ABSTRACT
Shrub willows (Salix species) are widespread beyond the latitudinal and altitudinal treelines. Their ring width has been shown to be a reliable ecological indicator for changes in the harsh cold conditions in the Arctic, but little is known on their growth in alpine conditions. The shrubby Salix oritrepha grows above the treeline on the northeastern Tibetan Plateau (TP), making it an interesting woody species to explore responses of alpine communities to ongoing climate warming in this area. Since precipitation increases with increasing elevation (until 4670 m) in the study area, we hypothesize that the growth of S. oritrepha is mainly constrained by cold summer temperature. We sampled 35 S. oritrepha individuals above the juniper treeline (4200 m), and took basal wood cross sections for dendrochronological analyses. Few missing rings were detected at the shoot base when serial sectioning was applied. Ring width (RW) and basal area increment (BAI) standard chronologies were established. We found that BAI reflected a stronger climatic signal than RW. The radial growth was constrained by low July to August temperatures. We expect that climate warming would enhance the growth of alpine willows, which could alter the services provided by these high-elevation ecosystems.
Introduction
Shrubby willows (genus Salix) are widely distributed beyond the treeline in high latitudinal and altitudinal regions (CitationWu, 1983; CitationBlok et al., 2011; CitationSchweingruber et al., 2013). In these treeless areas, shrubs are the only woody species that have been shown to record changes in climatic conditions in their growth rings (CitationWalker, 1987; CitationSrur and Villalba, 2009; García-Cervigón et al., 2012; CitationGazol and Camarero, 2012; CitationLiang et al., 2012; CitationRayback et al., 2012; CitationMyers-Smith et al., 2015). However, studies on willows have mainly focused on the High Arctic where shrub encroachment has been taken as a response to climate warming (CitationSturm et al., 2001; CitationForbes et al., 2010; CitationSchmidt et al., 2010; CitationBlok et al., 2011). In contrast, little is known about the growth performance of alpine willow and their responses to climate (cf. CitationMyers-Smith et al., 2015).
The Tibetan Plateau (TP) is considered to be one of the largest, little disturbed alpine regions showing a high responsiveness to ongoing climate change (CitationZheng and Yao, 2006). However, little is known on how the radial growth of alpine shrubs responds to climate warming in the large treeless areas of the TP. Since shrubs dominate above the TP treeline, several studies have dealt with these woody species using their ring features as monitors of climate change effects on alpine ecosystem, particularly in the southeastern TP (CitationLiang and Eckstein, 2009; CitationLiang et al., 2012, Citation2015; CitationLi et al., 2013; CitationLu et al., 2015; CitationWang et al., 2015). In the southeastern TP, July temperature is the key factor limiting the radial growth of alpine rhododendron shrubs under subhumid climate conditions (CitationLiang and Eckstein, 2009; CitationLi et al., 2013; CitationLu et al., 2015). However, along the monsoon margin of the central TP, low precipitation may constrain growth of alpine juniper shrubs (Liang et al., 2012). For instance, in the Qilian Mountains, northeastern TP, the growth of the shrub Hippophae rhamnoides is constrained by cold June temperature but also by low precipitation of February to March of the current year (CitationXiao et al., 2007). These biogeographical disparities in climate-growth associations show the need to analyze these relationships in detail, and to look at these associations among alpine shrub willows, as it is a species that is well distributed.
Salix oritrepha (hereafter called Salix) is one of the most common shrubby willow species in the northeastern TP, dominating in alpine communities occurring above the Juniperus przewalskii treeline (CitationFang et al., 2011). Here, we aim to determine if the radial growth and wood formation of alpine Salix is mainly limited by temperature or by precipitation. Understanding this relationship is important because climate warming could promote upward shrubline shifts but also enhance the growth of alpine shrubs and their ability to uptake carbon as wood. This could lead to shrub encroachment and changes in related services of alpine ecosystems such as the regulation of carbon and water cycles or the maintenance of high levels of plant biodiversity and endemism (CitationGreenwood and Jump, 2014).
The specific objectives of this study are (1) to test the dendrochronological potential of Salix by comparing the radial-growth patterns along the shoot of the same individual (serial sectioning), and (2) to quantify the climate-growth relationships using ring width (RW) and basal area increment (BAI) mean series (chronologies). We hypothesize that the radial growth of alpine Salix is mainly limited by growing-season temperatures, that is, summer thermal conditions.
Material and Methods
Study Species
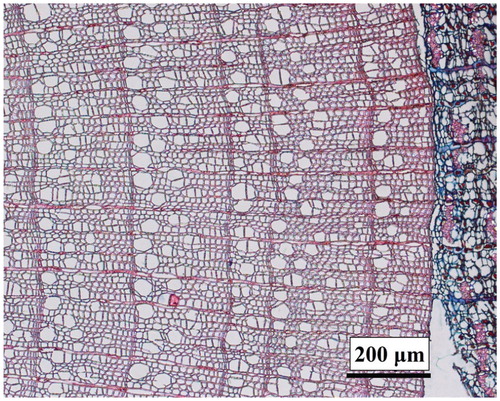
Salix oritrepha is a deciduous and shrubby willow that is widely distributed on the TP and usually grows polycormic (CitationFang et al., 2011).This species may also grow in a more erect shape attaining heights of 0.6–1.2 m. The Salix stems are usually round at the base. In the species' xylem, one to several rows of thick-walled fiber cells formed a welldefined ring boundary ().The wood anatomy is semidiffuse to diffuse-porous.
Study Area and Climate
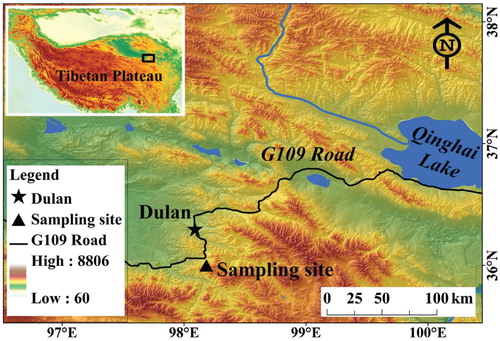
The study site (36°00′N, 98°11′E, 4270 m a.s.l.) is situated in the northeastern TP, about 32 km southeast from Dulan County ().This region is located at the eastern edge of the Qaidam Basin where J. przewalskii grow from 3850 m to 4200 m (CitationShao et al., 2005), and Salix appear above 4000 m elevation.
The study area is characterized by a typical continental arid climate. The annual mean temperature is 3.2 °C (1954–2014) at the meteorological station in Dulan (3191 m).July and January are the warmest and coldest months with mean temperatures of 15.8 °C and –9.8 °C, respectively. The mean annual total precipitation is 201 mm and 80% of the rainfall occurs between May and September. The annual mean pan (20 cm in diameter) evaporation is 1760 mm.
Meteorological data from the Qilian Mountains demonstrates that precipitation increases uphill and reaches a maximum at about 4670 m a.s.l. (CitationWang et al., 2009). Based on climatic data recorded in 2014 by an automatic weather station near the study site (4200 m a.s.l.), the annual mean temperature was –1.6 °C and the annual precipitation was 396 mm. The mean temperature from May to September was 5.9 °C and the total precipitation for that period was 331 mm. The mean temperatures in July and January were 9.2 °C and –12.0 °C, respectively.
Shrub Sampling and Chronology Establishment
In April 2015, we selected a well-drained site above the uppermost J. przewalskii individuals. A total of 35 Salix individuals were randomly selected and 2- to 3-cm-thick cross sections were cut at their bases.The samples were air dried and polished using sand papers of different grains until the ring boundaries were clearly visible. Then, the wood disks were visually cross-dated (CitationStokes and Smiley, 1996).The ring widths (RW) were measured along three radii per disk (every 120°) with a precision of 0.01 mm using the Lintab system (Rinntech, Heidelberg, Germany). The quality of the visual crossdating was checked by the program COFECHA (CitationHolmes, 1983). Digital photographs were taken under the microscope for each disk, and the shrub ring boundaries were vectored in each photograph in ArcGIS 10. Then the area of each shrub ring polygon was calculated as the BAI. By avoiding the effects of irregular rings, such a method is better than BAI calculation based on ring width (e.g., CitationBiondi and Qeadan, 2008).
The RW and BAI series were detrended and standardized by using the ARSTAN program (CitationCook and Krusic, 2005). A negative exponential function or a straight line was fitted to each individual RW or BAI series, and the resulting indices were averaged for all individuals using a biweight robust mean to obtain standard RW and BAI chronologies.
Serial Sectioning
To detect the presence of missing rings along the sampled shoots, we selected three individuals (B1, B2, and B3) to apply the serial-sectioning method following Kolishchuk (Citation1990) and CitationWilmking et al. (2012). Cross sections were collected at 20-cm intervals along the main shoot. The rings were crossdated among the cross sections and then the RW were measured along three radii (every 120°) per section.There was only one missing ring in 2013 at the base of one cross section.
Data Analysis
We calculated several statistics to describe the raw measurement and standardized chronologies: standard deviation (SD), first-order autocorrelation (AC), mean sensitivity (MS), mean series intercorrelation (Rbar), and expressed population signal (EPS).The AC measures the persistence in growth, whereas the MS quantifies the relative changes between years of RW or BAI (CitationFritts, 2001). The Rbar allows characterizing the strength of the common growth signal over a period (CitationBriffa and Jones, 1990).The EPS evaluates the degree to which the chronology represents a hypothetical chronology of an infinite number of cores, that is, how well replicated a chronology is; an EPS ≥ 0.85 is usually accepted to identify the reliable part of a ring width chronology (CitationWigley et al., 1984).
To determine which climatic factors were more strongly related to Salix growth, Pearson correlation coefficients were calculated between monthly climatic variables and RW and BAI standard chronologies from 1980–2014 in which EPS ≥ 0.85. The monthly climatic variables were monthly mean minimum and maximum temperatures, and total precipitation. As the relative air humidity is a fundamental indicator of hydroclimate conditions (CitationLendzion and Leuschner, 2008; CitationLiang et al., 2016), it was also employed for our analysis. The temporal window for the analysis encompassed from May of the previous year to current September (i.e., corresponding to the year of tree-ring formation). In addition to monthly variables, summer means or totals (July to August) were also used, as we assumed that maximum growth rates occurred in that period.
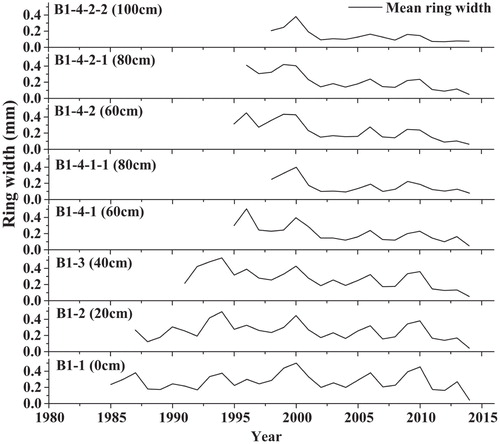
FIGURE 3. Serial sections of Salix oritrepha individual B1 showing a consistent year-to-year variability in the radial width between sections and within the same shoot. The codes indicate the name of each section. The numbers between brackets indicate the height above the ground of the sampled wood sections.
Results
As shown by the serial sectioning method, the growth rings throughout each shoot were obviously formed at the same time. The mean series of intercorrelation coefficients from the 21 cross sections of B1, B2, and B3 shoots was 0.64 (P < 0.001) (see an example for the B1 shoot in ). Therefore, we concluded that cross sections taken close to the soil level contain the maximum possible number of growth rings and can be used for dendrochronological research.
FIGURE 4. Standard chronologies of ring width (RW) and basal area increment (BAI). The first year, when the chronology is considered well replicated (Expressed Population Signal ≥ 0.85), is indicated with a vertical arrow.
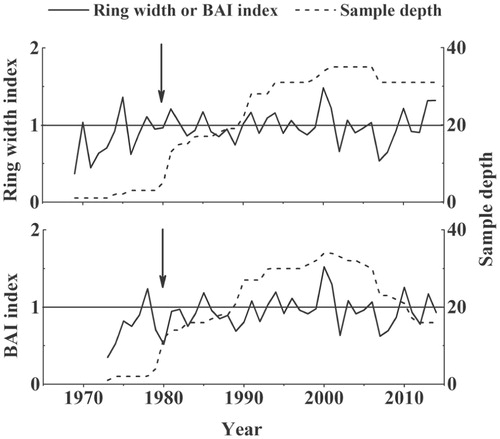
After successful cross-dating, we were able to develop a 42-year-long (1973–2014) BAI standard chronology and a 46-year-long (1969–2014) RW standard chronology (). The mean age of the individuals sampled was 27 years old. Their mean BAI and RW were 5.83 mm2 and 0.25 mm, respectively. The AC was relatively low, whereas the MS values of RWI and BAI Salix standard chronologies were moderate (0.26–0.29) ().The correlation between RW and BAI standard chronologies for the 1980–2014 period was high and significant (r = 0.82, P < 0.05).
July temperatures were positively correlated to RWI and BAI standard chronologies (in both cases: r > 0.40, p < 0.05; ). The temperature in August also presented a significantly positive effect on the BAI standard chronology. The BAI standard chronology showed higher correlation strength with July temperatures than that of RW standard chronology. Furthermore, the BAI was most responsive to the mean temperatures of July and August. Elevated January minimum temperatures were also positively related to Salix growth. Besides the summer and winter temperatures, summer moisture, particularly the July relative humidity, showed negative associations with Salix growth (in both cases: r < –0.40,p < 0.05; ).
TABLE 1 Statistics of the raw measurement and standard chronologies of ring width (RW) or basal area increment (BAI).
Discussion
The mean Salix RW is similar to that observed for J. przewalskii (0.28 mm) growing on the same slope (CitationZheng et al., 2008),but around three times lower than that of erect willows (S. alaxensis) in the western Canadian Arctic (CitationZalatan and Gajewski, 2006). The MS values are lower than those observed in most prostrate and erect willows growing in the Arctic (CitationWoodcock and Bradley, 1994; CitationSchmidt et al., 2010; CitationForbes et al., 2010;CitationBlok et al., 2011; CitationBuchwal et al., 2013). The Rbar of RW and BAI standard chronologies indicate a high common growth signal, probably relating to climate factors, which is confirmed by the EPS reaching values above the threshold of 0.85 since 1980.
July temperature is a dominant climatic factor in controlling the growth of Salix shrubs. This is in accordance with findings reported for nearby J. przewalskii forming the juniper treeline (CitationZheng et al., 2008; CitationZhu et al., 2008), and also for other alpine shrub species found across the TP (CitationLiang and Eckstein, 2009; CitationLi et al., 2013; CitationLu et al., 2015).This finding is also in agreement with what has been found for some shrub willows and other woody species growing in the Arctic (CitationForbes et al., 2010; CitationHallinger et al., 2010; CitationWeijers et al., 2010;CitationBlok et al., 2011; CitationBuchwal et al., 2013; CitationJørgensen et al., 2015).The high temperature in July may enhance the rate of carbon uptake and use for building new woody tissue (CitationRossi et al., 2008). The remarkable highest correlation between BAI and mean temperatures in July and August suggests that summer temperature is the main growth-limiting factor. The significant positive effect of August temperatures on the BAI standard chronology could be explained by a longer xylogenesis phase of cell enlargement during the late growing season (CitationRen et al., 2015). In addition, higher January minimum temperature could also enhance the Salix growth, possibly implying that very low winter temperature may threaten the survival of this species above the treeline. Tow winter temperature can kill the fine roots and buds of alpine shrubs through direct frost damage or freeze-thaw related cavitation (CitationField and Brodribb, 2001), albeit the redundant narrow vessels should provide hydraulic safety to these species. In any case, our findings indicate that cold winters may limit the growth of shrub species above the alpine treeline.
FIGURE 5. Correlations (Pearson coefficients) calculated between the standard chronologies of ring width (RW) and basal area increment (BAI) indices and monthly climatic data (mean maximum and minimum temperatures; total precipitation, mean air relative humidity). The temporal window includes months of the previous (May to December) and current (January to September) years; that is, they are prior and concurrent to ring formation. The last code (JA) in axis x indicates the mean or summed values of July and August. The horizontal dashed lines show the 0.05 significance levels.
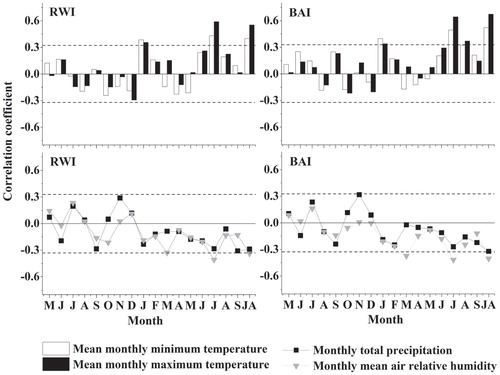
The negative effect of July relative humidity on shrub growth suggests that low summer precipitation does not constrain the radial growth of alpine shrub willows. Conversely, abundant summer rainfall is accompanied by the thick cloud cover that reduces air temperature, thereby negatively affecting shrub growth (CitationBuchwal et al., 2013; CitationStine and Huybers, 2015). In addition, we found significant negative correlations between relative humidity and July mean temperatures, providing additional evidence for this idea. A significant negative relationship between the BAI and relative air humidity in March could be related to a delayed start of shrub xylogenesis due to wet and cloudy spring conditions.
Eccentric or discontinuous rings occur frequently in shrub species (CitationBär et al., 2006; CitationAu and Tardif, 2007). In comparison with RW, a direct measurement of growth and conducting area such as BAI better captures the irregular geometry of radial growth in shrubs (CitationLatte et al., 2015).Thus, it could be better using BAI to describe alpine shrub growth dynamics than RW, as the correlation analyses between BAI/RW and climate show.
As one of the most widely distributed alpine deciduous shrubs in the northeastern TP (CitationFang et al., 2011), S. oritrepha is an indicator species in alpine ecosystem. This study is the first survey of growth trends with S. oritrepha as a model deciduous shrub species in the TP. In spite of the short time span of the chronology compared with other shrub dendrochronological studies performed in the TP (CitationLiang and Eckstein, 2009; Liang et al., 2012; CitationLi et al., 2013; CitationLu et al., 2015), it still provides valuable information on climatic drivers of alpine ecosystems.
Conclusions
The growth of the alpine shrub Salix oritrepha is limited by cold summer temperature on the Tibetan Plateau, which is a similar climate-growth response as observed in the nearby junipers (Juniperus przewalskii) forming the treeline. The BAI standard chronology captures a stronger summer temperature signal than the RW standard chronology. Climatic warming could enhance the radial growth of these alpine shrub willows, thus contributing to a potential upslope shift and promoting shrub encroachment in treeless areas of the TP.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41525001, 41471158) and the National Basic Research Program of China (2012FY111400).
References Cited
- Au, R. , and Tardif, J. C. , 2007: Allometric relationships and dendroecology of the dwarf shrub Dryas integrifolia near Churchill, subarctic Manitoba. Canadian Journal of Botany , 85: 585–597.
- Bär, A. , Bräuning, A. , and Löffler, J. , 2006: Dendroecology of dwarf shrubs in the high mountains of Norway—A methodological approach. Dendrochronologia , 24: 17–27.
- Biondi, F. , and Qeadan, F. , 2008: A theory-driven approach to tree-ring standardization : defining the biological trend from expected basal area increment. Tree-Ring Research , 64: 81–96.
- Blok, D. , Sass-Klaassen, U. , Schaepman-Strub, G. , Heijmans, M. M. P. D. , Sauren, P. , and Berendse, F. , 2011: What are the main climate drivers for shrub growth in Northeastern Siberian tundra? Biogeosciences , 8: 1169–1179.
- Briffa, K. R. , and Jones, P. D. , 1990: Basic chronology statistics and assessment. In Cook, E. R. , and Kairiukstis, L. A. (eds.), Methods of Dendrochronology: Applications in the Environmental Sciences. Dordrecht, Netherlands: Kluwer, 137–152.
- Buchwal, A. , Rachlewicz, G. , Fonti, P. , Cherubini, P. , and Gärtner, H. , 2013: Temperature modulates intra-plant growth of Salix polaris from a High Arctic site (Svalbard). Polar Biology , 36: 1305–1318.
- Cook, E. R. , and Krusic, P. J. , 2005: ARSTAN v. 41d: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics. Tree-Ring Laboratory, Lamont-Doherty Earth Observatory of Columbia University, Palisades, New York, U.S.A.
- Fang, J. , Wang, Z. , and Tang, Z. , 2011: Atlas of Woody Plants in China: Species Distribution and Climate. Springer (jointly published with Higher Education Press, Beijing).
- Field, T. S. , and Brodribb, T. , 2001: Stem water transport and freeze-thaw xylem embolism in conifers and angiosperms in a Tasmanian treeline heath. Oecologia , 127: 314–320.
- Forbes, B. C. , Fauria, M. M. , and Zetterberg, P. , 2010: Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Global Change Biology , 16: 1542–1554.
- Fritts, H. C. , 2001: Tree Rings and Climate . Caldwell, New Jersey: Blackburn, 567 pp.
- García-Cervigón, A. I. , Olano, J. M. , Eugenio, M. , and Camarero, J. J. , 2012: Arboreal and prostrate conifers coexisting in Mediterranean high mountains differ in their climatic responses. Dendrochronologia , 30: 279–286.
- Gazol, A. , and Camarero, J. J. , 2012: Mediterranean dwarf shrubs and coexisting trees present different radial-growth synchronies and responses to climate. Plant Ecology , 213: 1687–1698.
- Greenwood, S. , and Jump, A. S. , 2014: Consequences of treeline shifts for the diversity and function of high altitude ecosystems. Arctic, Antarctic, and Alpine Research , 46: 829–840.
- Hallinger, M. , Manthey, M. , and Wilmking, M. , 2010: Establishing a missing link: warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytologist , 186: 890–899.
- Holmes, R. L. , 1983: Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bulletin , 43: 51–67.
- Jørgensen, R. H. , Elallinger, M. , Ahlgrimm, S. , Friemel, J. , Kollmann, J. , and Meilby, H. , 2015: Growth response to climatic change over 120 years for Alnus viridis and Salix glauca in West Greenland. Journal of Vegetation Science , 26: 155–165.
- Kolishchuk, V. G. , 1990: Dendroclimatological study of prostrate woody plants. In Cook, E. R. , and Kairiukstis, L. A. (eds.), Methods of Dendrochronology: Applications in the Environmental Sciences. Dordrecht, Netherlands: Kluwer, 51–55.
- Latte, N. , Beeckman, H. , Bauwens, S. , Bonnet, S. , and Lejeune, P. , 2015: A novel procedure to measure shrinkagefree tree-rings from very large wood samples combining photogrammetry, high-resolution image processing, and CIS tools. Dendrochronologia , 34: 24–28.
- Lendzion, J. , and Leuschner, C. , 2008: Growth of European beech (Fagus sylvatica L.) saplings is limited by elevated atmospheric vapour pressure deficits. Forest Ecology and Management , 256: 648–655.
- Li, Z. , Liu, G. , Fu, B. , Zhang, Q. , Ma, K. , and Pederson, N. , 2013: The growth-ring variations of alpine shrub Rhododendron przewalskii reflect regional climate signals in the alpine environment of Miyaluo Town in Western Sichuan Province, China. Acta Ecológica Sinica , 33: 23–31.
- Liang, E. , and Eckstein, D. , 2009: Dendrochronological potential of the alpine shrub Rhododendron nivale on the south-eastern Tibetan Plateau. Annals of Botany , 104: 665–670.
- Liang, E. , Lu, X. , Ren, P. , Li, X. , Zhu, L. , and Eckstein, D. , 2012: Annual increments of juniper dwarf shrubs above the tree line on the central Tibetan Plateau: a useful climatic proxy. Annals of Botany , 109: 721–728.
- Liang, E. , Liu, W. , Ren, P. , Dawadi, B. , and Eckstein, D. , 2015: The alpine dwarf shrub Cassiope fastigiata in the Himalayas: Does it reflect site-specific climatic signals in its annual growth rings? Trees—Structure and Function , 29: 79–86.
- Liang, E. , Leuschner, C. , Dulamsuren, C. , Wagner, B. , and Hauck, M. , 2016: Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau. Climatic Change , 134: 163–176.
- Lu, X. , Camarero, J. J. , Wang, Y. , Liang, E. , and Eckstein, D. , 2015: Up to 400-year-old Rhododendron shrubs on the southeastern Tibetan Plateau: prospects for shrub-based dendrochronology. Boreas , 44: 760–768.
- Myers-Smith, I. H. , Hallinger, M. , Blok, D. , Sass-Klaassen, U. , Rayback, S. A. , Weijers, S. , Trant, A. J. , Tape, K. D. , Naito, A. T. , Wipf, S. , Rixen, C. , Dawes, M. A. , Wheeler, J. A. , Buchwal, A. , Baittinger, C. , Macias-Fauria, M. , Forbes, B. C. , Lévesque, E. , Boulanger-Lapointe, N. , Beil, I. , Ravolainen, V. , and Wilmking, M. , 2015: Methods for measuring Arctic and alpine shrub growth: a review. Earth-Science Reviews , 140: 1–13.
- Rayback, S. A. , Henry, G. H. R , and Lini, A. , 2012: Multiproxy reconstructions of climate for three sites in the Canadian High Arctic using Cassiope tetragona. Climatic Change , 114: 593–619.
- Ren, P. , Rossi, S. , Gricar, J. , Liang, E. Y. , and Cufar, K. , 2015: Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau? Annals of Botany , 115: 629–639.
- Rossi, S. , Deslauriers, A. , Gricar, J. , Seo, J. W. , Rathgeber, C. B. K. , Anfodillo, T. , Morin, H. , Levanic, T. , Oven, P. , and Jalkanen, R. , 2008: Critical temperatures for xylogenesis in conifers of cold climates. Global Ecology and Biogeography , 17: 696–707.
- Schmidt, N. M. , Baittinger, C. , Kollmann, J. , and Forchhammer, M. C. , 2010: Consistent dendrochronological response of the dioecious Salix arctica to variation in local snow precipitation across gender and vegetation types. Arctic, Antarctic, and Alpine Research , 42: 471–475.
- Schweingruber, F. H. , Hellmann, L. , Tegel, W. , Braun, S. , Nievergelt, D. , and Büntgen, U. , 2013: Evaluating the wood anatomical and dendroecological potential of Arctic dwarf shrubs. International Association of Wood Anatomists Journal , 34: 485–497.
- Shao, X. , Huang, L. , Liu, H. , Liang, E. , Fang, X. , and Wang, L. , 2005: Reconstruction of precipitation variation from tree rings in recent 1000 years in Delingha, Qinghai. Science in China Series D: Earth Sciences , 48: 939–949.
- Srur, A. M. , and Villalba, R. , 2009: Annual growth rings of the shrub Anarthrophyllum rigidum across Patagonia: interannual variations and relationships with climate. Journal of Arid Environments , 73: 1074–1083.
- Stine, A. R. , and Huybers, P. , 2015: Arctic tree rings as recorders of variations in light availability. Nature Communications , 5: 3836.
- Stokes, M. A. , and Smiley, T. L. , 1996: An Introduction to Tree-Ring Dating. Tucson: University of Arizona Press, 73 pp.
- Sturm, M. , Racine, C. , and Tape, K. , 2001: Climate change-increasing shrub abundance in the Arctic. Nature , 411: 546– 547.
- Walker, D. A. , 1987: Height and growth rings of Salix lanata ssp. richardsonii along the coastal temperature-gradient of Northern Alaska. Canadian Journal of Botany , 65: 988–993.
- Wang, N. L. , Zhang, S. B. , He, J. Q. , Pu, J. C. , Wu, X. B. , and Jiang, X. , 2009: Tracing the major source area of the mountainous runoff generation of the Heihe River in northwest China using stable isotope technique. Chinese Science Bulletin , 54: 2751–2757.
- Wang, Y. , Liang, E. , Ellison, A. M. , Lu, X. , and Camarero, J. J. , 2015: Facilitation stabilizes moisture-controlled alpine juniper shrublines in the central Tibetan Plateau. Global and Planetary Change , 132: 20–30.
- Weijers, S. , Broekman, R. , and Rozema, J. , 2010: Dendrochronology in the High Arctic: July air temperatures reconstructed from annual shoot length growth of the circumarctic dwarf shrub Cassiope tetragona. Quaternary Science Reviews , 29: 3831–3842.
- Wigley, T. M. L. , Briffa, K. R. , and Jones, P. D. , 1984: On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. Journal of Climate and Applied Meteorology , 23: 201–213.
- Wilmking, M. , Hallinger, M. , Van Bogaert, R. , Kyncl, T. , Babst, F. , Hahne, W. , Juday, G. P. , de Luis, M. , Novak, K. , and Völlm, C. , 2012: Continuously missing outer rings in woody plants at their distribution margins. Dendrochronologia , 30: 213–222.
- Woodcock, H. , and Bradley, R. S. , 1994: Salix arctica (Pall.): its potential for dendroclimatological studies in the High Arctic. Dendrochronologia , 12: 11–22.
- Wu, Z. , 1983: The Flora of Tibet. Beijing: Science Press (in Chinese).
- Xiao, S. C. , Xiao, H. L. , Kobayashi, O. , and Liu, P. X. , 2007: Dendroclimatological investigations of sea buckthorn (Hippophae rhamnoides) and reconstruction of the equilibrium line altitude of the July first glacier in the Western Qilian Mountains, northwestern China. Tree-Ring Research , 63: 15–26.
- Zalatan, R. , and Gajewski, K. , 2006: Dendrochronological potential of Salix alaxensis from the Kuujjua River area, western Canadian Arctic. Tree-Ring Research , 62: 75–82.
- Zheng, D. , and Yao, T. D. , 2006: Uplifting of Tibetan Plateau with Its Environmental Effects. Beijing: Science Press (in Chinese).
- Zheng, Y. , Liang, E. , Zhu, H. , and Shao, X. , 2008: Response of radial growth of Qilian juniper to climatic change under different habitats. Journal of Beijing Forestry University , 30: 7–12 ( in Chinese).
- Zhu, H. , Zheng, Y. , Shao, X. , Liu, X. , Xu, Y. , and Liang, E. , 2008: Millennial temperature reconstruction based on tree-ring widths of Qilian juniper from Wulan, Qinghai Province, China. Chinese Science Bulletin , 53: 3914–3920.