ABSTRACT
Auklets (Aethia spp.) are small seabirds, endemic to the North Pacific Ocean, that nest in rock crevices on islands in Alaska and Russia. Nesting habitats for least (A. pusilla) and crested (A. cristatella) auklet colonies in the southern part of their range (Aleutian and Kuril Islands) are becoming overgrown by vegetation, which is fertilized by the auklets, making rock crevices unavailable for breeding. Colonization of newly created volcanic habitats suggests that auklets are habitat-limited in the southern range. The largest colonies there of least and crested auklets exist on lava slopes <100 years old. We propose that in the south, volcanic activity is required to maintain auklet populations. In contrast, colonies in the northern Bering Sea and Sea of Okhotsk show no indication of habitat limitation. They occur in more persistent talus slope habitats maintained by weathering, slumping, frost heaving, and tumbling. Biological processes there are slower and vegetation communities not as developed. We propose a conceptual model describing the interaction of geological and biological processes that influence auklet demography. We conclude that least and crested auklets require episodic disturbance (provided by volcanoes, earthquakes, and rock fall deposits) to maintain access to nest crevices. Auklets thereby provide an example of disturbance-adapted, early successional species that self-inhibit if their habitat is not regularly disturbed.
Introduction
Ecological succession, the process of change in the species composition and structure of an ecological community, takes place after a disturbance damages or destroys the biota. Animal colonists of disturbed sites often rely on vegetation structure (CitationKritzinger and van Aarde, 1998) and therefore are seen as accommodating to the biotic habitat in colonization and succession (CitationFox, 1990) rather than being drivers of community dynamics. This perception has led to an emphasis on plant rather than animal succession, despite the pivotal role of animals in such key processes as soil development, dispersal, pollination, and food web development; or the direct process of animal replacements in heterotrophic succession (CitationMajer, 1989). Although animals, including seabirds, can drive community dynamics, including that of seabirds (CitationCroll et al., 2005; CitationSekercioglu, 2006; CitationMulder et al., 2011), these examples are often poorly documented.
There are several ways that large colonies of seabirds influence both abiotic and biotic aspects of their nesting habitats. Seabirds import marine nutrients to land where they defecate, reproduce, and die. Such inputs build soil fertility, support vegetative growth, and influence community dynamics such as succession (CitationMagnússon et al., 2009; CitationSigurdsson and Magnússon, 2009; CitationIvanov, 2013). However, dense bird colonies can also deplete flora and restrict vegetative growth through trampling and digging (CitationMulder and Keall, 2001; CitationWardle et al., 2007; CitationKhoreva and Mochalova, 2009; CitationEllis et al., 2011). Such alterations in habitat structure may affect the persistence of seabird colonies. Given the central role of seabird colonies in altering their environment, it is important to understand which factors promote and which inhibit their colonization and persistence.
Volcanic eruptions, rock falls, and other dramatic geological disturbances that destroy most or all of the biota, provide natural experiments to examine the influence of physical habitat variables on seabird colonization and persistence. Two regions where both seabirds and volcanic eruptions are common are the Aleutian and Kuril Islands, where 10–30 eruptions occur each decade (CitationMiller et al., 1998; http://www.avo.alaska.edu; CitationKotlyakov et al., 2009). Despite their destructive results, volcanic disturbances may be instrumental in providing new habitat for some biota.
The recent (2008) eruption of Kasatochi Volcano in the Aleutian Islands helped clarify the abiotic factors that are required for colonization and persistence of seabird colonies. Thick ash deposits from the Kasatochi eruption resulted in the burial and destruction of nearly all plant life on this island in the central Aleutian Archipelago (CitationTalbot et al., 2010). Eruptive deposits also resulted in the temporary loss of nesting habitats (from infilling with ash and pyroclastic debris) for the large colonies of least (Aethia pusilla) and crested (A. cristatella) auklets that were present before the eruption (CitationDrew et al., 2010; CitationWilliams et al., 2010). These small, gregarious birds are among the most abundant birds in the North Pacific Ocean (CitationSpringer and Roseneau, 1985; CitationStephensen and Irons, 2003) and they usually nest sympatrically in a limited number of dense colonies. In the southern part of the range (Aleutian and Kuril islands) these auklet colonies are primarily on recently active volcanic islands (CitationStephensen and Irons, 2003) and in the northern part of their range they are associated with block fields and rock fall deposits (Bering Sea and Sea of Okhotsk; CitationBedard, 1969; CitationGaston and Jones, 1998). The least and crested auklets rely on open rock crevices where they nest, but the auklets' promotion of plant succession may lead to the infilling of those crevices and the eventual demise of the colony (CitationBancroft et al., 2005). Thus, auklets may require periodic volcanic activity or other types of geologic disturbances to reduce vegetative cover and maintain adequate nesting habitat (CitationStephensen and Irons, 2003).
In this paper, we (1) discuss the geological processes that create and maintain auklet habitat; (2) summarize and contrast all known least and crested auklet populations in the northern Pacific; (3) examine evidence that auklets are habitat-limited in the southern part of their range, and that most of these southern colonies are threatened by vegetation encroachment; and (4) present a conceptual summary of the main processes that are likely to limit auklet distribution.
Study Area
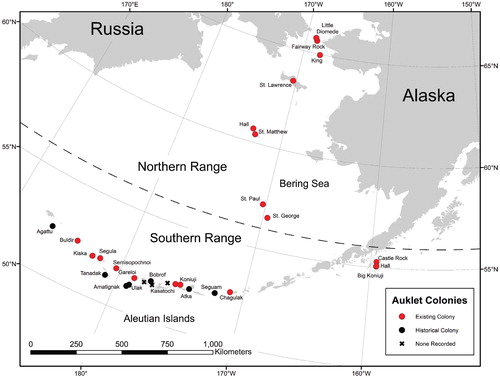
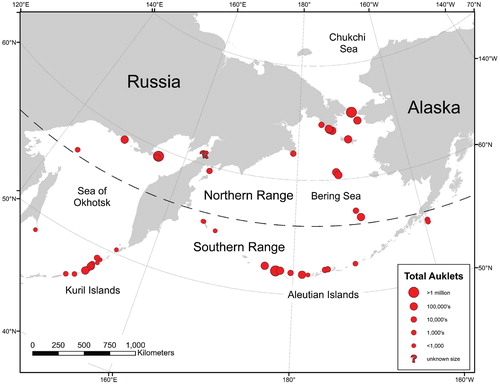
Least and crested auklets occur (usually together) on 20 islands in Alaska and at least 30 locations in Russia. Their breeding range includes in the south, the Aleutian and Kuril Islands, and in the north, islands in the Bering Sea, Sea of Okhotsk, and some mainland colonies in the Chukotka region (, ). Although estimates of colony sizes vary widely (CitationRenner et al., 2006), there are at least 4 million least auklets and 1.5 million crested auklets in Alaska (CitationUSFWS, 2004) and about 4.6 and 4.9 million, respectively, in Russia (). This paper focuses primarily on Alaskan colonies, where detailed data were available, but we present information from Russian colonies when possible.
Auklet colonies can be divided into two regions (north and south; see ) that exhibit different primary disturbance processes () as well as different rates of plant succession and climax stages. Colonies in the southern region (Aleutian and Kuril Islands) are primarily on active volcanoes where plant succession progresses more rapidly than in the north and reaches a complete cover of herbs and thick sod within a few hundred years. In contrast, in the northern part of their range (Bering Sea and Sea of Okhotsk islands, mainland Chukotskiy Peninsula), habitat is primarily created by mass movement (i.e., rock falls). These habitats may be actively maintained by freeze/thaw events, strong earthquakes, and erosion. In the northern region, plant succession takes place more slowly than in the south, lichens tend to dominate, and in many cases, a complete sod mat never develops. St. George in the Pribilof Islands is intermediate, where nesting habitat at the main colony is on rock fall deposits but where plant succession is encroaching into auklet nesting habitat. The few birds on nearby St. Paul nest in beach boulders that are subject to regular disturbance.
Creation and maintenance of auklet habitat
Least and crested auklets do not build or excavate nest sites, but must have rock crevices to breed (CitationBond et al., 2013; CitationJones, 2013). These crevices are created by lava flows, rock falls, and beach boulders (). Volcanic ash deposits (such as on Kasatochi in 2008) can fill and cover rock crevices, but rapid erosion in the wet maritime climate may re-expose them (CitationWalker et al., 2013).
FIGURE 1. Map of current colonies of least and crested auklets including population estimates. The marked latitude line (56°N) represents an approximate boundary between the southern range (where disturbance processes affecting auklets are primarily volcanic) and the northern range (primarily mass movement).
Volcanic habitat is common throughout the Aleutian and Kuril Islands (), but the long-term eruptive history and geomorphological characterization of eruptive products that are utilized by nesting seabirds at many locations are not known in detail. The largest auklet Alaskan colonies are found on the islands of Kiska, Gareloi, and Semisopochnoi, where lava flows erupted within the last 200 years provide habitat for >1 million birds on each island (CitationJones et al., 2001; CitationJones and Marais, 2004; CitationJones and Hart, 2006). Kiska Volcano erupted most recently in 1962, 1964, 1969, and 1990 and produced blocky lava flows ideal for auklet nests (CitationCoats et al., 1961); Gareloi has erupted at least 10 times since 1791 and several lava flows developed suitable crevices and cracks as they cooled; Semisopochnoi has erupted at least twice in 1873 and 1987 but these eruptions were minor ash-producing events that did not result in lava flows (http://www.avo.alaska.edu). Volcanic vents on the island of Segula most recently erupted about 200 years ago. Segula has an intermediate number of auklets (tens of thousands), apparently down from population estimates in 1979 (CitationEarly et al., 1979; H. Renner personal observation), likely due to nest sites overgrown with vegetation. A small auklet population is found on Koniuji, a small volcanic edifice near Kasatochi that last erupted within the past 1000–5000 years (CitationJicha, 2009).
The accumulation of rock fall debris creates auklet nesting habitat on both Aleutian (south) and Bering Sea (north) islands (). Rock fall deposits accumulate at the base of steep cliffs and can result from a single event or represent multiple events of many rock falls over decades or centuries. Rock falls can be triggered by earthquakes (CitationKeefer, 1984), cyclical freeze-thaw activity (CitationD'Amato et al., 2016), beach erosion [from wave action, or the action of salt crystals (CitationMottershead, 1989)], or biological weathering (CitationTrenhaile, 1987; CitationHall and Otte, 1990). Freeze/thaw activity is especially prominent where islands are underlain by permafrost (e.g., St. Lawence, Hall, and St. Matthew that support large [100,000 to 1 million birds; see ] auklet populations). Crevices in rock fall deposits can gradually fill in over time from settling of small rocks into the crevices or growth of vegetation and accompanying soil development. The relative age of rock fall deposits can be inferred from the degree of vegetation, soil, or ash cover. Medium-sized auklet populations occur on rock fall deposits on the islands of St. George, King, and Fairway Rock.
TABLE 1 Characteristics of known least and crested auklet colonies in Alaska (A) and Russia (B). Colonies are listed in descending order of least crested population within each location and substrate type. Note that rock fall habitats also occur on some volcanoes (e.g., Buldir, Kasatochi). Population size estimates (Pop. Est.) are approximate: huge = >1 million birds, large = 100,000–1 million, medium = 10,000–100,000, small = <10,000, tiny = <1000.
Table
FIGURE 2. Disturbance processes that create, maintain, and destroy crested and least auklet nesting habitat. Volcanic disturbances are the primary process in the southern range (Aleutian and Kuril Islands), and mass movement is the primary process in the northern range (Bering Sea and Sea of Okhotsk).
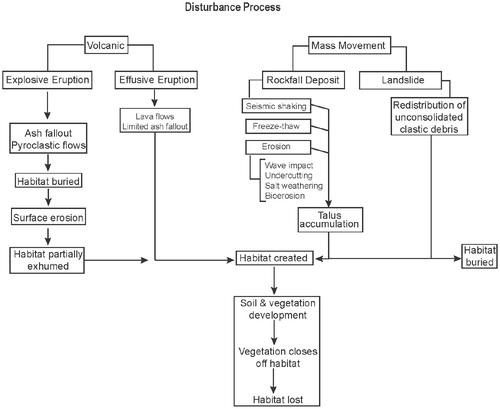
Beach boulders are a third habitat that sometimes supports auklet colonies, such as on St. Paul and Chagulak (). Beach boulders are the least stable habitat for auklets because they are subjected to tidal fluctuations, wave action and storm surges.
Plant Succession
During succession, vegetation cover generally increases, although not necessarily in a linear or continuous manner. On lava surfaces, lichens or mosses may be the initial colonists, followed by grasses and herbs. In more fertile microsites, such as cracks in the lava or in rock crevices where sediments accumulate, grasses and herbs may initiate succession. On Aleutian island habitats, a complete sod mat typically develops within several to many decades. Sod development may be accelerated by auklets bringing large quantities of marine-derived nutrients to their nest sites on land (CitationMaron et al., 2006; CitationMagnússon et al., 2009; CitationMulder et al., 2011; CitationZwolicki et al., 2013). Bank (Citation1953) suggested that biotic patterns in the Aleutians are unstable because underlying soil conditions are unstable, and that biological succession is altered by volcanic eruptions, wind erosion, and periodic moisture surpluses. In general, in the Aleutians, vegetation is unstable and biological succession unpredictable, as is typical of primary succession (CitationWalker and del Moral, 2003; CitationTalbot et al., 2010).
Vegetation Encroachment and Auklet Colony Decline
Several studies have suggested that vegetation growth may be a limiting factor for Aleutian island and southern Bering Sea auklet colonies (CitationRoby and Brink, 1986; CitationStephensen and Irons, 2003). Coupled with auklet colony mapping efforts in the last decade (see CitationRenner et al., 2006) and the disappearance of historic colonies, we now have substantial evidence that nesting habitat is ephemeral because of vegetative growth and the stochastic nature of habitat creating volcanic and other geologic disturbances.
Population monitoring of crevice-nesting auklets has numerous challenges (see CitationRenner et al., 2006, Citation2011; CitationRenner and Renner, 2010) and has taken place on only a few colonies. However, collective evidence suggests that auklet nest sites in the southern part of their range are compromised by vegetation growth. Vegetation encroachment has been described on nearly all Aleutian and southern Bering Sea auklet colonies, leading to either observed or inferred reductions in the distribution and number of auklet colonies. Ashfall, soil development, peat accumulation (augmented by marine-derived nutrients from bird excrement), and gradual plant growth blocks access to suitable nesting habitat, presumably followed by declines in nesting populations. Of course, it is possible that auklet colonies decline for some reason other than vegetative encroachment and the area subsequently becomes overgrown. We know that as plant succession advances, auklets maintain access holes through vegetation mats, allowing them entry to nesting crevices for an unknown period of time (H. Renner, personal observation). Shrinking colonies alone do not imply habitat limitation; however, evidence of rapid auklet expansion into new habitats (see below) strengthens the case for habitat limitation.
Southern Range
In the Aleutian Islands and Kuril, large colonies exist primarily on lava flows created in the past 48–200 years (). Vegetation encroachment and population declines in occupied areas have been noted on Gareloi (CitationJones and Hart, 2006); Kiska Island (CitationJones et al., 2001); Segula (CitationRenner and Reynolds, 2006); and Semisopochnoi (CitationJones and Marais, 2004). Although the rock fall deposits that host auklets on Buldir are likely more than 100 years old (CitationMiller et al., 1998), habitat limitation has been indicated here as well (CitationKnudtson and Byrd, 1982).
On Kasatochi, although observations were too brief prior to the 2008 eruption to document change in colony extent over multiple decades, vegetation increased on auklet plots over one decade from a mean of 20% bare, 65% short vegetation, and 15% tall vegetation in 1997 to 15% bare, 45% short, and 40% tall in 2007 (CitationDrummond and Larned, 2007). The decrease in cover and increase in height of the vegetation (and increase in total area vegetated) in 10 years suggests a future trend not favorable to the maintenance of rock crevices. We have no data on vegetation cover on the small auklet colony on Koniuji, and the few thousand auklets on Chagulak nest among beach boulders that are periodically affected by waves, which inhibit the accumulation of fine sediment and soil development.
On Kiska, a photograph of the colony site at Sirius Point in the 1940s shows that much of the area consisted of bare lava flows (CitationStephensen and Irons, 2003). By 2001, the lava flow was covered with vegetation and hosted only low densities of nesting auklets, presumably because rock crevices became inaccessible (CitationJones et al., 2001). Instead, auklets nested at higher densities on a younger lava flow that formed between 1962 and 1969 (CitationMiller et al., 1998) that was only sparsely vegetated with lichens. On the older lava flow, vegetative cover increased significantly between two surveys in 1986 and 2001: grass cover increased 216%, ferns 215%, and Carex sp. by 54%, while bare rock decreased by 70%, and mosses and lichen by 64% (CitationJones et al., 2001).
We have few data on vegetation changes in the Kuriles, but limited observations support a similar pattern in this highly volcanic region. A 1924 eruption on Raikoke Island destroyed vegetative cover; by 1963, vegetation was still sparse and 100,000 auklets were estimated on slopes up to 200 m a.s.l. (CitationVelizhanin, 1971). By 1996, dense plant communities were present on the island (CitationTakahashi et al., 2002), and auklets are now limited mainly to beach boulders in much smaller numbers.
In the southern Bering Sea (intermediate between the northern and southern range), some large colonies are also becoming overgrown. On St. George, the inland colony of least auklets at Ulakaia Hill is presumed to have declined by over an order of magnitude since 1900 due to vegetation encroachment (CitationRoby and Brink, 1986; CitationRenner et al., 2006).
Northern Range
Colonies north of the Pribilof Islands (St. George and St. Paul) experience limited vegetation encroachment. Three colonies in the central Bering Sea (two on St. Matthew and one on Hall) were mapped in 2005 (CitationRenner and Jones, 2005); no evidence was noted of vegetation encroachment into nesting habitat. The physical habitat there consists of actively eroding rock fall deposits (angular cobble to boulder size material) of some antiquity based on the relatively stable cover of crustose lichens, although flatter areas had thicker vegetation.
Few recent studies have taken place on the massive colonies in the northern Bering Sea. However, Searing (Citation1977) suggested that least auklet populations on St. Lawrence were apparently stable or increasing at inland colonies, and Bedard (Citation1969) noted no evidence for a loss of nesting habitat due to vegetation encroachment in areas where rock fall deposits were used. Talan Island in the northern Sea of Okhotsk is well studied and has undergone large population declines between 1989 and 2008 (CitationKondratyev et al., 1992; CitationAndreev et al., 2010). However, the cause of decline does not appear to be habitat limitation; previously used nest sites have been vacant for several years before eventually succumbing to overgrowth (E. Yu. Golubova, personal commun.). Big and Little Diomedes may host the largest colony in the northern region but have had few recent ornithological investigations (but see CitationBiderman and Drury, 1978; CitationZubakin et al., 1992; CitationKonyukhov et al., 1998). Although auklet colonies on St. Lawrence, King, and the Diomede islands are in very old rock (Cretaceous and Tertiary age), there is little vegetative cover, presumably because of the severe climate at that latitude (CitationStephensen and Irons, 2003). In addition, the rock fall deposits are relatively fresh in appearance and are most likely young and still forming.
Colony Extinction and Unused habitats
The ornithological record in Alaska is sparse before about 1970. Nevertheless, we found limited additional evidence that at least some historical auklet colonies in the Aleutian Archipelago have completely disappeared (). Murie (Citation1959) found least auklet remains on Amatignak, Tanadak, and Bobrof islands, with reports from Native Alaskans corroborating that they were once abundant on Bobrof. Additionally, he found a “sizeable” colony of crested auklets nesting on Seguam. Bent (Citation1919) described a least auklet colony on Kiska at North Head (near Kiska Harbor) which was absent in 2001 (CitationJones et al., 2001). Turner (Citation1886; as cited in CitationNelson, 1887) reported least auklets near “Semichi” (Nizki, Alaid, or Shemya islands) and breeding on Agattu as well as plentiful crested auklets in the Near Islands. Hartert (Citation1910) described auklets as “plentiful” at Atka, Attu, and Agattu as well as a few near Unalaska. In some cases, disappearance of these colonies was attributed to the introduction of the arctic fox (Alopex lagopus). Yet without more details it is difficult to disentangle the relative contribution of introduced predators and habitat loss to extirpation of auklets. However, auklets have not returned to any of these islands since eradication of foxes up to 5 decades ago even though suitable nesting habitat is abundant. Natural plant communities have been altered on many Aleutian Islands by a history of fox introductions changing the nutrient subsidies (CitationMaron et al., 2006). Because the exact locations on these islands of the historical auklet colonies on these islands are unknown, we cannot ascribe them to a particular substrate (lava, rock fall, or beach boulders). Archaeological evidence of auklet bones in middens on islands that do not currently have breeding auklets (e.g., Shemya and Amchitka; CitationCausey et al., 2005) may indicate historical colonies on those islands, but we cannot rule out the possibility that hunters brought them back from other islands.
A further puzzle is why several active Aleutian volcanoes within the range of current large colonies and with suitable nesting habitat do not host least or crested auklets (). These volcanoes include Tanaga, Kanaga, Great Sitkin, and Korovin. Tanaga Volcano erupted as recently as 1914, although some volcanic surfaces (and rock fall deposits) on the island are likely older. Kanaga Volcano erupted four times in the last century (CitationMiller et al., 1998; CitationWaythomas et al., 2010). Great Sitkin Volcano has erupted six times in the last century (CitationSimons and Mathewson, 1947; CitationJones, 1951; CitationAssociated Press, 1974) but no lava reached the lower slopes of the volcano. Rock fall deposits on the north coast of Great Sitkin could provide suitable nesting habitat for seabirds as they have elsewhere in the Aleutian Islands, but no known colonies are present there. Korovin Volcano on Atka has also had as many as three eruptions in the 1900s, with both ash and lava produced (CitationSmithsonian Institution, 1987; CitationMcGimsey et al., 2003). Reasons for the lack of nesting of least and crested auklets on these islands are unknown. It is possible that the fracture spacing and morphology of lava flows at these locations is insufficient to accommodate large numbers of nesting birds. In addition, non-native arctic foxes were only recently (in the past 20 years) eradicated from Great Sitkin, Kanaga, and Tanaga, so predation may be a major factor.
New habitat Colonization
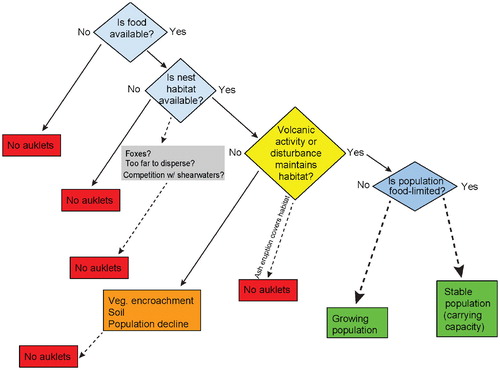
Where food is abundant, nesting habitat availability appears to be a major driver of distribution and abundance. We describe four instances from Alaska where new nesting habitat has been created, allowing auklets to begin breeding in previously unoccupied areas. However, the lack of accurate census methods does not allow us to determine if the colonization stems from birds abandoning old habitat or expanding due to population increases.
St. George is home to an inland colony of least auklets, estimated in 2008 to be 88,000 birds (CitationRenner and Renner, 2010). Least auklets also nest in small clusters in nearly all available habitat around the coast of the island (H. Renner, personal observation). In 1987, construction and dredging activities began for a boat harbor and seawall (CitationU.S. Army, 1988); previously no auklets nested at the site, although a few were noted in driftwood piles and boulders on a nearby beach (CitationRoby and Brink, 1986; CitationClimo, 1987). Least auklets immediately moved into the stockpiled talus-like rock (riprap) during the first season that it was available and within a few years thousands of auklets were observed nesting in the riprap comprising the harbor and seawall.
Kiska likely hosts Alaska's largest auklet colony on its north side at Sirius Point, with more than 1 million birds. Historical reports (e.g., CitationMurie, 1959) indicate that the primary nesting habitat on Kiska is a lava flow inferred to be several centuries old (CitationCoats et al., 1961). From 1962 to 1969, an effusive eruption created a large lava flow immediately adjacent to the original colony. Over the next 10 years, this new lava flow provided excellent nesting habitat and was rapidly colonized by both auklet species; this new colony eventually became larger than the pre-eruption site.
Kasatochi formerly supported tens to hundreds of thousands of nesting least and crested auklets. Auklets nested and socialized on beach boulders and rock fall deposits, including on rock fall deposits that were partially vegetated (CitationScharf et al., 1996). Kasatochi had no previously known historical eruptions prior to 2008. All nesting habitat was covered by ash in the 2008 eruption, but since that time surface erosion has exposed some former habitat, and more significantly, new rock fall deposits were created in formerly unoccupied areas (G. Drew, personal commun.). Since the eruption, the numbers of nesting auklets on the island have increased by an order of magnitude each year, and now approach pre-eruption numbers (J. C. Williams, personal observation).
Buldir experienced a February 1975 earthquake (M7.6), which caused a small rock slide near the main auklet colony. By June 1975, auklets were nesting in this new area (CitationKnudtson and Byrd, 1982). Eggs were also found on the surface of rock fall deposits each year of the 1974–1976 study (CitationKnudtson and Byrd, 1982) and in 2000–2003 (M. Renner, personal observation), which suggests an excess of adults that were unable to find nest sites.
In Russia, the data on colonization of new habitats by auklets are poor. In the southwestern part of the range, a new crested auklet colony (up to 1500 birds) was created in the 1980s—birds began to breed in basements of houses in a fur seal hunter settlement on Tyuleniy Island. Since 1998, a small number of least auklets have bred there also (CitationTrukhin and Kuzin, 1996).
A June 2009 eruption of Sarychev Peak volcano covered half of Matua Island (Kuriles) (CitationRybin et al., 2011), apparently exterminating part of the crested auklet colony and the colonies of other species. Quantitative information regarding the impact on the bird population is unavailable, but during shipboard observations in June 2011, the evening movements of auklets around the island were not less than those of previous years (CitationArtukhin, 2016). In 2016, auklets bred in old remaining colonies, but did not inhabit new volcanic areas yet (A. N. Ivanov, personal commun.).
Dispersal
Due to rapid vegetation succession in the southern part of their range, least and crested auklets must be adaptable to the availability of new habitat to maintain stable or increasing populations. During recorded times, we only have evidence of auklets establishing new colonies on the same islands as existing colonies, despite the occurrence of sometimes severe disturbance events (e.g., Kasatochi). Because some past volcanic eruptions have been significantly larger than historical events, there are probably instances of complete habitat destruction with little or no short-term reestablishment of habitat, suggesting that auklets must be able to establish colonies on new islands to persist.
The gregarious and social nature of least and crested auklets likely controls the dispersal potential of these species, leading to considerable inertia to disperse to a new island (CitationMunilla et al., 2016). When movement occurs, it is likely to be rapid. Dispersal on an island with an existing colony that exhibits long-term stability is presumably more common and has been observed at Kiska and St. George.
Other Factors Limiting auklet populations
Seabirds are central-place foragers during the breeding season and thus require access to concentrations of food within foraging distance of suitable nesting habitat (). Least and crested auklets are obligate planktivores that feed primarily on calanoid copepods; their distribution in Alaska is limited by access to concentrations of food resources in upwelling zones and cold, salty water (summarized in CitationStephensen and Irons, 2003). Areas of upwelling and high biological productivity occur throughout the central and western Aleutian Islands (CitationHunt et al., 1993; CitationStabeno et al., 2003; CitationUSFWS, 2004) and these areas are the primary food sources for the large auklet colonies (CitationSpringer et al., 1996). In the northern Bering Sea, auklet colonies are supplied via ocean circulation patterns with a rich and abundant food supply of zooplankton from the Bering Sea shelf to the south (CitationPiatt and Springer, 2003), and auklet colony distribution depends on the distance between foraging and breeding areas (CitationKonyukhov et al., 1998).
Competition from other birds or predation by mammals may limit auklet distributions, such as from suitable nesting habitat on islands east of Kasatochi in the central Aleutians. Renner et al. (Citation2008) proposed that crested auklets may be limited by summertime interference competition from short-tailed shearwaters (Puffinus tenuirostris), which feed in flocks numbering in the millions and can disrupt other species' foraging. Predation by arctic and red foxes may affect auklet populations (CitationMurie, 1959; CitationWilliams et al., 2003) as well as other seabird nest sites (CitationArtukhin et al., 2001). Native arctic foxes co-occur with all colonies in the Bering Sea, as they are accessible by sea ice. Red foxes also recently reached and established a breeding population on St. Matthew (CitationKlein and Sowls, 2015). In addition, arctic or red foxes (neither being native to islands in the southern range) were introduced at all current Aleutian auklet colonies except Buldir and Chagulak (CitationBailey, 1993) and some islands of the Kuril Chain (CitationKostenko et al., 2004). It is possible that fox predation was responsible for extirpations of small colonies or failure to establish new colonies, but we know of no areas where new colonies developed after foxes were eradicated. Finally, beach boulder habitats are not ideal nesting habitat. Buldir hosts the only large auklet colony in the Aleutians that is supported by beach boulder habitat. Vegetation mats grow on higher elevation boulder-covered slopes whereas boulders found at the shoreline are affected by wave action.
Population Dynamics and Management Implications
Geologic disturbances occasionally have dramatic effects on seabird populations. For example, the endangered Hutton's shearwater (Puffinus huttoni) lost 20–30% of its breeding population during the November 2016 M7.8 earthquake in New Zealand (CitationMcSweeney, 2016). A 2016 eruption of Mt. Curry (South Sandwich Islands) threatened the world's largest chinstrap penguin (Pygoscelis antarcticus) colony by burying it in ash (CitationBritish Antarctic Survey, 2016). Penguin colony population changes on the Antarctic Peninsula were linked to multiple phases of near-devastation by the Deception Island volcano, with 400–1100 year intervals needed for sustainable recovery (CitationRoberts et al., 2017). However, crevice-nesting species such as least and crested auklets appear to have another type of relationship with disturbance, whereby they depend on it to sustain their populations. Unlike the numerically dominant least and crested auklets, other auklets in the Bering Sea (e.g., parakeet and whiskered auklets; A. psittacula and A. pygmaea) do not seem limited by nesting habitat, likely because they are less gregarious and nest in small dispersed clusters nearly ubiquitously—often in beach boulders and cliff cracks.
Managers who monitor and maintain auklet populations will benefit from understanding the critical role of broad-scale disturbance in population regulation. In a recent study, vegetation was removed from volcanic substrate on portions of Gareloi Island (CitationMajor et al., 2017) as a potential technique to improve crevice-nesting habitat for crested auklets. The experiment resulted in low to limited short term success, perhaps because the effects on auklet populations of disturbance and succession may take place at a slower time scale than we are able to observe with current monitoring data. Major et al. (Citation2017) further suggested that least auklets may be faster to colonize new habitat than crested auklets, although they did not test this in their study.
The available geological data are insufficient to address the frequency of volcanic eruptions that produced lava >100 years ago. These data do not allow us to draw any firm conclusions about the frequency of lava eruptions necessary to maintain auklet nesting habitat, the rates of vegetation development and succession in detail, or the time required for a site to become completely overgrown and thus rendered uninhabitable for auklets. Each of these factors may be highly variable among islands. Another challenge is that we know very little about the time-frame for the development, use, and abandonment of rock crevices in the northern Bering Sea, which could be on the order of thousands of years where periods of active frost-wedging are synchronous with increases in the frequency of freezing and thawing in a periglacial climate. Because many of the northern colony sites do not receive regular input of fine sediment, such as volcanic ash, it may take much longer for northern rock falls to become vegetated, overgrown, and no longer suitable for nesting than volcanic sites in the southern areas. Vegetation succession in the southern areas requires that least and crested auklets must be adaptable to the availability of new habitat to maintain stable or increasing populations. Although insufficient data exist to constrain the timescales of nesting habitat formation, evolution, and demise, where the substrate age is known (e.g., Kiska, Gareloi) we observe robust colonies that have persisted for 50–226 years.
Conclusions
Least and crested auklet populations strongly influence their environments in the Aleutian Islands and the northern Bering Sea. They are keystone species whose movements and population fluctuations have implications for soil development, plant succession, and food webs. They interact with their terrestrial habitat in at least three ways. First, geological disturbances (volcanic eruptions, rock falls) are required to sustain auklet nesting habitat and allow stable or growing populations (). Second, in the southern part of their breeding range, marine-derived nutrients from auklets accelerate plant growth and soil development at the nest sites, apparently depriving them of the rock crevices they need to nest. Third, in the northern part of their breeding range, auklet colonies show no indication of habitat limitation as a cause of decline. Instead, the northern populations inhabit more persistent rock fall deposits that are maintained by weathering, slumping, frost-heaving, and tumbling. Biological processes in the north are slower and vegetation growth is less vigorous than in the south. Other biological factors such as food availability, competition, and predation also regulate which islands may be suitable for colony formation. What remains puzzling is the lack of auklet populations where both rock crevices and nearby food sources exist. Future studies on dispersal and colonization would be beneficial in understanding auklets' relative ability to occupy new or previously used habitat after its creation. We recommend a monitoring program including regular mapping of the geographic extent of colonies (e.g., CitationRenner et al., 2006) as well as documenting unoccupied areas.
Acknowledgments
We thank many colleagues whose conversations and decades spent observing auklets in the region contributed to the ideas in this paper as well as much unpublished data. In particular, we thank Vernon Byrd, David Irons, Ian Jones, Kim Nelson, John Piatt, and Martin Renner. Gary Drew, George Hunt, Game McGimsey, Aaron Shiels, Leslie Slater, and two anonymous reviewers provided helpful reviews of the manuscript. Marc Romano and Don Dragoo assisted with figure development. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the United States Fish and Wildlife Service.
References Cited
- Andreev, A. V. , Golubova, E. Yu. , Zubakin, V. A. , and Kharitonov, S. P. , 2010: The number of seabirds on Talan Island: the 20-year trend assessment. Bulletin of the North-East Scientific Center, Russian Academy of Sciences, Far East Branch , 2: 30–42 (in Russian).
- Andreev, A. V. , Kharitonov, S. P. , and Sleptsov, Yu. A. , 2012: Seabirds of Saint Jonah's Island (the Sea of Okhotsk). Russian Journal of Zoology , 91(7): 843–855 (in Russian).
- Artukhin, Yu. B. , 1999: Cadastre of seabird colonies of the Commander Islands. In Poyarkov, N. D. (ed.), The Biology and Conservation of the Birds of Kamchatka , vol. 1. Moscow: Dialog-MGU, 25–35, 139–144 (in Russian).
- Artukhin, Yu. B. , 2016: Middle Kuril Islands. In Artukhin, Yu. B. (ed.), Marine Important Bird Areas of the Russian Far East. Moscow: BirdsRussia, 110–113.
- Artukhin, Yu. B. , Trukhin, A. M. , Kornev, S. I. , and Purtov, S. Yu. , 2001: Cadastre of seabird colonies of the Kuril Islands. In Artukhin, Yu. B. , and Gerasimov, Yu. N. (eds.), The Biology and Conservation of the Birds of Kamchatka , vol. 3. Moscow: BCC Press, 3–59 (in Russian).
- Associated Press , 1974: Sitkin Island volcano puts on bright show: Fairbanks Daily News-Miner , 21 February 1974, p. 1.
- Bailey, E. P. , 1993: Introduction of foxes to Alaskan Islands—History, effects on avifauna, and eradication. USFWS, Resource Publication 193, 54 pp.
- Bancroft, W. J. , Garkaklis, M. J. , and Roberts, J. D. , 2005: Burrow building in seabird colonies: a soil-forming process in inland ecosystems. Pedobiologia , 49: 149–165.
- Bank, T. P. , 1953: Biological succession in the Aleutians. Pacific Science , 7: 493–503.
- Bedard, J. , 1969: The nesting of the crested, least, and parakeet auklets on St. Lawrence Island, Alaska. Condor , 71: 386–398.
- Bent, A. C. , 1919: Life histories of North American diving birds. U.S. National Museum Bulletin , 107, 245 pp.
- Biderman, J. O. , and Drury, W. H. , 1978: Ecological studies in the northern Bering Sea: studies of seabirds in the Bering Strait. In Environmental Assessment of the Alaskan Continental Shelf, Annual Reports of Principal Investigators, vol. 3. Boulder, Colorado: BLM/NOAA OCSEAP, 751–838.
- Bond, A. L. , Jones, I. L. , Seneviratne, S. , and Muzaffar, S. B. , 2013: Least Auklet (Aethia pusilla). In Rodewald, P. G. (ed.), The Birds of North America. Ithaca, New York: Cornell Lab of Ornithology. https://birdsna.org/Species-Account/bna/species/leaauk. doi: http://dx.doi.org/10.2173/bna.69.
- British Antarctic Survey , 2016: Penguin colonies at risk from eruption of volcano. Science Daily, 6 July 2016. https://www.sciencedaily.com/releases/2016/07/160706114615.htm.
- Causey, D. , Corbett, D. G. , Lefevre, C. , West, D. L. , Savinetsky, A. B. , Kiseleva, N. K. , and Khassanov, B. F. , 2005: The palaeoenvironment of humans and marine birds of the Aleutian Islands: three millennia of change. Fisheries Oceanography , 14: 259–276.
- Climo, L. A. , 1987: The effect of blast-generated noise on breeding seabirds at St. George Island, Alaska. Unpublished USFWS report, St. George, Alaska, 54 pp.
- Coats, R. R. , Nelson, W. H. , Lewis, R. G. , and Powers, H. A. , 1961: Geologic reconnaissance of Kiska Island, Aleutian Islands, Alaska: In Investigations of Alaskan volcanoes. U.S. Geological Survey Bulletin B 1028-R: 563–581.
- Croll, D. A. , Maron, J. L. , Estes, J. A. , Danner, E. M. , and Byrd, G. V. , 2005: Introduced predators transform subarctic islands from grassland to tundra. Science , 307: 1959–1961.
- D'Amato, J. , Hantz, D. , Guerin, A. , Jaboyedoff, M. , Baillet, L. , and Mariscal, A. , 2016: Influence of meteorological factors on rockfall occurrence in a middle mountain limestone cliff. Natural Hazards and Earth System Sciences , 16: 719–735.
- Drew, G. S. , Dragoo, D. E. , Renner, M. , and Piatt, J. F. , 2010: At-sea observations of marine birds and their habitats before and after the 2008 eruption of Kasatochi Volcano, Alaska. Arctic, Antarctic, and Alpine Research , 42: 325–334.
- Drummond, B. A. , and Larned, A. L. , 2007: Biological monitoring in the central Aleutian Islands, Alaska in 2007: summary appendices. Unpublished USFWS report, Homer, Alaska, 154 pp.
- Early, T. , Beall, J. , Henry, W. , and Taber, A. , 1979: Results of bird and mammal surveys of the western Aleutian Islands. Unpublished USFWS report, Adak, Alaska, 140 pp.
- Ellis, J. C. , Bellingham, P. J. , Cameron, E. K. , Croll, D. A. , Kolb, G. S. , Kueffer, C. , Mittlehauser, G. H. , Schmidt, D. , Vidal, E. , and Wait, D. A. , 2011: Effects of seabirds on plant communities. In Mulder, C. P. H. , Anderson, W. B. , Towns, D. R. , and Bellingham, P. J. (eds.), Seabird Islands: Ecology, Invasion, and Restoration. Oxford: Oxford University Press, 177–211.
- Fox, B. J. , 1990: Changes in the structure of mammal communities over successional time scales. Oikos , 59: 321–329.
- Gaston, A. J. , and Jones, I. L. , 1998: The Auks. Oxford: Oxford University Press, 349 pp.
- Hall, K. , and Otte, W. , 1990: A note on biological weathering on nunataks of the Juneau Icefield, Alaska. Permafrost and Periglacial Processes , 1: 189–196.
- Hartert, E. , 1910: Die Vögel der paläarkischen Fauna. Systematische Übersicht der in Europa, Nord-Asien und der Mittelmeerregion vorkommenden Vögel , Heft I. Berlin: R. Friedländer & Sohn, 832 s.
- Hunt, G. L., Jr. , Harrison, N. M. , and Piatt, J. F. , 1993: Foraging ecology as related to the distribution of planktivorous auklets in the Bering Sea. In Vermeer, K. , Briggs, K.T. , Morgan, K. H. , and Siegel-Causey, D. (eds.), The Status, Ecology, and Conservation of Marine Birds of the North Pacific. Ottawa: Environment Canada, 18–26.
- Ivanov, A. N. , 2013: Ornithogenical Geosystems of Northern Pacific Islands. Moscow: Nauchny Mir, 228 pp. (in Russian).
- Jicha, B. R. , 2009: Holocene volcanic activity at Koniuji Island, Aleutians. Journal of Volcanology and Geothermal Research , 185: 214–222.
- Jones, A. E. , 1951: Aleutian volcanic activity. The Volcano Letter , 514: 6.
- Jones, I. L. , 2013: Crested auklet (Aethia cristatella). In Rodewald, P. G. (ed.), The Birds of North America. Ithaca, New York: Cornell Lab of Ornithology. https://birdsna.org/Species-Account/bna/species/creauk, doi: http://dx.doi.org/10.2173/bna.70.
- Jones, I. L. , and Hart, K. A. , 2006: A survey of inland least and crested auklet breeding colonies at Gareloi Island in the Delarof Islands, Aleutian Islands, Alaska during 2006. Memorial University of Newfoundland, St. John's, Canada, unpublished report, 31 pp.
- Jones, I. L. , and Marais, J. F. , 2004: Survey of the least and crested auklet colony near Sugarloaf Head, Semisopochnoi Island, Aleutian Islands, Alaska, in 2004. Memorial University of Newfoundland, St. John's, Canada, unpublished report, 34 pp.
- Jones, I. L. , Gray, C. M. , Dussureault, J. , and Sowls, A. L. , 2001: Auklet demography and Norway rat distribution and abundance at Sirius Point, Kiska Island, Aleutian Islands, Alaska in 2001. Memorial University of Newfoundland, St. John's Canada, unpublished report, 25 pp.
- Keefer, D. K. , 1984: Landslides caused by earthquakes. Geological Society of America Bulletin, 95: 406–421.
- Khoreva, M. G. , and Mochalova, O. A. , 2009: Plants and birds on the Okhotsk Sea coast: equilibrium, crisis, adaptations. Contemporary Problems of Ecology , 1: 119–125.
- Klein, D. R. , and Sowls, A. , 2015: Red foxes replace arctic foxes on a Bering Sea island: consequences for nesting birds. Alaska Park Science , 14: 31–39.
- Knudtson, E. P. , and Byrd, G. V. , 1982: Breeding biology of crested, least, and whiskered Auklets on Buldir Island, Alaska. Condor , 84: 197–202.
- Kondratyev, A. Ya. , Zubakin, V. A. , and Kharitonov, S. P. , 1992: Methods for population number estimation of some seabird species (Aethia cristatella, Aethia pusilla). In Chernyavskiy, F. B. , and Kondratyev, A. Ya. (eds.), Coastal Ecosystems of the Northern Part of the Sea of Okhotsk. Talan Island. Magadan: IBPS DVO RAN, 137–152 (in Russian).
- Kondratyev, A. Ya. , Zubakin, V. A. , Kharitonov, S. P. , Tarkhov, S. V. , and Kharitonova, I. A. , 1993: Study of bird seashore colonies on Matykil and Kokontse islands (Yamskiye Islands) and Pyagina Peninsula. Bulletin of Moscow Society of Naturalists, Biological Series , 98(5): 21–32 (in Russian).
- Konyukhov, N. B. , Bogoslovskaya, L. S. , Zvonov, B. M. , and van Pelt, T. I. , 1998: Seabirds of the Chukotka Peninsula, Russia. Arctic , 51(4): 315–329.
- Kostenko, V. A. , Nesterenko, V. A. , and Trukhin, A. M. , 2004: Mammals of the Kuril Archipelago. Vladivostok: Dalnauka, 186 pp. (in Russian).
- Kotlyakov, V. M. , Baklanov, P. Yu. , Komedchikov, N. N. , et al. (eds.), 2009: Atlas of the Kuril Islands. Moscow, Vladivostok: IPTs “DIK,” 516 pp. (in Russian).
- Kritzinger, J. J. , and van Aarde, R. J. , 1998: The bird communities of rehabilitating coastal dunes at Richards Bay, KwaZulu-Natal. South African Journal of Science , 94: 71–78.
- Magnússon, B. , Magnússon, S. H. , and Fridriksson, S. 2009: Developments in plant colonization and succession on Surtsey during 1999–2008. Surtsey Research , 12: 57–76.
- Majer, J. D. , 1989: Long-term colonization of fauna in reclaimed land. In Majer, J. D. (ed.), Animals in Primary Succession. The Role of Fauna in Reclaimed Lands. Cambridge: Cambridge University Press, 143–174.
- Major, H. L. , Buxton, R. T. , Schacter, C. R. , Conners, M. G. , and Jones, I. L. , 2017: Habitat modification as a means of restoring crested auklet colonies. Journal of Wildlife Management , 81: 112–121.
- Maron, J. L. , Estes, J. A. , Croll, D. A. , Danner, E. M. , and Elmendorf, S. C. , 2006: An introduced predator alters Aleutian Island plant communities by thwarting nutrient subsidies. Ecological Monographs , 76: 3–24.
- McGimsey, R. G. , Neal, C. A. , and Girina, O. , 2003: 1998 volcanic activity in Alaska and Kamchatka: summary of events and response of the Alaska Volcano Observatory. U.S. Geological Survey Open-File Report, OF 03-0423, 35 pp.
- McSweeney, P. , 2016: At risk bird, Hutton's shearwater, has lost an estimated quarter of their population. http://www.stuff.co.nz/national/nz-earthquake/86547893/at-risk-bird-huttons-shearwater-has-lost-an-estimated-quarter-of-their-population.
- Miller, T. P. , McGinsey, R. G. , Richter, D. H. , Riehle, J. R. , Nye, C. J. , Yount, M. E. , and Dumoulin, J. A. , 1998: Catalog of the historically active volcanoes of Alaska. U.S. Geological Survey Open File Report 98-582, Anchorage, Alaska, and Alaska Division of Geological and Geophysical Surveys, Fairbanks, Alaska, 104 pp.
- Mottershead, D. N. , 1989: Rates and patterns of bedrock denudation by coastal salt spray weathering: a seven‐year record. Earth Surface Processes and Landforms , 14: 383–398.
- Mulder, C. P. H. , and Keall, S. N. , 2001: Burrowing seabirds and reptiles: impacts on seeds, seedlings and soils in an island forest in New Zealand. Oecologia , 127: 350–360.
- Mulder, C. P. H. , Anderson, W. B. , Towns, D. R. , and Bellingham, P. J. (eds.), 2011: Seabird Islands: Ecology, Invasion, and Restoration. Oxford: Oxford University Press, 498 pp.
- Munilla, I. , Genovart, M. , Paiva, V. H. , and Velando, A. , 2016: Colony foundation in an oceanic seabird. PLOS One , 11: e0147222.
- Murie, O. J. , 1959: Fauna of the Aleutian Islands and Alaska Peninsula. North American Fauna , 61, 364 pp.
- Nechaev, V. A. , 1991: Birds of Sakhalin Island. Vladivostok: DVO AN SSSR, 748 pp. (in Russian).
- Nelson, E. W. , 1887: Report upon natural history collections made in Alaska between the years 1877 and 1881. Arctic Ser. Pub. No. 3, issued in connection with the Signal Service, U.S. Army. Washington, D.C.
- Piatt, J. F. , and Springer, A. M. , 2003: Advection, pelagic food webs, and seabird biogeography in Beringia. Marine Ornithology , 31: 141–154.
- Renner, H. M. , and Jones, I. L. , 2005: Mapping distribution and relative density of auklets at selected colonies on Hall and St. Matthew Islands, 2005. USFWS, Homer, Alaska, unpublished report, 25 pp.
- Renner, H. M. , and Renner, M. 2010: Counting the countless: estimating the number of least auklets attending the colony on St. George Island, Alaska. Western Birds , 41: 168–173.
- Renner, H. M. , and Reynolds, J. H. , 2006: Mapping Distribution and Relative Density of Nesting Least and Crested Auklets at Segula Island, Alaska, May–June 2006. Homer, Alaska: U.S. Fish and Wildlife Service, Alaska Maritime National Wildlife Refuge Report, AMNWR 06/08, 26 pp.
- Renner, H. M. , Renner, M. , Reynolds, J. H. , Harding, A. M. A. , Jones, I. L. , Irons, D. B. , and Byrd, G. V. , 2006: Colony mapping: a new technique for monitoring crevice-nesting seabirds. Condor , 108: 423–434.
- Renner, H. M. , Reynolds, J. H. , Sims, M. , and Renner, M. , 2011: Evaluating the power of surface attendance counts to detect long-term trends in populations of crevice-nesting auklets. Environmental Monitoring and Assessment , 177: 665–679.
- Renner, M. , Hunt, G. L., Jr. , Piatt, J. F. , and Byrd, G. V. , 2008: Seasonal and distribution patterns of seabirds along the Aleutian Archipelago. Marine Ecology Progress Series , 357: 301–311.
- Roberts, S. , Monien, P. , Foster, L. , Loftfield, J. , Hocking, E. , Schnetger, B. , Pearson, E. , Juggins, S. , Fretwell, P. , Ireland, L. , Ochyra, R. , Howarth, A. , Allen, C. , Moreton, S. , Davies, S. , Brumsack, H. , Bentley, M. J. , and Hodgson, D. , 2017: Past penguin colony responses to explosive volcanism on the Antarctic Peninsula. Nature Communications , 8: doi: http://dx.doi.org/10.1038/ncomms14914.
- Roby, D. D. , and Brink, K. L. , 1986: Decline of breeding least auklets on St. George Island, Alaska. Journal of Field Ornithology , 57: 57–59.
- Rybin, A. , Chibisova, M. , Webley, P. , Steensen, T. , Izbekov, P. , Neal C ., and Realmuto, V. , 2011: Satellite and ground observations of the June 2009 eruption of Sarychev Peak volcano, Matua Island, Central Kuriles. Bulletin of Volcanology , 73: 1377–1392.
- Scharf, L. , Williams, J. C. , and Thomson, G. L. , 1996: Biological monitoring in the central Aleutian Islands. USFWS, Adak, Alaska, unpublished report, 120 pp.
- Searing, C. F. , 1977: Some aspects of the ecology of cliff-nesting seabirds at Kongkok Bay, St. Lawrence Island, Alaska, during 1976. In Environmental Assessment of the Alaskan Continental Shelf. Annual Report. Boulder, Colorado: BLM, NOAA, OSEAP, 263–412.
- Sekercioglu, C. H. , 2006: Increasing awareness of avian ecological function. Trends in Ecology and Evolution , 21: 465–471.
- Sigurdsson, B. D. , and Magnússon, B. , 2009: Ecosystem respiration, vegetation development and soil nitrogen in relation to breeding density of seagulls on a pristine volcanic island, Surtsey, Iceland. Biogeosciences Discussions , 6: 8393–8409.
- Simons, F. S. , and Mathewson, D. E. , 1947: Geology of Great Sitkin Island. U.S. Geological Survey Alaskan Volcano Investigations Report 0002, p. 55–67.
- Smithsonian Institution , 1987: Atka. Scientific Event Alert Network Bulletin , 12(3): unpaginated.
- Springer, A. M. , and Roseneau, D. G. , 1985: Copepod-based food webs: auklets and oceanography in the Bering Sea. Marine Ecology Progress Series , 21: 229–237.
- Springer, A. M. , Piatt, J. F. , and van Vliet, G. , 1996: Seabirds as proxies of marine habitats in the western Aleutian arc. Fisheries Oceanography , 5: 45–55.
- Stabeno, P. J. , Schumacher, J. D. , and Ohtani, K. , 2003: The physical oceanography of the Bering Sea. In Laughlin, T. R. , and Ohtani, K. (eds.). Dynamics of the Bering Sea: a Summary of Physical, Chemical, and Biological Characteristics, and a Synopsis of Research on the Bering Sea. North Pacific Marine Science Organization (PICES), University of Alaska Sea Grant, AK-SG-99-03, 1–28.
- Stephensen, S. W. , and Irons, D. B. , 2003: Comparison of colonial breeding seabirds in the eastern Bering Sea and Gulf of Alaska. Marine Ornithology , 31: 167–173.
- Takahashi, H. , Barkalov, V. Y. , Gage, S. , Joneson, S. , Ilushko, M. , and Zhuravlev, Y. N. , 2002: A floristic study of the vascular plants of Raikoke, Kuril Islands. Acta Phytotaxonomica et Geobotanica , 53(1): 17–33.
- Talbot, S. S. , Talbot, S. L. , and Walker, L. R. , 2010: Post-eruption legacy effects and their implications for long-term recovery of the vegetation on Kasatochi Island. Arctic, Antarctic, and Alpine Research , 42: 285–296.
- Trenhaile, A. S. , 1987: The Geomorphology of Rock Coasts. Oxford: Oxford University Press, 384 pp.
- Trukhin, A. M. , 2006: The state of the seabird colony on Tyuleniy Island, Sea of Okhotsk, under raising number of seals. Russian Journal of Ornithology , 15(328): 794–798 (in Russian).
- Trukhin, A. M. , and Kuzin, A. E. , 1996: Long-standing dynamic of species composition and abundance of seabirds nesting on Tyuleniy Island (The Sea of Okhotsk). In Litvinenko, N. M. (ed.), Birds of the Wetlands of the Southern Russian Far East and their Protection. Vladivostok: Dalnauka, 214–221 (in Russian).
- Turner, L. M. , 1886: Part V. Birds. Contributions to the natural history of Alaska. Arctic series of publications in connection with the Signal Service, U.S. Army, No. 2. Washington, D.C., 115–196.
- U.S. Army , 1988: St. George, Alaska harbor dredging final detailed project report, 45 pp.
- USFWS , 2004: Beringian Seabird Colony Catalog—Computer database and Colony Status Record archives. U.S. Fish and Wildlife Service, Migratory Bird Management, Anchorage, Alaska 99503.
- Velizhanin, A. G. , 1971: Colonial seabirds of the Kuril Islands. Ph.D. thesis, Biological Institute SO AN SSSR, Novosibirsk, 249 pp. (in Russian).
- Velizhanin, A. G. , 1975: Seabird colonies of the Yamskiye Islands. Hunting and the Hunting Economy , 7: 18–19 (in Russian).
- Velizhanin, A. G. , 1977: New data on the seabirds of the Far East. Russian Journal of Zoology , 56(7): 1077–1083 (in Russian).
- Vyatkin, P. S. , 2000: Nest cadastre of colonial seabirds of the coasts of Koryak Highland and Eastern Kamchatka. In Poyarkov, N. D. (ed.), The Biology and Conservation of the Birds of Kamchatka , vol. 2. Moscow: Rosselkhozakademiya, 7–15 (in Russian).
- Walker, L. R. , and del Moral, R. , 2003: Primary Succession and Ecosystem Rehabilitation. Cambridge: Cambridge University Press, 456 pp.
- Walker, L. R. , Sikes, D. S. , DeGange, A. R. , Jewett, S. C. , Michaelson, G. , Talbot, S. L. , Talbot, S. S. , Wang, B. , and Williams, J. C. , 2013: Biological legacies: direct early ecosystem recovery and food web reorganization after a volcanic eruption in Alaska. Écoscience, 20: 240–251.
- Wardle, D. A. , Bellingham, P. J. , Fukami, T. , and Mulder, C. P. H. , 2007: Promotion of ecosystem carbon sequestration by invasive predators. Biology Letters , 3: 479–482.
- Waythomas, C. F. , Scott, W. E. , Prejean, S. G. , Schneider, D. J. , Izbekov, P. , and Nye, C. J. , 2010: The 7–8 August 2008 eruption of Kasatochi Volcano, central Aleutian Islands, Alaska. Journal of Geophysical Research , 15: B00B06, 23 pp. doi: http://dx.doi.org/10.1029/2010JB007437.
- Williams, J. C. , Byrd, G. V. , and Konyukhov, N. B. , 2003: Whiskered Auklets Aethia pygmaea, foxes, humans and how to right a wrong. Marine Ornithology , 31: 175–180.
- Williams, J. C. , Drummond, B. A. , and Buxton, R. T. , 2010: Initial effects of the August 2008 volcanic eruption on breeding birds and marine mammals at Kasatochi Island, Alaska. Arctic, Antarctic, and Alpine Research , 42: 306–314.
- Yakhontov, V. D. , 1974: Seabird colonies of Penzhinskaya Bay and their status. In Byome, R. L. , and Flint, V. E. (eds.), Proceedings of the 6th All-Union Ornithological Conference, pt. 1. Moscow: Moscow State University, 251–252 (in Russian).
- Yakhontov, V. D. , 1979: Birds of the Penzhina region. In Krechmar, A. V. , and Chernyavskiy, F. B. (eds.), Birds of the Northeast Asia. Vladivostok: DVNTs AN SSSR, 135–164 (in Russian).
- Zelenskaya, L. A. , 2009: The number and distribution of birds on Matykil Island (the Yamskiye Islands, the Sea of Okhotsk. Russian Journal of Zoology , 88(5): 546–555 (in Russian).
- Zubakin, V. A. , Kondratyev, A. Ya. , Konyukhov, N. B. , and Panov, E. N. , 1992: On the numbers of seabirds on the Big Diomede Island. In Kondratyev, A. Ya. (ed.), Study of Colonial Seabirds in the USSR. Magadan: IBPS DVO RAN, 12–13 (in Russian).
- Zwolicki, A. K. , Zmudczyńska-Skarbek, K. M. , Iliszko, L. , and Stempniewicz, L. , 2013: Guano deposition and nutrient enrichment in the vicinity of planktivorous and piscivorous seabird colonies in Spitsbergen. Polar Biology , 36: 363–372.