Abstract
Aim: The assessment of tumor response to therapy is of critical importance as it permits for a prospective end point evaluation and provides a guide to clinicians for making future treatment decisions. However, current practices in early evaluation of chemotherapy are insufficient. Amantadine is a substrate for SSAT-1. The present pilot study tests the hypothesis that SSAT-1 activity within the tumor, as measured by plasma acetylamantadine concentrations, can be used to monitor patient response to therapy. Results: In cases with evidence of disease response, there was a reduction in the plasma acetylamantadine concentration at 4 h by approximately 32%. There was a mean increase of approximately 34% at the 4 h collection in the nonresponders. Conclusion: Although large-scale studies are required these findings suggest that the amantadine test could allow for determination of the efficacy of therapeutic interventions earlier, providing an effective test to assess response to treatment and for better management of patients.
Lay abstract
Aim: It is very important to get early information on the effectiveness of tumor treatment such that clinicians have a better understanding and decide on the next treatment regimen. Current methods are not sufficient to assess whether chemotherapy is effective early during a treatment cycle. We have previously used the presence of the acetylated metabolite of the drug amantadine in urine of patients diagnosed with lung cancer as a biomarker for disease. In the present study, our goal was to test the hypothesis that tumor responsiveness to therapy could be assessed by monitoring changes in the levels of the acetylated form of amantadine in the blood during the course of treatment. Results: In 70% of the patients we were able to relate disease progression or remission/stability to the levels of the acetylated form of amantadine in the blood. Conclusion: Although a larger study with a greater number of patients is required, our test could be used as a simple and effective tool to assess response to treatment and to better tailor treatment of the patient as well as reduce side effects and costs.
Graphical abstract
According to the WHO, cancer is the second leading cause of death in the world. In 2018, cancer accounted for an estimated 9.6 million deaths, approximately 1 in 6 deaths. The most common types of cancer in men are lung, prostate, colorectal, stomach and liver cancer, while breast, colorectal, lung, cervical and thyroid cancer are the most common among women. Current projections predict a substantial increase in cancer rates in the general population over time. Currently, one out of four deaths in the USA is attributable to cancer, making it the second leading cause of death behind cardiac disease. It is expected to overtake cardiovascular disease as the leading cause of death within the next 10–15 years [Citation1]. While the American Cancer Society recommends that those over 40 years of age undergo a yearly cancer check-up, this recommendation is often not followed. Consequently, patients can present to their physicians with symptoms of cancer at a later stage of development and thus survival tends to be poorer [Citation2]. Chemotherapy is one of the main treatment choices for malignant disease at an advanced stage. The objectives are usually palliative in intent: that is, to maintain or improve symptoms and quality of life, with the additional benefit of improving survival expectation, although mostly falling short of cure. Evaluation of response is often challenging as it can require several months to assess regression using conventional techniques, which include physical examination, serial radiographic studies and/or conventional laboratory biochemistry studies. Indeed, the WHO, RECIST 1.0 and RECIST 1.1 criteria for end point evaluation, in other words, progression-free survival or overall survival has been suggested to be unsatisfactory [Citation3].
Pseudoprogression is considered as an unconventional imaging pattern of tumor response in which tumors initially exhibit features of progression, with a subsequent radiologic tumor response that is evident on serial imaging with sustained therapy [Citation4]. Some caution has to be exercised in the assessment of the treatment response in patients receiving immune checkpoint inhibitors [Citation4–6] as pseudoprogression is initially radiologically indistinguishable from true disease progression. In approximately 5% of patients diagnosed with non-small-cell lung cancer receiving anti-PD-1 therapy, radiologic pseudoprogression has been reported to occur [Citation7,Citation8], however; there is a paucity of information regarding the incidence of clinically suspected pseudoprogression [Citation9]. Indeed, pseudoprogression is a challenge for both radiologists and clinicians, as there are no valid biochemical or radiological markers that can help to differentiate between true progression or hyperprogression and pseudoprogression [Citation10]. While pseudoprogression can be radiographically observed in the first weeks after the start of treatment, it can also be seen even months after treatment initiation [Citation11].
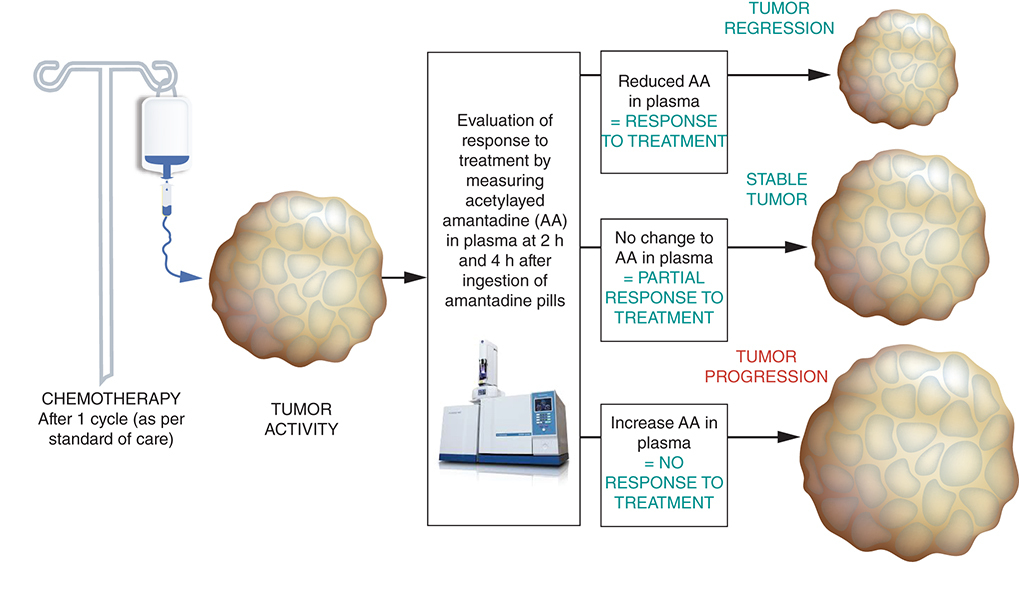
Changes in serum tumor markers provide early indications but are not available for many common types of cancer such as lung cancer. As a result, many patients endure side effects of therapy for months before clinicians are able to determine if therapy is being effective in achieving the intended therapeutic outcomes. Hence, there is a need for a marker or a new response evaluating method that can provide a better guide to assess response to treatment and to better tailor treatment in individual cases. Our team has designed a test which has the potential to enable earlier and accurate response to therapy in patients with advanced cancer. We discovered that the US FDA approved antiviral agent, amantadine, appears to be a specific substrate for acetylation by spermidine/spermine N1-acetyltransferase (SSAT-1) [Citation12,Citation13]. SSAT-1 activity in cancer cells is several-fold higher as compared with normal cells [Citation14]. Amantadine acetylation can be used to determine SSAT-1 cellular activity by determining excretion of acetylamantadine (AA) and may indicate the presence of cancer. A patent was granted in December 2004, with the claim that excretion of AA in urine will serve as a diagnostic screening test for cancer in humans [Citation15].
SSAT-1, the rate-limiting enzyme in the polyamine catabolic pathway, is widely distributed in mammalian tissues. It plays a regulatory role in spermidine and spermine homeostasis and normally is present in small amounts in mammalian cells [Citation16–18]. However, in cancer cells, there is increased expression of SSAT-1 preventing polyamine concentrations from reaching levels that would be toxic [Citation19]. Increased production of polyamines in cancer results in increased levels of polyamines and N1-acetylspermidine in urine, reflecting increased SSAT-1 activity [Citation20,Citation21]. Cancers with increased urinary N1-acetylspermidine include lung cancer, gastric carcinoma, ovarian cancer, acute myelocytic leukemia, lymphoma, breast cancer, liver cancer, renal cancer, colorectal cancer and prostate cancer [Citation21–25].
This pilot proof of concept study in lung cancer patients evolved from previous studies performed to determine the urinary levels of AA in normal healthy volunteers and in patients with a cancer diagnosis. Our goal was to test the hypothesis that downregulation of SSAT-1 activity as reflected by AA excretion and/or plasma levels of AA, will occur earlier than changes that can be detected by chest computed tomography (CT) used to evaluate efficacy of systemic therapy in patients with advanced lung cancer; this reduction in SSAT-1 activity would be subsequently related to a reduction in tumor volume. In essence, in this pilot study, we sought to determine if AA concentrations, as well as other associated metabolites, can be a prognostic biomarker for response to prediction of chemotherapy as well as a positive/negative indicator of the prognosis for patients with a lung cancer diagnosis, a disease for which reliable protein markers do not exist.
Materials & methods
Subjects & study design
Volunteers were informed that the study was examining the possibility that therapeutic response could be monitored by changes in urinary excretion of AA and that such changes would be present before response to therapy could be detected by conventional assessment methods. We recently conducted a pilot study exploring this hypothesis.
Eligibility: patients >18 years of age with biopsy-proven adenocarcinoma, squamous-cell carcinoma of the lung or advanced-stage small-cell lung cancer, who were being initiated on systemic chemotherapy, were eligible for this study provided they had hematologic, renal and hepatic function sufficient to tolerate conventional doses of systemic chemotherapy, measurable or evaluable disease by RECIST criteria and performance score <3 (using the Eastern Cooperative Oncology Group scale). Patients with prior surgery or radiation therapy were allowed, provided there was residual measurable or evaluable disease which had not been treated with radiation therapy. Patients with prior postoperative adjuvant therapy were also considered eligible for this trial.
Exclusion criteria: Alcohol consumption within 5 days, previous adverse reaction to amantadine, significant liver and kidney disease, chronic drug therapy other than oral contraceptives, patients currently pregnant or lactating. Patient volunteers were asked to fast overnight prior to the day of their first scheduled chemotherapy and to ingest an oral dose of 200 mg of amantadine HCl in the morning within an hour of arriving in clinic (C1). Urine and blood specimen was collected between 2 and 4 h after amantadine ingestion. The sampling protocol was repeated 3 weeks later prior to the second scheduled cycle of chemotherapy (C2).
Statistical considerations
This is a preliminary pilot assessment study. A total of 20 patients – ten with adenocarcinoma of the lung and ten with small-cell histology – should be sufficient to assess a signal of sensitivity to justify future larger-scale studies and is based on an expected response rate to conventional systemic therapy of 70% for small-cell lung cancer and 40% for adenocarcinoma.
Analytical procedures
Plasma and urine were analyzed for AA by validated GLP-compliant HPLC methods using d3-AA as the internal standard for quantitation at Biopharmaceuticals Research Inc. (BC, Canada) as described elsewhere [Citation14,Citation26,Citation27]. Health Canada authorized Biomark AA assay standard under application number: 229838 on 7 October 2014 (investigational testing authorization).
Chemotherapy regimen & clinical assessment
There was no restriction of selection of the systemic therapy choice for patient treatment. Regimens included cis-platin + pemetrexed (1 case), carboplatin + pemetrexed (4 cases), cis-platin + etoposide (1 case), carboplatin + etoposide (1 case) and pembrolizumab (1 case). The clinical assessment consisted of a follow-up clinical exam and CT scans, which were performed after 3 months of therapy.
Results
We present data following interim analysis. The patient demographics as well as the type and staging of lung cancer are shown in . Males represented 71% (5/7) of the study cohort and there were 29% (2/7) cases with diagnosed small-cell lung cancer (SCLC) and 71% (5/7) with diagnosed adenocarcinoma. Two out of the seven cases were diagnosed with stage III lung cancer, while the remainder were diagnosed with stage IV of the disease. It should be noted that some of the patients were unable to void and thus a complete urine collection could not be performed. However, a near complete blood sample was attained, which allowed for analysis of plasma AA concentrations. Accordingly, shows the plasma AA concentrations between 2 and 4 h after amantadine ingestion and prior to initial chemotherapy (C1) and prior to second scheduled cycle of chemotherapy (C2). In addition, assessment of disease progression/remission was undertaken for correlative purposes. It can be seen from that in two patients (BMRT-04 and BMRT-06) a reduction in the 4 h plasma concentration of 37 and 29%, respectively, was associated with partial disease remission. A decrease (33%) in the 4 h plasma concentration of AA was also observed in BMRT-03, but with an initial stable disease assessment. In contrast, in two patients (BMRT-05 and BMRT-07), disease progression was associated with an increase in the 4 h plasma concentration of 42 and 27%, respectively. While a decrease in the 4 h plasma AA concentration was observed in two patients (BMRT-01 and BMRT-08), clinical assessment revealed progression of disease. summarizes the net change in the 4 h plasma AA concentration in responders and nonresponders to initial cycle of chemotherapy. It can be seen that a decrease (~32%) in the mean value of the 4 h plasma AA concentration in the responders (BMRT-04, BMRT-04 and BMRT-06) was observed, while an increase (~34%) in the mean value of the 4 h plasma AA concentration in the nonresponders (BMRT-05 and BMRT-07) was observed.
Table 1. Patient demographics, type of lung cancer and staging.
Table 2. Plasma acetylamantadine concentration and disease assessment of lung cancer patients subsequent to one cycle of chemotherapy.
Table 3. Plasma acetylamantadine concentration in responders and nonresponders to initial cycle of chemotherapy.
Discussion
Despite the development of new cancer therapeutic agents as well as imaging modalities, the evaluation of the response to chemotherapy remains insufficient [Citation3]. During the first few months of treatment the standard monitoring therapeutic efficacy consisting of serial CT scans may not provide clear clinical guidance. Misinterpretation of scans can lead to inappropriate discontinuation of a potentially effective therapy; conversely, an ineffective treatment could be continued hoping for a delayed response that may never come. A situation that could be further confounded with additional chest CT scans and difficult with limited availability of and access to these examinations. A biomarker with rapid kinetics could offer an earlier indication of treatment efficacy to help clarify therapeutic/management decisions in such cases. Further, subsequent to our test, treatment could be tailored more efficiently, which would have a significant impact on quality of life. Also, since monthly treatment costs are estimated to be between $1000 and $12,000, depending on the types of drugs used; targeting the right drug earlier could lead to cost savings. From a clinical perspective, the development of a reliable test that can assess the effects of chemotherapy and can be performed earlier in the treatment cycle would be highly beneficial as it would avoid prolonged use and toxicity in patients and thus assist in the decision to modify the treatment regimen. Furthermore, assessing response early means security in knowing that the treatment is having the desired effect. Alternatively, early evidence of lack of response allows transition to second-line options for systemic therapy which could then be initiated without delay. Delays in transitioning treatment means a greater burden of disease and reduced ability of patients to tolerate second-line systemic therapy. Thus, researchers, clinicians and experts within the field need to direct their attention to identify reliable, reproducible and cost-effective measures to evaluate response to chemotherapy that can be implemented worldwide.
Currently there are no blood or serum biomarkers employed routinely to assess treatment response in lung cancer. Circulating (or cell-free) tumor DNA (ctDNA, cfDNA) holds promise as a cancer biomarker. Its utility in monitoring therapeutic response has been explored for various treatment modalities, including immunotherapy. Because cfDNA can be distinguished based on the presence of tumor-specific somatic mutations, it is expected to have greater specificity than other serum protein markers [Citation28]. Despite the promise of immunotherapy, the drugs are not effective for all patients. Indeed, recently, it was reported that only 13% of patients who receive checkpoint inhibitors actually benefit from the much-heralded treatment [Citation29,Citation30].
This pilot study was undertaken to determine whether amantadine metabolism to AA can detect changing activity of SSAT-1 that is present in malignancy during a chemotherapy regimen. This study is the first to report the ability to detect and quantify plasma concentrations of AA. The data obtained demonstrate a potential correlation between acetylation of amantadine and the response to the initial cycle of systemic chemotherapy in treatment of lung cancer. It is interesting to note that the two patients that responded to the initial chemotherapy regimen, which was associated with a reduction in the plasma AA concentration, were diagnosed with stage III lung cancer, while the two patients deemed as nonresponders to initial chemotherapy, were diagnosed with stage IV lung cancer.
The concept of ‘stable disease’ is important. If the disease appears stable, it reflects some kind of response to the therapy as the disease would be expected to progress if it were not responding. In addition, this category includes both light responders (less than 30% of response) and light nonresponders (less than 30% of progression) which could explain the high variability expected from this group. Based on RECIST criteria, responses were categorized as complete response, partial response and stable disease, which are in decreasing importance with respect to clinical benefit. In contrast, the category of disease progression is distinct, has the worst prognosis meaning treatment is not being effective. A mixed response is a category where uncertainty remains.
It is pointed out that there were no apparent toxicities or side effects identified with the addition of amantadine to systemic therapy in this study. This observation provides the basis for a larger study to generate more information regarding types of cancer and response. The literature reports a high rate of hypermetabolism in lung cancer, although different histologies and subtypes (adenocarcinomas and neuroendocrine tumors) of lung cancers have differing levels of hypermetabolism. Indeed, high variability would be expected in the metabolism in SCLC and in pulmonary carcinoids, as would also occur in adenocarcinoma with pure lepidic predominant pattern versus solid predominant pattern; possibilities that warrants further investigation. Advanced lung cancer is frequently associated with cachexia, which may have an impact. We propose to extend the study to determine the impact of comorbid disease on the assay. Overall, in our pilot study and interim analysis, in five out of seven patients we were able to correlate plasma AA concentration to clinical outcome. In conclusion, a larger trial is justified to validate the present observation and explore the impact on different lung cancer histologies and the various systemic therapy regimens.
Conclusion & future perspective
It is well understood that biomarkers that detect cancer and monitor response to treatment will be highly beneficial. The findings of this preliminary study have identified a possible test that could be used for monitoring patients after chemo-radiation therapy or curative surgery to evaluate disease/tumor status. Our test for response evaluation following chemotherapy in lung cancer patients could be used as an adjunct or prior to imaging modalities [Citation31]. The test also has the potential to detect proliferation of new cancer cells (relapse). Small-cell is a high-grade tumor with a high proliferation rate. Some other lung cancer types (adeno, squamous, etc.) may be lower grade, lower proliferation/metabolism. Thus, it is possible that a better correlation with the aggressivity of the tumor, in other words, the proliferation rate and plasma or urinary levels of AA may exist. This warrants further investigation.
In this preliminary study and interim analysis, a reduction in the plasma concentration of acetylamantadine (AA) was associated with disease remission subsequent to initial chemotherapy in patients diagnosed with stage III lung cancer.
Conversely, an increase in the plasma concentration of AA was associated with disease progression subsequent to initial chemotherapy in patients diagnosed with stage IV lung cancer.
Disease status and plasma AA concentration was evident in 5/7 (71%) patients.
While a decrease in the plasma AA concentration was observed in the three other patients, clinical assessment revealed stable disease or progression of disease.
The amantadine test could potentially serve as a novel, simple to use and low-cost test for assessing the response to treatment or surgical removal of tumor.
These possibilities warrant future large-scale studies.
Author contributions
DS Sitar, AW Maksymiuk and RA Bux designed the study, were involved in the data analysis, contributed to writing the paper and had primary responsibility for final content as well as procuring financial support for the clinical studies. DS Sitar and AW Maksymiuk were also responsible for the development of the overall research plan. AW Maksymiuk and P Joubert provided medical oversight for the clinical study at the Canada site. PS Tappia wrote the initial draft of the paper and was, in part, responsible for regulatory approvals and study implementation. DS Sitar was involved in the development of the LC/MS/MS assay for detection of acetylated amantadine. D Moyer and G Huang served as liaison between BioMark and the analytical laboratory. D Miller participated in the data analysis and writing of the manuscript. B Ramjiawan provided the expertise for regulatory and institutional review board approvals and contributed to the design of the study. All authors approved the manuscript.
Clinical trial data disclosure
The authors certify that this manuscript reports original clinical trial data. The data will be made publicly available at NIH Clinicaltrials.gov website (identifier: NCT02331290) when the study is completed.
Ethical conduct of research
The authors state the study was approved by the University of Manitoba Research Ethics Board (ethics file #: HS18750:B2014:136). Additionally, this study was conducted under Good Clinical Practice and Good Laboratory Practice conditions in accordance with the standards established by the Canadian Tri-Council Policies and the International Conference on Harmonization (ICH). The study protocol was reviewed and approved by Health Canada (file # HC6-24-c124939) and it is listed on the NIH Clinicaltrials.gov website (identifier: NCT02331290). Volunteers were informed that the study was examining the possibility that therapeutic response could be monitored by changes in urinary excretion of AA and that such changes would be present before response to therapy could be detected by conventional assessment methods.
Financial & competing interests disclosure
RA Bux is the President and CEO of BioMark Diagnostics Inc. D Moyer is Clinical Research Manager at BioMark Diagnostics Inc. G Huang is Project Director at BioMark Diagnostics Inc. AW Maksymiuk, DS Sitar, H Bach, PS Tappia and B Ramjiawan are minor shareholders of BioMark Diagnostics Inc. This study was supported, in part, by Biomark Diagnostics Inc. (BC, Canada), Maunders-McNeil Foundation (AB, Canada) and the University of Manitoba. Biopharmaceutical Research Inc. (BC, Canada) completed the LC/MS/MS analyses. Infrastructural support was provided by the St Boniface Hospital Foundation and the University of Manitoba. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- YoungRC. Cancer statistics, 2002: progress or cause for concern?CA Cancer J. Clin.52(1), 6–7 (2002).
- SmithRA , CokkinidesV , von EschenbachACet al.American Cancer Society guidelines for the early detection of cancer. CA Cancer J. Clin.52(1), 8–22 (2002).
- HwangK-E , KimH-R. Response evaluation of chemotherapy for lung cancer. Tuberc. Respir. Dis.80, 136–142 (2017).
- WolchokJD , HoosA , O'DaySet al.Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res.15, 7412–7420 (2009).
- HoosA , EggermontAM , JanetzkiSet al.Improved endpoints for cancer immunotherapy trials. J. Natl Cancer Inst.102, 1388–1397 (2010).
- ChiouVL , BurottoM. Pseudoprogression and immune-related response in solid tumors. J. Clin. Oncol.33, 3541–3543 (2015).
- GettingerSN , HornL , GandhiLet al.Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol.33, 2004–2012 (2015).
- BorghaeiH , Paz-AresL , HornLet al.Nivolumab versus docetaxel in advanced non squamous non-small-cell lung cancer. N. Engl. J. Med.373, 1627–1639 (2015).
- KatzSI , HammerM , BagleySJet al.Radiologic pseudoprogression during anti-PD-1 therapy for advanced non-small-cell lung cancer. J. Thorac. Oncol.13(7), 978–986 (2018).
- HochmairMJ , SchwabS , BurghuberOC , KrenbekD , ProschH. Symptomatic pseudo-progression followed by significant treatment response in two lung cancer patients treated with immunotherapy. Lung Cancer113, 4–6 (2017).
- KongBY , MenziesAM , SaundersCABet al.Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment Cell Melanoma Res.29(5), 572–577 (2016).
- BrasAP , HoffHR , AokiFY , SitarDS. Amantadine acetylation may be affected by acetyltransferases other than NAT1 or NAT2. Can. J. Physiol. Pharmacol.76(7–8), 701–706 (1998).
- BrasAP , JanneJ , PorterCW , SitarDS. Spermidine/spermine N(1)-acetyltransferase catalyzes amantadine acetylation. Drug Metab. Dispos.29(5), 676–680 (2001).
- MaksymiukAW , SitarDS , AhmedRet al.Spermidine/spermine N1-acetyltransferase-1 (SSAT-1) as a diagnostic biomarker in human cancer. Future Sci. OA4(10), FSO345 (2018).
- SitarDS , BrasAP: US6,811,967B2B2 Method for assaying nonspermine/spermidine activity of spermidine/spermine N1-acetyltransferase (SSAT). (2004)
- MatsuiI , WiegandL , PeggAE. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J. Biol. Chem.256(5), 2454–2459 (1981).
- PeggAE , SeelyJE , PösöH , della RagioneF , ZagonIA. Polyamine biosynthesis and interconversion in rodent tissues. Fed. Proc.41(14), 3065–3072 (1982).
- SeilerN. Functions of polyamine acetylation. Can. J. Physiol. Pharmacol.65(10), 2024–2035 (1987).
- BettuzziS , DavalliP , AstancolleSet al.Tumor progression is accompanied by significant changes in the levels of expression of polyamine metabolism regulatory genes and clusterin (sulfated glycoprotein 2) in human prostate cancer specimens. Cancer Res.60(1), 28–34 (2000).
- RussellDH. Increased polyamine concentrations in the urine of human cancer patients. Nat. New Biol.233(39), 144–145 (1971).
- SuhJW , LeeSH , ChungBC , ParkJ. Urinary polyamine evaluation for effective diagnosis of various cancers. J. Chromatogr. B Biomed. Sci. Appl.688(2), 179–186 (1997).
- TakenoshitaS , MatsuzakiS , NakanoGet al.Selective elevation of the N1-acetylspermidine level in human colorectal adenocarcinomas. Cancer Res.44(2), 845–847 (1984).
- KingsnorthAN , WallaceHM. Elevation of monoacetylated polyamines in human breast cancers. Eur. J. Cancer Clin. Oncol.21(9), 1057–1062 (1985).
- PineMJ , HubenRP , PeggAE. Production of N1-acetyl spermidine by renal cell tumors. J. Urol.141(3), 651–655 (1989).
- SessaA , PerinA. Increased synthesis of N1-acetylspermidine in hepatic preneoplastic nodules and hepatomas. Cancer Lett.56(2), 159–163 (1991).
- MaksymiukAW , TappiaPS , SitarDSet al.Use of amantadine as substrate for SSAT-1 activity as a reliable clinical diagnostic assay for breast and lung cancer. Future Sci. OA5(2), FSO365 (2018).
- TappiaPS , MaksymiukAW , SitarDSet al.Predictive value and clinical significance of increased SSAT-1 activity in healthy adults. Future Sci. OA5(7), FSO400 (2019).
- GoldbergSB , NarayanA , KoleAJet al.Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin. Cancer Res.24(8), 1872–1880 (2018).
- KhanM , LinJ , LiaoGet al.Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small-cell lung cancer. Medicine (Baltimore)97(33), e11936 (2018).
- SchmidS , FruhM. Immune checkpoint inhibitors and small-cell lung cancer: what's new?J. Thorac. Dis.10(Suppl. 13), S1503–S1508 (2018).
- TrinidadLópez C , DeLa Fuente Aguado J , OcaPernas Ret al.Evaluation of response to conventional chemotherapy and radiotherapy by perfusion computed tomography in non-small-cell lung cancer (NSCLC). Eur. Radiol. Exp.3(1), 23 (2019).
