areas surveyed, effects of microtopography upon local microclimate may be more important than larger scale environmental variables such as altitude or slope (CitationScherrer and Körner, 2010; CitationWundram et al., 2010). Thus, the scale used was potentially too small for detecting effects of the large-scale abiotic factors used in this study. Nevertheless, spatial factors at the same time accounted for a large part of the explained variance in our study, indicating that the results were influenced by factors such as nutrient availability or seed dispersal. In this regard, more research effort is needed and future investigations should also account for soil conditions and moisture in order to confirm the detected patterns. An influence of abiotic factors was found at the drier Guarda site but not in Mesocco, where mean annual precipitation is much higher. This result is consistent with previous studies suggesting an important role of water availability in shaping distribution of vegetation composition (CitationHodkinson et al., 1999; CitationMichalet et al., 2002).
Conclusions
Our results suggest that shrub encroachment driven by land abandonment or underuse is a major factor affecting the diversity of plants and insects on subalpine summer pastures. However, as they are based on only two pastures, further research is needed to confirm these conclusions. Agri-environment schemes involving direct payments for ecological quality are important instruments to counteract the abandonment or underuse of marginal land that eventually leads to shrub encroachment (CitationStrijker, 2005; CitationMack and Flury, 2008). In Switzerland, a scheme based upon floristic quality has been introduced to help conserve biodiversity at lower altitudes (CitationKampmann et al., 2012). A similar scheme for summer pastures at higher altitudes started in 2014. As vascular plants are an adequate surrogate for butterfly and grasshopper diversities in these areas (CitationKoch et al., 2013), it can be expected that these insect groups would also benefit from such a scheme. However, given the preference of the plant and grasshopper groups for different successional stages, we recommend the maintenance of patches with different degrees of shrub encroachment. In the context of habitat fragmentation, adequate size of patches and habitat connectivity should also be ensured.
Acknowledgments
We thank Serge Buholzer, Nina Richner, Anna Stäubli, and Rafael Wüest for help in the field. Thank you also to the landowners that permitted access to the field sites. We are grateful to Felix Herzog, Rafael Wüest, and four anonymous reviewers for valuable criticism. The study is affiliated to the AlpFUTUR project (www.alpfutur.ch) and was partly funded by the Swiss Federal Office for the Environment (FOEN), Armasuisse, Kanton Graubünden, Ricola, and Sophie und Karl Binding Stiftung.
References Cited
- Alewell, C. , and Bebi, P. , 2011: Forest development in the European Alps and potential consequences on hydrological regime. In Bredemeier, M. , Cohen, S. , Gobold, D. L. , Lode, E. , Pichler, V. , and Schleppi, P. (eds.), Forest Management and the Water Cycle. Dordrecht, Heidelberg, London, New York: Springer, 111–126.
- Anthelme, F. , Grossi, J. , Brun, J. , and Didier, L. , 2001: Consequences of green alder expansion on vegetation changes and arthropod communities removal in the northern French Alps. Forest Ecology and Management , 145: 57–65.
- Anthelme, F. , Michalet, R. , Barbaro, L. , and Brun, J. , 2003: Environmental and spatial influences of shrub cover (Alnus viridis DC.) on vegetation diversity at the upper treeline in the inner western Alps. Arctic, Antarctic, and Alpine Research , 35: 48–55.
- Anthelme, F. , Villaret, J. , and Brun, J. , 2007: Shrub encroachment in the Alps gives rise to the convergence of sub-alpine communities on a regional scale. Journal of Vegetation Science , 18: 355–362.
- Archer, S. , Boutton, T. W. , and Hibbard, K. A. , 2001: Trees in grasslands: biogeochemical consequences of woody plant expansion. In Schulze, E. D. , Heimann, M. , Harrison, S. , Holland, E. , Lloyd, J. , Prentice, I. , and Schimel, D. (eds.), Global biogeochemical cycles in the climate system. San Diego: Academic Press, 115–138.
- Baldock, D. , Beaufoy, G. , Brouwer, F. , and Godeschalk, F. , 1996: Farming at the Margins: Abandonment or Redeployment of Agricultural Land in Europe. London and The Hague: Institute for European Environmental Policy (London) and the Netherlands Agricultural Research Centre.
- Balmer, O. , and Erhardt, A. , 2000: Consequences of succession on extensively grazed grasslands for Central European butterfly communities: rethinking conservation practices. Conservation Biology , 14(3): 746–757.
- Bätzing, W. , 2005: Die Alpen-Geschichte und Zukunft einer europäischen Kulturlandschaft. München: C.H. Beck.
- Baur, B. , Baur, H. , Roesti, C. , and Roesti, D. , 2006a: Die Heuschrecken der Schweiz. Bern, : Haupt.
- Baur, P. , Bebi, P. , Gellrich, M. , and Rutherford, G. , 2006b: WaSAlp-Waldausdehnung im Schweizer Alpenraum. Eine quantitative Analyse naturräumlicher und sozio-ökonomischer Ursachen unter besonderer Berücksichtigung des Agrarstrukturwandels. Schlussbericht. Birmensdorf, Switzerland: Eidgenössische Forschungsanstalt WSL, 65 pp., http://www.wsl.ch/projects/WaSAlp.
- Bischof, N. , 1984: Pflanzensoziologische Untersuchungen von Sukzessionen aus gemähten Magerrasen in der subalpinen Stufe der Zentralalpen. Beiträge zur geobotanischen Landesaufnahme der Schweiz , 60: 128 pp.
- Borcard, D. , and Legendre, P. , 2002: All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecological Modelling , 153: 51–68.
- Borcard, D. , Legendre, P. , and Drapeau, P. , 1992: Partialling out the spatial component of ecological variation. Ecology , 73(3): 1045–1055.
- Brändli, U. , 2000: Waldzunahme in der Schweiz: gestern und morgen. Informationsblatt Forschungsbereich Landschaft , 45: 1–4.
- Bühler-Cortesi, T. , 2009: Schmetterlinge-Tagfalter der Schweiz. Bern: Haupt Verlag.
- Cavallero, A. , Aceto, P. , Gorlier, A. , Lombardi, G. , Lonati, M. , Martinasso, B. , and Tagliatori, C. , 2007: I tipi pastorali delle Alpi piemontesi: vegetazione e gestione dei pascoli delle Alpi occidentali. Bologna: A Perdisa Editore.
- Chemini, C. , and Rizzoli, A. , 2003: Land use change and biodiversity conservation in the Alps. Journal of Mountain Ecology , 7 (Supplement): 1–7.
- Connell, J. H. , and Slatyer, R. O. , 1977: Mechanisms of succession in natural communities and their role in community stability and organization. The American Naturalist , 111: 1119–1144.
- Crivelli, F. , 2011: Historische Entwicklung der Landnutzung in den Sömmerungsgebieten der Region Moesa seit 1880. Zürich: Masterarbeit am Departement Umweltwissenschaften and der ETH, 173 pp.
- Delarze, R. , and Gonseth, Y. , 2008: Lebensräume der Schweiz. Bern: Ott Verlag.
- DeLuca, T. H. , and Zackrisson, O. , 2007: Enhanced soil fertility under Juniperus communis in arctic ecosystems. Plant Soil , 294: 147–155.
- Dullinger, S. , Dirnböck, T. , and Grabherr, G. , 2003: Patterns of shrub invasion into high mountain grasslands of the northern calcareous Alps, Austria. Arctic, Antarctic, and Alpine Research , 35: 434–441.
- El-Bana, M. I. , Nijs, I. , and Kockelbergh, F. , 2002: Microenvironmental and vegetational heterogeneity induced by phytogenetic nebkhas in an arid coastal ecosystem. Plant and Soil , 247: 283–293.
- Eldridge, D. J. , Bowker, M. A. , Maestre, F. T. , Roger, E. , Reynolds, J. F. , and Whitford, W. G. , 2011: Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecology Letters , 14: 709–722.
- Erhardt, A. , 1985: Diurnal Lepidoptera: sensitive indicators of cultivated and abandoned grassland. Journal of Applied Ecology , 22: 849–861.
- Fartmann, T. , Krämer, B. , Stelzner, F. , and Poniatowski, D. , 2012: Orthoptera as ecological indicators for succession in steppe grassland. Ecological Indicators , 20: 337–344.
- Freléchoux, F. , Meisser, M. , and Gillet, F. , 2007: Succession secondaire et perte de diversité végétale après réduction du broutage dans un pâturage boisé des Alpes centrales suisses. Botanica Helvetica , 117: 37–56.
- Ganskopp, D. , and Vavra, M. , 1987: Slope use for cattle, feral horses, and bighorn sheep. Northwest Science , 61: 74–81.
- Graff, P. , Agular, M. R. , and Chaneton, E. J. , 2007: Shifts in positive and negative plant interactions along a grazing intensity gradient. Ecology , 88: 188–199.
- Guillaume Blanchet, F. , Legendre, P. , and Borcard, D. , 2008: Forward selection of explanatory variables. Ecology , 89: 2623–2632.
- Güsewell, S. , Jewell, P. L. , and Edwards, P. J. , 2005: Effects of heterogenous habitat use by cattle on nutrient availability and litter decomposition in soils of an Alpine pasture. Plant and Soil , 268: 135–149.
- Hodkinson, I. D. , Webb, N. R. , Bale, J. S. , and Block, W. , 1999: Hydrology, water availability and tundra ecosystem function in a changing climate: the need for a closer integration of ideas? Global Change Biology , 5: 359–369.
- Hohl, M. , 2006: Spatial and Temporal Variation of Grasshopper and Butterfly Communities in Differently Managed Semi-Natural Grasslands of the Swiss Alps. Ph.D. thesis, Swiss Federal Institute of Technology Zürich.
- Huxman, T. E. , Wilcox, B. P. , Breshears, D. D. , Scott, R. L. , Snyder, K. A. , Small, E. E. , Hultine, K. , Pockman, W. T. , and Jackson, R. B. , 2005: Ecohydrological implications of woody plant encroachment. Ecology , 86: 308–319.
- Jewell, P. L. , Güsewell, S. , Berry, N. R. , Käuferle, D. , Kreuzer, M. , and Edwards, P. J. , 2005: Vegetation patterns maintained by cattle grazing on a degraded mountain pasture. Botanica Helvetica , 115: 109–124.
- Kampmann, D. , Lüscher, A. , Konold, W. , and Herzog, F. , 2012: Agri-environment scheme protects diversity of mountain grassland species. Land Use Policy , 29: 569–576.
- Koch, B. , Edwards, P. J. , Blanckenhorn, W. U. , Buholzer, S. , Walter, T. , Wüest, R. O. , and Hofer, G. , 2013: Vascular plants as surrogates of butterfly and grasshopper diversity on two Swiss subalpine summer pastures. Biodiversity and Conservation , 22: 1451–1465.
- Kohler, F. , Gillet, F. , Reust, S. , Wagner, H. H. , Gadallah, F. , Gobat, J. , and Buttler, A. , 2006: Spatial and seasonal patterns of cattle habitat use in a mountain wooded pasture. Landscape Ecology , 21(2): 281–295.
- Lauber, K. , and Wagner, G. , 2001: Flora Helvetica. Bern: Haupt.
- Legendre, P. , and Gallagher, E. D. , 2001: Ecologically meaningful transformations for ordination of species data. Oecologia , 129: 271–280.
- Legendre, P. , Borcard, D. , Guillaume Blanchet, F. , and Dray, S. , 2012: PCNM: MEM spatial eigenfunction and principal coordinate analyses: R package version 2.1-2. Available at http://R-Forge.R-project.org/projects/sedar/.
- Livingston, R. B. , 1972: Influence of birds, stones and soil on the establishment of Pasture Juniper, Juniperus communis, and Red Cedar, J. virginiana in New England pastures. Ecology , 53: 1141–1147.
- MacDonald, D. , Crabtree, J. R. , Wiesinger, G. , Dax, T. , Stamou, N. , Fleury, P. , Gutierrez-Lazpita, J. , and Gibon, A. , 2000: Agricultural abandonment in mountain areas of Europe: Environmental consequences and policy response. Journal for Environmental Management , 59: 47–69.
- Mack, G. , and Flury, C. , 2008: Wirkung der Sömmerungsbeiträge. Agrarforschung , 15(10): 500–505.
- Mallik, A. U. , Bell, F. W. , and Gong, Y. , 1997: Regeneration behavior of competing plants after clear cutting: implications for vegetation management. Forest Ecology and Management , 95: 1–10.
- Marini, L. , Fontana, P. , Klimek, S. , Battisti, A. , and Gaston, K. J. , 2009: Impact of farm size and topography on plant and insect diversity of managed grasslands in the Alps. Biological Conservation , 142: 394–403.
- Marini, L. , Bommarco, R. , Fontana, P. , and Battisti, A. , 2010: Disentangling effects of habitat diversity and area on orthopteran species with contrasting mobility. Biological Conservation , 143: 2164–2171.
- McKinley, D. C. , Norris, M. D. , Blair, J. M. , and Johnson, L. C. , 2008: Altered ecosystem processes as a consequence of Juniperus virginiana L. encroachment into North American tallgrass prairie. In Van Auken, O. W. (ed.), Western North American Juniperus Communities. New York and Berlin: Springer, Ecological Studies, 170–187.
- Michalet, R. , Gandoy, C. , Joud, D. , Pagès, J. , and Choler, P. , 2002: Plant community composition and biomass on calcareous and siliceous substrates in the Northern French Alps: Comparative effects of soil chemistry and water status. Arctic, Antarctic, and Alpine Research , 34: 102–113.
- Moore, I. , Grayson, R. , and Ladson, A. , 1991: Digital terrain modelling: a review of hydrological, geomorphological, and biological applications. Hydrological Processes , 5: 3–30.
- Moser, D. , Gygax, A. , Bäumler, B. , Wyler, N. , and Palese, R. , 2002: Rote Liste der gefährdeten Farn- und Blütenpflanzen der Schweiz. Bundesamt für Umwelt, Wald und Landschaft, Bern; Zentrum des Datenverbundnetzes der Schweiz Flora, Chambésy; Conservatoire et Jardin botaniques de la Ville de Genève, Chambésy. BUWAL-Reihe “Vollzug Umwelt,” 118 pp.
- Niering, W. A. , 2005: Vegetation dynamics (succession and climax) in relation to plant community management. Conservation Biology , 1: 287–295.
- Öckinger, E. , Schweiger, O. , Crist, T. O. , Debinski, D. M. , Krauss, J. , Kuussaari, M. , Petersen, J. D. , Pöyry, J. , Settele, J. , Summerville, K. S. , and Bommarco, R. , 2010: Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecology Letters , 13: 969–979.
- Oksanen, J. , Guillaume Blanchet, F. , Kindt, R. , Legendre, P. , Minchin, P. R. , O'Hara, R. B. , Simpson, G. L. , Solymos, P. , Stevens, M. H. H. , and Wagner, H. , 2012: vegan: Community Ecology Package: R package version 2.0-4. Available at http://CRAN.R-project.org/package=vegan.
- Peres-Neto, P. R. , Legendre, P. , Dray, S. , and Borcard, D. , 2006: Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology , 87: 2614–2625.
- Poldini, L. , Oriolo, G. , and Francescato, C. , 2004: Mountain pine scrubs and heaths with Ericaceae in the south-eastern Alps. Plant Biosystems , 138: 53–85.
- Pornaro, C. , Schneider, M. K. , and Macolino, S. , 2013: Plant species loss due to forest succession in alpine pastures depends on site conditions and observation scale. Biological Conservation , 161: 213–222.
- Putz, F. E. , and Canham, C. D. , 1992: Mechanisms of arrested succession in shrublands: root and shoot competition between shrubs and tree seedlings. Forest Ecology and Management , 49: 267–275.
- Pykälä, J. , Luoto, M. , Heikkinen, R. K. , and Kontula, T. , 2005: Plant species richness and persistence of rare plants in abandoned semi-natural grasslands in northern Europe. Basic and Applied Ecology , 6: 25–33.
- R Core Team , 2012: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org.
- Reinhard, M. , Bächlin, R. , Graeter, P. , Lehner, P. , and Spicher, A. , 1962: Geologischer Atlas der Schweiz. Ninth edition. Bern: Kümmerly and Frey.
- Rudmann-Maurer, K. , Weyand, A. , Fischer, M. , and Stöcklin, J. , 2008: The role of landuse and natural determinants for grassland vegetation composition in the Swiss Alps. Basic and Applied Ecology , 9: 494–503.
- Samways, M. J. , Hamer, M. , and Veldtman, R. , 2012: Development and future of insect conservation in South Africa. In New, T. R. (ed.), Insect Conservation: Past, Present and Prospects. Berlin: Springer, 245–278.
- Scherrer, D. , and Körner, C. , 2010: Infra-red thermometry of alpine landscapes challenges climatic warming projections. Global Change Biology , 16: 2602–2613.
- Schirmel, J. , Mantilla-Contreras, J. , Blindow, I. , and Fartmann, T. , 2011: Impacts of succession and grass encroachment on heathland Orthoptera. Journal of Insect Conservation , 15: 633–642.
- Schütz, M. , Wildi, O. , Krüsi, B. O. , Märki, K. , and Nievergelt, B. , 2000: From tall-herb communities to pine forests: distribution patterns of 121 plant species during a 585-year regeneration process. In Schütz, M. , Krüsi, B. O. , and Edwards, P. J. (eds.), Succession research in the Swiss National Park, 237–255.
- Schweizerische Meteorologische Anstalt , 2008: Annalen der Schweizerischen Meteorologische Anstalt. Zürich: Bundesamt für Meteorologie und Klimatologie.
- Shreeve, T. G. , 1995: Butterfly mobility. In Pullin, A. S. (ed.), Ecology and Conservation of Butterflies. London: Chapman and Hall, 37–45.
- Stefanescu, C. , Peñuelas, J. , and Filella, I. , 2009: Rapid changes in butterfly communities following the abandonment of grasslands: a case study. Insect Conservation and Diversity , 2: 261–269.
- Stöcklin, J. , Bosshard, A. , Klaus, G. , Rudmann-Maurer, K. , and Fischer, M. , 2007: Landnutzung und biologische Vielfalt in den Alpen. Zürich: Hochschulverlag AG an der ETH Zürich, 191 pp.
- Strijker, D. , 2005: Marginal lands in Europe-cause of decline. Basic and Applied Ecology , 6: 99–106.
- Szövényi, G. , 2002: Qualification of grassland habitats based on their Orthoptera assemblages in the Köszeg Mountains (W-Hungary). Entomologia Experimentalis et Applicata , 104: 159–163.
- Tague, C. , 2005: Heterogeneity in hydrologic processes: a terrestrial hydrologic modeling perspective. In Lovett, G. M. , Jones, C. , Turner, M. G. , and Weathers, K. C. (eds.), Ecosystem Functioning in Heterogeneous Landscapes. New York and Berlin: Springer, 119–136.
- Tasser, E. , Walde, J. , Tappeiner, U. , Teutsch, A. , and Noggler, W. , 2007: Land-use changes and natural reforestation in the Eastern Central Alps. Agriculture, Ecosystems and Environment , 118: 115–129.
- Van Auken, O. W. , 2009: Causes and consequences of woody plant encroachment into western North American grasslands. Journal of Environmental Management , 90: 2931–2942.
- Väre, H. , Lampinen, R. , Humphries, C. , and Williams, P. , 2003: Taxonomic diversity of vascular plants in the European alpine areas. In Nagy, L. , Grabherr, G. , Körner, C. , and Thompson, D. B. A. (eds.), Alpine Biodiversity in Europe. Berlin: Springer-Verlag, 133–148.
- Wallis de Vries, M. F. , Poschlod, P. , and Willems, J. H. , 2002: Challenges for the conservation of calcareous grasslands in northwestern Europe: integrating the requirements of flora and fauna. Biological Conservation , 104: 265–273.
- Welch, D. , and Scott, D. , 1995: Studies in the grazing of heather moorland in north-east Scotland. VI. 20-year trends in botanical composition. Journal of Applied Ecology , 32: 596–611.
- Werthemann, A. , and Imboden, A. , 1982: Die Alp- und Weidewirtschaft in der Schweiz: Zusammenfassung der Alpkatastererhebungen. Bern: Bundesamt für Landwirtschaft.
- Wettstein, S. , 2001: Der Einfluss abiotischer Faktoren auf die Morphologie der Grünerle. Botanica Helvetica , 111: 31–44.
- Wiedmer, E. , and Senn-Irlet, B. , 2006: Biomass and primary productivity of an Alnus viridis stand-a case study from the Schächental valley, Switzerland. Botanica Helvetica , 116: 55–64.
- Woodcock, C. E. , Macomber, S. , and Kumar, L. , 2002: Vegetation mapping and monitoring. In Skidmore, A. (ed.), Environmental Modelling with GIS and Remote Sensing. London: Taylor and Francis, 97–120.
- Wundram, D. , Pape, R. , and Löffler, J. , 2010: Alpine soil temperature variability at multiple scales. Arctic, Antarctic, and Alpine Research , 42: 117–128.
- Zoller, H. , Bischof, N. , Erhardt, A. , and Kienzle, U. , 1984: Biocoenosen von Grenzertragsflächen und Brachland in den Berggebieten der Schweiz: Hinweise zur Sukzession, zum Naturschutzwert und zur Pflege. Phytocoenologia , 12: 373–394.
APPENDIX
TABLE A1 Results of variance partitioning with a redundancy analysis (RDA) for plants, butterflies, and grasshoppers in Mesocco (n = 49) and Guarda (n = 48). Pure (B = biotic, A = abiotic, S = spatial), joint (∩ indicates shared variation) and unexplained fractions of variance (adjusted R 2; R 2 Adj), and significance values for pure fractions (p; significant values in bold) are reported.
